Abstract
The pathogenesis of human immunodeficiency virus type 1 (HIV-1)-associated dementia (HAD) is mediated mainly by mononuclear phagocyte (MP) secretory products and their interactions with neural cells. Viral infection and MP immune activation may affect leukocyte entry into the brain. One factor that influences central nervous system (CNS) monocyte migration is matrix metalloproteinases (MMPs). In the CNS, MMPs are synthesized by resident glial cells and affect the integrity of the neuropil extracellular matrix (ECM). To ascertain how MMPs influence HAD pathogenesis, we studied their secretion following MP differentiation, viral infection, and cellular activation. HIV-1-infected and/or immune-activated monocyte-derived macrophages (MDM) and human fetal microglia were examined for production of MMP-1, -2, -3, and -9. MMP expression increased significantly with MP differentiation. Microglia secreted high levels of MMPs de novo that were further elevated following CD40 ligand-mediated cell activation. Surprisingly, HIV-1 infection of MDM led to the down-regulation of MMP-9. In encephalitic brain tissue, MMPs were expressed within perivascular and parenchymal MP, multinucleated giant cells, and microglial nodules. These data suggest that MMP production in MP is dependent on cell type, differentiation, activation, and/or viral infection. Regulation of MMP expression by these factors may contribute to neuropil ECM degradation and leukocyte migration during HAD.
Pathogenic mechanisms for human immunodeficiency virus type 1 (HIV-1)-associated dementia (HAD) revolve around the secretion of neurotoxic products by immune-activated mononuclear phagocytes (MPs), which include microglia and perivascular and parenchymal brain macrophages (17, 24). Immune-activated MPs, a critical pathological correlate of HAD (25), secrete a number of neurotoxins including eicosanoids (arachidonic acid and its metabolites), quinolinic acid, platelet activating factor, tumor necrosis factor alpha (TNF-α), nitric oxide, and matrix metalloproteinases (MMPs) (5, 15, 19, 41, 42). All can affect HAD pathogenesis (5, 15, 26, 28, 58, 61). Indeed, MP products often lead to alterations in adhesion molecules, cytokines, and chemokines in endothelial cells, MPs, and astrocytes.
MMPs produced as a consequence of MP activation can compromise blood-brain barrier (BBB) integrity and influence monocyte transmigration into the brain. MMPs are proteolytic enzymes responsible for the maintenance, turnover, and integrity of the extracellular matrix (ECM) (30). MMP dysregulation can affect tissue remodeling and organ homeostasis (38, 65). In response to inflammation and cellular activation, MMPs are synthesized by a variety of cell types (4, 49) including endothelial and epithelial cells (13, 64), leukocytes (14, 23, 34, 46), neural cells (38), and hepatocytes (47). Cytokines (11), cell differentiation (6, 48, 60), and viral infection (11, 55) affect the regulation of MMPs. When produced in abundance, MMPs affect cell signaling triggered by integrins (53). Methods for detection of MMP enzymatic activities are highly divergent, and each assay system (molecular, enzyme-linked immunosorbent assay [ELISA], and zymography) measures distinct aspects of their function (43).
To explore how MMPs are regulated during HAD, we studied their biological activities in the natural target cells of HIV-1 in brain, the MPs. Pure populations of primary human monocyte-derived macrophages (MDMs) and microglia were used to study the effects of cellular differentiation and immune activation on MMP production. CD40 ligand (CD40L) was utilized to induce MP activation, because it has been shown to be upregulated in plasma and on CD4+ T lymphocytes during HAD. Importantly, ligation of the CD40 receptor on human endothelial cells upregulates the expression of leukocyte adhesion molecules (37). In addition, CD40L can effect microglial activation in Alzheimer's disease (57). MMPs were detected by reverse transcriptase PCR (RT-PCR), ELISA, and gelatin zymography tests. Differentiating monocytes and cultured microglia produced high levels of MMP-9. Microglial cells generated higher levels of MMPs de novo. CD40L-induced immune activation potentiated MMP production early after MP differentiation and/or cell cultivation. HIV-1 infection of MPs reduced MMP production, while CD40L activation increased it. Analysis of human autopsy tissue by RT-PCR analysis showed that the levels of both MMP-9 and MMP-2 transcripts were increased during HIV-1 encephalitis (HIVE) and that these increases correlated with the CD14 mRNA levels, indicating that the upregulation observed in HIVE brain tissue is mainly due to the infiltrating MPs. Immunohistochemical analysis performed on brain tissue from patients who died of HIVE demonstrated MMP antigen expression in infected brain tissue. Both MMP-9 and MMP-2 were found in multinucleated giant cells, microglial nodules, and in close proximity to brain microvessels. An understanding of the processes involved in MMP production should provide unique insights into what controls monocyte infiltration across the BBB and the degradation of neuropil ECM during HAD.
MATERIALS AND METHODS
Isolation and propagation of monocytes.
Peripheral blood mononuclear cells were obtained from HIV-1, HIV-2, and hepatitis B-seronegative donors by leukopheresis, and then monocytes were purified by countercurrent centrifugation (16). Cell suspensions were >98% pure monocytes by Wright staining, nonspecific esterase, granular peroxidase, and CD68 immunostaining. Cells were cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% heat-inactivated pooled human serum, 10 μg of ciprofloxacin per ml (Sigma), 50 μg of gentamicin per ml (Sigma), and 1,000 U of macrophage colony-stimulating factor (MCSF) per ml (a generous gift from Genetics Institute, Cambridge, Mass.) for 7 days and thereafter maintained in MCSF-free medium. All reagents were prescreened and found negative for endotoxin (<10 pg/ml; Associates of Cape Cod, Woods Hole, Mass.) and mycoplasma contamination (Gen-probe II, Gen-probe, San Diego, Calif.).
Isolation and propagation of microglia.
Human fetal microglial cells were prepared as described previously with some modifications (21, 22). Briefly, fetal brain tissue (gestational age, 14 to 19 weeks) was obtained after elective abortion procedures performed in full compliance with National Institutes of Health and University of Nebraska Medical Center ethical guidelines. The tissue was digested with 0.25% trypsin (Sigma) for 30 min at 37°C. The resultant single-cell suspension was cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum, penicillin-streptomycin (50 μg/ml), and 100 μg of neomycin per ml for 7 days. Released microglia were collected and purified by preferential adhesion and identified as >98% pure by CD68 immunostaining. Purified microglial cells were cultured in the presence of MCSF (1,000 U/ml) for 7 days in parallel with monocytes derived from elutriation.
Viral strains and MP activators.
The viral strains HIV-1ADA (16) and HIV-1DJV (27) have been described previously. HIV-1YU-2 (33), HIV-1JR-FL (31), and HIV-1SF162 (7) were obtained from the AIDS Research and Reference Program, National Institute of Allergy and Infectious Diseases. HIV-1MSCSF was recently isolated at the Center for Neurovirology and Neurodegenerative Disorders from cerebrospinal fluid of an HAD patient (18).
All HIV-1 isolates were propagated on MDMs. Soluble trimeric CD40L was a generous gift from Immunex Corp., Seattle, Wash.
HIV-1 infection of MDMs and microglia.
Monocytes and microglia were cultured on 96-well plates (Costar Corp., Cambridge, Mass.) at a density of 105 cells/well for 7 days prior to viral infection. The cell-free viral inoculum was standardized for all experiments by RT activity (2 × 105 cpm/106 cells). Adherent monolayers of monocytes and microglia were incubated with virus for 4 h at 37°C. Culture medium was exchanged twice weekly. RT was determined every 2 to 3 days with 10 μl of culture supernatant (21).
Immune stimulation of MDMs and microglia.
Adherent monolayers of monocytes and microglia were stimulated on specific days following cell culture. For all experiments, cells were maintained in MCSF-free medium after 7 days. Serum-free medium was placed into the MP cultures prior to zymography for collection of supernatant samples. Select cells were treated with 2 μg of CD40L per ml for the entire experiment. Untreated cells were used as controls.
RT-PCR tests.
Levels of MMP RNA in uninfected and infected CD40L-treated MDM, untreated controls, and human brain autopsy tissue were determined after reverse transcription with antisense primers and PCR amplification of the cDNA. RNA for the cellular gene actin served as an internal standard. Data were quantitated by the Molecular Dynamics PhosphorImager Storm system, with results normalized to actin products. Amplified DNAs were identified by Southern blotting (10). Briefly, total cellular RNA (0.7 μg) in 2.5 μl was mixed with 0.5 μg of antisense primers. The mixture was heated at 70°C for 10 min and then cooled to 4°C. RT (200 U/μl; Life Technologies) and 1.5 μl (each) of the four deoxynucleotide triphosphates (10 mM; Perkin-Elmer) were added. RT reactions were performed at 37°C for 15 min and were terminated by heating the sample to 95°C. For PCR amplification of the cDNAs, 0.5 μg of sense primers and 0.25 μg of antisense primers were added, with 1 μl each of the four deoxynucleotide triphosphates and 0.5 μl of Amplitaq DNA polymerase (5 U/μl; Perkin-Elmer). Denaturation was performed at 95°C for 2 min followed by 28 amplification cycles (94°C for 30 s, 50°C for 30 s, 72°C for 1 min each). Products were reannealed at 72°C for 5 min. The final reaction mix was held at 4°C.
ELISA determinations of MMPs.
MMP-1, -2, -3, and -9 were assayed with ELISA kits (Amersham, Arlington Heights, Ill., and Oncogene Research Laboratories, Cambridge, Mass.) following the manufacturer's instructions. For experiments involving HIV-1 infection, the values obtained were normalized to the cell number on the basis of MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) activity. Statistical analyses were performed with GraphPad Prism 2.0, with one-way analysis of variance (ANOVA), and Newman-Keuls posttest.
Gelatin zymography.
Zymography was performed as described previously (10). Samples were analyzed on sodium dodecyl sulfate (SDS)-polyacrylamide gels containing gelatin (0.8 mg/ml). Prior to zymography, volumes of samples were normalized on the basis of MTT activity or total protein content. Enzyme activity was visualized as substrate degradation by negative staining with Coomassie brilliant blue R250. Commercially available MMP-9 and MMP-2 were utilized as positive controls. The gelatinase activity was quantified as densitometric units (DU) by using the GelExpert software supplied with the Nucleovision gel imaging system (Nucleo Tech Corp., Hayward, Calif.).
Immunohistochemical assays.
Immunohistochemistry was performed on 5-μm-thick paraffin tissue sections as described previously (44). Human MDMs or microglia were identified with anti-CD68 KP-1 (1:100; Dako) monoclonal antibodies. Astrocytes were localized by glial fibrillary acidic protein (GFAP) immunoreactivity (1:200; Dako). Antibodies to HLA-DR, CR3/43 (Boehringer Mannheim), and HIV-1 p24 (Dako) were used at 1:25, 1:40, and 1:10 dilutions, respectively. Antibodies to MMP-2 and MMP-9 (Labvision Corp., Fremont, Calif.) were used at 1:50 and 1:10 dilutions, respectively. The avidin-biotin immunoperoxidase staining was performed with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.) with 3, 3′-diaminobenzidine (DAB) as the chromogen. The sections were counterstained with Mayer's hematoxylin.
RESULTS
MMP-1, -2, -3, and -9 regulation in MDMs and microglia.
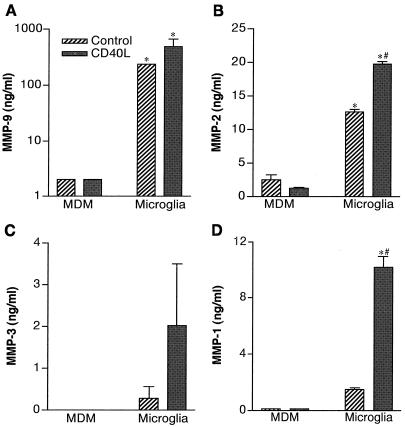
We first studied MMP production by MPs following cell differentiation, viral infection, and immune activation. Cross-validating measurements of MMPs included RT-PCR, ELISA, and zymography tests. Freshly elutriated monocytes and human microglial cells were used to determine how MP heterogeneity affects MMP expression. Monocytes were cultured for 1 day and then stimulated with CD40L (2 μg/ml). MMP levels were measured by ELISA in culture fluids at 72 h after CD40L addition. The levels of MMP-9 (Fig. 1A), MMP-2 (Fig. 1B), MMP-3 (Fig. 1C), and MMP-1 (Fig. 1D) in both monocytes and microglia are shown. CD40L-stimulated microglia generated significantly larger amounts of all MMPs than similarly treated MDMs (Fig. 1; P < 0.01). MMP-3 was detected only in culture supernatant of microglia (Fig. 1C). Microglia demonstrated a significant upregulation in MMP-2 and -1 following CD40L stimulation (Fig. 1B and D). Production of MMP-9 and MMP-1 by CD40L-stimulated microglia increased 10-fold and 2-fold, respectively. Thus, activated microglia are capable of secreting higher levels of MMPs than replicate MDMs. This finding infers that microglial cells, when subjected to the appropriate signals, could contribute to degradation of the neuropil ECM during HAD.
FIG. 1.
MMP production in monocytes and human fetal microglia. Monocytes and microglia were isolated and then cultivated in medium with or without CD40L (2 μg/ml). Culture supernatants were collected at 72 h after CD40L stimulation, and MMP levels were determined by ELISA. Values represent the mean of triplicate determinations ± standard error. MMP-9 (A), MMP-2 (B), MMP-3 (C), and MMP-1 (D) levels were compared. Statistical tests were performed with GraphPad Prism 2.0, using one-way ANOVA with Newman-Keuls posttest. These demonstrated that microglia generated significantly higher levels of all MMPs when compared to monocytes (∗, P < 0.01). The effect of CD40L stimulation of microglial cells was also statistically significant for MMP-2 and -1 (#; P < 0.001).
MP differentiation affects MMP production.
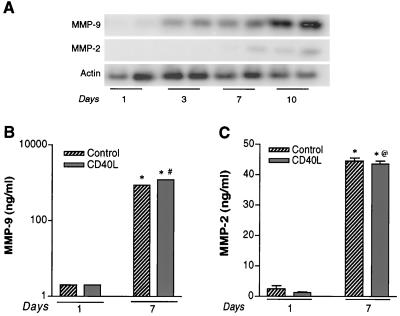
Increased infiltration of immune-activated monocytes, from blood to brain, characterizes HAD. Importantly, monocyte transendothelial migration into brain occurs during macrophage differentiation. To address whether differential MMP production between monocytes, MDMs, and microglia reflects cellular differentiation, we examined MMP production during cell differentiation. MDMs were cultured for 10 days. At days 1, 3, 7, and 10, MDMs were subjected to complete replacement of medium, and MMP gene expression was analyzed by RT-PCR after an additional 72 h, actin served as the internal standard for the RT-PCR assays. Figure 2A demonstrates that expression of both MMP-2 and MMP-9 increased during MDM differentiation. MMP-9 mRNA increased 4.5-, 7.5-, and 22.5-fold at days 3, 7, and 10, respectively, after cell culture. The levels of MMP-2 transcripts were 1.2-, 2.6-, and 9.2-fold higher at days 3, 7, and 10, respectively. The data demonstrate that cell differentiation influences MMP production in MDM.
FIG. 2.
Effect of MDM differentiation on MMP-9 and MMP-2 expression. (A) MDMs were cultured as adherent monolayers. Complete exchange with serum-free medium was performed on days 1, 3, 7, and 10. Total cellular RNA was analyzed for levels of MMP-9 and -2 by RT-PCR. Each time point was assayed in duplicate. Actin was utilized as an internal standard. A time-dependent increase in the levels of MMP RNA is shown. To assess the effect of immune activation in conjunction with differentiation, MDMs were stimulated with CD40L (2 μg/ml) on culture days 1 and 7. Culture supernatant samples were collected at 72 h after stimulation for MMP-9 (B) and MMP-2 (C) and tested by ELISA according to the manufacturer's instructions. At day 7 significantly larger amounts of both MMP-9 and -2 were generated compared to those from day 1 (∗, P < 0.001). MMP-9 production by MDMs was upregulated by CD40L at 7 days of cell cultivation (#, P < 0.001). MMP-2 did not show significant differences following cell activation (@, P > 0.05). Values represent the mean of triplicate determination ± standard error. Statistical analysis was performed with GraphPad Prism 2.0, using one-way ANOVA with Newman-Keuls posttest.
Brain MPs become immune-activated during HAD and influence disease progression. This can occur through a variety of mechanisms, although proinflammatory cytokines, β-chemokines, and/or CD40L are likely candidates. To determine the influence of immune activation on MMP production by MPs, total MMP content of macrophage culture supernatants was measured in the presence and absence of CD40L. Figure 2B and C show the levels of MDM MMP-9 (Fig. 2B) and MMP-2 (Fig. 2C) produced after 1 and 7 days of cell culture with or without 2 μg of CD40L stimulation per ml. The levels of MMP-9 produced by MDMs at day 1 in control and CD40L-activated cells were negligible, as determined by ELISA. MMP-2 levels in MDMs were unchanged with or without CD40L at 1 day (Fig. 2C). After 7 days of culture, unstimulated MDMs generated 886 and 1,212 ng of MMP-9 per ml before and after CD40L activation. The differences in MMP production between days 1 and 7 were statistically significant (P < 0.001). For MMP-2, the levels were 44 ng/ml for control MDMs and 43 ng/ml with CD40L activation. These data implied that the MMP-2 produced by MDMs had reached a saturation point 7 days after cell cultivation. However, for MMP-1, only immune-activated cells showed low, but detectable, amounts of enzyme (data not shown). These data support the idea that cell differentiation is a principal driving force for MMP production in MDMs.
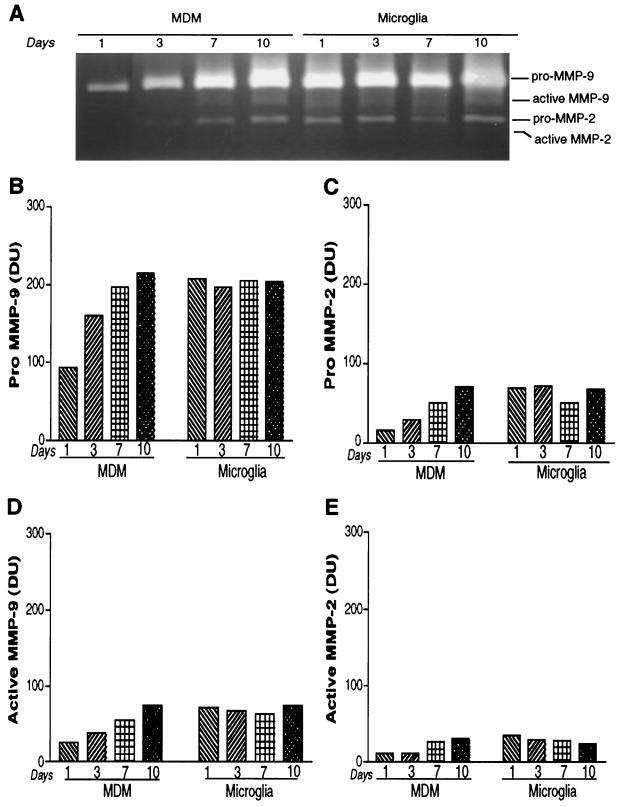
To compare subtle differences in the levels of MMPs and to ascertain the relative production of active MMP-9 and -2 from MPs, gelatin zymography was used. In our experimental systems, zymography proved more sensitive than ELISA, with a limit of detection of 3 pg. The zymograms obtained were analyzed with densitometric scanning with band intensity expressed as DU. For the purpose of comparison, all sample volumes were normalized by total protein content. To confirm the data set, each zymogram was performed with incremental sample volumes on at least three separate gels. In addition, representative samples were analyzed in triplicate and treated as independent samples. A total of six individual MDM donors and three microglia donors were studied. Figure 3 summarizes the data obtained for the effects of cell differentiation (as obtained in tissue culture) over time on pro- and active MMP-9 and -2.
FIG. 3.
MDMs secrete pro- and active MMP-9 and MMP-2. MDMs and microglia were cultured as adherent monolayers. Complete medium exchange was performed at the time points indicated, and culture supernatants were tested by zymography after 72 h. A representative zymogram obtained from days 1, 3, 7, and 10 is shown in panel A. Levels of pro-MMP-9 (92 kDa) (B) and pro-MMP-2 (72 kDa) (C) and their active forms (active MMP-9 [D] and active MMP-2 [E], respectively) were quantified densitometrically and compared. Data are representative of a total of six MDM donors and two microglia donors performed three or more times on multiple gels.
The levels of MMP-9 and -2 produced by MDMs within 72 h of culture were low and increased incrementally with time. This led to a more than twofold increase in the pro-MMP-9 levels (Fig. 3A and B). Microglia, in contrast, generated high levels of MMPs de novo, which did not change significantly over time in culture. This pattern of MMP production was reflected in pro-MMP-9 and pro-MMP-2 and their active forms (Fig. 3B, C, D, and E). This also suggests that differentiation of cells in vitro not only led to the increase in the total MMP production, but also led to an increase in levels of their active forms. The production of large amounts of pro-MMPs would simply represent a pool of enzyme molecules available for activation upon receipt of the appropriate stimuli, while the generation of the active forms would represent enzyme activity directly available to the cells that are attempting to traverse the BBB.
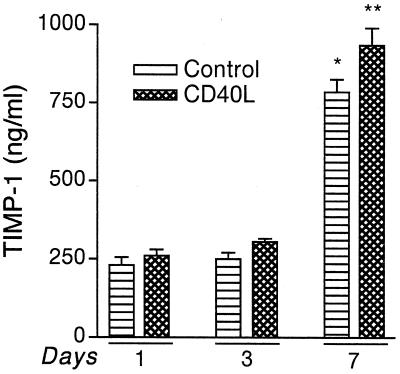
Regulation of TIMP-1 during monocyte differentiation.
Our initial observations led us to investigate the levels of MP tissue inhibitor of metalloproteinase-1 (TIMP-1), because TIMPs regulate MMP activity in the extracellular milieu. TIMP-1 and -2 are the two types of inhibitors produced by MP. TIMP-2 generally is expressed constitutively, whereas TIMP-1 is inducible. We determined the levels of TIMP-1 during differentiation in the presence and absence of CD40L (Fig. 4). Levels of TIMP-1 increased with differentiation, together with MMP-9. TIMP-1 production increased 3.4-fold from day 1 to day 7 (P < 0.001). Also, CD40L led to a significant increase in TIMP-1 levels in MDMs stimulated at day 7 in culture (P < 0.001). Thus, during brain tissue infiltration in HAD, the increase in MMPs is perhaps balanced with the higher levels of TIMP-1. It is noteworthy, however, that the TIMP-1 ELISA measured total TIMP-1, including both free TIMP-1 and that complexed with MMP-1, -2, -3, and -9.
FIG. 4.
Secretory levels of TIMP-1 produced by MDMs. Freshly elutriated monocytes were cultured as adherent monolayers. At days 1, 3, and 7, cells were stimulated with 2 μg of CD40L per ml for 72 h, and TIMP-1 levels in the culture supernatant were analyzed by ELISA. Each sample was analyzed in triplicate. Levels of TIMP-1 were upregulated 3.4-fold from day 1 to day 7 (∗, P < 0.001). In addition, CD40L stimulation led to a statistically significant increase in TIMP-1 levels in cells stimulated on day 7 of culture (∗∗, P < 0.001). Representative data obtained from a total of three donors are shown. Values represent the mean of triplicate determinations ± standard error. Statistical analysis was performed with GraphPad Prism 2.0, using one-way ANOVA with Newman-Keuls posttest.
MMP production in MDM following HIV-1 infection.
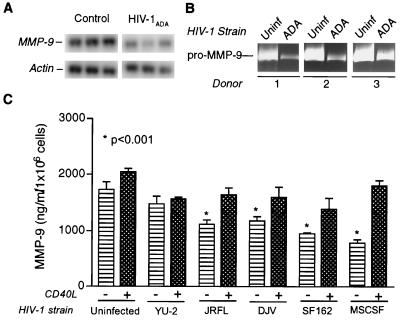
Next, we examined the effects of HIV-1 infection on the production of MMP-9 and -2 by macrophages. MDMs were cultivated for a period of 7 days to permit cell differentiation. Cells were then infected with HIV-1ADA. MMP-9 expression was determined by RT-PCR and normalized to the levels of actin (Fig. 5A). Several donors were analyzed, and representative data are shown. The observed down-modulation of MMPs by HIV-1ADA was statistically significant (P < 0.05). Importantly, the data demonstrating a down-modulation of MMP-9 stand in contrast to previously published observations by Dhawan et al. (11) in which HIV-1ADA-infected MDMs demonstrated a twofold increase in pro-MMP-9. We confirmed our observations in subsequent experiments by gelatin zymography, which is currently a sensitive tool for detection of subtle changes in MMP levels. Figure 5B shows three experiments performed with independent MDM donors that demonstrated similar trends of down-regulation of MMP-9 following viral infection. In the study performed by Dhawan et al. (11), pro-MMP-9 levels were measured 12 h after complete exchange of medium. In our systems, the MMP-9 levels were measured 48 h after medium exchange. Thus, the differences in the observations could reflect the differences in the experimental systems used. The proteins, once secreted, would lead to a cumulative effect as a function of time, whereas the transcripts would be upregulated transiently. In our experimental system, both the mRNA and the protein levels of MMP-9 at 48 h were significantly down-regulated. We thus measured MMP-9 levels in culture supernatant samples obtained from MDMs infected with multiple other central nervous system (CNS) donors (Fig. 5C). We utilized HIV-1YU-2, HIV-1JR-FL, and HIV-1DJV as brain isolates and HIV-1SF162 and HIV-1MSCSF as those obtained from cerebrospinal fluid samples. All demonstrated significant down-regulation compared to uninfected cells (P < 0.001). This down-regulation by infection with HIV-1DJV, HIV-1JR-FL, HIV-1MSCSF, and HIV-1SF162 was significantly reversed with CD40L activation (P < 0.01).
FIG. 5.
Regulation of MMP expression in HIV-1-infected MDM. (A) Adherent monolayers of MDMs were maintained for 7 days prior to infection with HIV-1ADA. Triplicate wells of infected or uninfected control cells had complete medium exchange at day 5 postinfection. Cells were maintained for an additional 48 h. MDMs were harvested in TRIzol, and total RNA was extracted. MMP mRNA was detected by RT-PCR. Representative data for MMP-9 mRNA are shown. Actin was utilized as the internal standard for semiquantitative comparison. HIV-1ADA significantly down-regulated the levels of MMP-9 mRNA (A, ∗, P < 0.05). (B) MDMs were infected as described for panel A. MMP levels in culture supernatant samples from HIV-1-infected and uninfected cells were analyzed by zymography. Culture supernatant volumes were normalized on the basis of endpoint MTT activity. Three independent donors are represented. (C) Secretory profiles of MMP-9 in culture supernatant samples derived from uninfected and HIV-1-infected cells with or without CD40L stimulation were analyzed by ELISA. Three CNS HIV-1 isolates (HIV-1YU-2, HIV-1DJV, and HIV-1JR-FL) and two cerebrospinal fluid HIV-1 isolates (HIV-1SF162 and HIV-1MSCSF) were utilized. All isolates except for HIV-1YU-2 led to a significant down-regulation in MMP-9 levels in infected cells (∗, P < 0.001). CD40L stimulation led to a statistically significant upregulation in MMP-9 levels in uninfected and HIV-1-infected cells, with the exception of HIV-1YU-2 (P < 0.01). Statistical analysis was performed with GraphPad Prism 2.0, using one-way ANOVA with Newman-Keuls posttest.
MMPs expressed in HIV-1-infected human brain tissue.
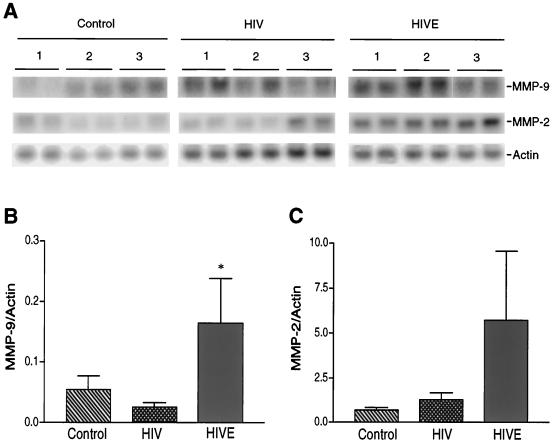
In order to correlate our laboratory observations with those in an infected person, we investigated the levels of MMP-9 and -2 in brain tissue from human autopsy specimens. This was performed by semiquantitative comparisons of MMP transcripts by RT-PCR analysis and immunohistochemical staining for MMP antigens. Brains of patients who died of HIVE, HIV-1 disease without neurological complications and controls (patients with systemic diseases not related to brain dysfunction) were examined. Total RNA was extracted from brain tissue derived from three control, five HIV-1-seropositive, and seven HIVE subjects. In most cases, RNA extractions were performed on two distinct areas of the brain—the frontal cortex and the basal ganglia. For semiquantitative comparisons, actin was utilized as the internal control. The data are summarized in Fig. 6. Representative RT-PCR data from three independent donors from each group are shown in Fig. 6A, and a summary of the comparisons obtained for MMP-9 and -2 with all patients and control subjects is shown in Fig. 6B and C, respectively. HIVE led to statistically significant increases in MMP-9 transcripts compared to those in controls (P < 0.05). The levels of MMP-2 transcripts were higher than those in controls, although they were not statistically significant. A correlation determined by RT-PCR showed that the levels of both MMP-9 and -2 in HIVE patients correlated with CD14 levels normalized to actin products (correlation coefficient, r2 = 0.9593 for MMP-9 and r2 = 0.9412 for MMP-2). Comparisons between control and HIV-1-seropositive donors yielded correlation coefficients below 0.5. These data suggest that the higher levels of MMP transcripts in HIVE brain tissue were a result of the increased numbers of immune-competent MP in these patients. In order to colocalize MMP antigens with specific cell types, we next performed immunohistochemical analysis with antibodies to MMP-9 and -2 on areas adjacent to the brain regions utilized for RNA extraction. Serial sections were utilized to identify specific cell types expressing MMPs in brain parenchyma.
FIG. 6.
Levels of MMP transcripts in human brain tissue. Total cellular RNA was extracted from frontal cortex and basal ganglia tissue obtained from three controls, five HIV-seropositive patients, and four HIVE patients. MMP-9 and -2 transcripts were detected by RT-PCR as described in Materials and Methods. Actin was used as an internal control for semiquantitative comparisons. Each sample was analyzed in duplicate determinations. Panel A illustrates three representative donors each from control, HIV-positive, and HIVE groups. Panels B and C summarize the data obtained with all patients investigated for MMP-9 and MMP-2 levels, respectively. Values represent the mean of duplicate determinations ± standard error. Statistical analysis was performed with GraphPad Prism 2.0, using one-way ANOVA with Newman-Keuls posttest. Levels of MMP-9 between HIVE and control brain tissue were significantly different (∗, P < 0.05).
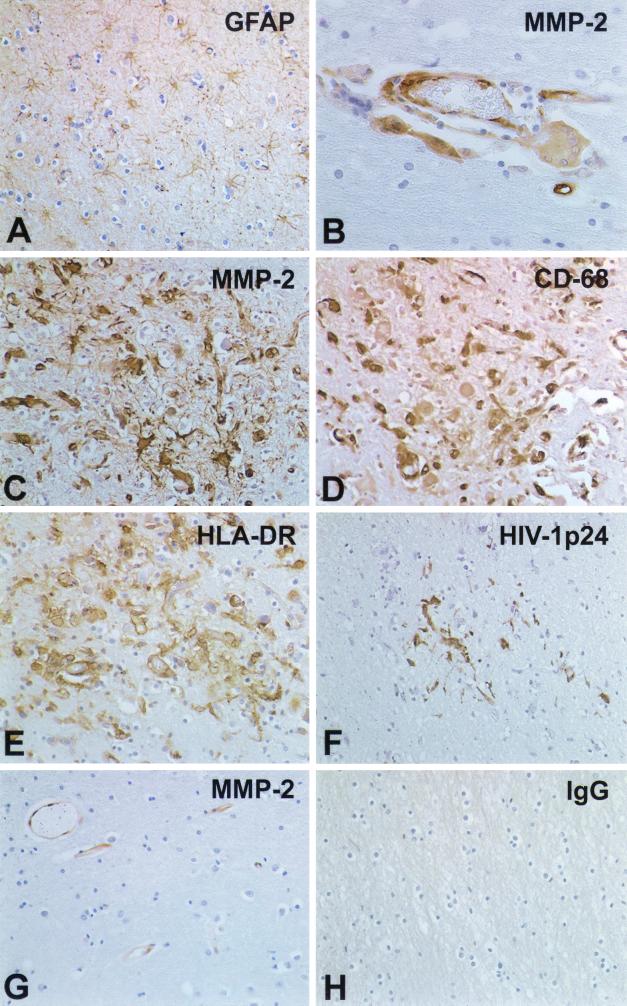
For the immunohistochemical analyses, we examined a total of 15 brain autopsy samples, including 6 from patients with severe HIVE, 3 from patients with mild HIVE, 3 from HIV-1 seropositive patients without HIVE, and 3 from controls with neither HIV-1 infection nor encephalitis (Fig. 7 and 8). Four of the HIVE, three of the HIV-1-seropositive, and one of the control samples were kindly provided via the Manhattan Brain Bank. Results of the MMP-2 cell localization and extent of antigen expression are summarized in Fig. 7. Panels A to F and H show results from HIVE brain tissue, and panel G shows results from control brain tissue. Neuropathological analyses of HIVE brain tissue showed a direct relationship between microglial accumulation and activation (detected as HLA-DR expression and transformation of ramified microglia into amoeboid form), formation of microglial nodules, the intensity of macrophage infiltration, and MMP expression within infiltrating inflammatory cells, perivascular macrophages, and microglial nodules. These changes were accompanied by a pronounced astrogliosis. The amount of plump reactive astrocytes was significantly increased compared to that in controls (Fig. 7A). In addition, a significant accumulation of microglial cells and clear signs of microglia activation (HLA-DR staining) were present in areas with reactive astrogliosis (Fig. 7E). In parallel, perivascular macrophages (cells with abundant cytoplasm) and monocytes that had migrated into the brain (smaller round cells) expressed high levels of HLA-DR. They were localized immediately at the abluminal surface of brain microvascular endothelial cells, a major component of the BBB. Perivascular cells and some microglia were HIV-1 p24 immunoreactive (Fig. 7F). Endothelial cells and smooth muscle cells expressing MMP-2 antigens were frequently observed (Fig. 7B and G). Occasional single and multinucleated giant MPs were also MMP-2 immunoreactive in close proximity to microvessels (Fig. 7B). Microglial nodules present in the brain parenchyma were identified by immunostaining with CD68 (a mononuclear phagocyte marker) (Fig. 7D) and for HLA-DR (Fig. 7E). Cells in microglial nodules were positive for MMP-2 (Fig. 7C) and HIV-1 p24 (Fig. 7F). HIVE serial sections immunostained with mouse immunoglobulin G (IgG) for nonspecific control did not demonstrate any immunoreactivity (Fig. 7H). Specificity of MMP-2 and MMP-9 antibodies was confirmed with human placental sections as positive controls (data not shown). Brain tissue derived from control HIV-1-seropositive patients and HIV-1-negative control subjects showed nonspecific changes with minimal expression of HLA-DR in less than 10% of microglia located in white matter. Astrogliosis was absent, and GFAP-positive astrocytes showed long slender processes. Moderate expression of MMP-2 was found on endothelial cells and on a few smooth muscle cells in controls without neurological disease (Fig. 7G). Neither parenchymal microglia nor occasional perivascular macrophages showed expression of MMP-2.
FIG. 7.
Cellular localization of MMP-2 in human brain tissue. Fifteen brain autopsy samples were analyzed, including those from 3 control subjects (without CNS disease), 6 patients with severe HIVE, 3 patients with mild HIVE, and 3 HIV-1-seropositive patients without nervous system disease. In the patients with severe HIVE, prominent astrogliosis as identified by GFAP immunoreactivity was observed (A). HIV-1-infected multinucleated giant cells (B) expressed MMP-2 and were prominent in areas with significant monocyte infiltration. Serial sections stained with MMP-2 (C), CD68 (D), HLA-DR (E), and HIV-1 p24 (F) demonstrated activated, HIV-1-infected cells in microglial nodules positive for MMP-2. Control mouse IgG served as the negative control (H). Few MMP-2 antigen-positive endothelial cells were observed in the control samples (G). Sections are stained with GFAP (A), MMP-2 (B, C, and G), HLA-DR (E), HIV-1 p24 (F), and control IgG (H). Immunoreactivity was detected with a Vectastain Elite kit with DAB as a substrate. Tissue sections were counterstained with Mayer's hematoxylin. Original magnification, panels A and C to H, ×200; panel B, ×400.
FIG. 8.
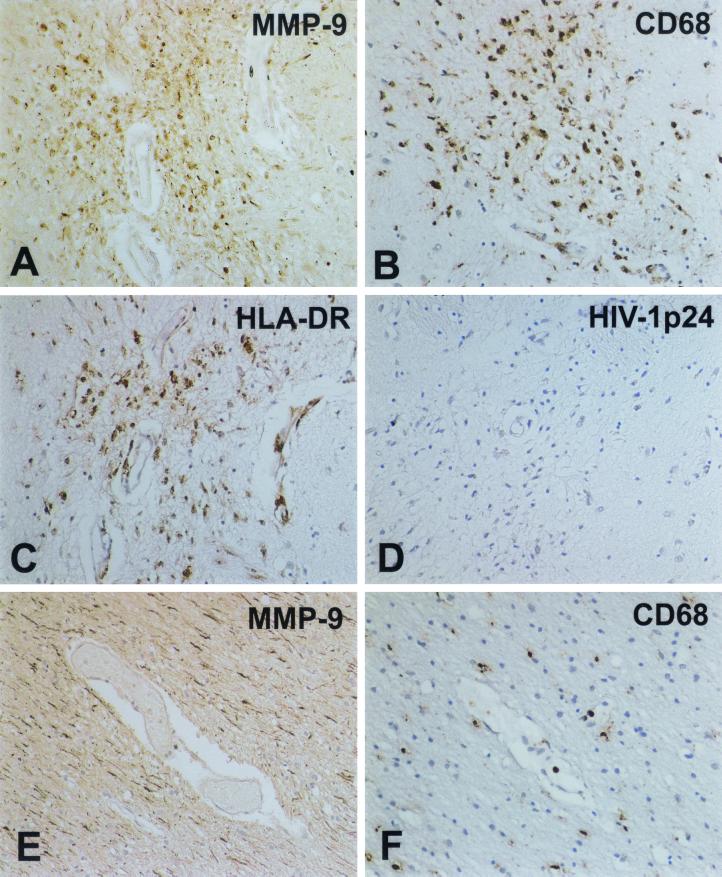
MMP-9 localization in human brain tissue. Expression of MMP-9 was evaluated for all subjects as described for MMP-2. MMP-9 expression was detected on microglial nodules in brain tissue of HIV-1-seropositive patients devoid of neurological complications (A). Serial sections stained with CD68 (B [MP marker]) showed that some of these cells demonstrating MMP-9 reactivity were activated MPs. Importantly, the majority of MMP-9-positive cells expressed HLA-DR, a marker for activation (C), yet were minimally infected, as demonstrated by a lack of HIV-1 p24 detection (D). Interestingly, in a nonneurological control subject, MMP-9 immunoreactivity was observed mainly on linear axon-like processes (E) and was absent on MPs (F). Staining for MMP-9 (A and E), CD68 (B and F), HLA-DR (C), and HIV-1 p24 (D) is shown. Original magnifications: A to E, ×200; F, ×100.
Figure 8 summarizes the data obtained for MMP-9. MMP-9 expression (Fig. 8A) in HLA-DR-positive (Fig. 8C) microglial cells was observed in patients with mild cases of HIVE and HIV-1-seropositive patients. These cells were readily positive for CD68 (a marker for MP) (Fig. 8B). It is important to note that MMP-9 immunoreactivity colocalized well with a marker of immune activation (HLA-DR [Fig. 8C]). However, localization by HIV-1 p24 on serial sections showed minimal viral infection (Fig. 8D). Interestingly, all samples examined demonstrated additional MMP-9 localization in axon-like linear structures (Fig. 8A and E). A nonneurological control sample showed MMP-9 staining mainly in linear structures (Fig. 8E). The identity of these linear tracks of MMP-9 immunoreactivity is yet to be confirmed. These studies confirm the importance of MMPs in conjunction with immune activation of MPs in the brain and its implication for the progression of HAD. In toto, analysis of human brain tissue showed that HIVE is associated mainly with MP activation, traffic of activated macrophages, and astrogliosis and that these activities were correlated with expression of MMPs.
DISCUSSION
Our results demonstrate that heterogeneity exists among MPs with regard to MMP regulation. Under steady-state conditions, MMPs maintain the brain's ECM through remodeling of the neuropil. MMPs play an important role in BBB compromise. Migration of T lymphocytes and monocytes across basement membranes and their infiltration into the neuropil are ensured by the production of MMPs (32). Indeed, several studies have convincingly demonstrated the role of MMPs in affecting BBB permeability and endothelial cell function (50–52). Bacterial collagenases disrupt ECM and destroy the integrity of the BBB (51). Alterations in monocyte-endothelial cell interactions are also dependent on MMPs (11). Notably, the interactions between monocytes with both microvascular endothelial cells and resident astrocytes form a tripartite MMP-producing complex that can affect cell recruitment into brain. During CNS disease, MMP production is enhanced, leading to the degradation of the ECM and loss of its homeostatic remodeling function. Also, monocyte infiltration in the brain increases during HAD progression, and these newly recruited macrophages would be responsible for enhancement of MMPs and consequent disease progression. Indeed, our data show that perivascular leukocytes associated with HIVE express both MMP-2 and MMP-9. Our works and those of others support a relationship between macrophage numbers and neuropil alterations. For example, in Alzheimer's disease, matrix-degrading enzymes have been implicated in the development of senile dementia (3). Moreover, in studies of 27 individuals with advanced HIV-1 infection, it was shown that 9 of 9 who had HIVE were completely devoid of labeled ECM. Eight of 18 subjects without HIVE had substantial losses of ECM, and only 2 manifested a normal ECM complement. The data showed that a severe compromise in ECM integrity occurs in the neuropil during HIVE (1).
Under homeostatic conditions, microglia are present as terminally differentiated ramified cells that can undergo dedifferentiation during injury. As proposed for HAD, microglia can be recruited to sites of neural injury and undergo proliferative responses (12). It is of interest that brain MPs (microglia, as compared to MDMs) differ in their capacity to secrete MMPs. This might enable the cells to perform dual functions in disease. First, differentiated brain macrophages could enhance continued penetration of monocytes through the BBB. This hypothesis is supported by the presence of perivascular macrophages in areas of diseased brain with microgliosis and signs of MP activation. Second, the ability of macrophages and/or microglia to directly degrade the brain ECM may affect neurodegeneration. Given the fact that monocytes differentiate during brain migration, MMP production by infiltrating cells would continue. Once inside the brain, MP activation would allow the secretion of chemokines and other inflammatory molecules, serving to amplify secretory neurotoxic activities resulting in further brain monocyte migration. Our data were obtained by utilizing fetal microglial cells and comparing them to adult MDMs, and the possibility that the intrinsic MMP profiles in these cells may be different remains open. However, we have previously demonstrated comparisons consistent with the data presented in this work (20, 22, 44)
Interestingly, we also found a concomitant increase in TIMP-1 production. TIMP-1 preferentially binds to pro-MMP-9; however, it can inhibit the activity of all active MMPs. In addition, TIMP-1 is the inducible tissue inhibitor of MMPs, as compared to TIMP-2, which is believed to have a constitutive expression pattern. These studies clearly demonstrate that a balance between the levels of MMPs and their tissue inhibitors in the BBB microenvironment determines ECM breakdown mediated by infiltrating monocytes. Ultimately, MP activation, differentiation, and/or viral infection would result in the amplification of brain inflammation and progressive neural impairment.
The role of MP immune activation in HIV neuropathogenesis is underscored by our observation that CD40L stimulation can reverse the MMP-9 down-regulation induced by viral infection. Our data show that CD40L activation of differentiating MPs leads to significant increases in MMP production. Although the biological source of CD40L is unclear, it remains an important physiological immune activator relevant to HAD. Human vascular endothelial cells express CD40 antigen and produce functional CD40L (37). The notion that CD40L produced by endothelial cells leads to an upregulation in MP MMPs is attractive, although, perhaps oversimplified. Indeed, the interactions between transmigrating monocytes and endothelial cells involve astrocytes, an integral part of the BBB. Since astrocytes also express CD40 receptor, they too are candidates for CD40L-mediated activation. In addition, astrocytes and endothelial cells, as well as MPs, are capable of generating MMPs. There is likely a complex intercellular dialogue that is mediated, only in part, by MMP production by MPs. Indeed, the restoration of high levels of MMP-9 in MPs after CD40L stimulation reflects the critical importance of immune activation in HIVE pathogenesis and the relevance of our laboratory findings to what is observed in an infected human host.
Our results demonstrate a consistent down-regulation in MMP-9, as determined by multiple assays, including RT-PCR, ELISA, and gelatin zymography. Importantly, our assays examine MMP-9 production on a single-cell basis, and the results were confirmed in multiple donors. These results appear to be at odds with previously published reports (9, 11, 36, 54, 59). These studies assayed biological material such as patient plasma or cerebrospinal fluid to show net increases in MMP-9 production, but could not detect whether higher production by each cell was occurring. Indeed, the studies performed by Liuzzi et al. (36) and Sporer et al. (54) demonstrated increased pleiocytosis in the cerebrospinal fluid concomitant with MMP-9 levels. Thus, we propose that the MMP-9 levels during disease reflect increases in numbers of immune-competent MPs in the brain due to increased trafficking of activated macrophages and resident microglial activation. It has been previously shown by Glass et al. (25) that the best histopathologic correlate of HAD is the number of inflammatory macrophages in the CNS. The phenomenon of down-regulation of MMP-9 production by HIV-1 infection is nonetheless intriguing. MMP-9 production in MPs involves the signal transduction pathways mediated by the mitogen-associated protein (MAP) kinase. Interestingly, the infection of host cells by HIV-1 is also linked to MAP kinase pathways (2, 62, 63). In addition, works published on the phosphorylation of HIV-1 matrix show that HIV-1 virions package the cellular MAP kinase enzyme in the viral particles, which is responsible for the phosphorylation of matrix and the nuclear import of the viral preintegration complex (29, 56). We propose that due to the packaging of the cellular MAP kinase molecules in budding virions, the MMP-9 production is down-regulated due to a compromise in available kinase activity in the cellular milieu. This is further supported by the fact that activation of infected cells with CD40L, which is known to upregulate the MAP kinase pathway, reverses the effect of HIV-1-mediated down-regulation of MMP-9. This hypothesis is currently under investigation.
We also demonstrated that MMP-2 expression in HIVE brain tissue was observed on microglial nodules in the subcortical white matter, perivascular and multinucleated macrophages, and microvascular endothelial cells. Interestingly, MMP-9 expression was most commonly observed on longitudinal tracks and was rarely observed on microglial nodules associated with severe encephalitis. In addition, MMP-9-positive microglial nodules were occasionally observed in HIV-1-infected individuals devoid of neurological disease. However, MMP-2 was not detected. MMP-9 was detected in perivascular macrophages in brain tissue with subtle signs of HIVE. Our observations also suggest that even a minimal increase in cell numbers and, more importantly, the state of MP immune activation might be sufficient to induce neurological dysfunction. In support of this idea is the observation that widespread microglial activation and accompanying reactive astrogliosis are found in the areas with the most pronounced dendritic alterations (39). Synaptic loss, also linked to MP activation, is now the best correlate of clinical cognitive deterioration in HAD (40). Three-dimensional stereological measures show a significant correlation between reduced synaptic density and poor neuropsychological performance. We also observed MMP-9 immunoreactivity on axon-like linear tracks in white matter. These data underscore the significance of traffic of activated macrophages into the brain and immune activation of microglia in HAD pathogenesis.
Our works, along with those of others, imply that multiple mechanisms are operative for MMP production and its regulation during neurodegenerative disease. Both microglia and infiltrating monocytes can produce MMPs in response to a variety of immune stimuli and/or viral infection. Either factor is readily available in the BBB or CNS microenvironment. Given this information, we speculate that in HAD, MMP inhibitors might prevent the influx of inflammatory cells through the BBB and help prevent damage to the neuropil's ECM. Indeed, such inhibitors have been effectively used in experimental autoimmune encephalitis, the animal model for multiple sclerosis (8, 35) and the SCID mouse model for HIVE (45). Thus, treatment with MMP inhibitors may aid in repression of the immune effector responses of macrophages in addition to their direct effects on MMP activity. Altogether these observations strongly suggest a role for MMPs in disease progression. Thus, the potential to halt their production may influence the course and severity of cognitive dysfunction in HIV-1-infected individuals.
ACKNOWLEDGMENTS
We thank Elaine Thomas and Immunex Corp. for kindly providing CD40L, Susan Morgello, director of the Manhattan Brain Bank, for the brain tissue sections used in these works, and the Center for Neurovirology Neurodegenerative Disorders Brain Bank for providing human brain RNA. We also thank Julie Ditter and Robin Taylor for outstanding administrative and secretarial support.
These studies were supported grants from the National Institute of Neurological Disorders and Stroke (R01 NS31492-01, PO1 MH57556-01, R01 NS34239-01, and R01 NS36126-01) and the Elizabeth Glazer Pediatric AIDS Foundation.
REFERENCES
- 1.Belichenko P V, Miklossy J, Celio M R. HIV-I induced destruction of neocortical extracellular matrix components in AIDS victims. Neurobiol Dis. 1997;4:301–310. doi: 10.1006/nbdi.1997.0143. [DOI] [PubMed] [Google Scholar]
- 2.Biggs T E, Cooke S J, Barton C H, Harris M P, Saksela K, Mann D A. Induction of activator protein 1 (AP-1) in macrophages by human immunodeficiency virus type-1 NEF is a cell-type-specific response that requires both hck and MAPK signaling events. J Mol Biol. 1999;290:21–35. doi: 10.1006/jmbi.1999.2849. [DOI] [PubMed] [Google Scholar]
- 3.Bignami A, LeBlanc A, Perides G. A role for extracellular matrix degradation and matrix metalloproteinases in senile dementia? Acta Neuropathol. 1994;87:308–312. doi: 10.1007/BF00296747. [DOI] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H. Role of inflammatory mediators in tissue destruction. J Periodontal Res. 1993;28:500–510. doi: 10.1111/j.1600-0765.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 5.Bukrinsky M I, Nottet H S L M, Schmidtmayerova H, Dubrovsky L, Mullins M E, Lipton S A, Gendelman H E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busiek D F, Ross F P, McDonnell S, Murphy G, Matrisian L M, Welgus H G. The matrix metalloproteinase matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992;267:9087–9092. [PubMed] [Google Scholar]
- 7.Cheng-Mayer C, Levy J A. Distinct biological and serological properties of human immunodeficiency viruses from the brain Ann. Neurol. 1988;23:S58–S61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 8.Clements J, Cossins J, Wells G, Corkill D, Helfrich K, Wood L, Piggot R, Stabler G, Ward G, Gearing A, Miller K. Matrix metalloproteinases expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol. 1997;74:85–94. doi: 10.1016/s0165-5728(96)00210-x. [DOI] [PubMed] [Google Scholar]
- 9.Conant K, McArthur J C, Griffin D E, Sjulson L, Wahl L M, Irani D N. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter B T. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan S, Weeks B S, Soderland C, Schnaper H W, Toro L A, Asthana S P, Hewlett I K, Stetler-Stevenson W G, Yamada S S, Yamada K M, et al. HIV-1 infection alters monocyte interactions with human microvascular endothelial cells. J Immunol. 1995;154:422–432. [PubMed] [Google Scholar]
- 12.Dickson D, Mattiace L, Kure K, Hutchins K, Lyman W, Brosnan C. Microglia in human disease, with an emphasis on the acquired immune deficiency syndrome. Lab Investig. 1991;64:135–156. [PubMed] [Google Scholar]
- 13.Duszyk M, Shu Y, Sawicki G, Radomski A, Man S F, Radomski M W. Inhibition of matrix metalloproteinase MMP-2 activates chloride current in human airway epithelial cells. Can J Physiol Pharmacol. 1999;77:529–535. [PubMed] [Google Scholar]
- 14.Fujisawa T, Kato Y, Terada A, Iguchi K, Kamiya H. Matrix metalloproteinase-9 in peripheral blood eosinophils. Int Arch Allergy Immunol. 1999;120:65–69. doi: 10.1159/000053598. [DOI] [PubMed] [Google Scholar]
- 15.Gelbard H A, Nottet H S L M, Swindells S, Jett M, Dzenko K A, Genis P, White R, Wang L, Choi Y-B, Zhang D, Lipton S A, Tourtellotte W W, Epstein L G, Gendelman H E. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994;68:4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gendelman H E, Orenstein J M, Martin M A, Ferrua C, Mitra R, Phipps T, Wahl L A, Lane H C, Fauci A S, Burke D S, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gendelman H E, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of AIDS dementia complex. AIDS. 1997;11:S35–S45. [PubMed] [Google Scholar]
- 18.Gendelman H E, Zheng J, Coulter C L, Ghorpade A, Che M, Thylin M, Rubocki R, Persidsky Y, Hahn F, Reinhard J, Jr, Swindells S. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated dementia. J Infect Dis. 1998;178:1000–1007. doi: 10.1086/515693. [DOI] [PubMed] [Google Scholar]
- 19.Genis P, Jett M, Bernton E W, Boyle T, Gelbard H A, Dzenko K, Keane R W, Resnick L, Mizrachi Y, Volsky D J, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghorpade A, Gard L C. Efficient processing and export of human growth hormone by heat labile enterotoxin chain B signal sequence. FEBS Lett. 1993;330:61–65. doi: 10.1016/0014-5793(93)80920-p. [DOI] [PubMed] [Google Scholar]
- 21.Ghorpade A, Nukuna A, Che M, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L, Gendelman H E. Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J Virol. 1998;72:3340–3350. doi: 10.1128/jvi.72.4.3340-3350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuna A, Bock P, Che M, Limoges J, Gendelman H E, Mackay C R. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs D F, Shanley T P, Warner R L, Murphy H S, Varani J, Johnson K J. Role of matrix metalloproteinases in models of macrophage-dependent acute lung injury. Evidence for alveolar macrophage as source of proteinases. Am J Respir Cell Mol Biol. 1999;20:1145–1154. doi: 10.1165/ajrcmb.20.6.3482. [DOI] [PubMed] [Google Scholar]
- 24.Giulian D, Vaca K, Noonan C A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 25.Glass J D, Fedor H, Wesselingh S L, McArthur J C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlation with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 26.Griffin G E, Wesselingh S L, McArthur J C. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- 27.Heinzinger N, Baca-Regen L, Stevenson M, Gendelman H E. Efficient synthesis of viral nucleic acids following monocyte infection by HIV-1. Virology. 1995;206:731–735. doi: 10.1016/s0042-6822(95)80097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heyes M, Gravell M, London W, Eckhaus M, Vickers J, Yergey J, April M, Blackmore D, Markey S. Sustained increases in cerebrospinal fluid quinolinic acid concentrations in rhesus macques naturally infected with simian retrovirus type-D. Brain Res. 1990;531:148–158. doi: 10.1016/0006-8993(90)90768-7. [DOI] [PubMed] [Google Scholar]
- 29.Jacque J M, Mann A, Enslen H, Sharova N, Brichacek B, Davis R J, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleiner D E J, Tuuttila A, Tryggvason K, Stetler-Stevenson W G. Stability analysis of latent and active 72-kDa type IV collagenase: the role of tissue inhibitor of metalloproteinases-2 (TIMP-2) Biochemistry. 1993;32:1583–1592. doi: 10.1021/bi00057a024. [DOI] [PubMed] [Google Scholar]
- 31.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 32.Leppert D, Waubant E, Galardy R, Bunnet N, Hauser S. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995;154:4379–4389. [PubMed] [Google Scholar]
- 33.Li Y, Kappes J C, Conway J A, Price R W, Shaw G M, Hahn B H. Molecular characterization of human immunodeficiency virus type I cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichthinghagen R, Seifert T, Kracke A, Marckmann S, Wurster U, Heidenreich F. Expression of matrix metalloproteinase-9 and its inhibitors in mononuclear blood cells of patients with multiple sclerosis. J Neuroimmunol. 1999;99:19–26. doi: 10.1016/s0165-5728(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 35.Liedtke W, Cannella B, Mazzaccaro R J, Clements J M, Miller K M, Wucherpfennig K W, Gearing A J, Raine C S. Effective treatment of models of multiple sclerosis by matrix metalloproteinase inhibitors. Ann Neurol. 1998;44:35–46. doi: 10.1002/ana.410440110. [DOI] [PubMed] [Google Scholar]
- 36.Liuzzi G, Mastroianni C, Santacroce M, Fanelli M, D'Agostino C, Vullo V, Riccio P. Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological diseases. Neurovirology. 2000;April:156–163. doi: 10.3109/13550280009013159. [DOI] [PubMed] [Google Scholar]
- 37.Mach F, Schonbech U, Sukhova G K, Bourcier T, Bonnefoy J-Y, Pober J S, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda A, Sobel R A. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Masliah E, Ge N, Morey M, DeTeresa R, Terry R D, Wiley C A. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Investig. 1992;66:285–291. [PubMed] [Google Scholar]
- 40.Masliah E, Heaton R K, Marcotte T D, Ellis R J, Wiley C A, Mallory M, Achim C L, McCutchan J A, Nelson J A, Atkinson J H, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 41.Nottet H S, Jett M, Flanagan C R, Zhai Q H, Persidsky Y, Rizzino A, Bernton E W, Genis P, Baldwin T, Schwartz J, et al. A regulatory role for astrocytes in HIV-1 encephalitis. An overexpression of eicosanoids, platelet-activating factor, and tumor necrosis factor-alpha by activated HIV-1-infected monocytes is attenuated by primary human astrocytes. J Immunol. 1995;154:3567–3581. [PubMed] [Google Scholar]
- 42.Nottet H S L M, Persidsky Y, Sasseville V G, Nukuna A N, Bock P, Zhai Q H, Sharer L R, McComb R, Swindells S, Soderland C, Rizzino A, Gendelman H E. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- 43.Parsons S, Watson S, Brown P, Collins H, Steele R. Matrix metalloproteinases. Br J Surg. 1997;84:160–166. [PubMed] [Google Scholar]
- 44.Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu X J, Stins M, Fiala M, Way D, Kim K S, Witte M H, Weinand M, Carhart L, Gendelman H E. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persidsky Y, Limoges J, Rasmussen J, Zheng J, Gearing A, Gendelman H E. Reduction in glial immunity and neuropathology by a PAF antagonist and an MMP and TNFalpha inhibitor in SCID mice with HIV-1 encephalitis. J Neuroimmunol. 2001;114:57–68. doi: 10.1016/s0165-5728(00)00454-9. [DOI] [PubMed] [Google Scholar]
- 46.Pugin J, Widmer M C, Kossodo S, Liang C M, Preas H L N, Suffredini A F. Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol. 1999;20:458–464. doi: 10.1165/ajrcmb.20.3.3311. [DOI] [PubMed] [Google Scholar]
- 47.Quondamatteo F, Knittel T, Mehde M, Ramadori G, Herken R. Matrix metalloproteinases in early human liver development. Histochem Cell Biol. 1999;112:277–282. doi: 10.1007/s004180050448. [DOI] [PubMed] [Google Scholar]
- 48.Rifas L, Fausto A, Scott M J, Avioli L V, Welgus H G. Expression of metalloproteinases and tissue inhibitors of metalloproteinases in human osteoblast-like cells: differentiation is associated with repression of metalloproteinase biosynthesis. Endocrinology. 1994;134:213–221. doi: 10.1210/endo.134.1.8275936. [DOI] [PubMed] [Google Scholar]
- 49.Romanic A M, Madri J A. Extracellular matrix-degrading proteinases in the nervous system. Brain Pathol. 1994;4:145–156. doi: 10.1111/j.1750-3639.1994.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg G A. Matrix metalloproteinases in brain injury. J Neurotrauma. 1995;12:833–842. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg G A, Estrada E, Kelley R O, Korneld M. Bacterial collagenase disrupts extracellular matrix and opens blood-brain barrier in rat. Neurosci Lett. 1993;160:117–119. doi: 10.1016/0304-3940(93)90927-d. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg G A, Estrada E Y, Dencoff J E, Stetler-Stevenson W G. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- 53.Seltzer J L, Lee A Y, Akers K T, Sudbeck B, Southon B, Southon E A, Wayner E A, Eisen A Z. Activation of 72-kDa type IV collagenase/gelatinase by normal fibroblasts in collagen lattices is mediated by integrin receptors but is not related to lattice contraction. Exp Cell Res. 1994;213:365–374. doi: 10.1006/excr.1994.1211. [DOI] [PubMed] [Google Scholar]
- 54.Sporer B, Paul R, Koedel U, Grimm R, Wick M, Goebel F D, Pfister H-W. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus infected patients. J Infect Dis. 1998;178:854–857. doi: 10.1086/515342. [DOI] [PubMed] [Google Scholar]
- 55.Stefansson S, Aimes R T, Seward N B, Alexander D S, Quigley J P. Native TIMP-free 70 kDa progelatinase (MMP-2) secreted at elevated levels by RSV transformed fibroblasts. J Cell Physiol. 1994;161:419–428. doi: 10.1002/jcp.1041610304. [DOI] [PubMed] [Google Scholar]
- 56.Stevenson M. HIV nuclear import: what's the flap? Nat Med. 2000;6:626–628. doi: 10.1038/76191. [DOI] [PubMed] [Google Scholar]
- 57.Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson M P, Flavell R A, Mullan M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- 58.Tyor W R, Glass J D, Griffin J W, Becker P S, McArthur J C, Bezman L, Griffin D E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 59.Weeks B S. The role of HIV-1 activated leukocyte adhesion mechanisms and matrix metalloproteinase secretion in AIDS pathogenesis. Int J Mol Med. 1998;1:361–366. doi: 10.3892/ijmm.1.2.361. [DOI] [PubMed] [Google Scholar]
- 60.Welgus H G, Senior R M, Parks W C, Kahn A J, Ley T J, Shapiro S D, Campbell E J. Neutral proteinase expression by human mononuclear phagocytes: a prominent role of cellular differentiation. Matrix Suppl. 1992;1:363–367. [PubMed] [Google Scholar]
- 61.Wesselingh S L, Power C, Glass J D, et al. Intracerebral cytokine messenger RNA expression in acquired immunedeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- 62.Yang X, Chen Y, Gabuzda D. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappaB. J Biol Chem. 1999;274:27981–27988. doi: 10.1074/jbc.274.39.27981. [DOI] [PubMed] [Google Scholar]
- 63.Yang X, Gabuzda D. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J Virol. 1999;73:3460–3466. doi: 10.1128/jvi.73.4.3460-3466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeo S J, Kim J H, Lee H J, Kook Y H. Influenza A virus infection modulates the expression of type IV collagenase in epithelial cells. Arch Virol. 1999;144:1361–1370. doi: 10.1007/s007050050592. [DOI] [PubMed] [Google Scholar]
- 65.Yong V W, Krekoski C A, Forsyth P A, Bell R, Edwards D R. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]