Abstract
Background
Independent and valid prognostic predictors for locally advanced gastric cancer (LAGC) patients with non-elevated serum tumor markers (Triple-negative: CA199 < 37U/ml, CEA < 5 µg/ml and CA125 < 35U/ml) before and after neoadjuvant chemotherapy (NACT) remain unclear.
Methods
A total of 352 LAGC patients treated with NACT(NLAGC) from two centers were included. Of the 156 were Triple-negative patients. CA72-4 trajectory groupings was defined as longitudinal changes in CA72-4 levels before and after NACT to identify different potential subgroups and to compare recurrence-free survival (RFS) and overall survival (OS) among subgroups. The predictive performance of the nomogram-trajectory was evaluated using the area under the receiver operating characteristic curve(AUC), decision curve analysis, and C-index.
Results
In the Triple-negative patients, the Stable group had significantly worse 3-year OS than the Normal, Elevated, and Descend groups(3-year OS: 53.9% vs. 77.9% vs. 73.5% vs. 87.7%;P = 0.002). Cox multivariate analysis showed that CA72-4 trajectory groupings (Stable group: HR:3.442, 95%CI[1.574–7.528], P = 0.002) was an independent prognostic risk factor. In addition, the C-index and AUC values based on the nomogram-trajectory were significantly higher than those of ypTNM staging (C-index: 0.788 vs. 0.719,P < 0.001;AUC: 0.800 vs. 0.667,P < 0.001). Furthermore, The survival analysis revealed that the 3-year OS of the Low-Risk group of nomogram scores was significantly better than that of the High-Risk group(3-year OS:84.7% vs. 29.1%). And the Low-Risk group had the lower cumulative risk of recurrence (P < 0.001).
Conclusion
The CA72-4 trajectory groupings were an independent prognostic factor for NLAGC Triple-negative patients. The predictive efficacy of the Nomogram-trajectory was significantly better than the ypTNM.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12937-9.
Keywords: Gastric cancer, Neoadjuvant chemotherapy, Triple-negative, Tumor marker, Independent predictor
Introduction
Gastric cancer (GC) is the fourth leading cause of cancer-related deaths worldwide [1], yet the 5-year survival rate for patients treated with surgery alone is only 20–30% [2]. Previously, the MAGIC trial [3] and the FLOT4 trial [4] demonstrated that perioperative chemotherapy as part of GC treatment resulted in better overall survival (OS). Similar results were obtained during the FNCLCC and FFCD multicenter phase III trial [5] in 2011. Thus, surgical resection combined with perioperative systemic chemotherapy has become the standard treatment option for LAGC.
The 8th edition of the AJCC staging system for gastric cancer primarily stratifies patients based on the depth of tumor invasion in the gastric wall (T stage) and the number of positive lymph nodes (N stage) [6]. However, the current prognostic assessment of patients with NLAGC remains challenging [7]. The existing ypTNM staging system has limitations in predicting prognosis in the neoadjuvant chemotherapy population [8]. This staging system focuses solely on the pathological findings of the tumor and does not account for tumor markers, which reflect residual tumor burden and can significantly impact prognosis. Therefore, its predictive value is limited for patients receiving neoadjuvant chemotherapy.
Previous studies [9–13] have shown that gastric cancer patients with elevated tumor markers have worse postoperative survival. For instance, Kambara Y et al. found that the recurrence-free survival (RFS) and OS in patients with stage III GC with CA19-9 ≥ 46.3 U/mL were significantly lower than those with CA19-9 < 46.3 U/mL [10]. Meanwhile, Zhang et al. [14] found that early postoperative normalization of CEA or CA19-9 was an important prognostic indicator for stage N3 GC. They also observed that elevated levels of tumor markers in the early postoperative period correlated with a worse prognosis in patients with N3a and N3b stage GC. Such findings have also been confirmed in the population of NLAGC patients [15–18]. Sun et al. [15] found that high preoperative serum CA19-9 levels in NLAGC patients were associated with a relatively higher risk of death and that high pre-neoadjuvant CEA levels (> 50 ng/mL) were indications of clinical disease progression after neoadjuvant chemotherapy. In a study by xiao-Huan Tang et al., they found that elevated CEA /CA19-9 levels before or after NACT were significantly associated with prognosis, while patients with normalized CEA/CA19-9 also showed longer survival than non-neoadjuvant patients.
Previous studies have shown that compared to imaging changes, tumor marker changes provide a more sensitive assessment of neoadjuvant therapy efficacy in patients. These marker changes typically precede imaging changes by 2–3 months [19], and thus, they are valuable in the prognostic assessment of NLAGC patients. Currently, three tumor markers, carcinoembryonic antigen (CEA), carbohydrate antigen (CA19-9), and carbohydrate antigen (CA 125), are routinely measured in GC patients in Eastern and Western countries to evaluate tumor progression and patient prognosis. However, for Triple-negative patients( normal levels of traditional tumor markers [CA19-9, CEA, CA125]), the normalization of these traditional tumor marker levels make it challenging to provide robust and straightforward prognostic information. Previous studies have shown that CA72-4, as a biomarker, also has significant predictive value for postoperative survival in patients with various malignancies such as gastric cancer, colorectal cancer, and pancreatic cancer [20–25]. However, most of these studies focus on non-neoadjuvant populations, with relatively limited exploration of prognosis in neoadjuvant patients. Additionnally, the current studies about CA72-4 on gastric malignant tumors mainly focus on Eastern countries, with approximately 81% from China and 98% from East Asia [20], while it is rarely applied in clinical practice in Western countries. Its prognostic predictive value in such special populations (Triple-negative patients) with normal CA19-9, CEA, and CA125 before and after NACT has not been reported.
Therefore, considering the dynamic impact of chemotherapy on tumor burden during neoadjuvant chemotherapy, relying solely on preoperative or postoperative CA72-4 levels may not fully capture the true prognostic status of neoadjuvant patients. We explored the predictive prognostic value of CA72-4 trajectory grouping metrics for Triple-negative NLAGC patients based on data from two large tertiary care medical centers in China. We also developed a Nomogram model based on CA72-4 trajectory groupings to further improve the prognostic prediction accuracy and provide a reference for individualized follow-up and treatment of patients.
Materials and methods
Study population
In this study, we prospectively collected and retrospectively analyzed the clinicopathological data of 414 patients diagnosed with locally advanced gastric cancer who underwent D2 radical gastrectomy after neoadjuvant chemotherapy. The data were gathered from January 2016 to December 2021 at Fujian Medical University Union Hospital and from December 2017 to March 2020 at Zhangzhou Hospital of Fujian Medical University. The inclusion criteria were as follows: (1) cT2-4NxM0 before NACT; (2) absence of prior malignant tumors; (3) no distant metastasis or neighboring organ invasion; (4) D2 radical gastrectomy after NACT. The exclusion criteria included: (1) prior gastrectomy; (2) acute cardiovascular events (e.g., cerebral or coronary artery injury) within three months; (3) history of emergency surgery; and (4) insufficient clinical or follow-up data. After applying the exclusion criteria, a total of 352 patients were included in this study (eFig. 1). The protocol for the present study was approved by the Research Ethics Board (REB) of Fujian Medical University Union Hospital and Zhangzhou Affiliated Hospital of Fujian Medical University.
Perioperative chemotherapy regimens and surgical intervention
In this retrospective cohort study, the perioperative chemotherapy (NACT + AC) regimens include (1) platinum-based regimens (platinum + capecitabine/platinum + S-1/platinum + 5-FU); (2) paclitaxel regimens (paclitaxel + capecitabine/paclitaxel + S-1/paclitaxel + 5-FU); and (3) other regimens (paclitaxel + platinum/single-agent S-1, and so on). The above neoadjuvant / adjuvant chemotherapy regimens and dose have been previously reported [26]. All perioperative chemotherapy regimens and dosages were formulated by experienced oncologists at each center and adjusted accordingly based on tumor response and adverse reactions to chemotherapy drugs. The surgical method was GC resection combined with D2 lymph node dissection, and the extent of the lymphadenectomy was determined based on the Japanese GC treatment guidelines 2014 (version 4th).
Data collection and definition
Tumor marker data from the databases of the two hospitals were collected for this study. Hematological test data were obtained from the analysis of serum samples by the laboratory departments of the two centers using a fully automated immunochemical analysis system. Routine blood tests, including CEA, CA19-9, CA125, and CA72-4, were conducted within 7 days before the initiation of NACT and within 7 days before the surgery of patients. Based on clinical reference, the upper limit of the tumor markers (CEA, CA19-9, and CA125) were 5 ng/mL, 37 U/mL, and 35 U/mL, respectively [27].
We defined patients in the Triple-negative group as those who exhibited expression levels of all three markers below the established thresholds before and after neoadjuvant chemotherapy, whereas patients with secretion values of one or more tumor markers (CA19-9, CEA, CA125) above the upper limit before and after neoadjuvant chemotherapy were included in the Positive group.
Tumor regression grade (TRG) refers to the pathological response to neoadjuvant chemotherapy. Tumor (T) and node (N) stages were classified based on the 8th edition of the AJCC TNM staging system [6].
Follow-up and primary outcomes
Follow-up appointments were conducted every 3 months for the first 2 years, every 6 months for the subsequent 3 years, and once per year after year 5. Follow-up was conducted both online and in person, and it included physical examinations, laboratory (CA72-4, CEA, CA19-9, CA125, AFP, blood routine and biochemistry), and imaging tests (gastrointestinal endoscopy, abdominal computed tomography). The primary outcome was OS. OS was defined as the time from surgery to death from any cause or last follow-up. Recurrence was determined by medical history, physical examination, imaging, cytology, and tissue biopsy. RFS was defined as the time from surgery to recurrence due to tumor or last follow-up.
Statistical analysis
SPSS version 25 (IBM, Armonk, NY, USA) and R version 4.3.3 (http://www.r-project.org) were utilized for correlation analysis. The “ggcoxzph” function was employed to visualize the relationship between continuous variables and survival time, determining if these variables could be stratified by cutoff values. Normally distributed continuous variables were expressed as mean ± standard deviation (SD); otherwise, they were expressed as median (interquartile range). The optimal breakpoints for predicting endpoint events were obtained using X-tile software or R. Categorical variables were analyzed using the chi-square or Fisher’s exact test and expressed as percentages. X-tile, proposed by Camp et al. from Yale University [28], calculates statistical outcomes for different CA72-4 cutoff values using the Kaplan-Meier method and employs the Log-Rank test to select the CA72-4 threshold that maximizes the difference between groups. A heatmap was used to assess the correlation between factors through color coding. The Cox proportional hazards model identified independent risk factors for long-term prognosis, including all variables with P < 0.05 from the univariate analysis in the multivariate analysis. The performance of developed models was assessed using the area under the receiver operating characteristic curve (AUC), time-dependent ROC curves, calibration curves, and decision curve analysis. Survival outcomes were described using the Kaplan–Meier method, and differences between curves were analyzed using the log-rank test.
Results
Group-based categorization of trajectory of CA72-4 levels
The optimal cut-off value (3.7 U/mL) for the serum tumor marker CA72-4 was determined before neoadjuvant chemotherapy using X-tile software (eFig. 2 A–2 C). Based on the measurements of serum CA72-4 before and after neoadjuvant chemotherapy, the CA72-4 trajectory groupings were categorized into (1) Normal group: consistently below the cutoff value; (2) Elevated group: below the cutoff value before neoadjuvant chemotherapy and above the cutoff value after neoadjuvant chemotherapy; (3) Descend group: above the cutoff value before neoadjuvant chemotherapy and below the cutoff value after neoadjuvant chemotherapy; (4) Stable group: consistently above the cut-off value (eFig. 3).
Clinicopathological characteristics
A total of 352 patients were enrolled in this study, of which 156 patients (44.3%) were in the NLAGC Triple-negative group and 196 patients (56.7%) were in the Positive group. eTable 1 contains a comparison of baseline data between the two groups of patients. No statistically significant differences were observed during the comparison of clinicopathologic data between the two groups, except for the pre-NACT CA72-4 levels (Pre-NACT CA72-4: <3.7 U/L vs. ≥ 3.7 U/mL, P < 0.001) and group-based of the trajectory of CA72-4 levels (Normal group vs. Elevated group vs. Descend group vs. Stable group, P = 0.005).
Table 1 contains the baseline characteristics of the four trajectory groups of the Triple-negative group, with 80 (51.3%) in the Normal group, 25 (16.0%) in the Elevated group, 22 (14.1%) in the Descend group, and 29 (18.6%) in the Stable group. Except for statistical differences in sex (P = 0.034), no significant differences were observed among the 4 groups in age, ECOG scale, ypT, ypN, tumor location, lymphovascular invasion, neurological invasion, TRG grade, NACT regimens, NACT cycle, adjuvant chemotherapy regimens, adjuvant chemotherapy administration status, postoperative complications, length of hospital stay, operative time, and intraoperative blood loss (all P > 0.05).
Table 1.
Baseline comparisons in the Triple-negative group (grouped according to the group-based trajectory of CA72-4)
| characteristic | Total | Normal N = 80 |
Elevated N = 25 |
Descend N = 22 |
Stable N = 29 |
p-value1 |
|---|---|---|---|---|---|---|
| Age, n(%) | 0.127 | |||||
| ≤ 60 | 63(40.4) | 31(38.8) | 14(56) | 5(22.7) | 13(44.8) | |
| > 60 | 93(59.6) | 49(61.3) | 11(44) | 17(77.3) | 16(55.2) | |
| Sex, n(%) | 0.034 | |||||
| Male | 112(71.8) | 56(70) | 18(72) | 21(95.5) | 17(58.6) | |
| Female | 44(28.2) | 24(30) | 7(28) | 1(4.5) | 12(41.4) | |
| ECOG scores, n(%) | 0.995 | |||||
| 0 | 97(62.2) | 50(62.5) | 15(60) | 14(63.6) | 18(62.1) | |
| 1/2 | 59(37.8) | 30(37.5) | 10(40) | 8(36.4) | 11(37.9) | |
| ypT, n(%) | 0.058 | |||||
| 0/1 | 24(15.4) | 12(15) | 9(36) | 3(13.6) | 0 | |
| 2 | 33(21.2) | 16(20) | 2(8) | 6(27.3) | 9(31) | |
| 3 | 60(38.4) | 33(41.3) | 7(28) | 8(36.4) | 12(41.4) | |
| 4 | 39(25) | 19(23.7) | 7(28) | 5(22.7) | 8(27.6) | |
| ypN, n(%) | 0.774 | |||||
| 0 | 62(39.8) | 31(38.8) | 11(44) | 10(45.5) | 10(34.5) | |
| 1 | 36(23.1) | 20(25) | 6(24) | 6(27.3) | 4(13.8) | |
| 2 | 30(19.2) | 17(21.2) | 3(12) | 3(13.6) | 7(24.1) | |
| 3 | 28(17.9) | 12(15) | 5(20) | 3(13.6) | 8(27.6) | |
| Location, n(%) | 0.560 | |||||
| Upper | 65(41.7) | 35(43.8) | 9(36) | 7(31.8) | 14(48.3) | |
| Middle | 37(23.7) | 16(20) | 6(24) | 8(36.4) | 7(24.1) | |
| Lower | 43(27.6) | 26(32.5) | 7(28) | 5(22.7) | 5(17.3) | |
| Mixed | 11(7) | 3(3.7) | 3(12) | 2(9.1) | 3(10.3) | |
| Lymphovascular Invasion, n(%) | 0.388 | |||||
| No | 96(61.5) | 53(66.3) | 16(64) | 13(59.1) | 14(48.3) | |
| Yes | 60(38.5) | 27(33.7) | 9(36) | 9(40.9) | 15(51.7) | |
| Neural Invasion, n(%) | 0.400 | |||||
| No | 68(43.6) | 33(41.3) | 13(52) | 12(54.5) | 10(34.5) | |
| Yes | 88(56.4) | 47(58.7) | 12(48) | 10(45.5) | 19(65.5) | |
| TRG grade, n(%) | 0.625 | |||||
| 0/1 | 39(25) | 18(22.5) | 8(32) | 7(31.8) | 6(20.7) | |
| 2/3 | 117(75) | 62(77.5) | 17(68) | 15(68.2) | 23(79.3) | |
| NACT regimens, n(%) | 0.995 | |||||
| Platinum based | 64(41) | 32(40) | 11(44) | 8(36.4) | 13(44.8) | |
| Paclitaxel based | 63(40.4) | 33(41.3) | 10(40) | 9(40.9) | 11(37.9) | |
| Others | 29(18.6) | 15(18.7) | 4(16) | 5(22.7) | 5(17.3) | |
| NACT cycles, Median (IQR) | 4(3–4) | 4(3–4) | 3(3–4) | 4(3–4) | 3(3–4) | 0.735 |
| AC regimes, n(%) | 0.986 | |||||
| Platinum based | 55(41.4) | 27(40.9) | 11(45.8) | 7(33.3) | 10(45.4) | |
| Paclitaxel based | 52(39.1) | 26(39.4) | 9(37.5) | 9(42.9) | 8(36.4) | |
| Others | 26(19.5) | 13(19.7) | 4(16.7) | 5(23.8) | 4(18.2) | |
| AC, n(%) | 0.178 | |||||
| No | 19(12.2) | 11(13.8) | 1(4) | 1(4.5) | 6(20.7) | |
| Yes | 137(87.8) | 69(86.3) | 24(96) | 21(95.5) | 23(79.3) | |
| Complication, n(%) | 0.349 | |||||
| No | 140(89.7) | 75(93.8) | 22(88) | 19(86.4) | 24(82.8) | |
| Yes | 16(10.3) | 5(6.3) | 3(12) | 3(13.6) | 5(17.2) | |
| LOS, Median (IQR) | 8(7–10) | 8(7–10) | 8(7–9) | 8(7–9) | 9(7–12) | 0.282 |
| Operative time, Median (IQR) |
185 (157–220) |
190 (160–225) |
180 (150–210) |
199 (165–210) |
180 (155–221) |
0.277 |
| Bleed, Median (IQR) | 35(30–50) | 35(30–50) | 30(30–50) | 40(30–50) | 30(30–60) | 0.861 |
1: One-way ANOVA; Pearson’s Chi-squared test; Fisher’s exact test
Bold values indicated that the P value < 0.05
Abbreviation ECOG score: Eastern Cooperative Oncology Group (ECOG) Performance Status score; ypT: Tumor (T pathological stage) after neoadjuvant chemotherapy; ypN: Nodes pathological stage after neoadjuvant chemotherapy; TRG: Tumor Regression Grade; NACT: Neoadjuvant chemotherapy; CA72-4: Carbohydrate antigen 72 − 4; LOS: length of stay
Survival outcomes of triple-negative and positive patients
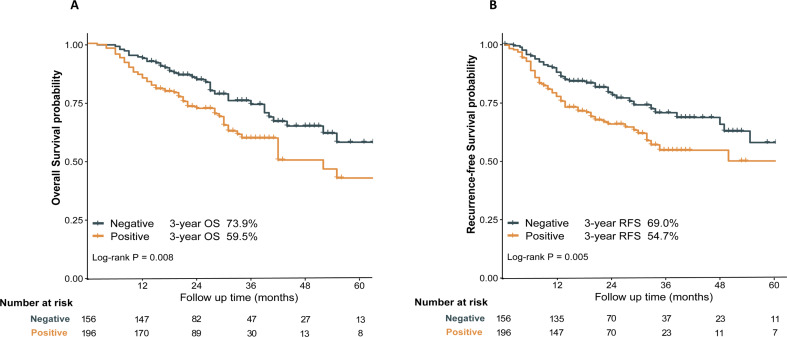
The median follow-up in this multicenter cohort study was 34.9 months, 95% CI [32.3–37.4]. A total of 107 deaths were recorded during this follow-up period, including 39 deaths in the Triple-negative group and 68 deaths in the Positive group. Kaplan–Meier curve analysis showed that the 3-year OS of patients in the Triple-negative group was significantly better than that of the Positive group (3-year OS: 73.9% vs. 59.5%, P = 0.008) (Fig. 1A). Similarly, in terms of RFS, the 3-year RFS of patients in the Triple-negative group was significantly better than that of the Positive group (3-year RFS: 69.0% vs. 54.7%, P = 0.005) (Fig. 1B).
Fig. 1.
Overall survival (A), recurrence-free survival (B) between Triple-negative and Positive groups
Correlation analysis and oncological outcomes of group-based trajectories in triple-negative patients
Heatmap displays some visual correlations among variates (eFig. 4) and Pearson correlation analysis revealed that high positive correlation between the pre-NACT CA72-4 and the group-based of trajectory of CA72-4 levels (r = 0.925, p < 0.001) and moderate positive correlation between post-NACT CA72-4 and Group-based of the trajectory of CA72-4 levels (r = 0.661, p < 0.001)(eTable 5). However, no statistically significant correlations were observed between the group-based of the trajectory of CA72-4 levels and other prognostically relevant clinicopathological features (All P > 0.05).
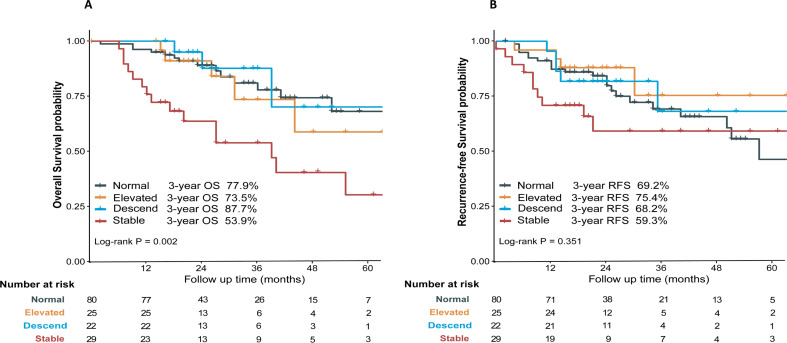
Analysis of the correlation between OS/RFS and dynamic changes in CA72-4 trajectories in the Triple-negative group showed that the 3-year OS of patients in the Stable group was significantly lower than that of patients in the Normal, the Elevated, and Descend groups (3-year OS: 53.9% vs. 77.9% vs. 73.5% vs. 87.7%, respectively; P = 0.002), whereas no statistically significant difference was observed in the comparison of 3-year OS between the Normal, Elevated, and Descend groups (all P > 0.05) (Fig. 2A). Meanwhile, no statistically significant difference in RFS was observed between any of the CA72-4 trajectory subgroups and the 3-year postoperative RFS of patients (3-year RFS: Normal vs. Elevated vs. Descend vs. Stable, 69.2% vs. 75.4% vs. 68.2% vs. 59.3%, respectively, P = 0.351) (Fig. 2B).
Fig. 2.
Kaplan–Meier Survival curves of overall survival/recurrence-free survival based on the group-based trajectory of CA72-4 levels
Univariate and multivariate analyses of factors associated with overall survival and recurrence-free survival in triple-negative patients
Univariate Cox regression analysis revealed that pre-NACT CA72-4, post-NACT CA72-4, and group-based of trajectory of CA72-4 levels were prognostic factors for determining OS of patients in the Triple-negative group. In the Cox multivariate analysis of model I, after including only pre-NACT CA72-4 and post-NACT CA72-4 levels, we found that pre-NACT CA72-4 (≥ 3.7: HR: 1.408, 95%CI [0.686–2.889], P = 0.351) and post-NACT CA72-4 levels (≥ 3.7: HR: 1.843, 95%CI [0.848–4.007], P = 0.123) were not independent prognostic risk factors for OS of patients in the Triple-negative group; contrastingly, in Model II, when only the group-based of trajectory of CA72-4 levels was included, we found that the CA72-4 trajectory subgroup (Stable: HR: 3.442, 95%CI [1.574–7.528], P = 0.002), ECOG scores (1/2: HR: 2.048, 95%CI [1.052–3.985], P = 0.035), ypT (ypT3: HR: 8.714, 95%CI [2.261–33.591], P = 0.001), ypT4 (HR: 9.121, 95%CI [2.623–31.719], P = 0.002), and ypN (ypN3: HR: 2.866, 95%CI [1.155–7.108], P = 0.023) were independent prognostic risk factors for OS of patients in the Triple-negative group (Table 2 ).
Table 2.
Univariate and multivariate analyses of factors associated with overall survival in the Triple-negative group
| characteristic | Overall Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis I a | Multivariable analysis IIb | |||||||
| HR 1 | 95%CI 1 | P-value | HR 1 | 95%CI 1 | P-value | HR 1 | 95%CI 1 | P-value | |
| Age | |||||||||
| ≤ 60 | REF | ||||||||
| > 60 | 1.011 | 0.535–1.910 | 0.973 | ||||||
| Sex | |||||||||
| male | REF | ||||||||
| female | 0.974 | 0.473–2.007 | 0.943 | ||||||
| ECOG scores | |||||||||
| 0 | REF | REF | REF | ||||||
| 1/2 | 2.034 | 1.070–3.868 | 0.030 | 1.984 | 1.018–3.870 | 0.044 | 2.048 | 1.052–3.985 | 0.035 |
| ypT | |||||||||
| 0–2 | REF | REF | REF | ||||||
| 3 | 6.362 | 2.048–19.767 | 0.001 | 8.202 | 2.150-32.284 | 0.002 | 8.714 | 2.261–33.591 | 0.001 |
| 4 | 7.367 | 1.954–27.784 | 0.003 | 8.447 | 2.483–28.734 | 0.001 | 9.121 | 2.623–31.719 | 0.002 |
| ypN | |||||||||
| 0 | REF | REF | REF | ||||||
| 1 | 1.077 | 0.409–2.839 | 0.881 | 1.227 | 0.435–3.458 | 0.699 | 1.326 | 0.464–3.791 | 0.599 |
| 2 | 2.829 | 1.197–6.687 | 0.018 | 2.665 | 1.092-6.500 | 0.031 | 2.399 | 0.974–5.909 | 0.057 |
| 3 | 3.632 | 1.532–8.614 | 0.003 | 2.928 | 1.189–7.209 | 0.019 | 2.866 | 1.155–7.108 | 0.023 |
| Location | |||||||||
| upper | REF | ||||||||
| middle | 0.866 | 0.428–1.967 | 0.825 | ||||||
| lower | 0.689 | 0.441–2.255 | 0.994 | ||||||
| mixed | 0.978 | 0.412–2.338 | 0.974 | ||||||
| LVI | |||||||||
| no | REF | ||||||||
| yes | 1.470 | 0.769–2.810 | 0.244 | ||||||
| NI | |||||||||
| no | REF | ||||||||
| yes | 1.213 | 0.634–2.319 | 0.560 | ||||||
| TRG grade | |||||||||
| 0/1 | REF | REF | REF | ||||||
| 2//3 | 1.644 | 0.766–3.532 | 0.202 | 1.196 | 0.495–2.889 | 0.691 | 0.989 | 0.382–2.558 | 0.982 |
| Post-AC | |||||||||
| no | REF | ||||||||
| yes | 0.602 | 0.280–1.296 | 0.195 | ||||||
| Postoperation complication | |||||||||
| no | REF | REF | REF | ||||||
| yes | 2.491 | 1.177–5.273 | 0.017 | 2.061 | 0.902–4.711 | 0.086 | 2.055 | 0.906–4.664 | 0.085 |
| CA72-4-related Ia | |||||||||
| Pre-NACT CA72-4 | |||||||||
| < 3.7 | REF | REF | NA | NA | NA | ||||
| ≥ 3.7 | 2.126 | 1.116–4.053 | 0.022 | 1.408 | 0.686–2.889 | 0.351 | NA | NA | NA |
| Post-NACT CA72-4 | |||||||||
| < 3.7 | REF | REF | NA | NA | NA | ||||
| ≥ 3.7 | 2.482 | 1.298–4.744 | 0.006 | 1.843 | 0.848–4.007 | 0.123 | NA | NA | NA |
| CA72-4-related IIb | |||||||||
| Group-based trajectory of CA72-4, n(%) | |||||||||
| normal | REF | NA | NA | NA | REF | ||||
| elevated | 1.247 | 0.449–3.466 | 0.672 | NA | NA | NA | 1.543 | 0.528–4.513 | 0.428 |
| descend | 0.819 | 0.235–2.852 | 0.753 | NA | NA | NA | 0.751 | 0.201–2.809 | 0.670 |
| stable | 3.443 | 1.659–7.145 | 0.001 | NA | NA | NA | 3.442 | 1.574–7.528 | 0.002 |
1:HR = Hazard Ratio, CI = Confidence Interval
Bold values indicated that the P value < 0.05
a: model I: Including only pre-NACT CA72-4 and post-NACT CA72-4 levels
b: model II: Only the group-based of trajectory of CA72-4 levels was included
Abbreviation REF: Reference; ECOG score: Eastern Cooperative Oncology Group (ECOG) Performance Status score; ypT: Tumor (T pathological stage) after neoadjuvant chemotherapy; ypN: Nodes pathological stage after neoadjuvant chemotherapy; TRG: Tumor Regression Grade; NACT: Neoadjuvant chemotherapy; CA72-4: Carbohydrate antigen 72 − 4
The Cox univariate and multivariate regression analyses associated with RFS are shown in eTable 2. The ECOG scores (1/2: HR: 2.599, 95%CI [1.332–5.074], P = 0.005), ypT (HR: 2.874, 95%CI [1.030–8.023], P = 0.044), ypT4 (HR: 3.681, 95%CI [1.153–11.754], P = 0.028) was an independent prognostic risk factor for postoperative RFS of patients in the Triple-negative group, whereas pre-NACT CA72-4, post-NACT CA72-4, and group-based of trajectory of CA72-4 levels were not associated with patient postoperative RFS (all P > 0.05).
Nomogram construction and performance comparison
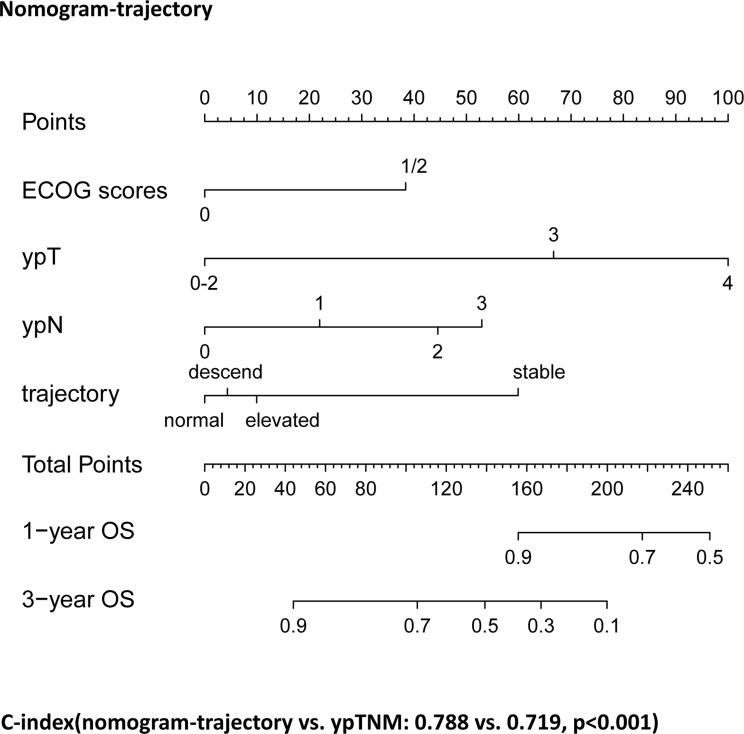
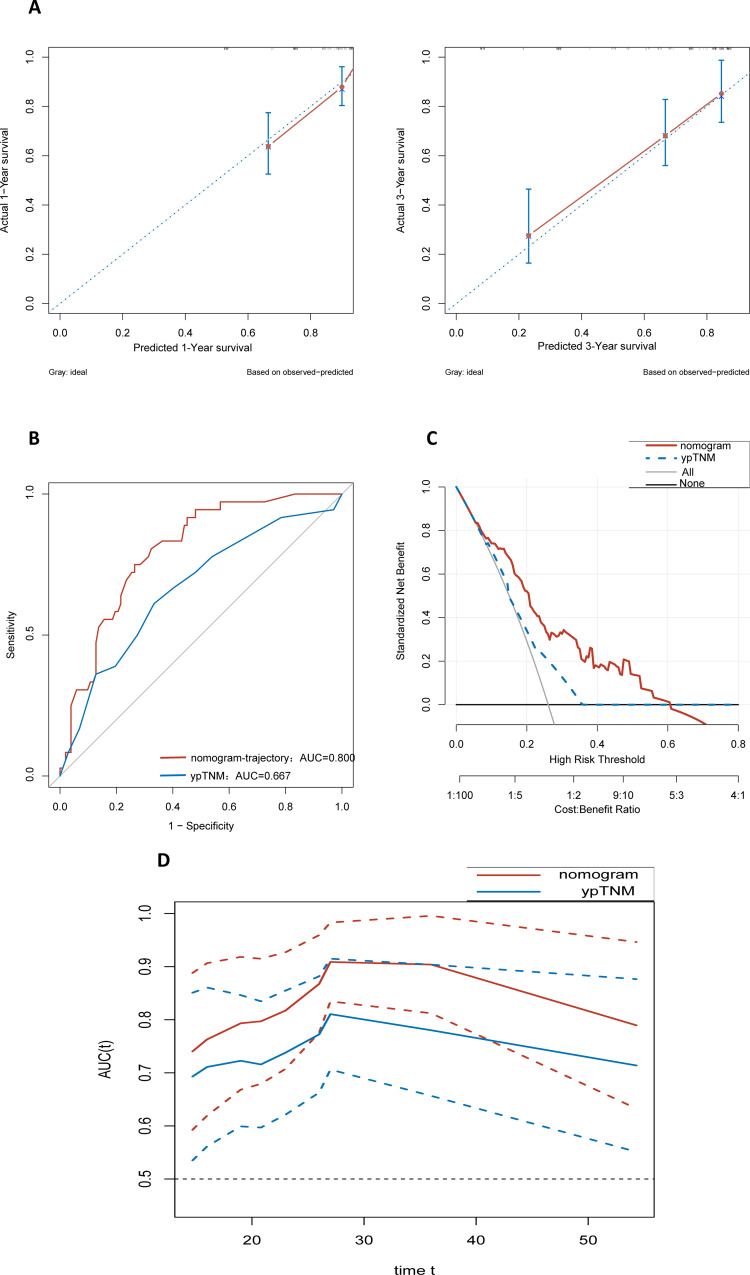
Based on the results of the multivariate Cox regression analysis of model II, we developed a nomogram (Nomogram-trajectory) that incorporated the ECOG scores, ypT, ypN, and group-based of trajectory of CA72-4 levels to predict the postoperative long-term OS of patients in the Triple-negative group who underwent both NACT and surgery (Fig. 3). We plotted calibration curves for 1- and 3-year predictions using the Nomogram-trajectory to evaluate the calibration of this model. The subsequent analysis revealed that the actual curve aligned with the virtual curve, demonstrating a high degree of consistency (Fig. 4A).
Fig. 3.
Establishment of Nomogram-trajectory
Fig. 4.
Nomogram-trajectory performance evaluation: the calibration curve of the Nomogram-trajectory prediction model for assessing the accuracy and consistency of the prediction model (A), 3-year area under the receiver operating characteristic curve values and C-index of the Nomogram-trajectory prediction model and traditional ypTNM staging (B), decision curve analysis curve of Nomogram-trajectory vs. ypTNM (C), time-dependent receiver operating characteristic (Nomogram-trajectory vs. ypTNM) (D)
Additionally, the results indicate that both the C-index and AUC values of the nomogram-trajectory significantly surpass those of the traditional ypTNM staging (C-index: Nomogram-trajectory vs. ypTNM: 0.788 vs. 0.719, P < 0.001; AUC: Nomogram-trajectory vs. ypTNM: 0.800 vs. 0.667, P < 0.001) (Fig. 4B). Decision curve analysis showed that within the most reasonable threshold probability range (0.1–0.6), the overall net benefit of Nomogram-trajectory was greater than that of ypTNM staging (Fig. 4C), indicating that Nomogram-trajectory is better at predicting OS compared to the traditional ypTNM staging.
The time-dependent ROC curves showed that the AUC values of the Nomogram-trajectory were superior than those of the traditional ypTNM staging within 0–60 months ( Fig. 4D ).
Effect of overall mortality risk score on the overall survival in Triple-negative patients
The overall mortality risk score (OMRS) for individual patients was calculated by summing the scores for each variable in the column-line graph (eTable 3). The OMRS ranges from 0 to 251. The optimal breakpoint of OMRS for predicting the endpoint event using the maxstat function in R is 153 (eFig. 5 A). Patients in the Triple-negative group with OMRS scores <153 were defined as the Low-Risk group (Nomogram-low), and patients with OMRS scores ≥ 153 were defined as the High-Risk group (Nomogram-high). Kaplan–Meier analysis showed that the 3-year OS was significantly better in the Low-Risk group than in the High-Risk group (3-year OS: Low-Risk group vs. High-Risk group: 84.7% vs. 29.1%, P < 0.001), and the cumulative risk of recurrence was also significantly lower in the Low-Risk group than that in the High-Risk group (P < 0.001) (eFig. 5B-5 C).
Discussion
To the best of our knowledge, this multicenter retrospective cohort study is the first to investigate the impact on the long-term prognostic predictors of the NLAGC Triple-negative (normal levels of traditional tumor markers [CA19-9, CEA, CA125]) patients. Through our study, we found that the group-based trajectory of CA72-4 levels before and after neoadjuvant chemotherapy were independent prognostic factors for postoperative OS in Triple-negative patients with NLAGC. In addition, the 3-year OS of patients was significantly worse in the Stable group, with CA72-4 levels consistently higher than 3.7 U/mL. The Nomogram-trajectory prediction model was developed based on the group-based trajectory of CA72-4 levels, and it demonstrated superior prognostic efficacy over the existing ypTNM stage.
The high expression of serum CA72-4, a high molecular weight mucin (220–400 kDa), usually implies the persistence and proliferation of tumor cells [20]. Previously, many scholars have investigated and discovered the outstanding predictive value of serum CA72-4 as a biomarker for assessing postoperative survival in patients with non-neoadjuvant gastrointestinal malignancies [20–23]. M J Gaspar et al. [29] found that the incidence of CA72-4 positivity was higher in patients with advanced tumors (p = 0.04), lymph node invasion (p = 0.02), hepatic metastases (p = 0.02), and peritoneal involvement (p = 0.03), and the risk of death in patients with elevated levels of serum CA72-4 was 4.2 times higher than that of patients with low levels of serum CA72-4. In addition, a post hoc analysis based on the correlation between serum CA72-4 and GC in the Chinese population, conducted by Chen XZ et al., showed that when serum CA72-4 alone was used to diagnose GC, the sensitivity and specificity of CA72-4 were 49% and 96%, respectively, which were better than those of other tumor markers [30]. Through further studies, Tong et al. found that CA72-4 before and after NACT was an independent prognostic factor for neoadjuvant patients with locally advanced GC, and it could be used to predict ypN and ypTNM staging in patients with locally advanced GC who underwent NACT and radical resection [18].
Since the Triple-negative NLAGC population is rarer than the elevated tumor marker population, and there is a lack of reports exploring the prognosis of these patients. A Mata analysis [19] by the Japanese Gastric Cancer Association found that the positive rate of CA72-4 in patients with LAGC was as high as 86.3% in patients with stage III-IV progressive gastric cancer. In addition, another prospective study [31] recruited 66 gastric cancer patients (including 27 patients with stage I and II gastric cancer, and the rest with stage III and IV gastric cancer), and found that there was a statistically significant difference in the preoperative CA72-4 positivity rate between patients with early gastric cancer (stage I and II) and those with advanced gastric cancer (stage III and IV) (0% vs. 71.8%). Therefore, serum CA72-4 may be a potential prognostic predictor in the Triple-negative NLAGC population.
Meanwhile, to assess whether the continuous variables in this study could be stratified by cutoff values, we plotted the residuals by using the “ggcoxzph” function to visualize the relationship between these variables and survival time. The output showed that the residuals for age, Pre-CA72-4, and Post-CA72-4 were roughly horizontally distributed over time, with non-significant p-values, indicating that the proportional hazards assumption of the Cox model was met(eFig. 6 A-6 C). However, when we used X-tile software to obtain the optimal cutoff value of CA72-4, we found that the optimal cutoff value of CA72-4 was 3.7 U/mL, which was significantly lower than the currently existing upper limit value of CA72-4 of 6.9 U/mL in China. The reason may be that the Triple-negative population of patients with advanced gastric cancer has its own lower tumor markers produced during tumor progression compared with positive patients, thus leading to the emergence of a lower upper limit value of CA72-4 would be a better prediction of survival prognosis in such patients. However, the specific pathologic mechanisms still need to be discovered by further research.
Meanwhile, to rule out the possibility that different neoadjuvant chemotherapy regimens could cause varying levels of CA72-4, we compared the baseline characteristics of different neoadjuvant regimens (eTable 4) and found that the different treatment regimens did not result in significant differences in CA72-4 expression among LAGC patients (P = 0.640). Additionally, there were no significant statistical differences in other clinicopathological data between the groups (All P > 0.05).
Furthermore, we found that neither pre- nor post-NACT CA72-4 alone was an independent prognostic factor for the OS and RFS in NLAGC Triple-negative patients. Whereas when evaluating the dynamic changes in CA72-4 trajectories before and after NACT, we found that the 3-year OS of patients in the Stable group with consistently elevated CA72-4 levels was significantly worse (3-year OS: Normal vs. Elevated vs. Descend vs. Stable, 53.9% vs. 77.9% vs. 73.5% vs. 87.7%, respectively, P = 0.002). The possible reason is that: The CA72-4 levels in the Stable Group remained consistently higher than those in the Elevated Group both before and after neoadjuvant chemotherapy (NACT)(eFig.7). This suggests that the Stable Group may have greater tumor invasiveness, which could be associated with a poorer prognosis. Conversely, the Elevated Group, with lower initial CA72-4 levels, indicates a lighter tumor burden before treatment and relatively better overall condition. However, due to poorer sensitivity to chemotherapy, these patients might experience an increase in tumor burden post-treatment, leading to a less favorable prognosis. Despite this, the initially lower tumor burden may offer some prognostic advantage compared to the Stable Group. In contrast, the Stable Group’s already high CA72-4 levels prior to neoadjuvant therapy indicate a higher baseline tumor burden and more aggressive tumor biology. The persistently high CA72-4 levels post-treatment suggest a poor response to chemotherapy, combining high initial tumor burden with inadequate treatment response, which results in a worse prognosis for the Stable Group compared to the Elevated Group. Further analysis revealed that the CA72-4 trajectory subgroup was an independent prognostic risk factor for the OS of patients in the Triple-negative group with NLAGC, an observation that has not been previously reported.
Considering that the traditional AJCC 8th edition staging system only considers the pathological results of the tumor but ignores the prognostic impact of tumor markers and other indicators reflecting the tumor load. Zhong et al. also indicates that the current ypTNM staging system has limitations in predicting prognosis for patients undergoing neoadjuvant chemotherapy [32]. Relying solely on ypTNM staging may lead to significant prognostic deviations for these patients. To address this limitation, many studies have attempted to integrate tumor burden indicators with traditional staging systems. As shown in the study by Ruopeng Zhang et al. and corroborated by our previous research, integrating tumor markers with the traditional AJCC 8th edition staging system significantly improves prognostic accuracy compared to the conventional staging system alone [26, 33] Based on this, in this study, we developed a column-line graphical model (Nomogram-trajectory) based on the ECOG scores, ypT, ypN, and group-based trajectory of CA72-4 levels to predict postoperative long-term OS of patients in the Triple-negative group undergoing neoadjuvant therapy and surgery. We found that Nomogram-high (High-Risk) and Nomogram-low (Low-Risk) scores correlated with the postoperative survival benefit of the patients. The 3-year OS of patients in the Low-Risk group was significantly better than that of patients in the High-Risk group (3-year OS: Low-Risk group vs. High-Risk group: 84.7% vs. 29.1%, P < 0.001). The nomogram prediction model developed based on the CA72-4 trajectory grouping demonstrated more robust and superior predictive performance in predicting the OS of patients compared with ypTNM staging. Therefore, the Nomogram-trajectory model can offer individualized risk assessment, guide postoperative follow-up, enhance prognostic accuracy, and enable dynamic monitoring and treatment adjustments, providing potential reference for physicians in making scientifically sound and precise individualized treatment plans in patient management.
Our study had several limitations. First, this study was a multicenter retrospective study, there could still be an inevitable selection bias. Second, since the tumor markers before and after neoadjuvant (CEA, CA125 and CA199) both negative were rare with LAGC patients, the results still need a larger prospective, multicenter clinical study for further validation. Third, the use of different neoadjuvant chemotherapy regimens could impact the study results. Although no differences were observed after comparing baseline data, the study did not assess the toxicity of the chemotherapy regimens, the timing of administration, or the potential impact of dose reductions. Fourth, this study cohort comprised an Eastern population of NLAGC patients, and further research is needed to determine the efficacy of the developed nomogram model in Western populations. Nonetheless, this study is the first to explore the independent prognostic indicators affecting NLAGC Triple-negative patients and highlight the potential predictive efficacy of dynamic change of CA72-4 levels in this population.
Conclusion
The group-based of trajectory of CA72-4 levels before and after NACT is an independent prognostic risk factor for patients with Triple-negative GC. The predictive efficacy of the nomogram-trajectory developed is significantly better than that of the existing ypTNM stage system. Therefore, the group-based of trajectory of CA72-4 levels before and after NACT may be an effective tool for clinicians to make clinical decisions about NLAGC Triple-negative patients. However, the results need to be further validated in prospective multicenter clinical studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients, their families and all investigators involved in the present study.
Author contributions
Xie JW and Huang CM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Zheng HL, Zhang LK, and Lv CB contributed equally to this work and should be considered co-first authors. Concept and design: Zhang LK, Huang CM, and Xie JW. Acquisition, analysis, or interpretation of data: Zheng HL, Zhang LK, Lv CB and Xu BB. Drafting of the manuscript: Zheng HL, Zhang LK, Huang CM and Xie JWStatistical analysis: Zheng HL, Zhang LK, Huang CM and Xie JW. Administrative, technical, or material support: Lin JX, Zheng CH, and Xie JW. Supervision: Zheng HL,Huang CM and Xie JW. Manuscript preparation: All authors. Manuscript editing: All authors. Manuscript review: All authors. Additional contributions: We thank who have devoted a lot to this study, including nurses, pathologists, further-study doctors, statisticians, reviewers and editors. Thanks for Feng-Qiong Liu, Experimental Center of School of Public Health, Fujian Medical University.
Funding
This research was funded by Fujian Provincial Medical “Building High-level Hospitals, High-level Clinical Medical Centers and Key Clinical Specialty Projects” ([2021] No. 76).
Data availability
The dataset generated for this current study are not publicly available due additional research questions to be answered, but is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Association Declaration of Helsinki- ethical principles for medical research, and and the study obtained approval from the Independent Ethics Committee of the Fujian Medical University Union Hospital and Zhangzhou Affiliated Hospital of Fujian Medical University. All patients and their legal guardians gave their informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ling-Kang Zhang, Hua-Long Zheng and Chen-Bin Lv contributed equally to this work and should be considered co-first authors.
Contributor Information
Chang-Ming Huang, Email: hcmlr2002@163.com.
Jian-Wei Xie, Email: xjwhw2019@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000;26(4):243–55. [DOI] [PubMed] [Google Scholar]
- 3.2022;33:1005–20. 5. diagnosis, treatment and follow-up. Ann Oncol, Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. 6. [DOI] [PubMed]
- 4.Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fuorouracil plus leucovorin, oxaliplatin, and docetaxel versus fuorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57. [DOI] [PubMed] [Google Scholar]
- 5.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, In H, Sano T, et al. Stomach. In: Amin MB, editor. AJCC Cancer Staging Manual. 8th ed. New York: Springer-; 2016. [Google Scholar]
- 7.Tsagkalidis V, Blaszczyk MB, In H. Interpretation of Tumor Response Grade following preoperative therapy for gastric Cancer: an overview. Cancers (Basel). 2023;15(14):3662. Published 2023 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y, Li T, Liang X, et al. Association of Adjuvant Chemotherapy with Survival in patients with stage II or III gastric Cancer. JAMA Surg. 2017;152(7):e171087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng K, Yang L, Hu B, et al. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS ONE. 2015;10:e0124151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kambara Y, Miyake H, Nagai H, et al. CA19-9 is a significant prognostic marker of patients with stage III gastric cancer. Eur J Surg Oncol. 2020;46:1918–24. [DOI] [PubMed] [Google Scholar]
- 11.Shibata C, Nakano T, Yasumoto A, et al. Comparison of CEA and CA19-9 as a predictive factor for recurrence after curative gastrectomy in gastric cancer. BMC Surg. 2022;22:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song YX, Huang XZ, Gao P et al. Clinicopathologic and prognostic value of serum carbohydrate antigen 19 – 9 in gastric cancer: a meta-analysis. Dis Markers. 2015; 2015:549843. [DOI] [PMC free article] [PubMed]
- 13.Ren G, Li R, Zheng G, et al. Prognostic value of normal levels of preoperative tumor markers in colorectal cancer. Sci Rep. 2023;13(1):22830. Published 2023 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Qu H, Sun G et al. Early postoperative tumor marker responses provide a robust prognostic indicator for N3 stage gastric cancer. Medicine (Baltimore). [DOI] [PMC free article] [PubMed]
- 15.Sun Z, Zhang N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J Surg Oncol. 2014;12:397. Published 2014 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu C, Zhang Y, Xu J et al. Prognostic significance of serum tumor marker normalization in the perioperative period for patients with advanced gastric cancer. Ann Transl Med. [DOI] [PMC free article] [PubMed]
- 17.Tang XH, Wu XL, Gan XJ, et al. Using normalized Carcinoembryonic Antigen and Carbohydrate Antigen 19 to predict and monitor the efficacy of Neoadjuvant Chemotherapy in locally advanced gastric Cancer. Int J Mol Sci. 2023;24(15):12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong Y, Zhao Y, Shan Z, Zhang J. CA724 predicts overall survival in locally advanced gastric cancer patients with neoadjuvant chemotherapy. BMC Cancer. 2021;21(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17(1):26–33. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Zhang P, Zhang K, Huang C. The application of CA72-4 in the diagnosis, prognosis, and treatment of gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188634. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, Zhao X, Hu J, et al. Single-nanoparticle Differential Immunoassay for Multiplexed Gastric Cancer Biomarker Monitoring. Anal Chem. 2022;94(37):12899–906. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Li S, Wei L, Liang X, Zhang H, Liu J. The correlation between pre-operative serum tumor markers and lymph node metastasis in gastric cancer patients undergoing curative treatment. Biomarkers. 2013;18(7):632–7. [DOI] [PubMed] [Google Scholar]
- 23.Wu T, Wang CH, Wang W, Liu LL, Yun JP, Zhou ZW. Association of preoperative and postoperative CA72-4 with gastric cancer outcome. J Surg Oncol. 2021;123(8):1699–707. [DOI] [PubMed] [Google Scholar]
- 24.Kuang J, Gong Y, Xie H, et al. The prognostic value of preoperative serum CA724 for CEA-normal colorectal cancer patients. PeerJ. 2020;8:e8936. Published 2020 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, Zhu Y, Liu L. CA724 is a novel factor for predicting the unresectability in pancreatic adenocarcinoma. Int J Clin Exp Pathol. 2015;8(11):15112–7. Published 2015 Nov 1. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LK, Zheng HL, Zheng HH, et al. Effects of tumor marker regression load score on long-term prognosis of gastric cancer patients undergoing radical surgery after neoadjuvant chemotherapy. Eur J Surg Oncol. 2024;50(6):108367. [DOI] [PubMed] [Google Scholar]
- 27.Zheng CY, Wu J, Chen CS, et al. A scoring model for predicting early recurrence of gastric cancer with normal preoperative tumor markers: a multicenter study. Eur J Surg Oncol. 2023;49(11):107094. [DOI] [PubMed] [Google Scholar]
- 28.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9. [DOI] [PubMed] [Google Scholar]
- 29.Gaspar MJ, Arribas I, Coca MC, Díez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19 – 9 and CA 72 – 4 in gastric carcinoma. Tumour Biol. 2001;22(5):318–22. [DOI] [PubMed] [Google Scholar]
- 30.Chen XZ, Zhang WK, Yang K, et al. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep. 2012;39(9):9031–9. [DOI] [PubMed] [Google Scholar]
- 31.Cidón EU, Bustamante R. Gastric cancer: tumor markers as predictive factors for preoperative staging. J Gastrointest Cancer. 2011;42(3):127–30. [DOI] [PubMed] [Google Scholar]
- 32.Zhong Q, Chen QY, Parisi A, et al. Modified ypTNM staging classification for gastric Cancer after Neoadjuvant Therapy: a multi-institutional study. Oncologist. 2021;26(1):e99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang R, Chen X, Chen G, et al. Combined use of tumor markers in gastric Cancer: a Novel Method with Promising Prognostic Accuracy and Practicality. Ann Surg Oncol. 2023;30(13):8561–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated for this current study are not publicly available due additional research questions to be answered, but is available from the corresponding author on reasonable request.