Abstract
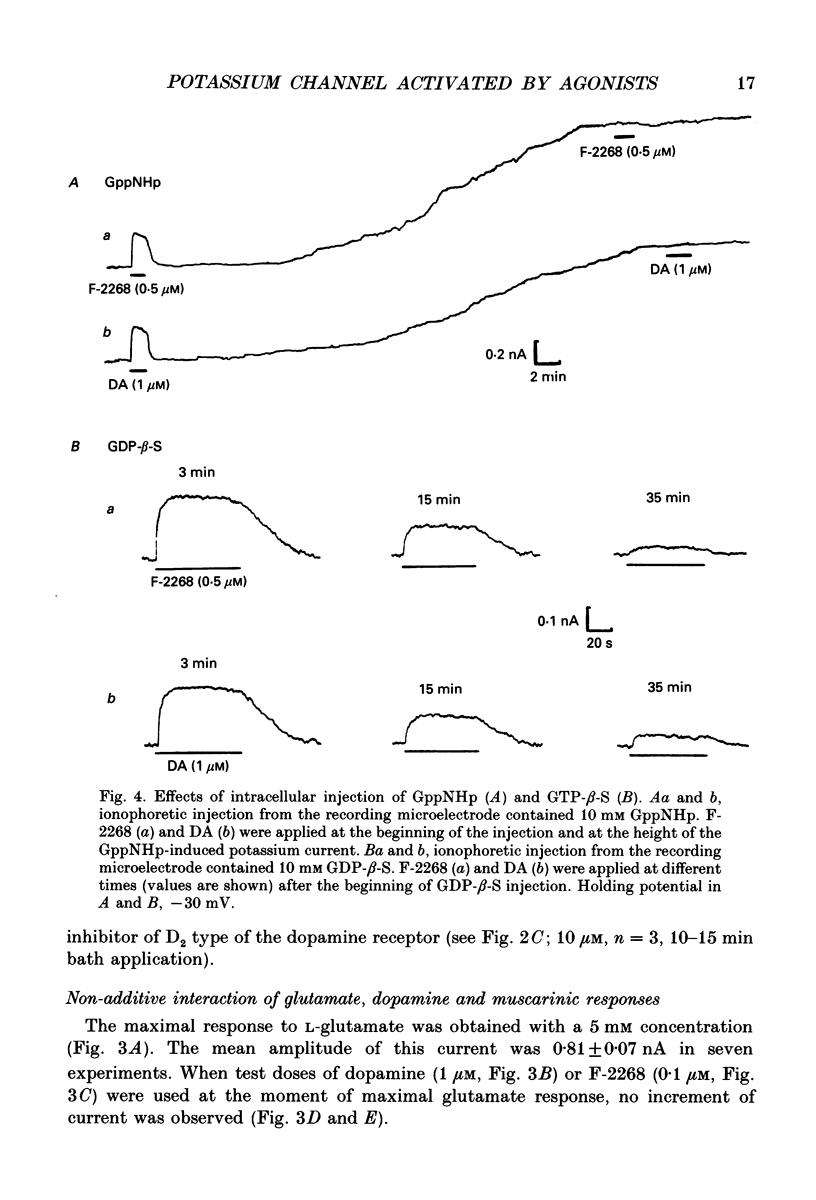
1. The potassium currents evoked in isolated and identified neurones of molluscan pedal ganglia by either glutamate, dopamine or the muscarinic agonist F-2268 were investigated using voltage and patch clamp techniques. 2. Potassium currents induced by either dopamine or F-2268 could be blocked by pertussis toxin, as well as by a prolonged intracellular injection of the G protein inhibitor, GDP-beta-S. Loading the neurones with the G protein activator, GppNHp, on the other hand, induced a potassium current. This current was not additive to the currents evoked by agonist application. 3. Intracellular injection of the calcium buffer BAPTA failed to affect any of the agonist-induced currents, although it effectively blocked the after-hyperpolarization following directly evoked action potentials. 4. The activity of the potassium channels seen in cell-attached patches was greatly enhanced by application to the bath of either glutamate, dopamine, or F-2268. 5. The only effect of an addition of agonists to the bath was to increase the open probability (Po) of the K+ channel already active in the control conditions. The identity of the spontaneously active and agonist-activated channels was concluded from the identity of their channel conductances, rectification properties and current amplitudes. 6. Phorbol-12,13-dibutyrate, when applied to the bath, induced an increase in open time and caused an increase in Po, as did the agonists. Staurosporine completely prevented changes of Po induced by the phorbol ester but not those induced by the agonists. 7. The same inwardly rectifying potassium channel may be opened by both the receptor-linked G protein (with glutamate, dopamine, F-2268) and by protein kinase C (with phorbol ester) activation. 8. Strong evidence was obtained against the involvement of any known secondary messenger systems (formation of nucleotides, phosphoinositide turnover and subsequent activation of protein kinase C, formation of nitric oxide, metabolism of arachidonic acid) in the transduction mechanism of F-2268-, dopamine- and glutamate-induced responses. 9. Since none of the known secondary messenger systems seems to affect the activation by agonists applied to receptors outside the patch of channels located under the patch electrode, it appears that some as yet undescribed linking system must exist that could connect the spatially separated receptor-G protein complex and the potassium channel.
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Anichkov S. V., Losev N. A. The relationship of structure and pharmacological action of some cholinolytics. Gen Pharmacol. 1980;11(1):31–32. doi: 10.1016/0306-3623(80)90008-7. [DOI] [PubMed] [Google Scholar]
- Ascher P., Chesnoy-Marchais D. Interactions between three slow potassium responses controlled by three distinct receptors in Aplysia neurones. J Physiol. 1982 Mar;324:67–92. doi: 10.1113/jphysiol.1982.sp014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VYu, Gapon S. A., Magazanik L. G. Different types of glutamate receptors in isolated and identified neurones of the mollusc Planorbarius corneus. J Physiol. 1991 Aug;439:15–35. doi: 10.1113/jphysiol.1991.sp018654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VYu, Gapon S. A., Magazanik L. G. Transduction mechanism for glutamate-induced potassium current in neurones of the mollusc Planorbarius corneus. J Physiol. 1992 Sep;455:33–50. doi: 10.1113/jphysiol.1992.sp019289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V. Guanosine 5'-triphosphate analogue activates potassium current modulated by neurotransmitters in Aplysia neurones. J Physiol. 1988 Dec;407:15–40. doi: 10.1113/jphysiol.1988.sp017401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M. A cellular logic for G protein-coupled ion channel pathways. FASEB J. 1991 May;5(8):2175–2179. doi: 10.1096/fasebj.5.8.1708737. [DOI] [PubMed] [Google Scholar]
- Brown A. M., McCrohan C. R. Properties of cyclic AMP-dependent inward current in two identified neurons of the snail Lymnaea stagnalis. Comp Biochem Physiol C. 1992;101(1):131–136. doi: 10.1016/0742-8413(92)90210-x. [DOI] [PubMed] [Google Scholar]
- Christie M. J., North R. A. Agonists at mu-opioid, M2-muscarinic and GABAB-receptors increase the same potassium conductance in rat lateral parabrachial neurones. Br J Pharmacol. 1988 Nov;95(3):896–902. doi: 10.1111/j.1476-5381.1988.tb11719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshenko P. A., Kostyuk P. G., Luk'yanetz E. A. Modulation of calcium current by calmodulin antagonists. Neuroscience. 1988 Dec;27(3):1073–1080. doi: 10.1016/0306-4522(88)90211-4. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Francis G. S., Cohn J. N. Heart failure: mechanisms of cardiac and vascular dysfunction and the rationale for pharmacologic intervention. FASEB J. 1990 Oct;4(13):3068–3075. doi: 10.1096/fasebj.4.13.2210153. [DOI] [PubMed] [Google Scholar]
- Ger B. A., Zeimal E. V. Pharmacological study of two kinds of cholinoreceptors on the membrane of identified completely isolated neurones of Planorbarius corneus. Brain Res. 1977 Jan 31;121(1):131–139. doi: 10.1016/0006-8993(77)90443-7. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D., Yakel J. L. Muscarinic enhancement of the voltage-dependent calcium current in an identified snail neuron. J Physiol. 1991 Mar;434:85–105. doi: 10.1113/jphysiol.1991.sp018460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., Kado R. T. Voltage-current relationship of a carbachol-induced potassium-ion pathway in Aplysia neurones. J Physiol. 1975 Mar;245(3):713–725. doi: 10.1113/jphysiol.1975.sp010870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachman A. N., Samoilova M. V., Snetkov V. A. Odinochnyi kalievyi kanal s anomal'nym vypriamlenium v neironakh molliuska. Neirofiziologiia. 1989;21(1):31–38. [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Cyclic AMP-induced slow inward current in depolarized neurons of Aplysia californica. J Neurosci. 1990 Oct;10(10):3194–3207. doi: 10.1523/JNEUROSCI.10-10-03194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Synaptic block of a calcium-activated potassium conductance in Aplysia neurones. J Physiol. 1985 Dec;369:439–474. doi: 10.1113/jphysiol.1985.sp015910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko N. I., Kostyuk P. G., Shcherbatko A. D. The effect of intracellular cAMP injections on stationary membrane conductance and voltage- and time-dependent ionic currents in identified snail neurons. Brain Res. 1983 Jun 6;268(2):321–338. doi: 10.1016/0006-8993(83)90499-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Sasaki K., Sato M., Shozushima M., Takashima K. Dopamine-induced depolarizing responses associated with negative slope conductance in LB-cluster neurones of Aplysia. J Physiol. 1988 Dec;407:199–213. doi: 10.1113/jphysiol.1988.sp017410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Sasaki K., Takashima K., Sato M. Desensitization of dopamine receptors observed in Aplysia ganglion cells. Jpn J Physiol. 1987;37(2):327–331. doi: 10.2170/jjphysiol.37.327. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Greengard P. Lipoxygenase metabolites of arachidonic acid in neuronal transmembrane signalling. Trends Pharmacol Sci. 1990 Sep;11(9):367–373. doi: 10.1016/0165-6147(90)90182-8. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Burgess G. M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989 Jun;10(6):218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Selyanko A. A., Smith P. A., Zidichouski J. A. Effects of muscarine and adrenaline on neurones from Rana pipiens sympathetic ganglia. J Physiol. 1990 Jun;425:471–500. doi: 10.1113/jphysiol.1990.sp018114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman M. S., Sekiguchi K., Nishizuka Y. Modulation of ion channel activity: a key function of the protein kinase C enzyme family. Pharmacol Rev. 1989 Jun;41(2):211–237. [PubMed] [Google Scholar]
- Shen K. Z., North R. A., Surprenant A. Potassium channels opened by noradrenaline and other transmitters in excised membrane patches of guinea-pig submucosal neurones. J Physiol. 1992 Jan;445:581–599. doi: 10.1113/jphysiol.1992.sp018941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Levitan I. B. Intraneuronal guanylyl-imidodiphosphate injection mimics long-term synaptic hyperpolarization in Aplysia. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4689–4692. doi: 10.1073/pnas.73.12.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A., Siegelbaum S. A. Opposing modulation of S-K+ channel activity by cyclic AMP and arachidonic acid metabolites. Prog Clin Biol Res. 1990;334:323–338. [PubMed] [Google Scholar]


