Abstract
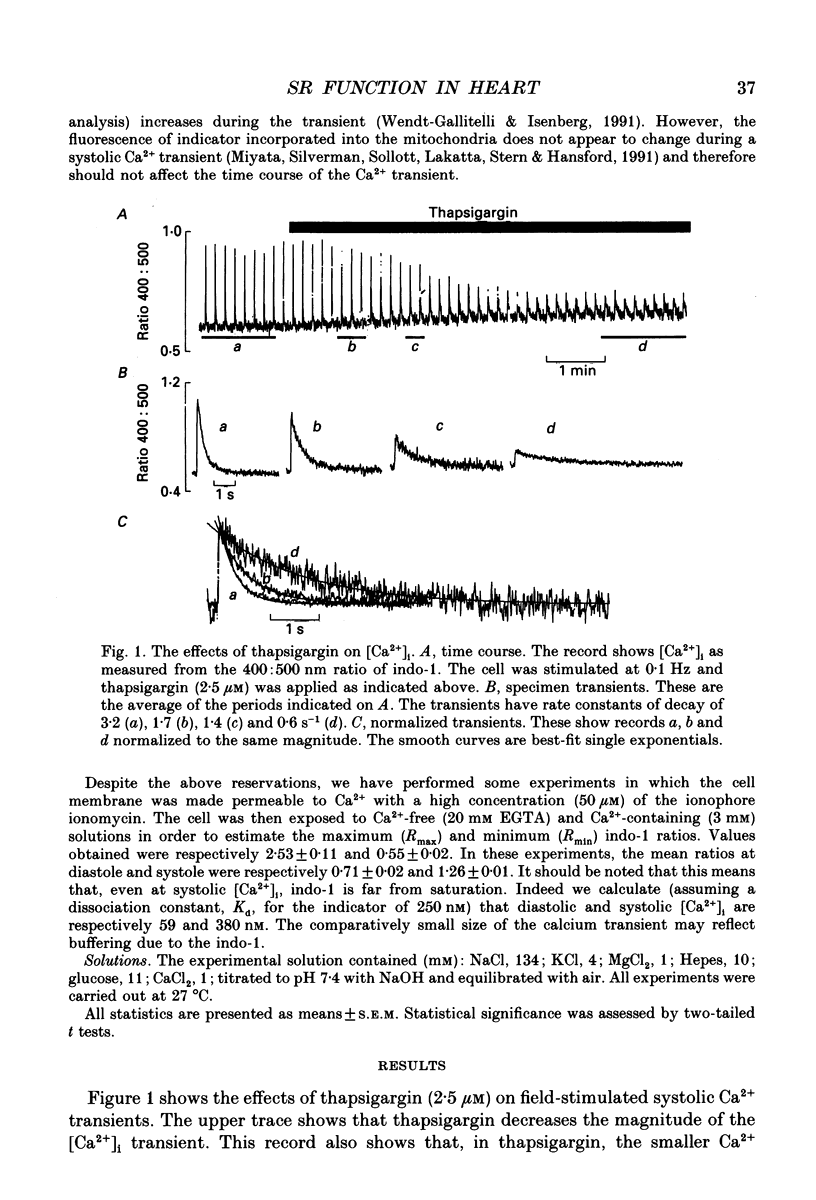
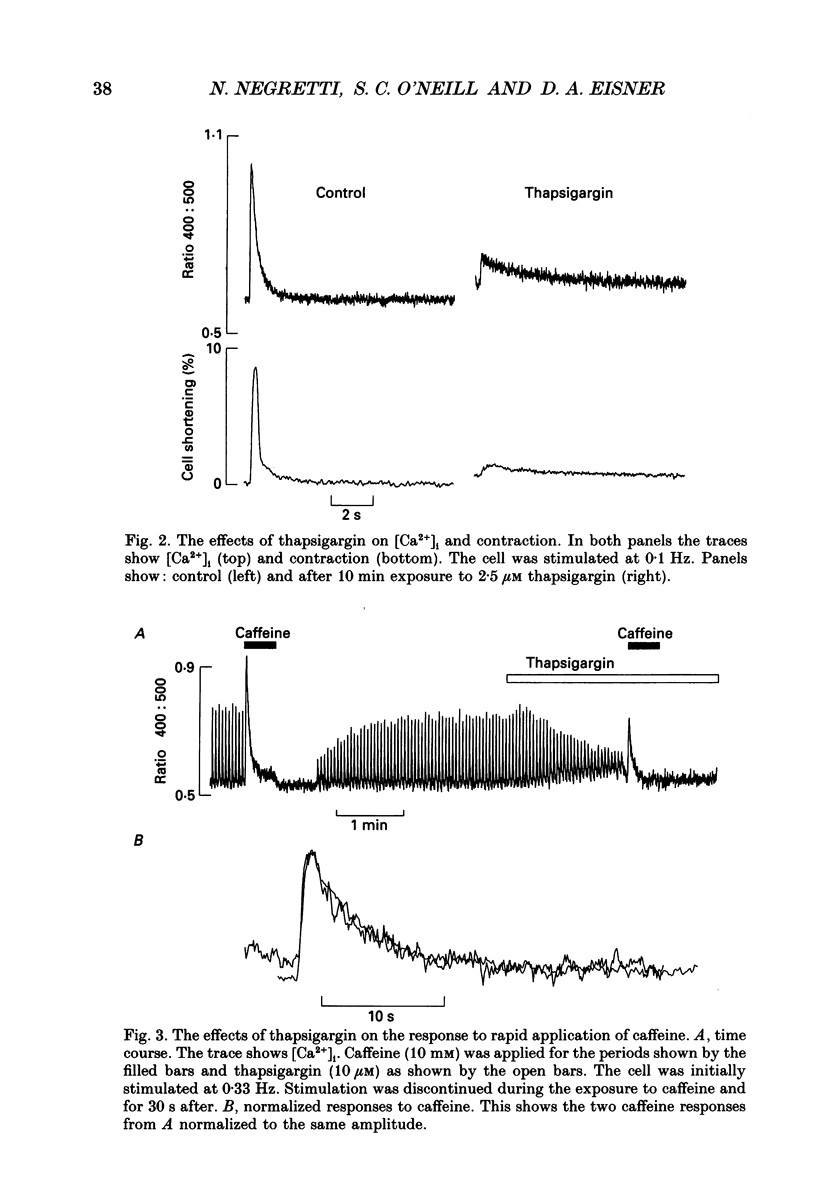
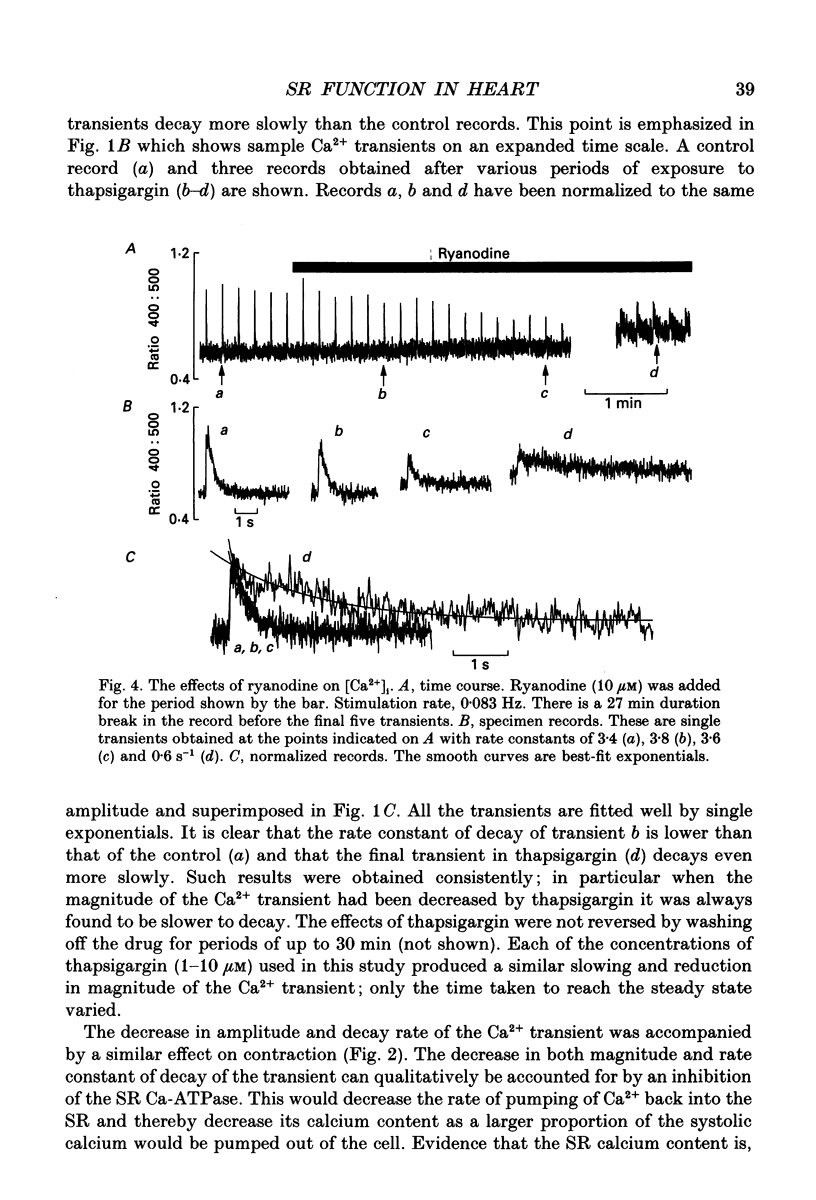
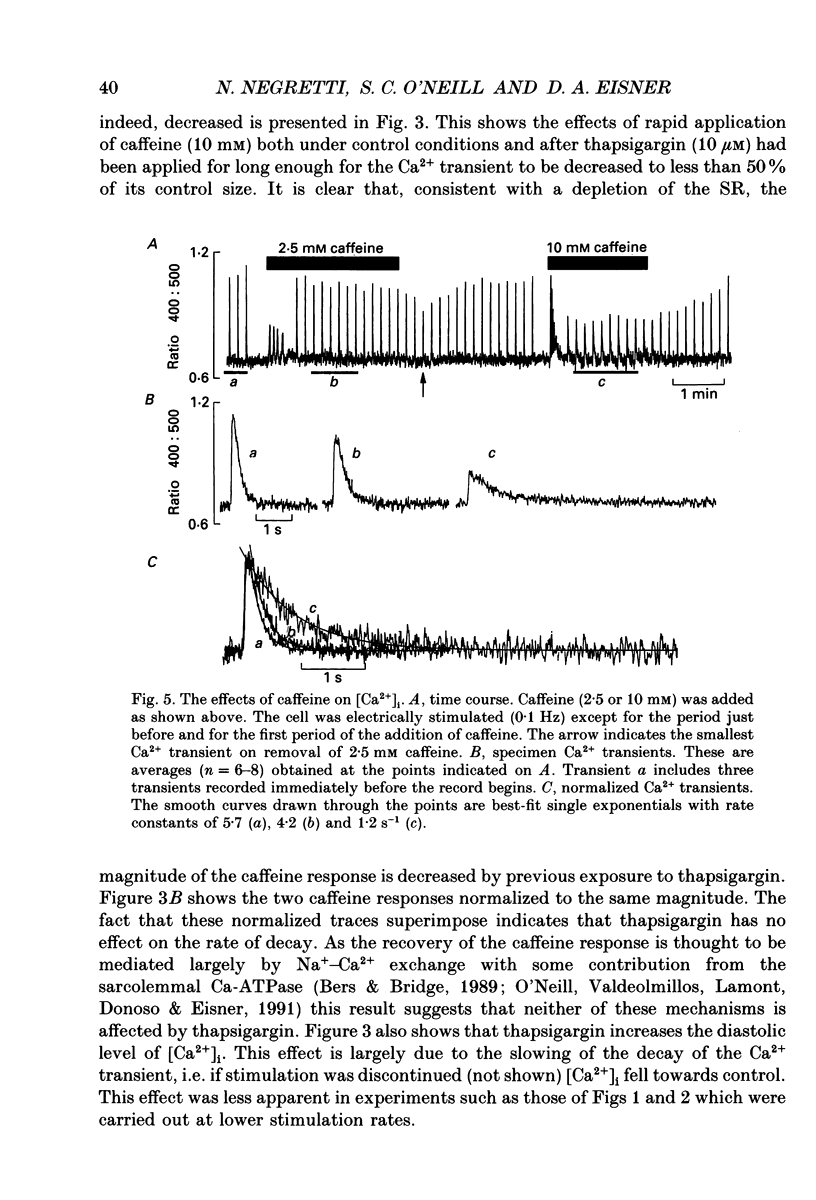
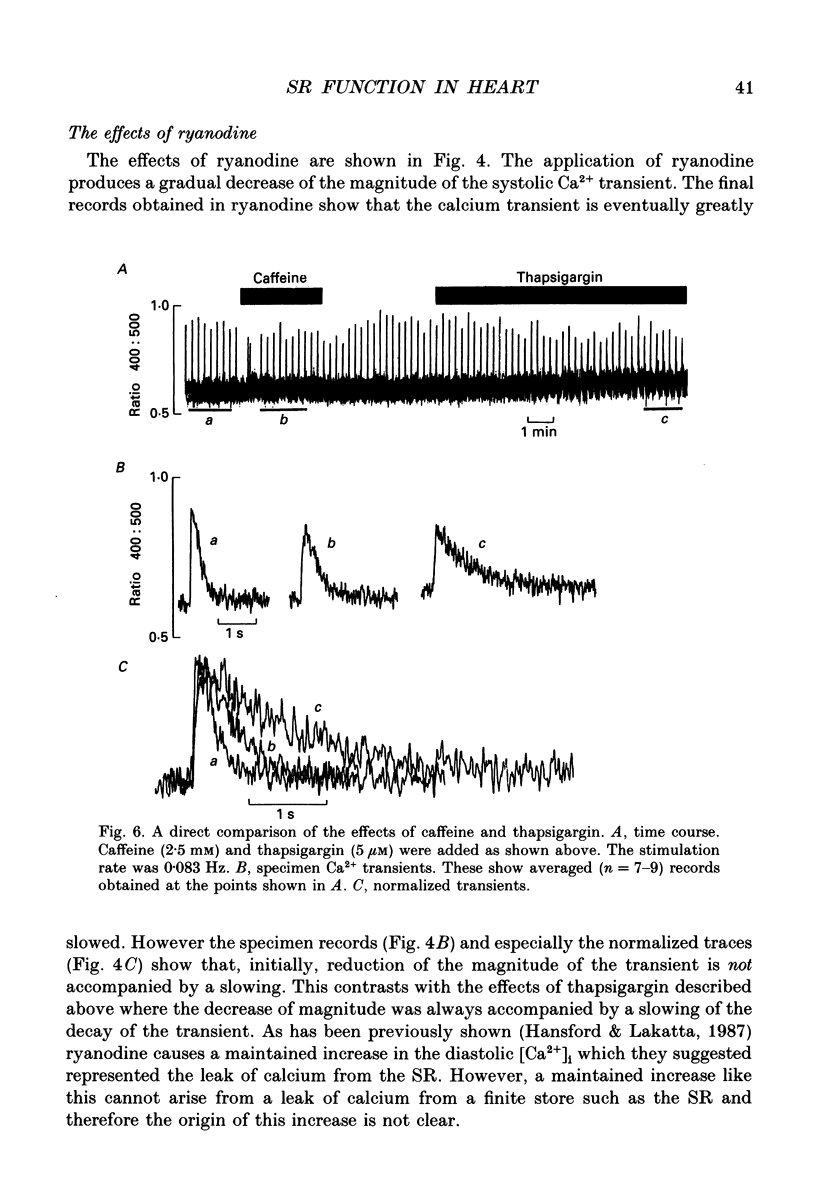
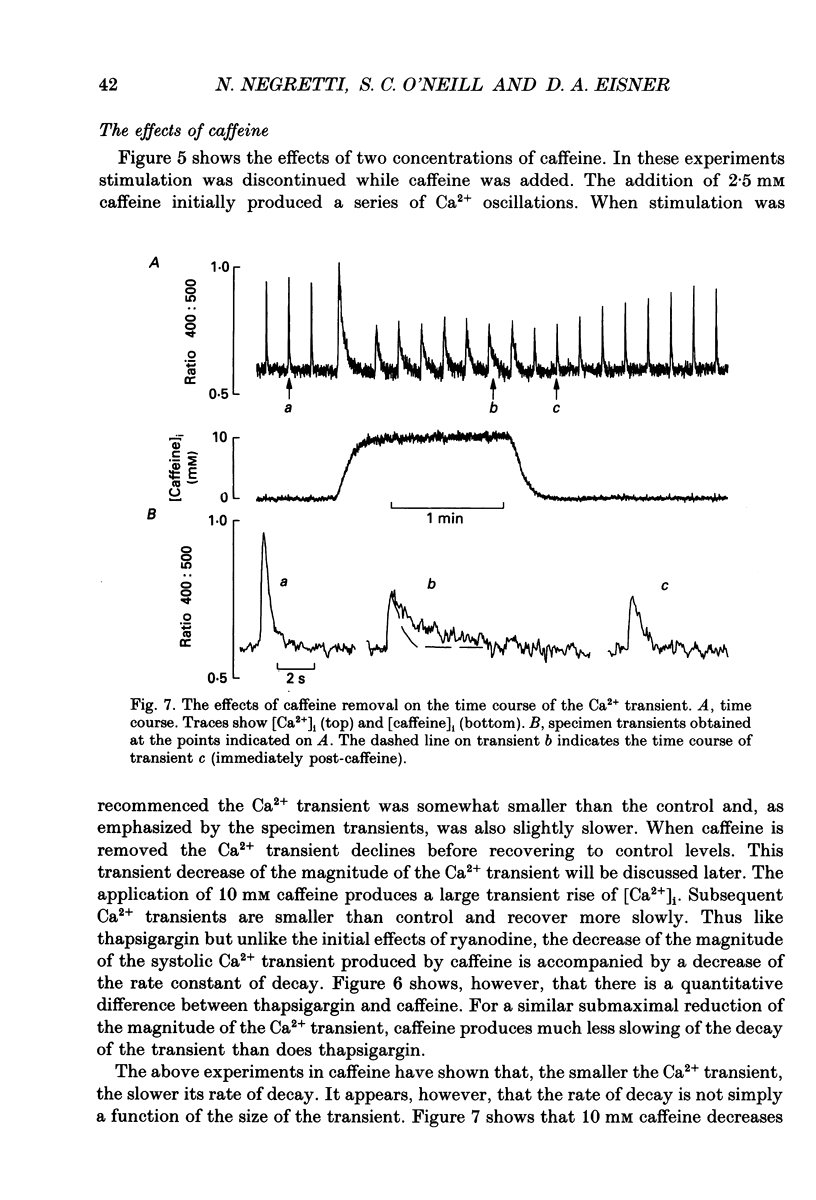
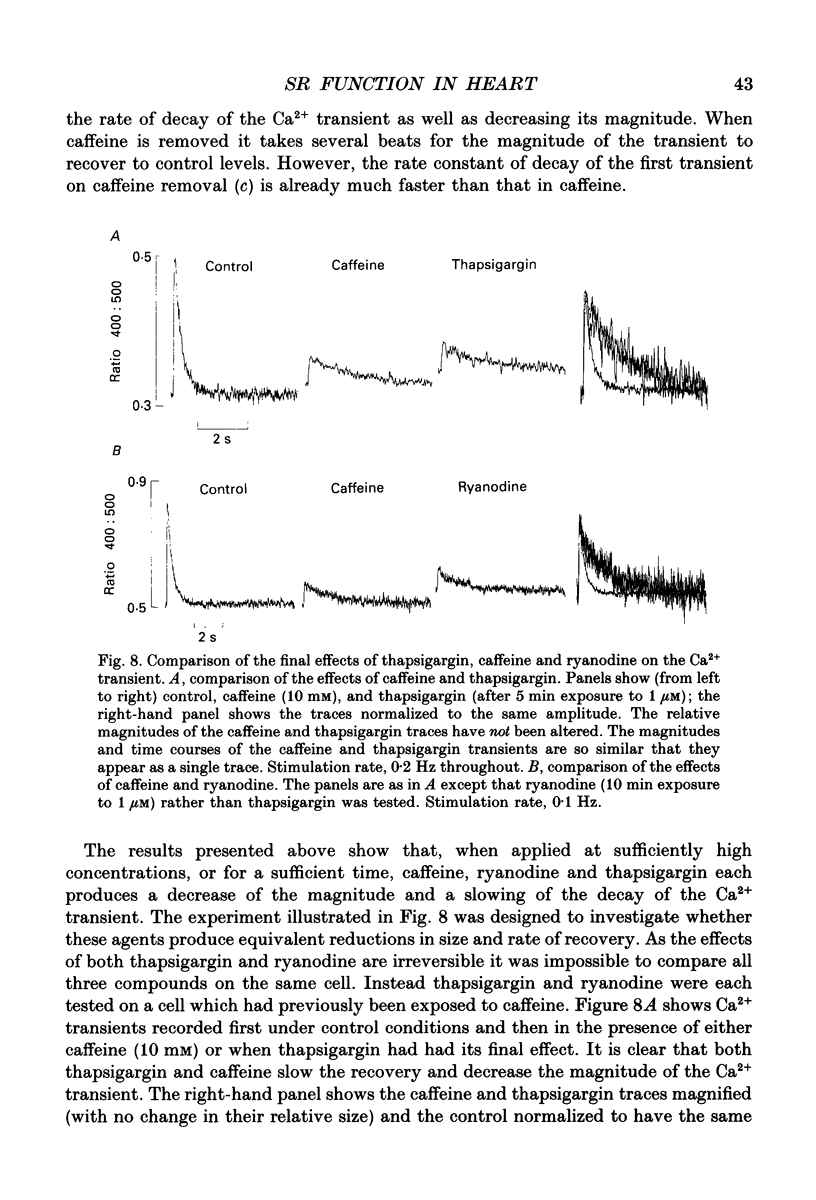
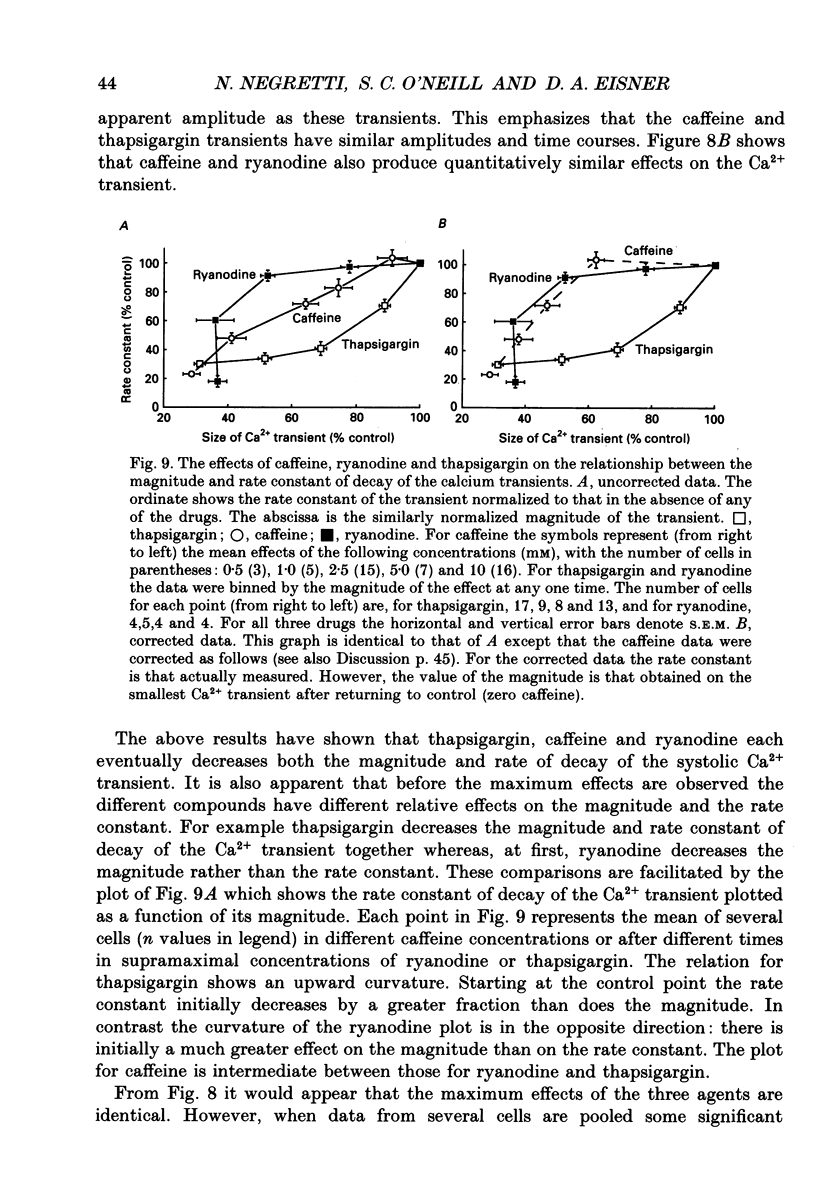
1. The effects of thapsigargin, ryanodine and caffeine were examined on systolic Ca2+ transients in indo-1-loaded rat ventricular myocytes. 2. Thapsigargin (1-10 microM) decreased the magnitude of the Ca2+ transient. This was accompanied by a decrease of the rate constant of decay of the transient. 3. Ryanodine (1-10 microM) decreased the magnitude of the Ca2+ transient. Initially there was no change in the rate of decay but further reduction of the magnitude was accompanied by a slowing. 4. Caffeine (0.5-10 mM) decreased the magnitude of the Ca2+ transient and its rate of decay. These effects were graded with caffeine concentration. 5. For a given submaximal reduction of the magnitude of the Ca2+ transient, the effect on the rate of decay was greatest for thapsigargin, least for ryanodine and intermediate for caffeine. 6. The above data are reproduced by a model in which all three agents decrease the magnitude of the Ca2+ transient by decreasing the calcium content of the sarcoplasmic reticulum (SR) (thapsigargin by inhibiting the Ca2+ pump and ryanodine and caffeine by increasing the leak of Ca2+ from the SR). The decreased contribution of the SR will thereby slow relaxation. The fact that thapsigargin inhibits the SR Ca2+ pump accounts for the observation that, for a given decrease of amplitude, it has more effect than the other agents on the rate of decay. The difference between caffeine and ryanodine is suggested to arise because caffeine potentiates Ca2+ release from the SR and thereby attenuates the effect of the decreased SR calcium content on the magnitude of the Ca2+ transient.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Baró I., O'Neill S. C., Eisner D. A. Changes of intracellular [Ca2+] during refilling of sarcoplasmic reticulum in rat ventricular and vascular smooth muscle. J Physiol. 1993 Jun;465:21–41. doi: 10.1113/jphysiol.1993.sp019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ Res. 1989 Aug;65(2):334–342. doi: 10.1161/01.res.65.2.334. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in guinea-pig cardiac cells: exchange current and changes in intracellular Ca2+. J Physiol. 1989 Jul;414:499–520. doi: 10.1113/jphysiol.1989.sp017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Olson C. B., Jewell B. R., Bravený P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Evidence for a dual mechanism of action. Circ Res. 1972 Apr;30(4):367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Wier W. G. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of caffeine on the intracellular [Ca2+] transient, membrane currents, and contraction. J Gen Physiol. 1984 Mar;83(3):417–433. doi: 10.1085/jgp.83.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S., Bloebaum P., Snowdowne K. W. Sarcoplasmic reticulum interacts with the Ca(2+) indicator precursor fura-2-am. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1153–1162. doi: 10.1016/s0006-291x(86)80403-x. [DOI] [PubMed] [Google Scholar]
- Inesi G., de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem. 1989 Apr 5;264(10):5929–5936. [PubMed] [Google Scholar]
- Kijima Y., Ogunbunmi E., Fleischer S. Drug action of thapsigargin on the Ca2+ pump protein of sarcoplasmic reticulum. J Biol Chem. 1991 Dec 5;266(34):22912–22918. [PubMed] [Google Scholar]
- Meissner G., Henderson J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987 Mar 5;262(7):3065–3073. [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Mitchell R. D., Simmerman H. K., Jones L. R. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988 Jan 25;263(3):1376–1381. [PubMed] [Google Scholar]
- Miyata H., Silverman H. S., Sollott S. J., Lakatta E. G., Stern M. D., Hansford R. G. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991 Oct;261(4 Pt 2):H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- O'Neill S. C., Donoso P., Eisner D. A. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S. C., Eisner D. A. A mechanism for the effects of caffeine on Ca2+ release during diastole and systole in isolated rat ventricular myocytes. J Physiol. 1990 Nov;430:519–536. doi: 10.1113/jphysiol.1990.sp018305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S. C., Valdeolmillos M., Lamont C., Donoso P., Eisner D. A. The contribution of Na-Ca exchange to relaxation in mammalian cardiac muscle. Ann N Y Acad Sci. 1991;639:444–452. doi: 10.1111/j.1749-6632.1991.tb17331.x. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am J Physiol. 1989 Feb;256(2 Pt 2):H328–H333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990 Apr;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Valdeolmillos M., Eisner D. A., Allen D. G. Effects of rapid application of caffeine on intracellular calcium concentration in ferret papillary muscles. J Gen Physiol. 1988 Sep;92(3):351–368. doi: 10.1085/jgp.92.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Yue D. T., Marban E. Effects of ryanodine on intracellular Ca2+ transients in mammalian cardiac muscle. Fed Proc. 1985 Dec;44(15):2989–2993. [PubMed] [Google Scholar]


