Abstract
N6-methyladenosine (m6A) is a prevalent mRNA modification known for its implications in various cancer types, yet its role in chromophobe renal cell carcinoma (chRCC) remains largely unexplored. In this study, we performed m6A-SEAL-seq and RNA-seq analyses on tissues from three chRCC subjects, aiming to uncover m6A alterations in chRCC. Our findings revealed reduced expression levels of four m6A regulators in chRCC tissues and highlighted differences in m6A levels compared to normal tissues. Furthermore, we identified specific genes and cancer-related pathways affected by these differences, including notable candidates like NOTCH1 and FGFR1, implicated in chRCC development. Additionally, we developed a predictive model based on the expression level of m6A associated genes, demonstrating promising prognostic capabilities for patient survival prediction. Overall, our study provides valuable insights into the role of m6A in chRCC and its potential as a prognostic indicator.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12956-6.
Keywords: Chromophobe renal cell carcinoma, N6-methyladenosine, Transcriptome, Prognostic indicator
Introduction
According to the Global Cancer Observatory (GCO, https://gco.iarc.fr/en) database as of 2022, kidney cancer accounts for over 434 thousand new cases globally, with more than 155 thousand deaths attributed to the disease. Despite advancements in healthcare, kidney cancer remains a significant public health concern, ranking 16th in terms of incidence and 17th in terms of cancer-related mortality worldwide. The urgent need to decipher the molecular mechanisms underlying kidney cancer progression persists.
There are three primary types of kidney cancer: clear cell renal cell carcinoma (ccRCC), papillary renal cell carcinoma (PRCC), and chromophobe renal cell carcinoma (chRCC). Among these, ccRCC is the most prevalent form of kidney cancer. Significant advancements have been made in understanding the pathogenesis and therapeutic approaches for ccRCC [1–4]. Papillary renal cell carcinoma (PRCC) stands as the second most common type of renal carcinoma and is currently a well-studied subtype of renal cell carcinoma (RCC) [5, 6]. Whereas chRCC is an uncommon renal cell carcinoma (RCC) subtype, accounting for 5–10% of all cases of RCC [7]. Previous studies suggest that chRCC is a malignant neoplasm with a mortality rate of about 10%, and aggressive clinical course such as metastasis [8]. While the somatic genomic landscape of chRCC has revealed various genomic features [9], the understanding of the functional role of epitranscriptomics remains limited.
More than 170 types of modifications have been identified in the RNA molecule [10]. N6-methyladenine (m6A) emerges as the most abundant mRNA modification in eukaryotes, playing pivotal roles in various biological functions. These functions include the regulation of RNA stability [11–13], 3′-end processing [14, 15], alternative splicing [16, 17] and translation efficiency at the post-transcriptional level [13, 18, 19]. m6A is dynamically reversible in mammals and is governed by “writers” (methyltransferases), “erasers” (demethylases), and “readers” (binding proteins). The majority of m6A modifications are installed by the methyltransferase complex, primarily composed of the METTL3-METTL14 heterodimer [20, 21] and other subunits like WTAP [22–24], and removed by demethylases like FTO and ALKBH5 [25, 26]. m6A modification can also be recognized and bound by m6A binding proteins for the regulation of gene expression, like YTH domain family proteins [27–29]. The discovery of these diverse m6A regulators has significantly contributed to a deeper understanding of the physiological functions of m6A.
Numerous studies have underscored the close relationship between m6A modifications and tumor progression [30–33]. In pancreatic cancer, ALKBH5 inhibits cancer cell growth and progression by increasing PER1 mRNA levels through m6A removal, consequently inhibiting YTHDF2-dependent mRNA degradation [33]. In bladder cancer, METTL3 installs m6A on pri-miR221/222, expediting miRNA maturation and promoting cancer cell proliferation [34]. These findings highlight the pivotal roles of m6A modification in carcinogenesis through the regulation of RNA processing and metabolism, shedding light on new molecular mechanisms underlying cancer progression. m6A regulators exert significant impacts on clear cell renal cell carcinoma (ccRCC) [35]. METTL14, for instance, is downregulated in ccRCC tissue, and patients with lower METTL14 expression tend to exhibit worse prognoses [36]. Alterations in m6A regulators are associated with worse clinical characteristics [37]. In RPCC, a prognostic risk signature model incorporating three m6A regulatory genes, IGF2BP3, KIAA1429, and HNRNPC, accurately predicts survival outcomes [38]. The advent of high-throughput sequencing has enabled transcriptome-wide profiling of m6A distribution in various human carcinomas, offering insights into the molecular mechanisms underlying m6A modification and renal cell carcinoma (RCC). Transcriptome-wide m6A mapping in ccRCC has revealed the identification of unique m6A-related genes associated with cancer-related pathways and provided insights into potential mechanisms of m6A-mediated gene regulation [39]. However, the transcriptome-wide distribution of m6A in chRCC remains elusive. In this study, we utilized m6A-SEAL-seq to construct a landscape of m6A profiling in human chRCC [40], and identified novel m6A gene signature.
Methods
Patients and specimens
A total of three patients with chRCC were involved in our study. chRCC tissues and corresponding tumor-adjacent normal tissues were collected at the time of surgery from urology department, Peking University Third Hospital. All specimens were immediately separated into 1.5 ml RNase-free centrifuge tubes and stored at -80 ◦C before RNA isolation. The study was approved by The Beijing Haidian Hospital Medical Ethics Committee, and the written informed consents were obtained from all the participants.
RNA preparation
Total RNA was extracted from tissue specimens using TRIzol reagent (Magen) and poly(A)+ RNA was isolated from total RNA using oligo(dT) 25 Dynabeads (Thermo Fisher Scientific). RNA concentration was determined using a Nanodrop ultraviolet-visible light spectrophotometer (Thermo).
qRT–PCR
Poly(A)+ RNA was isolated from tissues using TRIzol reagent (Invitrogen) and oligo(dT)25 Dynabeads (Thermo Fisher Scientific). First-strand cDNA synthesis was carried out using SuperScript III (Thermo Fisher Scientific) and oligo(dT)20 primer. Each sampling was performed with at least three biological replicates. The relative expression levels were normalized using GADPH as the reference gene. All the primers were designed by multiPrime at https://multiprime.cn [41]. All primer sequences are provided in Supplementary Dataset 1.
m6A-SEAL-seq and library construction
Poly(A)+ RNA for each sample was fragmented by a magnesium RNA fragmentation module (NEB). In a poly(A)+ RNA m6A oxidation assay, the reaction was performed in 300 µl aliquots of aqueous solution containing 300 µM of (NH4)2Fe(SO4)2·6H2O, 2 mM of l-ascorbic acid, 300 µM of α-KG, 100 mM pH 7.0 HEPES, 0.2 µM FTO and 1 µg poly(A)+ RNA. After the FTO treatment at 37 °C for 5 min, RNA was purified by Oligo Clean & Concentrator column (Zymo Research). hm6A-modified RNA converted from m6A by FTO oxidation was treated by 200 mM freshly prepared DTT at 37 °C for 3 h in acidic aqueous solution (100 mM HEPES, pH 4.0). The product RNA was purified by ethanol precipitation. After ethanol precipitation, DTT treated RNA was washed by 75% ethanol and dissolved in 200 µl biotinylation buffer that contained 100 µM of MTSEA-XX-biotin (Biotum), 100 mM HEPES (pH 7.0), 1 mM EDTA and 20% DMF. The reaction was performed at 25 °C and 800 rpm in a ThermoMixer for 1 h. The product RNA was purified by phenol-chloroform extraction. 50 ng biotin-labeled RNA was saved as input, and the rest proceeded to affinity enrichment. After that, 20 µl Dynabeads MyOne Streptavidin C1 (Invitrogen) was washed twice by 200 µl 0.1 M NaOH to remove RNase contamination, and then washed with diethyl pyrocarbonate water to a neutral pH. The beads were resuspended in 100 µl binding solution containing 10 µl of high-salt wash buffer (100 mM Tris pH 7.5, 10 mM EDTA, 1 M NaCl, 0.05% Tween 20) and 90 µl diethyl pyrocarbonate water, and incubated with the biotinylated RNA for 1 h. The beads with biotinylated RNA were washed three times with 1 ml high salt wash buffer. 50 µl of 100 mM DTT was used to release the biotinylated RNA at 37 °C for 15 min on a ThermoMixer (800 rpm.). After collecting the supernatant, the second elution was performed with 50 µl of 100 mM DTT at 50 °C for 5 min to completely release the RNA. The twice-eluted RNA was combined and purified by ethanol precipitation. Library construction was performed using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina according to the manufacturer’s protocol. Libraries were sequenced on the Illumina HiSeq XTen platform with a paired-end model (PE150).
Analysis of m6A-seq data
Sequencing reads were trimmed and mapped to the reference genome (GRCh38) by using Cutadapt (v1.18) [42] and HISAT2 (v2.1.0) [43], respectively. The m6A-enriched regions in chRCC and normal tissues were identified using the MACS2 [44] peak-calling algorithm based on enrichment criteria (IP/Input) ≥ 2 and FDR < 0.05. Confident m6A peaks were subjected to Hypergeometric Optimization of Motif EnRichment tools (HOMER) [45] for Motif Discovery. Genes with differentially methylated m6A sites were identified by MeTDiff [46] based on enrichment criteria fold change ≥ 2 and FDR < 0.05. Tissue analysis, Gene ontology (GO) and pathway enrichment analyses were performed by using DAVID.
Analysis of RNA-seq data
Adapter and low-quality reads were trimmed by using Cutadapt (v1.18) [42], and trimmed reads were aligned to the reference genome (GRCh38) using HISAT2 (v2.1.0) [43]. The differential expression genes between chRCC and adjacent normal tissues were screened by R package (DEseq2) [47] based on a cutoff criterion of fold change ≥ 2 and FDR < 0.05.
Risk stratification and survival analysis
A cohort of 65 chRCC cases from The Cancer Genome Atlas (TCGA) database was used to illustrate the relationship between the differential expressed DMMGs and chRCC patients. We randomly chose 40 samples from 65 cases as a training set to predict signature model and the rest samples form a testing set to verify the model (make sure the training set and testing set both contain tumor samples and normal samples). Firstly, we used least absolute shrinkage and selection operator (LASSO) to select candidate genes (glmnet package) in training set. Secondly, we performed the multi-variates cox regression and removed genes not supported by PH hypothesis using the selected candidate genes in training set. Thirdly, we performed the second regression (survival package) to calculate the coefficients between candidate genes and 5-years survival using the remaining candidate genes in training set to build a Cox model. The concordance index (C-index) was calculated to evaluate the prognostic power. Risk score of each sample was calculated through the sum of the product of each candidate gene fpkm-uq and its coefficient in training set. The patients were then classified into high-risk or low-risk group using the risk score where the difference value of true positive and false positive reaches to the maximum as the cutoff value. The Kaplan-Meier survival curve (survminer package) was performed to evaluate the 5-years survival, and the sensitivity and accuracy of the cox model to predict clinical outcome were evaluated by the area under curve (AUC) of the receiver operating characteristic (ROC) curve (survival ROC package). At last, we test the signature model in the testing set, ccRCC dataset (a cohort of 602 cases from TCGA database) and PRCC dataset (a cohort of 318 cases from TCGA database).
Results
The expression pattern of m6A regulators in normal and chRCC tissue
In order to determine whether m6A modification functions in chRCC, we first analyzed the expression levels of 8 m6A regulators, including 3 key writer subunits, 3 readers, and 2 erasers (m6A writer subunits: METTLE14, METTL3, and WTAP; m6A readers: YTHDF1, YTHDF2, and YTHDF3; m6A erasers: ALKBH5 and FTO) in three patients. The qPCR results showed that the expression levels of WTAP, YTHDF2, FTO, and ALKBH5 were downregulated markedly in chRCC tissues compared with corresponding tumor-adjacent normal tissues (termed normal tissues) (Fig. 1A-H). Furthermore, upon comparison with The Cancer Genome Atlas (TCGA database), we observed a notable decrease in the expression of the m6A writer WTAP as well (Fig. S1). The aberrant expression of the m6A regulators in chRCC, suggesting m6A might be dysregulated in chRCC.
Fig. 1.
Relative expression level of known m6A-related genes in Normal and chRCC tissues by qPCR. A, METLL3, B, METTL14, C, WTP, D, YTHDF1, E, YTHDF2, F, YTHDF3, G, FTO, H, ALKBH5. Data are presented as means ± SE, n = 3 biological replicates. *P < 0.05 by t test (two-sided)
Overview of m6A methylation feature in normal and chRCC tissues
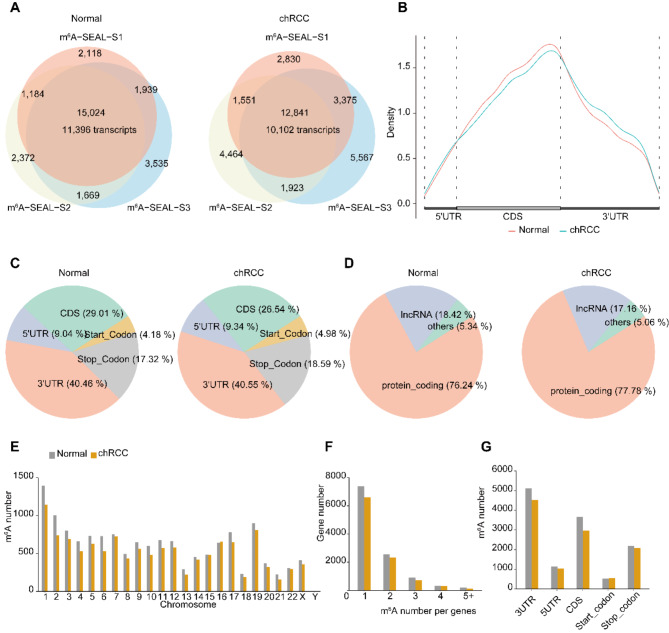
To investigate whether m6A methylation landscape changes between the normal and chRCC tissues, we next constructed m6A-SEAL-seq using three samples and explored the distribution of m6A modification [40]. Approximately 87.1–13.2 million reads were generated from each library and 82.8–12.8 million reads were mapped to GRCh38 genome (Supplementary Dataset 2). m6A peaks were called in each sample using MACS2, based on fold enrichment (IP/input) ≥ 2 and FDR ≤ 0.05). The m6A peaks identified in all three replicates were classified as “confident m6A peaks”. Finally, we identified 15,024 confident m6A peaks corresponding to 11,396 transcripts/genes in normal tissues, whereas 12,841 confident m6A peaks corresponding to 10,102 transcripts/genes in chRCC tissue (Fig. 2A). To evaluate the reliability and performance of m6A-SEAL-seq, we compared our confident m6A peaks of normal tissues with MeRIP-seq of normal kidney tissues from published sequencing data (GSE122744) [48]. 5686 out of 7261 (78.3%) of the m6A peaks in MeRIP-seq were identified as confident m6A peaks in our m6A-SEAL-seq (Fig. S2A), and the metagene profile results of m6A-SEAL-seq and MeRIP-seq indicated that that our confident m6A peaks were highly enriched around MeRIP-seq peaks (Fig. S2B). We additionally computed normalized read coverages from m6A-SEAL-seq around MeRIP-seq peaks, revealing their co-enrichment (Fig. S2C, D). These findings suggest that m6A-SEAL-seq provides accurate and reliable results.
Fig. 2.
Characterization of m6 A modification in Normal and chRCC tissues reveals that decreased methylation number in chRCC Tissues. A, overlap of three biological replicates of m6A-SEAL-seq peaks identifying ~ 15,000 high-confident m6A peaks corresponding to 11,396 unique transcripts in normal tissues (left); Overlap of three biological replicates of m6A-seal peaks identifying ~ 12,800 high-confident m6A peaks corresponding to 10,102 unique transcripts in chRCC tissues (right). B, Metagene profile illustrating the region distribution of m6A peaks across the indicated mRNA segments. C, Pie chart depicting the fraction of the confident m6A peaks in each of the five non-overlapping transcript segments (5’UTR, start codon, coding sequence [CDS], stop codon and 3’UTR) in normal tissues (left); Pie chart depicting RNA types of m6A peaks in normal tissues (right). D, Pie chart presenting the fraction of the confident m6A peaks in eCRCC tissues (left); Pie chart depicting RNA types of m6A peaks in chRCC tissues (right). E, The number of m6A peaks in human chromosomes. F, The number of m6A peaks per gene. G, The number of m6A peaks in five non-overlapping transcript segments
The metagene profiles results demonstrated that confident m6A peaks in normal and chRCC tissue were both highly located within Coding Sequence (CDS) (Fig. 2B). However, the distribution pattern of m6A peaks in chRCC shifted from CDS region to stop codon and 3’UTR. To further locate confident m6A peaks, we divided the transcripts into five non-overlapping regions and assigned the confident m6A peaks into these regions. The fraction of confident m6A peaks of normal and chRCC tissues (Fig. 2C) in these five regions showed that they were dominantly enriched in 3′ UTR (40.46%, 40.55%), CDS (29.01%, 26.54%) and stop codon (17.32%, 18.59%). The motifs analysis results revealed that in both normal and chRCC tissues, the identified motifs are GGAYN (Y = C/U, N = U/A/C/G), AAACK (K = G/U) and NBNAH (N = U/A/C/G, B = G/C/U, H = C/U/A), RAACW (R = G/A, W = U/A). These motifs closely resemble the well-known m6A binding motif, RRACH (R = G/A, H = A/C/U).
Further we asked which RNA molecules prefer to contain m6A modification. We assigned confident m6A peaks to GRCh38 genome and found that 76.24% and 77.78% were mRNA, 18.42% and 17.16% were long non-coding RNA (lncRNA) in normal and chRCC tissues (Fig. 2D), separately. We also noticed that the confident m6A peaks number in chRCC tissues were less than that in normal tissues. Then we assigned m6A peaks to chromosome, genes and five non-overlapping regions. We found that the number of confident m6A peaks in chRCC tissues decreased globally among each chromosome (Fig. 2E). By analyzing the distribution of m6A peaks per gene, we found that most of m6A-motified mRNAs contained one or two m6A peak, while a small number of them contained three or more (Fig. 2F). Furthermore, m6A number per gene in chRCC tissues was all less than that in normal tissues. We also counted the number of m6A peaks among the five non-overlapping regions in normal and chRCC tissues (Fig. 2G) and found the number of m6A peaks decreased in chRCC tissues (except start codon) too. These results suggested that m6A modification level decreased in cancer tissues.
Differentially methylated m6A genes (DMMGs) participate in multi-cancer related pathways
To dissect the role of m6A modification, we subsequently identified DMMPs between normal and chRCC tissues. Compared to the normal tissues, the hyper- and hypo-methylated peaks were respectively regarded as hyper and hypo group. As to hyper group, we identified 644 shared differentially hyper-methylated m6A peaks corresponding to 593 genes in the three biological replicates. While as to hypo group, we identified 1304 shared differentially hypo-methylated m6A peaks representing 1137 genes (Fig. 3A). We further identified the m6A motif in hyper- and hypo- methylated m6A peaks within chRCC tissues by HOMER, and it revealed that the top consensus motifs in the hyper- and hypo-methylated m6A peaks were both similar to RRACH (Fig. S4).
Fig. 3.
Tissue analysis, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses of coding genes containing hypomethylation m6A peaks in chRCC tissues. A, Overlap of three biological replicates of hypermethylation m6A peaks identifying ~ 644 high-confident hypermethylation m6A peaks corresponding to 593 unique transcripts in chRCC tissues (left); Overlap of three biological replicates of hypomethylation m6A peaks identifying ~ 1,304 high-confident hypomethylation m6A peaks corresponding to 1,137 unique transcripts in chRCC tissues (right). B, Metagene profile illustrating the region distribution of hypermethylation and hypomethylation m6A peaks across the indicated mRNA segments. C, Pie chart presenting the fraction of the confident hypomethylation m6A peaks five non-overlapping transcript segments; D, Pie chart presenting RNA types (that is, transcript species) of the confident hypomethylation m6A peaks identified in chRCC. E-G, UP_TISSUE (E), Gene ontology (GO) (F), KEGG (G) analysis of the confident hypomethylation m6A genes
Then we examined the distribution profiles of DMMPs across the transcripts, and found that the density of DMMPs tended to be around the stop codon in both hyper and hypo group. In hypo group, the m6A peaks were especially enriched in the CDS and 3’ UTR near the stop codon; whereas in the hyper group, m6A peaks were enriched in the 3’ UTR near the stop codon (Fig. 3B). Further examination revealed that the m6A peaks in both groups were dominantly enriched within 3′ UTR (54.6% and 56.68%), CDS (25.92% and 23.14%) and around stop codon (13.19% and 11.02%) (Fig. 3C and Fig. S3A). Almost all of the transcripts containing DMMPs were mRNA (80.44% and 70.61%), only ~ 14–18% were lncRNA and a small proportion were other RNAs (Fig. 3D and Fig. S3B).
To explore the potential role of hypo-methylated m6A peaks in chRCC, we took advantage of DAVID to examine the most preferential expression tissues of genes with hypo-methylated peaks. The results showed that genes containing hypo-methylated m6A sites preferentially expressed in epithelium, followed by brain, placenta and renal cell carcinoma (RCC) (Fig. 3E), indicating the correlation between these genes and RCC. Furthermore, we performed GO enrichment analysis to uncover the functions of these genes. The results revealed that genes with hypo-methylated m6A peaks were enriched in many biological processes involved in kidney development and cancer pathogenesis, including transcription, androgen receptor signaling pathway, GTPase activity and cell-cell adhesion (Fig. 3F). Pathway analysis showed that genes with hypo-methylated m6A peaks were mainly enriched in pathways in cancer (Fig. 3G). These results suggested that genes with hypo-methylated peaks may participate in various pathophysiologic aspects of kidney cancer through different pathways.
Subsequently, we explored the potential function of hyper-methylated m6A peaks using the same method. The results showed that genes containing hyper-methylated m6A sites preferentially expressed in brain, followed by epithelium, duodenum, fetal kidney and ovary (Fig. S3C) which also indicating these genes is important in kidney development. GO biological process analysis revealed that genes with hyper-methylated m6A peaks were significantly associated with cancer-related biological processes, including protein phosphorylation, positive regulation of cholesterol efflux, ubiquinone biosynthetic process, regulation of mitophagy and so on (Fig. S3D). Pathway analysis showed that genes with hyper-methylated m6A peaks were mainly enriched in ubiquitin mediated proteolysis, metabolic pathways and adherents’ junction (Fig. S3E). These results further illustrated that gene with DMMPs played crucial roles in kidney cancer.
Differentially expressed genes involved in kidney development and cancer occurrence
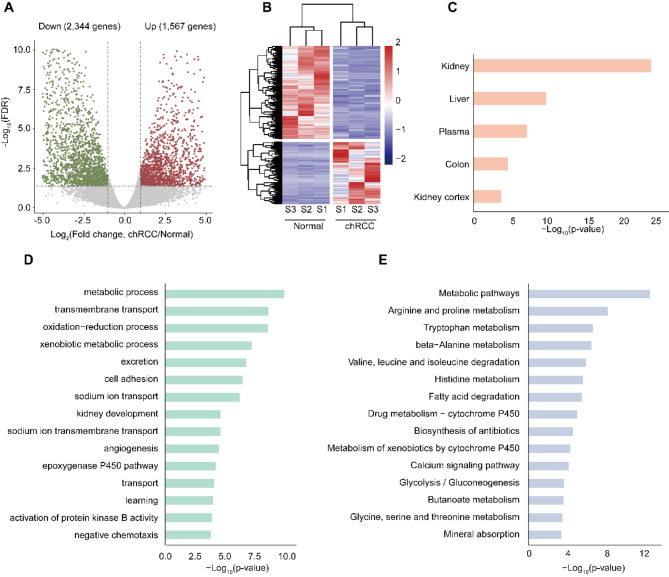
We next identified the global mRNA expression patterns in normal and cancer groups. The results showed that a total of 3,911 mRNAs were significantly dysregulated in cancer group compared with normal group, including 2,344 down-regulated mRNAs and 1,567 up-regulated mRNAs (fold change ≥ 2, P < 0.05) (Fig. 4A). Hierarchical clustering depicted differential expression profiles in all the samples. (Fig. 4B). We then took advantage of the DAVID to examine the preferentially expressed tissues of these genes. The results showed that the dysregulated genes preferentially expressed in kidney, followed by liver and plasma (Fig. 4C). Then we performed GO analysis and KEGG pathway analysis. GO enrichment analysis demonstrated that dysregulated genes were significantly enriched in kidney development and cancer related biological processes involving metabolic process, cell adhesion and kidney development (Fig. 4D). While the KEGG pathway analysis revealed that dysregulated genes were significantly associated with metabolic pathways and protein metabolism (Fig. 4E).
Fig. 4.
Differential expression genes in chRCC tissues compared with adjacent normal tissues. A, Volcano plots showing the differentially expressed genes in chRCC tissues compared with those in adjacent normal tissues. B, Heatmap plots showing the differentially expressed genes in chRCC tissues vs. those in adjacent normal tissues. C-E, UP_TISSUE (C), Gene ontology (GO) (D), KEGG (E) analysis of the differential expression genes
Novel m6A gene signature identified by m6A-SEAL-seq and RNA-seq data
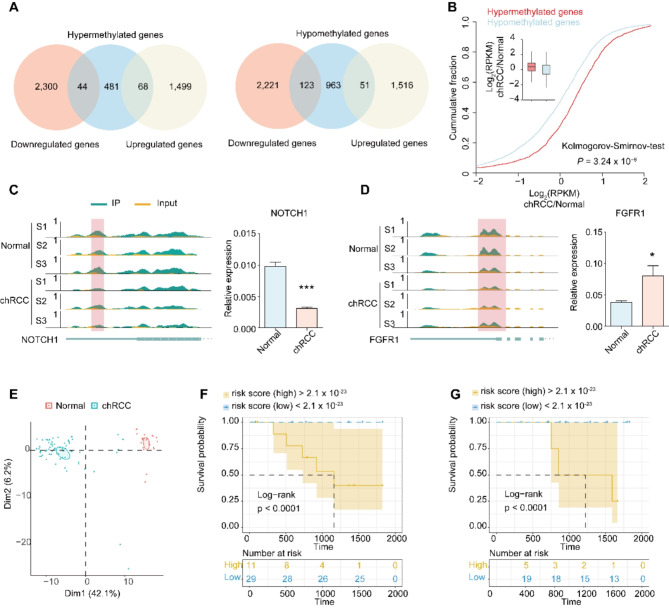
We comprehensively analyzed m6A-SEAL-seq and RNA-seq data in normal and chRCC tissues. The results demonstrated that among the 593 hyper-methylated genes detected by m6A-SEAL-seq, 44 targets tended to be down-regulated and 68 targets tended to be up-regulated (Fig. 5A, left); among the 1,137 hypo-methylated genes, 123 targets tended to be down-regulated and 51 targets tended to be up-regulated (Fig. 5A, right). Further, a significant increase of RPKM was observed in the hyper group compared to the hypo group (Fig. 5B).
Fig. 5.
Conjoint analysis of m6A-SEAL-seq and RNA-seq data. A, Overlap of hypermethylation genes with up-regulation genes and down-regulation genes (left). Overlap of hypomethylation genes with up-regulation genes and down-regulation genes (right). B, Cumulative distribution displaying the expression level changes in mRNAs with hypermethylation or hypomethylation m6A modification. C-D, Integrative genomics viewer (IGV) tracks showing the indicated m6A-seal reads distribution on target transcripts and the relative expression level in Normal and chRCC tissues. (C) NOTCH1, (D) FGFR1. F-G Survival analyses in the training set (F) and testing set (G). Log-rank p < 0.0001 showed a significant survival difference between the two sub-groups
To further analyze the role of DMMGs in cancers, we intersected DMMGs with Cancer Gene Census (CGC) database [49], a database consists of genes with strong indications of a role in cancer, and found 73 and 23 genes were annotated in hypo-methylated and hyper-methylated genes separately (Supplementary Dataset 3 and 4). Among these cancer related genes, 10 genes were differentially expressed genes, including NOCH1 and FGFR1. According to Integrative Genomics Viewer (IGV) software, the m6A modification level of NOTCH1 decreased significantly (Fig. 5C, left), then we measure the expression level of NOTCH1 and found in chRCC tissue, the expression level of NOTCH1 reduced significantly (Fig. 5C, right), suggesting NOTCH1 acts as a tumor suppressor gene in chRCC. Fibroblast growth factor receptor 1 (FGFR1) is a well-known oncogene. We found the m6A modification level of FGFR1 decreased significantly (Fig. 5D, left) and the expression level of FGFR1 increased in chRCC tissue (Fig. 5D, right). We observed differences in the expression patterns of these two genes, with both showing hyper-methylation in their modification levels but opposite expression patterns. This discrepancy indicates potential differences in m6A regulation pathways.
Thus, we conducted principal component analysis of the differential expressed DMMGs in 65 chRCC cases from The Cancer Genome Atlas (TCGA) database. Based on the expression of these genes, we could completely distinguish chRCC samples from normal samples (Fig. 5E). Cox regression screen and least absolute shrinkage and selection operator (LASSO) identified three m6A-dependent signatures (Supplementary Dataset 5) and defined a m6A-dependent cox model in the training set (Fig. S5A). Concordance index (C-index = 0.96) showed that the proposed model has a high prognostic power. In this model, we separated patients into high-risk or low-risk group according to their risk score, and patients with different 5-years survival could be distinguished completely between the two groups (Log-rank p < 0.0001) (Fig. 5F). AUC of ROC curve also confirmed the prognostic power of the m6A-dependent model (Fig. S5B). Then the proposed model was applied to the testing set for prediction. We calculated risk score of each patient in the testing set and assigned to high-risk or low-risk group according to the cut off value in the training set. The Kaplan-Meier survival curve and log-rank between the two groups showed significant difference (Fig. 5G), which demonstrated the high predictive ability of the m6A-dependent model. Furthermore, we also test the m6A-dependent model in ccRCC samples (a cohort of 602 cases from TCGA database) and PRCC dataset (a cohort of 318 cases from TCGA database). The results indicated that the m6A-dependent model is also suitable for ccRCC, but not PRCC (Fig. S5C, D).
Discussion
Despite having the lowest incidence and mortality rates among the three main types of kidney cancer, chRCC still contributes to thousands of deaths worldwide, according to statistics from the GCO in 2022. Over the past few years, there has been a growing effort to elucidate the mechanism of m6A modification in RCC, resulting in extensive accumulation of knowledge regarding the correlation between m6A modification and RCC. However, chRCC has been overlooked. Here, we demonstrated that m6A writer WTAP and m6A erasers FTO and ALKBH5 were downregulated in chRCC tissues, which may result in a different m6A modification pattern in chRCC tissues. By m6A-SEAL-seq, we confirmed that m6A modification pattern in chRCC tissues is distinct from that in normal tissues. The m6A peaks number and m6A peaks per gene in chRCC decreased and the distribution pattern of m6A peaks shifted to 3’UTR orientation in chRCC. Further functional studies showed that genes with hyper- or hypo-methylated peaks were mainly enriched in kidney development and cancer pathogenesis related pathway, which is a further proof of the fundamental role of m6A modification in chRCC. We also found m6A reader YTHDF2 were downregulated in chRCC tissues. The role of YTHDF2 in m6A-dependent gene regulation, particularly its impact on RNA stability, suggests the existence of m6A-dependent RNA degradation and gene dysregulation in chRCC. The down-regulation of NOTCH1 may be attributed to the down-regulation of YTHDF2. Additionally, the observed discrepancy in the expression pattern of FGFR1 indicates potential differences in m6A regulation pathways. Cumulative fraction of genes with hyper-methylated peaks and genes with hypo-methylated peaks indicated that these two group gene sets have different expression pattern in chRCC tissues, which may result from the dysregulation of m6A readers, such as YTHDF2.
In the present study, we depicted transcriptome-wide m6A profiling in normal and chRCC tissues by an antibody-free method. By comparing the m6A peaks in normal tissues and chRCC tissues, we revealed a total of 1730 DMMGs including 593 hyper- and 1137 hypo-methylated genes in chRCC tissues, which may be important factors in chRCC. For example, RARA, one of the hypo-methylated genes, has been demonstrated as a target of FTO in acute myeloid leukemia (AML). The m6A on RARA at UTRs influences the stability of RARA. The decreased m6A modification level of RARA destabilizes RARA mRNA, and then promotes tumorigenesis [50]. By CGC database analysis [49], we found 96 genes were causally implicated in cancers, such as ZEB1, which acts as an oncogene in ccRCC, PBRM1, ASXL2 and SETD2, which act as tumor suppressor genes in ccRCC. These results indicate m6A modification has a major influence on ccRCC, implying the roles of m6A modification in chRCC. Combined with our RNAseq data, we found 10 genes were differential expressed genes, including NOTCH1 and FGFR1. The level of m6A on NOTCH1 at 3’UTR and the expression level of NOTCH1 reduced significantly, which are similar to PER1 in pancreatic cancer and RARA in AML [33, 50]. PER1’s degradation is YTHDF2-dependent manner, while the mechanism underlying the degradation of NOTCH1 and RARA remains unknown. The level of m6A on FGFR1 at 3’UTR also decreased, but the expression level of FGFR1 increased significantly. It is reported that m6A reader IGF2BP3 enhances mRNA stability in an m6A-dependent manner [13, 51], which may account for the upregulation of FGFR1 in chRCC. Further functional studies are needed to clarify the molecular mechanisms of above-mentioned genes in the development of chRCC.
Despite the insights gained from our study, it is important to acknowledge a significant limitation: the small sample size. Our findings are based on data obtained from only three chRCC patients, which may not be sufficient to draw definitive conclusions about m6A modifications in chRCC. While the observed patterns in m6A regulation provide valuable initial insights, larger cohorts are needed to validate these results and ensure that they are broadly applicable to the wider chRCC patient population. Future studies with expanded sample sizes will be essential for confirming the role of m6A modifications in chRCC and for elucidating the underlying mechanisms with greater accuracy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
The project was conceptualized and designed by Baoguo Li, Lulin Ma and Guifang Jia. Experiments were conducted by Zhigang Chen, Wei Zhang, Yang Qian, Nan Zhang,Min Lu, Liyuan Ge, Cheng Liu and Xiaojun Tian. The sequencing data were analyzed by Junbo Yang and Zixin Chen. Zhigang Chen, Junbo Yang and Wei Zhang wrote the manuscript. Baoguo Li, Lulin Ma and Guifang Jia revised the manuscript. All authors reviewed the manuscript and attest that they meet the current ICMJE criteria for authorship.
Funding
This work was supported by the National Basic Research Program of China (2019YFA0802201 and 2017YFA0505201), Beijing Natural Science Foundation (Z200010) and Beijing Haidian Hospital research fund (KYQ 2022018).
Data availability
The raw sequence data of RNA-seq (input), m6A-SEAL-seq have been deposited in the Genome Sequence Archive in the National Genomics Data Center (NGDC), Beijing Institute of Genomics, Chinese Academy of Sciences, under accession number (PRJCA004912).
Declarations
Ethical approval
Our research was conducted in accordance with the principles of the Declaration of Helsinki. The study was approved by The Beijing Haidian Hospital Medical Ethics Committee under the Project number M202231 and approval number 2022 − 112. Written informed consent was obtained from all participants prior to collecting clinical specimens for research purposes. All specimens were stored securely and handled using appropriate procedures to maintain their integrity. De-identified data were used in all analyses to protect patient privacy.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhigang Chen, Junbo Yang and Wei Zhang contributed equally to this work.
Contributor Information
Guifang Jia, Email: guifangjia@pku.edu.cn.
Lulin Ma, Email: puth_malulin@163.com.
Baoguo Li, Email: libaoguo_hdyy@163.com.
References
- 1.Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. 2021;17:245–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-Sáez O, Gajate Borau P, Alonso-Gordoa T, Molina-Cerrillo J, Grande E. Targeting HIF-2 α in clear cell renal cell carcinoma: a promising therapeutic strategy. Crit Rev Oncol Hematol. 2017;111:117–23. [DOI] [PubMed] [Google Scholar]
- 3.Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018;70:127–37. [DOI] [PubMed] [Google Scholar]
- 4.Wolf MM, Kimryn Rathmell W, Beckermann KE. Modeling clear cell renal cell carcinoma and therapeutic implications. Oncogene. 2020;39:3413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCroskey Z, Sim SJ, Selzman AA, Ayala AG, Ro JY. Primary collision tumors of the kidney composed of oncocytoma and papillary renal cell carcinoma: a review. Ann Diagn Pathol. 2017;29:32–6. [DOI] [PubMed] [Google Scholar]
- 6.Rogala J, Kojima F, Alaghehbandan R, Agaimy A, Martinek P, Ondic O, Ulamec M, Sperga M, Michalova K, Pivovarcikova K, Pitra T, Hora M, Ferak I, Marečková J, Michal M, Hes O. Papillary renal cell carcinoma with prominent spindle cell stroma - tumor mimicking mixed epithelial and stromal tumor of the kidney: clinicopathologic, morphologic, immunohistochemical and molecular genetic analysis of 6 cases. Ann Diagn Pathol. 2020;44:151441. [DOI] [PubMed] [Google Scholar]
- 7.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of Tumours of the urinary system and male genital organs-Part A: renal, Penile, and testicular tumours. Eur Urol. 2016;70:93–105. [DOI] [PubMed] [Google Scholar]
- 8.Przybycin CG, Cronin AM, Darvishian F, Gopalan A, Al-Ahmadie HA, Fine SW, Chen YB, Bernstein M, Russo P, Reuter VE, Tickoo SK. Chromophobe renal cell carcinoma: a clinicopathologic study of 203 tumors in 200 patients with primary resection at a single institution. Am J Surg Pathol. 2011;35:962–70. [DOI] [PubMed] [Google Scholar]
- 9.Davis CF, Ricketts CJ, Wang M, Yang L, Cherniack AD, Shen H, Buhay C, Kang H, Kim SC, Fahey CC, Hacker KE, Bhanot G, Gordenin DA, Chu A, Gunaratne PH, Biehl M, Seth S, Kaipparettu BA, Bristow CA, Donehower LA, Wallen EM, Smith AB, Tickoo SK, Tamboli P, Reuter V, Schmidt LS, Hsieh JJ, Choueiri TK, Hakimi AA, Chin L, Meyerson M, Kucherlapati R, Park WY, Robertson AG, Laird PW, Henske EP, Kwiatkowski DJ, Park PJ, Morgan M, Shuch B, Muzny D, Wheeler DA, Linehan WM, Gibbs RA, Rathmell WK. C.J. Creighton, The somatic genomic landscape of chromophobe renal cell carcinoma, Cancer Cell, 26 (2014) 319–330. [DOI] [PMC free article] [PubMed]
- 10.Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, Vendeix FA, Fabris D, Agris PF. The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Res. 2011;39:D195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei LH, Song P, Wang Y, Lu Z, Tang Q, Yu Q, Xiao Y, Zhang X, Duan HC, Jia G. The m(6)a reader ECT2 controls Trichome morphology by affecting mRNA Stability in Arabidopsis. Plant Cell. 2018;30:968–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, Hu YC, Hüttelmaier S, Skibbe JR, Su R, Deng X, Dong L, Sun M, Li C, Nachtergaele S, Wang Y, Hu C, Ferchen K, Greis KD, Jiang X, Wei M, Qu L, Guan JL, He C, Yang J, Chen J. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou Y, Sun J, Wu B, Gao Y, Nie H, Nie Z, Quan S, Wang Y, Cao X, Li S. CPSF30-L-mediated recognition of mRNA m(6)a modification controls alternative polyadenylation of nitrate signaling-related gene transcripts in Arabidopsis. Mol Plant. 2021;14:688–99. [DOI] [PubMed] [Google Scholar]
- 15.Song P, Yang J, Wang C, Lu Q, Shi L, Tayier S, Jia G. Arabidopsis N(6)-methyladenosine reader CPSF30-L recognizes FUE signals to control polyadenylation site choice in liquid-like nuclear bodies. Mol Plant. 2021;14:571–87. [DOI] [PubMed] [Google Scholar]
- 16.Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T, Hamakubo T. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288:33292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, Wang X, Ma HL, Huang CM, Yang Y, Huang N, Jiang GB, Wang HL, Zhou Q, Wang XJ, Zhao YL, Yang YG. Nuclear m(6)a reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–19. [DOI] [PubMed] [Google Scholar]
- 18.Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millán-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N, De Braekeleer E, Ponstingl H, Hendrick A, Vakoc CR, Vassiliou GS, Kouzarides T. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature. 2017;552:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C, 6 N, Modulates methyladenosine, RNA M, Efficiency T. Cell. 2015;161:1388–99. [DOI] [PMC free article] [PubMed]
- 20.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–47. [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)a decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, Zhao X, Li A, Yang Y, Dahal U, Lou XM, Liu X, Huang J, Yuan WP, Zhu XF, Cheng T, Zhao YL, Wang X, Rendtlew Danielsen JM, Liu F, Yang YG. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 2012;8:e1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RP, Dai Q, Hao YJ, Yang X, Zhao WM, Tong WM, Wang XJ, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan HE, Klungland A, Yang YG, He C. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Theler D, Kaminska KH, Hiller M, de la Grange P, Pudimat R, Rafalska I, Heinrich B, Bujnicki JM, Allain FH, Stamm S. The YTH domain is a novel RNA binding domain. J Biol Chem. 2010;285:14701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, Lu Z, He C, Min J. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927–9. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Zhao D, Wu J, Shi Y. Structure of the YTH domain of human YTHDF2 in complex with an m(6)a mononucleotide reveals an aromatic cage for m(6)a recognition. Cell Res. 2014;24:1490–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, Cheng C, Li L, Pi J, Si Y, Xiao H, Li L, Rao S, Wang F, Yu J, Yu J, Zou D, Yi P. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Peng C, Chen J, Chen D, Yang B, He B, Hu W, Zhang Y, Liu H, Dai L, Xie H, Zhou L, Wu J, Zheng S. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X, Li K, Jiang W, Hu Y, Xiao W, Huang Y, Feng Y, Pan Q, Wan R. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Mol Cancer. 2020;19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, Yuan WB, Lu JC, Zhou ZJ, Lu Q, Wei JF, Yang H. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Zhang N, Zhang E, Wang T, Jiang L, Wang X, Zheng J. A novel m(6)a gene signature Associated with Regulatory Immune function for Prognosis Prediction in Clear-Cell Renal Cell Carcinoma. Front Cell Dev Biol. 2020;8:616972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Zhang H, Chen Q, Wan Z, Gao X, Qian W. Identification of METTL14 in Kidney Renal Clear Cell Carcinoma Using Bioinformatics Analysis, Dis Markers, 2019 (2019) 5648783. [DOI] [PMC free article] [PubMed]
- 37.Zhou J, Wang J, Hong B, Ma K, Xie H, Li L, Zhang K, Zhou B, Cai L, Gong K. Gene signatures and prognostic values of m6A regulators in clear cell renal cell carcinoma - a retrospective study using TCGA database. Aging. 2019;11:1633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Z, Jing C, Xiao C, Li T, Wang Y. Prognostic risk signature based on the expression of three m6A RNA methylation regulatory genes in kidney renal papillary cell carcinoma. Aging. 2020;12:22078–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Zhou C, Sun Y, He X, Xue D. M(6)a RNA modification modulates gene expression and cancer-related pathways in clear cell renal cell carcinoma. Epigenomics. 2020;12:87–99. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Xiao Y, Dong S, Yu Q, Jia G. Antibody-free enzyme-assisted chemical approach for detection of N(6)-methyladenosine. Nat Chem Biol. 2020;16:896–903. [DOI] [PubMed] [Google Scholar]
- 41.Xia H, Zhang Z, Luo C, Wei K, Li X, Mu X, Duan M, Zhu C, Jin L, He X, Tang L, Hu L, Guan Y, Lam DCC, Yang J. MultiPrime: A reliable and efficient tool for targeted next-generation sequencing, iMeta, n/a e143. [DOI] [PMC free article] [PubMed]
- 42.Martin M. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet J, 17 (2011).
- 43.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui X, Zhang L, Meng J, Rao MK, Chen Y, Huang Y. MeTDiff: a Novel Differential RNA methylation analysis for MeRIP-Seq Data. IEEE/ACM Trans Comput Biol Bioinform. 2018;15:526–34. [DOI] [PubMed] [Google Scholar]
- 47.Love MI, Huber W, Anders S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Shi X, Huang T, Zhao X, Chen W, Gu N, Zhang R. Dynamic landscape and evolution of m6A methylation in human. Nucleic Acids Res. 2020;48:6251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sondka Z, Bamford S, Cole CG, Ward SA, Dunham I, Forbes SA. The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat Rev Cancer. 2018;18:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, Qin X, Tang L, Wang Y, Hong GM, Huang H, Wang X, Chen P, Gurbuxani S, Arnovitz S, Li Y, Li S, Strong J, Neilly MB, Larson RA, Jiang X, Zhang P, Jin J, He C, Chen J. FTO plays an oncogenic role in Acute myeloid leukemia as a N(6)-Methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Y, Niu S, Wang Y, Duan L, Pan Y, Tong Z, Zhang X, Yang Z, Peng B, Wang X, Han X, Li Y, Cheng T, Liu Y, Shang L, Liu T, Yang X, Sun M, Jiang S, Zhang C, Zhang N, Ye Q, Gao S. DMDRMR-Mediated regulation of m(6)A-Modified CDK4 by m(6)a reader IGF2BP3 drives ccRCC progression. Cancer Res. 2021;81:923–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data of RNA-seq (input), m6A-SEAL-seq have been deposited in the Genome Sequence Archive in the National Genomics Data Center (NGDC), Beijing Institute of Genomics, Chinese Academy of Sciences, under accession number (PRJCA004912).