Abstract
Objective
Pulmonary infection is one of the leading causes of death in patients with ANCA-associated vasculitis (AAV). It is sometimes difficult to differentiate pulmonary infection from pulmonary involvement of vasculitis in AAV patients. Fiberoptic bronchoscopy and bronchoalveolar lavage fluid (BALF) assays are useful diagnostic methods. In addition to conventional microbiological tests (CMTs), metagenomic next-generation sequencing (mNGS) facilitates rapid and sensitive detection of various pathogens. The current study aimed to evaluate the advantages of additional BALF mNGS in the management of pulmonary infection in AAV patients.
Methods
27 patients with active AAV and suspected pulmonary infection whose BALF samples were tested by mNGS and CMTs and 17 active AAV patients whose BALF were tested by CMTs alone were retrospectively recruited. The results of microbiological tests, and adjustments of treatment following BALF mNGS, were described. The durations of antimicrobial treatment and in-hospital mortality in patients were compared.
Results
Among the 27 patients whose BALF samples were tested by mNGS, 25.9% of patients did not have evidence of pathogenic microorganism in their BALF samples, 55.6% had polymicrobial infections, including bacteria, fungi and viruses. Of these 27 patients, 40.7% did not have evidence of pathogenic microorganism in their BALF or serum samples according to CMTs. Patients in the BALF mNGS/CMT group received a significantly shorter duration of antibacterial and total antimicrobial treatment than patients in the CMT alone group (17.3 ± 14.7 vs. 27.9 ± 19.0 days, P = 0.044; 18.9 ± 15.0 vs. 29.5 ± 17.7 days, P = 0.040, respectively). Fewer patients in the BALF mNGS/CMT group died than in the CMT alone group (4/27 vs. 7/17, P = 0.049).
Conclusion
Compared with CMT alone, additional mNGS tests may shorten the duration of antimicrobial treatment and possibly decrease death from severe infection by providing precise and quick diagnosis of infection.
Keywords: Antineutrophil cytoplasmic antibody, Vasculitis, Pulmonary involvement, Pulmonary infection, Metagenomic next-generation sequencing
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) comprises granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA) [1, 2]. The lung and kidney are the two most involved organs in AAV. Pulmonary involvement occurs in 25–80% of AAV patients [3–6], including interstitial lung disease, cavity, nodules, and alveolar hemorrhage. Diffuse alveolar hemorrhage (DAH) is one of the most severe manifestations of AAV [7], significantly contributing to morbidity and mortality.
AAV is usually fatal when left untreated, with a 1-year mortality rate of approximately 80% [8]. Although the introduction of immunosuppressive therapy substantially improved the outcome of AAV, it was also associated with high rates of adverse events, especially secondary infection [9]. Consistent with studies from other groups [10, 11], we found that secondary infection was the leading cause of death during the first year after the diagnosis of AAV [12], and the majority of infectious complication was pulmonary infection [13]. Therefore, early recognition and appropriate treatment in time are of great importance.
However, it is sometimes difficult to differentiate pulmonary infection from pulmonary involvement of vasculitis in AAV patients due to similar clinical manifestations (e.g. fever, cough and hemoptysis), laboratory findings (e.g. elevated white blood cell and neutrophil counts in peripheral blood, C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]) and imaging features (patches, masses, ground-glass opacities and consolidations). Moreover, given that the treatment strategies for pulmonary involvement of active AAV and pulmonary infection are opposite, the challenging differential diagnosis presents difficult treatment decisions. Misdiagnosing pulmonary infection as active AAV pulmonary involvement and thus treating with aggressive immunosuppressive therapy could result in disastrous aggravation of infection. Fiberoptic bronchoscopy and bronchoalveolar lavage fluid (BALF) assays are useful diagnostic methods when a co-existing infection is suspected in AAV patients with pulmonary involvement. In addition to conventional microbiological tests (CMTs) of BALF, metagenomic next-generation sequencing (mNGS) is a nucleic acid sequencing technique with a high-throughput capacity and an approach that can theoretically detect any microorganism in the bio-sample, including BALF [14, 15]. Various studies have reported the application of mNGS to detect pathogens in pulmonary infection [16–19]. In the study by Peng et al., which involved immunocompromised patients with pulmonary infection, it was found that the diagnostic performance of BALF mNGS was similar to that of comprehensive CMTs, but when there is a lack of consideration of potential pathogens, the combination of mNGS and CMTs might be a better diagnostic strategy [18].
To our knowledge, there are few, if any, reports on the application of mNGS in AAV. Thus, the current study evaluated the advantages of additional BALF mNGS in the management of pulmonary infection in patients with AAV.
Materials and methods
Patients
AAV patients diagnosed at Peking University First Hospital between March 2019 and March 2022 were reviewed. Treatment protocols were described previously [20]. In brief, patients received the induction therapy typically including corticosteroids in combination with cyclophosphamide (CTX) or rituximab (RTX). Patients with severe pulmonary hemorrhage or acute renal failure requiring dialysis at diagnosis received additional methylprednisolone pulse and plasmapheresis. For maintenance therapy, daily oral azathioprine (AZA) or RTX was administered. Those who were clinically suspected of pulmonary infection and underwent fiberoptic bronchoscopy were retrospectively recruited. All patients met the Chapel Hill Consensus Conference criteria for AAV [1]. Patients with secondary vasculitis or with other comorbid renal diseases were excluded. 27 patients whose BALF samples were simultaneously available for mNGS tests besides CMTs (termed the BALF mNGS/CMT group), and 17 patients whose BALF was only tested by CMTs (termed the CMT alone group), were included in the study. This research was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of the Peking University First Hospital. Written informed consent was obtained from the patients or their guardians.
Data collection
All the clinical and laboratory data were collected from the medical records of the patients at admission and at the time of bronchoscopies, including age, sex, vital signs, diagnosis, ANCA serotype, disease phenotype, organ involvement, laboratory data (serum creatinine, CRP, ESR, white blood cell [WBC], neutrophil and lymphocyte counts, hemoglobin, platelet count, CD4 + T cells, serum albumin, lactate dehydrogenase [LDH] and arterial blood gas analysis), immunosuppressive therapies, antimicrobial treatment, concomitant treatment, respiratory support, response to treatment and intensive care unit (ICU) stay. The estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Eq. [21]. The risk scores CRB65 were calculated based on the initial available data, which involve mental status, respiratory rate, blood pressure and age [22].
Specimen collection
All bronchoscopies were performed by experienced bronchoscopists. The target lesion was decided according to the chest CT beforehand. A standard bronchoalveolar lavage was collected before any other bronchoscopy procedures to avoid possible contamination. Once collected, BALF specimens were immediately sent to the clinical microbiology laboratory for CMTs and to a commercial laboratory for mNGS.
Conventional microbiological tests
Comprehensive CMTs were performed on BALF, serum, sputum and throat swab samples, including culture, antigen detection, multiplex polymerase chain reaction (PCR), and direct microscopic examination of specimens according to standard procedures [23, 24]. Gram staining, acid-fast staining, hexamine silver staining, the galactomannan antigen detection test (GM test), cryptococcal capsular polysaccharide antigen test and Aspergillus antibody detection with BALF samples, interferon-gamma release assay (T-SPOT. TB) [1, 3], -β-D-glucan test (G test) and GM test with serum samples were performed to identify bacteria, including Mycobacterium tuberculosis, and fungi, including Pneumocystis carinii, Cryptococcus neoformans, Candida and Aspergillus.
mNGS of BALF
mNGS was performed once the sample was obtained. The total turnaround time is approximately 24 h (5 h for shipping and 19 h for testing). BALF samples were collected from patients for metagenomic analysis pipeline for microbial identification (MAPMI). Total DNA and RNA were extracted from clinical samples by using a MAPMI sample preparation kit (CapitalBio Technology, Beijing, China. Catalogue No. S60150) according to the manufacturer’s instructions. The extracted RNA was reverse-transcribed using random primers, and cDNA was pooled with DNA from the same clinical sample for sequencing library preparation. The pooled nucleic acid was enzymatically fragmented to a size of 200–300 base pairs (bp), and sequencing libraries were constructed through end repair, adapter ligation and PCR amplification. Qubit fluorometer (Thermo Fisher Scientific, MA, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, USA) were utilized to assess the quality and quantity of each library before sequencing. Sequencing templates were further prepared with the OneTouch2 System (Life Technologies, CA, USA) and sequenced on the BioelectronSeq 4000 sequencer (CapitalBio Technology) after quality control. A negative control sample of water was used in each run to monitor potential contamination. The original sequencing data were subjected to quality control, and reads with lengths less than 50 bp, low quality (Q < 30), or low complexity were removed. The remaining high-quality sequencing data were mapped to the human reference genome grch38 for the depletion of human host sequences using Bowtie2 software. Subsequently, the remaining reads were aligned to Microbial Genome Databases using the Burrows-Wheeler Aligner with the BWA-SW algorithm. Negative controls (NTC, sterile deionized water) and positive controls (known quantities of synthetic fragments) were included in each batch of experiments, following the same wet lab and bioinformatics procedures. For detected bacteria (excluding Mycobacterium tuberculosis), fungi (excluding Cryptococcus), and parasites, a positive result was considered if the coverage ranked in the top 10 of similar species/genera and was absent in NTC, or if the term reads per ten million (RPTM) ratio between the sample and NTC (RPTMsample/RPTMNTC) was greater than 10 when RPTMNTC was not zero. For viruses, Mycobacterium tuberculosis, and Cryptococcus, a positive mNGS result was indicated by the detection of at least one species-specific read and its absence in NTC, or a RPTMsample/RPTMNTC ratio greater than 5 when RPTMNTC was not zero. The report was addressed as the name of genus and species, confidence, copy number of sequences and relative abundance.
Statistical analysis
Data were expressed as the mean ± SD (for data that were normally distributed) or median and interquartile range (IQR; for data that were in skewed distribution) for continuous variables and number (%) for categorical variables as appropriate. Quantitative parameters were compared using Student’s t test (for data that were normally distributed) or the Mann-Whitney U test (for data that were in skewed distribution) as appropriate. Categorical variables were compared using the χ2 test. Logistic regression analysis was used to assess patient survival. A P value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 18.0 (Chicago, IL, USA).
Results
General data of the AAV patients
The clinical and laboratory data of the 27 patients in the BALF mNGS/CMT group and 17 patients in the CMT alone group are presented in Table 1. No significant difference in age, sex, laboratory data, the ratio of partial pressure to fraction of inspiration oxygen (PaO2/FiO2), CRB-65, immunosuppressive regimens, AAV disease activity or infection severity was found between these two groups of patients.
Table 1.
General data of the patients in the BALF mNGS/CMT group and CMT alone group
| Items | BALF mNGS/CMT group (n = 27) | CMTs group (n = 17) |
nominal P values |
|---|---|---|---|
| Age, years | 66.1 ± 9.2 | 61.6 ± 18.5 | 0.371 |
| Male, n (%) | 11(41%) | 11 (65%) | 0.126 |
| MPO-ANCA/PR3-ANCA, n | 18/9 | 12/5 | 0.786 |
| ANCA titers, AU/ml | 122.8 ± 111.1 | 108.3 ± 87.8 | 0.657 |
| Serum creatinine, umol/l | 329.4 ± 232.7 | 342.7 ± 249.0 | 0.858 |
| eGFR, ml/min/1.73m2 | 30.2 ± 31.2 | 30.3 ± 28.7 | 0.995 |
| CRP, mg/l | 31.6 (13.5/90.5) | 41.0 (5.0/107.2) | 0.990 |
| ESR, mm/1 h | 69.1 ± 44.8 | 58.3 ± 33.5 | 0.642 |
| PCT, ng/ml | 0.34 (0.13/1.52) | 0.37 (0.14/1.34) | 0.951 |
| WBC, *109/l | 11.4 ± 6.1 | 9.7 ± 3.8 | 0.292 |
| Neutrophils, *109/l | 9.8 ± 6.0 | 8.0 ± 3.6 | 0.256 |
| Lymphocytes, *109/l | 0.99 ± 0.57 | 0.91 ± 0.45 | 0.630 |
| CD4 + T cells, counts/ml | 472.1 ± 407 | 464.1 ± 441.3 | 0.967 |
| Hemoglobin, g/l | 99.1 ± 22.6 | 100.8 ± 23.6 | 0.817 |
| Blood platelet count, *109/l | 252.7 ± 132.9 | 229.7 ± 121.7 | 0.568 |
| Serum albumin, g/l | 32.2 ± 4.3 | 31.2 ± 3.6 | 0.438 |
| LDH, IU/l | 279.3 ± 111.9 | 248.8 ± 132.8 | 0.417 |
| PaO2/FIO2 | 237.2 ± 155.5 | 210.7 ± 134.9 | 0.977 |
| CRB-65, 0/1/2/3/4 | 7/11/5/2/2 | 4/6/3/3/1 | 0.633 |
| prednisolone (equivalent) dose, mg/day | 36.1 ± 18.4 | 29.0 ± 26.4 | 0.296 |
| CTX/RTX/AZA/none, n | 4/4/3/15 | 2/1/3/11 | 0.772 |
Abbreviation: AZA, azathioprine; BALF, bronchoalveolar lavage fluid; CMT, conventional microbiological tests; CRP, C-reactive protein; CTX, cyclophosphamide; eGFR, estimated glomerular filtration rate; ESR erythrocyte sedimentation rate; LDH, lactic dehydrogenase; mNGS, metagenomic next-generation sequencing; RTX, rituximab; WBC, white blood cell counts in peripheral blood
The results of microbiological tests in the BALF mNGS group
The results of microbiological tests in the 27 patients in the BALF mNGS/CMT group are described in Table 2. A total of 25.9% (7/27) of the patients did not have any evidence of pathogenic microorganism in their BALF samples according to BALF mNGS tests, while 40.7% (11/27) of the patients did not have any pathogenic microorganism in their BALF and serum samples according to CMTs tests. At the same time, a total of 55.6% (15/27) of patients had polymicrobial infections, including bacteria, fungi and viruses, according to both CMTs and BALF mNGS tests. 92.6% (25/27) of BALF mNGS tests were consistent with those of CMTs, but with detecting more pathogens than CMTs, as the positive rate was significantly higher according to BALF mNGS tests than CMTs tests (nominal P = 0.045). In two cases, the mNGS assay failed to detect a CMT-identified pathogen: patient No. 19, whose CMV-DNA was weak positive by conventional PCR assay but was not detected by mNGS assay in BALF samples; and patient No. 25, whose parainfluenza virus-IgM was positive in serum, while there was no parainfluenza virus detected by mNGS assay in BALF sample, potentially indicating an absence of parainfluenza viral infection of the lower respiratory tract.
Table 2.
The results of microbiological tests in patients in the BALF mNGS/CMT group
| Patient No. | Sex/Age | Conventional microbiological tests | mNGS tests | Symptomatic infection duration before bronchoscopy, days | Treatment before bronchoscopy | Treatment adjustment upon mNGS tests | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/80 | Fungal spores (sputum culture)/ BALF GM(+)/hMPV (throat swab PCR) | Aspergillus fumigatus | 12 | PE, prednisolone, antibiotics | voriconazole, prednisolone withdrawal | death | ||||
| 2 | M/63 | none | none | 0 | - | plasmapheresis, prednisolone, RTX | survival | ||||
| 3 | M/66 | Serum G(+)/Pneumocystis cysts (BALF smear)/RhV (throat swab PCR) | Pneumocystis jirovecii/RhV-A | 4 |
PE, MP pulse, prednisolone, RTX, antibiotics |
SMZ/TMP, prednisolone withdrawal | survival | ||||
| 4 | F/66 | Serum G(+)/Pneumocystis cysts (BALF smear)/BALF GM(+)/BALF CMV-DNA(+) | Bca/Hin/Aspergillus fumigatus/Pneumocystis jirovecii/CMV/HHV-7 | 5 | MP pulse, prednisolone, RTX, antibiotics | antibiotics upgrading, voriconazole, SMZ-TMP, prednisolone withdrawal | death | ||||
| 5 | M/65 | none | Sps/CMV | 6 |
MP pulse, prednisolone AZA |
antibiotics, immunosuppressive therapy continuance | survival | ||||
| 6 | F/48 | none | none | 4 | PE, prednisolone, RTX, antibiotics | antibiotics discontinuance | survival | ||||
| 7 | F/57 | Pae (BALF culture) | Pae/Sep | 9 | prednisolone, AZA | antibiotics, immunosuppressive therapy continuance | survival | ||||
| 8 | F/65 | Serum G(+)/Pneumocystis cysts (BALF smear) | Pneumocystis jirovecii/P. chrysogenum species complex/CMV/HHV-6B | 6 | prednisolone, CTX, antibiotics | SMZ-TMP, ganciclovir, prednisolone withdrawal | death | ||||
| 9 | M/71 | none | Pae/Nocardia pneumoniae /Candida albicans | 4 | prednisolone, AZA |
antibiotics, SMZ-TMP, immunosuppressive therapy continuance |
survival | ||||
| 10 | F/67 | none | none | 1 | prednisolone, antibiotics | antibiotics discontinuance, immunosuppressive therapy intensification | survival | ||||
| 11 | F/65 | Candida albicans (sputum culture) | Sho/ HCov-OC43/ Candida albicans | 9 | prednisolone, CTX, antibiotics | antibiotics upgrading, prednisolone withdrawal | death | ||||
| 12 | F/71 | Pae (sputum and BALF culture) | Pae | 13 |
PE, MP pulse, prednisolone, CTX |
antibiotics, prednisolone withdrawal | survival | ||||
| 13 | M/58 | Serum G(+) | Pneumocystis jirovecii | 4 | MP pulse, prednisolone, CTX, antibiotics | SMZ-TMP, prednisolone withdrawal | survival | ||||
| 14 | F/60 | Serum G(+)/BALF CMV-DNA(+) | Efa/ Pneumocystis jirovecii/CMV/EBV | 4 | prednisolone, antibiotics | antibiotics upgrading, SMZ-TMP, prednisolone withdrawal | survival, ESRD | ||||
| 15 | M/61 | none | Pae | 8 | prednisolone |
antibiotics, PE, immunosuppressive therapy continuance |
survival | ||||
| 16 | M/70 | BALF EBV-DNA(+) | Sps/EBV/ Candida albicans | 0 | - |
prednisolone, CTX, antibiotics |
survival | ||||
| 17 | F/77 | none | none | 1 | antibiotics | antibiotics discontinuance prednisolone, CTX | survival | ||||
| 18 | M/67 | Pae (sputum and BALF culture) | Pae/ Achromobacter xylosoxidans/RhV-A | 9 | prednisolone, antibiotics | antibiotics upgrading, prednisolone withdrawal | survival, ESRD | ||||
| 19 | M/82 | BALF CMV-DNA(+) | C. amycolatum | 0 |
prednisolone, ganciclovir |
antibiotics, immunosuppressive therapy continuance | survival | ||||
| 20 | F/54 | Candida tropicalis (sputum culture) | Bca/C. striatum/ Candida albicans/ Candida tropicalis | 5 |
PE, prednisolone |
antibiotics, prednisolone withdrawal | survival | ||||
| 21 | F/76 | none | none | 3 | prednisolone | immunosuppressive therapy intensification | survival | ||||
| 22 | F/44 | none | none | 4 | prednisolone | immunosuppressive therapy intensification | survival | ||||
| 23 | M/71 | BALF CMV-DNA(+) | Efa/Sho /HCov/EBV/CMV/Candida parapsilosis | 7 |
PE, prednisolone, antibiotics |
antibiotics adjustment, ganciclovir, prednisolone withdrawal |
survival, ESRD | ||||
| 24 | F/59 | none | Sau/Sma | 4 | prednisolone, antibiotics | antibiotics upgrading, prednisolone withdrawal | survival | ||||
| 25 | F/75 | Serum PIV IgM (+)/Pneumocystis cysts (BALF smear)/BALF EBV-DNA(+) | Pneumocystis jirovecii/ EBV/CMV/RSV | 0 | prednisolone, CTX | SMZ-TMP, ganciclovir, antibiotics, prednisolone withdrawal | survival | ||||
| 26 | F/75 | none | none | 2 | antibiotics, prednisolone | antibiotics discontinuance, immunosuppressive therapy intensification | survival | ||||
| 27 | F/72 | Sho (BALF culture)/ Serum G(+)/BALF GM(+) |
Sho/Kpn/Pneumocystis jirovecii/ Aspergillus terreus EBV/CMV |
0 | fluconazole, SMZ-TMP, antibiotics, prednisolone | voriconazole, antibiotics adjustment, prednisolone withdrawal | survival | ||||
Abbreviation: AZA, azathioprine; Bca, Moraxella catarrhalis; C. amycolatum, Corynebacterium amycolatum; CMV, cytomegalovirus; C. striatum, Corynebacterium striatum; CTX, cyclophosphamide; EBV, human gammaherpesvirus 4; Efa, Enterococcus faecium; ESRD, end-stage renal disease; HCov, human coronavirus; HHV, human herpesvirus; Hin, Haemophilus influenza; hMPV, human metapneumovirus; Kpn, Klebsiella pneumoniae; MP, methylprednisolone; mNGS, metagenomic next-generation sequencing; Pae, Pseudomonas aeruginosa; P. chrysogenum, Penicillium chrysogenum; PE, plasmapheresis; PIV, parainfluenza virus; RhV-A, rhinovirus A; RSV, respiratory syncytial virus; RTX, rituximab; Sau, Staphylococcus aureus; Sep, Staphylococcus epidermidis; Sho, Staphylococcus hominis; Sma, Stenotrophomonas maltophilia; SMZ-TMP, sulfamethoxazole and trimethoprim; Sps, Streptococcus pseudopneumoniae;
Subsequent treatment adjustments and outcomes are also summarized in Table 2.
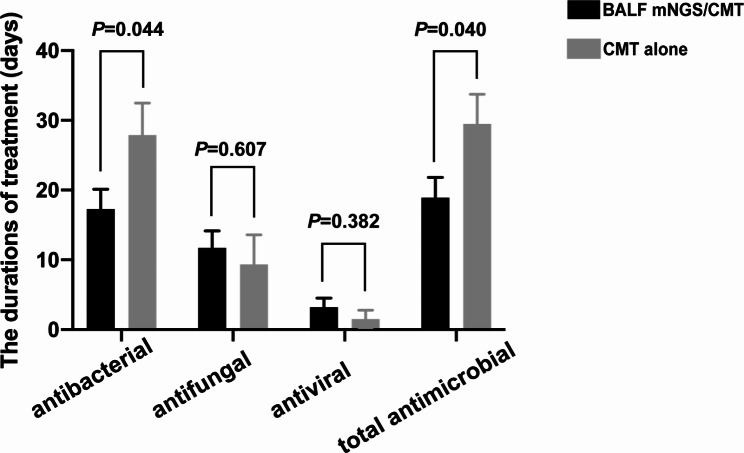
Shorter duration of antimicrobial treatment in the BALF mNGS/CMT group
The duration of antibacterial, antifungal, antiviral and total antimicrobial treatment in patients were compared between the BALF mNGS/CMT group and the CMT alone group. Patients in the BALF mNGS/CMT group received a significantly shorter duration of antibacterial and total antimicrobial treatment than patients in the CMT alone group (17.3 ± 14.7 vs. 27.9 ± 19.0 days, nominal P = 0.044; 18.9 ± 15.0 vs. 29.5 ± 17.7 days, nominal P = 0.040, respectively), while patients in the BALF mNGS/CMT group received comparable duration of antifungal and antiviral treatment to patients in the CMT alone group (11.7 ± 12.6 vs. 9.4 ± 17.4 days, nominal P = 0.607; 3.2 ± 6.8 vs. 1.5 ± 5.2 days, nominal P = 0.382, respectively) (Fig. 1).
Fig. 1.
Shorter duration of antimicrobial treatment in the BALF mNGS/CMT group
We then divided patients in the BALF mNGS/CMT group into two subgroups based on the median duration from occurrence of symptoms of suspected infection to bronchoscopy, i.e., < 5 days and ≥ 5 days, termed the early BALF mNGS/CMT group (n = 16) and late BALF mNGS/CMT group (n = 11), respectively. The early BALF mNGS/CMT group received significantly shorter antibacterial and total antimicrobial treatment than the late BALF mNGS/CMT group (11.3 ± 11.4 vs. 26.1 ± 14.8 days, nominal P = 0.007; 13.3 ± 12.9 vs. 27.1 ± 14.5 days, nominal P = 0.016, respectively).
Lower trend in-hospital mortality in the BALF mNGS/CMT group
The in-hospital mortality, ICU stay and duration of ventilation between the BALF mNGS/CMT group and the CMT alone group were compared. Patients in the BALF mNGS/CMT group had lower in-hospital mortality than patients in the CMT alone group (4/27 vs. 7/17, nominal P = 0.049 compared toP < 0.05). No deaths occurred in the early BALF mNGS/CMT group, while all 4 deaths occurred in the late BALF mNGS/CMT group. Infection was the cause of all observed deaths. Association between the different groups and all-cause mortality odds was not significant in logistic regression analysis (OR 0.059 [95% CI 0.003–1.145], P = 0.061), by additional putting the duration of antimicrobial treatment, CRB65 and sex in a multivariable model, however, a trend persisted.
Of note, all 7 patients without any pathogenic microorganisms in their BALF samples according to BALF mNGS tests received intensive immunosuppressive therapy, and none of them developed infection during hospitalization after the treatment.
There was no significant difference in the duration of ICU stay or ventilation in patients between these two groups. (Table 2).
Discussion
In the current study, we summarized the application of BALF mNGS tests in 27 active AAV patients who received immunosuppressive therapy and were suspected of pulmonary infection. We also compared the duration of antimicrobial treatment, as well as in-hospital mortality between these 27 patients and another 17 active AAV patients, diagnosed during the same period, whose samples were only tested by conventional microbiological tests. We found that the duration of antibacterial and total antimicrobial treatment was significantly shorter in patients whose BALF samples were available for mNGS tests. Due to precise antimicrobial treatment, a possibly lower proportion of patients died of severe infection in the BALF mNGS/CMT group, as compared with the CMT alone group. Moreover, patients would benefit more from early mNGS tests. To the best of our knowledge, this is the first report of applying mNGS in the management of pulmonary infection in patients with AAV.
According to our results, the mNGS test could generate benefits as a rule-out test for infection. By weighing the benefits and limitations of mNGS, Blauwkamp found that immunocompromised patients presenting with non-specific symptoms of infection could potentially benefit from this approach including rule-out testing for sepsis when the standard of care was too invasive or was not sensitive enough [25]. Furthermore, Qian’s study showed that the negative prediction accuracy rate was convincing when the mNGS result was negative. The authors also suggested that if there are indications of respiratory tract infection, respiratory tract samples were recommended for the mNGS test because the negative rates of sputum and BALF were much lower than other types of samples [26].
Early identification of infecting pathogens is crucial for targeted and appropriate antimicrobial therapy, but physicians typically prescribe antimicrobials empirically, based on clinical guidelines and local epidemiology, before microbiological test results are available. The mNGS test could provide more useful information for adjusting antimicrobial treatment, as we found that the duration of antibacterial and total antimicrobial treatment was significantly shorter in patients whose BALF samples were timely available for mNGS tests. First, mNGS test has advantages in turnaround time over conventional microbiological tests [27, 28]. Although microbiological culture is the standard diagnostic test for many pathogens, it is also time-consuming for the turnaround time to results of ≥ 2 days, and some pathogens are difficult to culture. Second, consistent with other immunocompromised patients with pulmonary infection [18], most AAV patients under immunosuppressive therapy with pulmonary infection had atypical polymicrobial infection, including bacterium, fungus and virus. As a relatively sensitive method with high detection rates, mNGS tests could provide evidence for adequate and precise antimicrobial treatment. Finally, mNGS tests could provide evidence for adjusting treatment strategies in time for physicians. For instance, patient No.4 (Table 2) in the current study received a second fiberoptic bronchoscopy and BALF mNGS test two weeks later when his condition worsened despite aggressive antimicrobial treatment. However, the results showed a much lower incidence of the pathogen RPTM, so the treatment strategy was adjusted from intensive antimicrobial treatment to enhanced immunosuppressive treatment. In this way, it was helpful to avoid unnecessary side-effects of antimicrobial treatment and overuse of antibiotics.
However, there were several limitations of the mNGS assay. Briefly, the limitations lay in distinction capability, cost issues, lack of regulatory consensus and limited information on the resistance profile of pathogens. First, as conventional microbiological methods, mNGS does not effectively distinguish between pathogens, colonizers, and contaminants, which could lead to misdiagnosis or misinterpretation. Second, the high cost (approximately $500 per test) of mNGS complicates its integration into routine testing, thus limiting its widespread application. Third, there is currently a lack of regulatory consensus regarding the development and validation of mNGS assays for use in clinical microbiology laboratories, which poses barriers to the promotion and application of the technology. Finally, as described in the Methods section, the pooled nucleic acid was enzymatically fragmented to a size of 200–300 bp, which means that the sequencing only covered a fraction of the whole genome of a pathogen, making it difficult to detect resistance determinants. Therefore, further antibiotic susceptibility testing will be needed to supplement pathogen identification and support antimicrobial treatment decisions. There were also several limitations of this study. On the one side, as a retrospective study, it might be difficult to fully control potential bias and confounding variables, even though there was no significant difference in disease activity of AAV, severity of infection or intensity of immunosuppressive therapy between the two groups. On the other side, the sample size was small to obtain more robust conclusions.
In conclusion, compared with conventional microbiological tests, using mNGS tests may facilitate shortening and decreasing antimicrobial treatment, and decreasing mortality of severe infection. Therefore, we recommended mNGS as a useful rule-out tool in differentiating pulmonary infection from pulmonary involvement of vasculitis in AAV patients, and mNGS may offer the potential for early implementation of accurate therapy in AAV patients with suspected pulmonary infection.
Acknowledgements
We gratefully acknowledge the contributions of Han Xia from the Department of Scientific Affairs, Hugobiotech Co., Ltd., who provided technical support.
Author contributions
Chen Wang and Zhi-Ying Li designed and planned the study. Chen wang handled the selection of suitable patients for the study, arranged the collection of clinical data, performed data analysis and drafted the manuscript. Zhan-Wei Hu conducted patients’ bronchoscopies. Zhi-Ying Li contributed to manuscript preparation. Ming-Hui Zhao and Min Chen guided the study as senior authors. Mark A. Little contributed great efforts during the revision of this manuscript. All the authors contributed to data interpretation and manuscript revision. The author(s) read and approved the final manuscript.
Funding
This study was supported by National key research and development program (2022YFC2502500/2022YFC2502502), National Natural Science Fund (82090020/82090021 and 82270754), Chinese Academy of Medical Sciences Research Unit (2019RU023), National High Level Hospital Clinical Research Funding (Multi-center Clinical Research Project of Peking University First Hospital, 2022CR52), National High Level Hospital Clinical Research Funding (High Quality Clinical Research Project of Peking University First Hospital, 2024HQ01), Beijing Physician Scientist Training Project (BJPSTP-2024-15) and Peking University Clinical Scientist Training Program, supported by “the Fundamental Research Funds for the Central Universities” (BMU2024PYJH009).
Data availability
Data collection has been conducted in accordance to local regulation. The authors declare their availability in providing data if requested by the referees or the editorial team of the journal. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
The research was in compliance with the Declaration of Helsinki and was approved by the clinical research ethics committee of the Peking University First Hospital. Written informed consent was obtained from the patients or their guardians.
Consent for publication
All authors have read the final draft of the article and approved its submission for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international chapel Hill Consensus Conference nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. [DOI] [PubMed]
- 2.Falk RJ, Jennette JC. ANCA small-vessel vasculitis. J Am Soc Nephrol. 1997;8(2):314–22. [DOI] [PubMed] [Google Scholar]
- 3.Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98(1):76–85. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116(6):488–98. [DOI] [PubMed] [Google Scholar]
- 5.Guillevin L, Durand-Gasselin B, Cevallos R, Gayraud M, Lhote F, Callard P, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42(3):421–30. [DOI] [PubMed] [Google Scholar]
- 6.Lane SE, Watts RA, Shepstone L, Scott DG. Primary systemic vasculitis: clinical features and mortality. QJM. 2005;98(2):97–111. [DOI] [PubMed] [Google Scholar]
- 7.West S, Arulkumaran N, Ind PW, Pusey CD. Diffuse alveolar haemorrhage in ANCA-associated vasculitis. Intern Med. 2013;52(1):5–13. [DOI] [PubMed] [Google Scholar]
- 8.Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41(4):776–84. [DOI] [PubMed] [Google Scholar]
- 9.Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69(6):1036–43. [DOI] [PubMed] [Google Scholar]
- 10.Kronbichler A, Kerschbaum J, Gopaluni S, Tieu J, Alberici F, Jones RB, et al. Trimethoprim-sulfamethoxazole prophylaxis prevents severe/life-threatening infections following rituximab in antineutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis. 2018;77(10):1440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartin-Ceba R, Golbin JM, Keogh KA, Peikert T, Sánchez-Menéndez M, Ytterberg SR, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single center. Arthritis Rheum. 2012;64(11):3770–8. [DOI] [PubMed] [Google Scholar]
- 12.Lai QY, Ma TT, Li ZY, Chang DY, Zhao MH, Chen M. Predictors for mortality in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: a study of 398 Chinese patients. J Rheumatol. 2014;41(9):1849–55. [DOI] [PubMed] [Google Scholar]
- 13.Li ZY, Chen M, Zhao MH. Severe infections following Rituximab Treatment in Antineutrophil cytoplasmic antibody-Associated Vasculitis. Kidney Dis (Basel). 2021;7(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietel M, Jöhrens K, Laffert MV, Hummel M, Bläker H, Pfitzner BM, et al. A 2015 update on predictive molecular pathology and its role in targeted cancer therapy: a review focussing on clinical relevance. Cancer Gene Ther. 2015;22(9):417–30. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the Leap from Research Laboratory to Clinic: challenges and opportunities for Next-Generation sequencing in Infectious Disease Diagnostics. mBio. 2015;6(6):e01888–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zinter MS, Dvorak CC, Mayday MY, Iwanaga K, Ly NP, McGarry ME, et al. Pulmonary metagenomic sequencing suggests missed infections in Immunocompromised Children. Clin Infect Dis. 2019;68(11):1847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Gao H, Meng H, Wang Q, Li S, Chen H, et al. Detection of pulmonary infectious pathogens from lung biopsy tissues by Metagenomic Next-Generation sequencing. Front Cell Infect Microbiol. 2018;8:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng JM, Du B, Qin HY, Wang Q, Shi Y. Metagenomic next-generation sequencing for the diagnosis of suspected pneumonia in immunocompromised patients. J Infect. 2021;82(4):22–7. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Zhang Y, Yang J, Liu Y, Chen J. Application of mNGS in the Etiological Analysis of Lower Respiratory Tract Infections and the prediction of Drug Resistance. Microbiol Spectr. 2022;10(1):e0250221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li ZY, Gou SJ, Chen M, Zhao MH. Predictors for outcomes in patients with severe ANCA-associated glomerulonephritis who were dialysis-dependent at presentation: a study of 89 cases in a single Chinese center. Semin Arthritis Rheum. 2013;42(5):515–21. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards L, Perrin K, Wijesinghe M, Weatherall M, Beasley R, Travers J. The value of the CRB65 score to predict mortality in exacerbations of COPD requiring hospital admission. Respirology. 2011;16(4):625–9. [DOI] [PubMed] [Google Scholar]
- 23.Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HY, Rhee CK, Choi JY, Lee HY, Lee JW, Lee DG. Diagnosis of cytomegalovirus pneumonia by quantitative polymerase chain reaction using bronchial washing fluid from patients with hematologic malignancies. Oncotarget. 2017;8(24):39736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4(4):663–74. [DOI] [PubMed] [Google Scholar]
- 26.Qian M, Zhu B, Zhan Y, Wang L, Shen Q, Zhang M, et al. Analysis of negative results of Metagenomics Next-Generation sequencing in clinical practice. Front Cell Infect Microbiol. 2022;12:892076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju CR, Lian QY, Guan WJ, Chen A, Zhang JH, Xu X, et al. Metagenomic next-generation sequencing for diagnosing infections in lung transplant recipients: a retrospective study. Transpl Int. 2022;35:10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parize P, Muth E, Richaud C, Gratigny M, Pilmis B, Lamamy A, et al. Untargeted next-generation sequencing-based first-line diagnosis of infection in immunocompromised adults: a multicentre, blinded, prospective study. Clin Microbiol Infect. 2017;23(8):574.e1-.e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data collection has been conducted in accordance to local regulation. The authors declare their availability in providing data if requested by the referees or the editorial team of the journal. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.