Abstract
Genetic diagnosis plays a crucial role in rare diseases, particularly with the increasing availability of emerging and accessible treatments. The International Rare Diseases Research Consortium (IRDiRC) has set its primary goal as: “Ensuring that all patients who present with a suspected rare disease receive a diagnosis within one year if their disorder is documented in the medical literature”. Despite significant advances in genomic sequencing technologies, more than half of the patients with suspected Mendelian disorders remain undiagnosed. In response, IRDiRC proposes the establishment of “a globally coordinated diagnostic and research pipeline”. To help facilitate this, IRDiRC formed the Task Force on Integrating New Technologies for Rare Disease Diagnosis. This multi-stakeholder Task Force aims to provide an overview of the current state of innovative diagnostic technologies for clinicians and researchers, focusing on the patient’s diagnostic journey. Herein, we provide an overview of a broad spectrum of emerging diagnostic technologies involving genomics, epigenomics and multi-omics, functional testing and model systems, data sharing, bioinformatics, and Artificial Intelligence (AI), highlighting their advantages, limitations, and the current state of clinical adaption. We provide expert recommendations outlining the stepwise application of these innovative technologies in the diagnostic pathways while considering global differences in accessibility. The importance of FAIR (Findability, Accessibility, Interoperability, and Reusability) and CARE (Collective benefit, Authority to control, Responsibility, and Ethics) data management is emphasized, along with the need for enhanced and continuing education in medical genomics. We provide a perspective on future technological developments in genome diagnostics and their integration into clinical practice. Lastly, we summarize the challenges related to genomic diversity and accessibility, highlighting the significance of innovative diagnostic technologies, global collaboration, and equitable access to diagnosis and treatment for people living with rare disease.
Keywords: Rare disease, Rare disease diagnosis, Innovative technologies, IRDiRC, Rare disease research, Genomics, Molecular diagnostics
Introduction
Accurate diagnosis is a cornerstone of rare disease (RD) patient care; however, a significant proportion of patients with RD remains undiagnosed [1]. This is harmful as a diagnosis is essential for accurate genetic counselling, including recurrence risk in future children and identification of family members at risk, for information sharing and understanding. Receiving an accurate diagnosis might remove feelings of guilt, lead to better access to community services, and allow for personalized management, including targeted therapy and prevention [1]. The window of therapeutic opportunity is often missed if diagnosis is delayed or not achieved at all [2]. To optimize health outcomes for individuals with RDs, establishing a diagnosis should be prioritized in accordance with the International Rare Diseases Research Consortium’s (IRDiRC) goals: “All patients coming to medical attention with a suspected RD will be diagnosed within one year if their disorder is known in the medical literature; all currently undiagnosable individuals will enter a globally coordinated diagnostic and research pipeline” [2]. Although genomic sequencing technologies have revolutionized our ability to diagnose Mendelian diseases, at least half of all patients remain without a diagnosis [3]. Given this large unmet medical need on the one hand and the rapid technological evolution on the other, IRDiRC launched the Task Force on Integrating New Technologies for Rare Disease Diagnosis to draft an article to support the advancement of diagnostic and research pipelines around the globe. To this end, our Task Force, comprising relevant RD stakeholders, has reviewed the most recent advances in innovative diagnostic technologies for clinicians and researchers. Highlighting the patient’s diagnostic journey, we outline different technologies, their strengths and limitations, and their current state of use, exemplified by case vignettes. Herein, we provide expert recommendations to support the diagnostic process and enable access to personalized therapy and care.
Challenges related to genomic diversity and implications related to the global (in-)equity in access, are discussed. The importance of FAIR and CARE data management [3], as well as genomics education and training beyond academia is highlighted. We end with future technological developments and the transition of these technologies from research to clinical practice. We hope that our article will support the goal of ‘leaving no patient behind,’ and ultimately contribute to improving the diagnosis and care of RD patients globally.
Methods
To develop this manuscript, we used a combination of expert opinion and literature review. The International Rare Diseases Research Consortium (IRDiRC) Task Force on Integrating New Technologies for Rare Disease Diagnosis is composed of renowned multi-stakeholder experts in RD diagnosis and research. The Task Force members were consulted to provide their expert opinions on the latest advances in innovative diagnostic technologies and their potential applications in the diagnosis of RDs.
In addition to collective expert opinion, we conducted a scoping literature review in September 2023 to identify the latest research and advancements in the field of RD diagnosis. We performed searches for relevant articles in PubMed, Google Scholar, ScienceOpen, and NIH National Library of Medicine, using keywords such as “rare disease diagnosis,” “genomics,” “multi-omics,” “functional testing,” “model systems,” “bioinformatics,” and “artificial intelligence.” We also reviewed relevant guidelines and recommendations from national and international RD organizations.
We assessed the advantages and limitations of each omics technology and the current state of utilization in clinical practice. We also explored the challenges associated with global differences in accessibility and proposed a stepwise approach to the application of these innovative technologies to support the diagnostic process and enable access to personalized therapy and care. The fact that the current review is scoping rather than systematic in nature poses a limitation; this is counterbalanced by the diversity in rare diseases expertise and backgrounds of the authors which enables a comprehensive and well-rounded overview of this topic.
The Task Force members also discussed the importance of FAIR data management, genomics education and training beyond academia, future technological developments, and the transition of these technologies from research to clinical practice. These factors were evaluated in light of the current lack of global equity of access to RD diagnosis and care.
Key findings of the task force
Patient perspective—patients at the forefront
Patients are the most important advocates for their disorders, providing essential information e.g., through patient registries [4], supporting other people affected by the same disorder, raising the funding required to further research into therapeutics, and making disease-specific information available in a clear and easy to understand form. Consideration of making key publications freely available for patients and participants is therefore essential [5].
Patients and patient advocates can also bring clinicians and scientists together to support research by establishing scientific advisory boards. This is particularly important for RDs, where there are often only a handful of people identified globally with a given disorder. For these ultra-rare conditions, there is limited interest from the industry in developing therapies due to the perception of a lack of Return On Investment (ROI) and the challenges of creating a development program unless significant additional incentives such as the Orphan Medicinal Products Regulations are in place, and socially responsible frameworks for public–private partnerships are developed and used [6]. More research is also required to understand the impact of an early diagnosis in RD versus one later in life.
Clinical trials often struggle to identify and enroll sufficient patients, and this could potentially be solved if effective, accurate, and patient-centric testing is widely available to increase diagnosis. The Global Alliance for Genomics and Health (GA4GH) is promoting the concept of a global genomic database federation to allow the secure sharing of genomic and healthcare data, increasing the probability of finding such rare patients [7]. Therefore, the key to using any innovative technology in diagnosing RDs is to have a patient-centric approach. All too often, the patient experience is either unaccounted for or considered too late in the design, which can cause delays or even the failure of initiatives [8]. An exemplar for patient involvement is the Participant Panel at Genomics England, which oversees what Genomics England and its partners do with their data [9]. The patient and participant are essential in many aspects of genomics/biotech and are central in planning research, advising on ethical issues, and aiding clinical trial readiness by having an organized and engaged patient community.
Innovative technologies for rare diseases
Deep phenotyping and reverse phenotyping
Deep phenotyping of patients suspected of having or diagnosed with a RD, optimally in a categoric and computer-readable format, has become standard practice in the last ten years. The Human Phenotype Ontology (HPO) [10] provides the most comprehensive resource for computational deep phenotyping and has become the de facto industry standard, used in analysis of exome and genome sequencing data [11, 12], as well as data integration in translational research and bioinformatics [13]. The ontology, maintained by the Monarch Initiative [14], provides a set of more than 15,500 terms describing human phenotypic abnormalities—arranged as a hierarchy. More than half of these terms have a plain language representation, allowing patients and families to become more effective partners in translational research [15]. Recently, translations into seven languages were made available.
Conversion of phenotype risk scores from the electronic health records to HPO terms enables differentiation of patients with Mendelian diseases from unaffected controls and assists in rare variant interpretation [16] or causative gene association [17]. Importantly, HPO data can be shared across platforms through the Phenopacket Schema, developed by GA4GH [18].
Reverse phenotyping is an approach in which specific clinical features are interrogated in a subsequent clinical examination based on the candidate genetic variants identified. It has been shown to increase RD diagnostic rate, particularly for disorders with high genetic heterogeneity and phenotypic complexity [19, 20], supporting the rationale for detailed clinical characterization at various stages of the diagnostic odyssey.
Where possible, deep phenotyping should be prospective (to the molecular test), objective to reduce (e.g., cognitive) bias, easily accessible (e.g., free and open access), and scalable for diversity, equity, and inclusion. However, when an expected phenotype is not reported, its absence should be confirmed through reverse phenotyping, as features that are part of the diagnosis may be overlooked or assumed to be familial.
Genomics
Genome Sequencing (GS) applies Next Generation Sequencing (NGS) to assess three billion bases of human DNA, while Exome Sequencing (ES) focuses on the ~ 2% of protein-coding DNA (including ~ 19,000 genes), enabling a more focused, interpretable, and lower cost, albeit less comprehensive approach. Further focused approaches target known disease-causing genes (Mendeliome or Clinome, typically defined by Online Inheritance in Man (OMIM) [21] or the Gene Curation Coalition (GenCC) [22]). However, ES excludes noncoding and copy-neutral structural variation, may have limited sensitivity in complex genomic regions such as high CG density sequences, and is limited to pre-defined gene transcript isoforms. The diagnostic yield of ES is reported to average 40% for intellectual disability [23] and immunologic conditions [24] but may be higher for more specific phenotypes such as metabolic [25], neuromuscular [26], vision loss, and sensory deficits [27]. Currently, GS increases this diagnostic yield over ES by < 10% [28]. This is likely to increase as continuing advancements in analytic technologies and gene-disease associations advance, highlighting the need for reanalysis of preexisting patient NGS data, as has already been demonstrated [29, 30].
Resources for RD definitions, gene mappings, and ontologies that enable NGS analysis and interpretation include Orphanet [31], UMLS [32], MonDO [33], and OMIM [21], and tools that aggregate data from these resources [34]. Selection of potential variants and elucidation of the genetic basis of the disease is done by filtration and prioritization of variants, with higher diagnostic yields via trio- or family-based analysis given the ability to phase variants and identify de novo variation [35]. In addition, when available, a genomic medicine team review, phenotypic validation, and functional assessment, are important to eliminate the semi-automated NGS analysis that may miss 15% of diagnosis and 4% of candidate disease-causing variants [36]. With the reduction in sequencing costs, advancement of analysis tools, and increasing accumulations of databases, it’s likely that GS will be a feasible tool for diagnosing RDs.
Long read sequencing
Advances in sequencing technologies have further expanded our ability to sequence from hundreds of base pairs with short read sequencing to tens of thousands of base pairs and occasionally millions of base pairs with long read sequencing [37]. These long reads provide improvements in the calling of short variants (SNVs and indels), particularly for genes with high homology with other regions of the genome, due to segmental duplications (i.e. CBS), paralogues (i.e. SMN1), or highly homologous gene families. Additionally, about three times as many structural variants are identifiable from long read sequencing, and there is improved resolution of tandem repeats. Haplotype phasing can support evaluation for compound heterozygous variant pairs without requiring DNA from the parents. DNA methylation can also be detected by this technology, which may be applicable to episignature assessment. Due to the need for high molecular weight DNA, analytic challenges including the lack of reference data, and higher costs, access to long read genome sequencing is currently more in research settings.
Several proof-of-principle studies have used long read sequencing technologies for RD diagnosis [38, 39]. Thus far, the majority of the genetic diagnoses achieved are also detectable by short read sequencing. For variants exclusively detectible by long read sequencing, it is challenging to differentiate between common from the rare (and ultra-rare) variants, further limiting the clinical utility of this approach at the moment [40, 41]. Applications of this technology in single cell and bulk RNA sequencing can enable gene transcript differentiation, which holds potential for use in RD diagnosis [42]. Another successful application of this technology involves targeted sequencing of genes strongly related to a patient’s phenotype without variants detectable by conventional analyses. The diagnostic power of long read sequencing is expected to increase as analysis approaches and reference data are developed, and this remains a promising area for increasing diagnosis.
DNA methylation (DNAm) episignatures
The classic definition of epigenetics is mitotically heritable changes in gene expression without alteration of the DNA sequence and includes DNA methylation at CpG motifs [43]. However, an increasing number and spectrum of rare disorders exhibit the so-called DNA methylation episignatures, defined as recurring, sensitive, and specific DNA methylation biomarkers associated with a common genetic or environmental etiology [44]. DNA methylation episignatures can be used to help resolve ambiguous clinical and genetic findings, including genetic variants of unknown significance, and evaluate undiagnosed patients with RDs [45, 46].
Episignatures are developed by computational machine learning models primarily using methylation microarray data from peripheral blood samples in cohorts of individuals with common genetic or environmental etiology. The ability to detect episignatures is contingent upon the intensity (effect size) and extent (number of differentially methylated CpGs) of the observed DNAm changes, which can range from tens of thousands of differentially methylated CpGs (Sotos and NSD1, Tatton-Brown-Rahman syndrome and DNMT3A) to only a few hundred (BAFopathies) [46, 47]. The presence of an episignature is considered strong functional evidence that can aid in the reclassification of VUSs to a likely pathogenic status. In contrast, the absence of an episignature is considered supportive but not definitive for lack of pathogenicity [48]. Current limitations of this technology may include technical batch effects of microarray analysis, limited detection in mosaic cases, still limited (but growing) number of RDs with the defined episignatures, and a limited number of clinical diagnostic laboratories providing this testing.
Application of this technology to the broader patient populations will depend on the rate of discovery of gene and disorder-specific episignatures. Larger-scale studies are necessary to assess the diagnostic yield and health system impact as either a first-line test or in unresolved cases post-genomic assessment. Finally, the development of clinical recommendations and guidelines for the use and application of DNA methylation episignature analysis is warranted and currently ongoing.
Data sharing
Data sharing initiatives have greatly enhanced our understanding of genetic variation in human populations and RDs. As new gene-disease relationships (GDR) are discovered and published in the literature, they are reviewed and added to databases like OMIM. This consideration applies to diagnostic laboratories and collaborative research initiatives like Clinical Genome Resource (ClinGen), Genomics England PanelApp, and PanelApp Australia, and are now shared through the Gene Curation Coalition (GenCC) database [22]. The ClinVar database specializes in collecting variant interpretations by clinical testing laboratories along with other submitters [49].
Many genetic causes of RD remain to be identified [50]; most RDs are exceedingly rare, often with a prevalence of < 1 in 1–100 million individuals. Individual systems were developed to support two-sided gene matching–between researchers or clinicians with the same gene candidate. Further advancements came from the launch of the Matchmaker Exchange (MME) in 2015, a federated network connecting multiple databases through a common application programming interface (API). Already, MME has > 13,000 unique genes from > 120,000 cases submitted by > 12,000 contributors in 98 countries across eight matchmaking nodes [51]. About half of the gene submissions receive a match, and approximately 15% of matches are “successful”, which is determined through follow-up email exchanges. Hundreds of gene-disease discoveries have been made through the MME through the engagement of large research initiatives, independent researchers and clinicians, and clinical testing laboratories [52–55].
Another type of matchmaking involves early efforts to support variant matching—to allow querying of sequenced datasets for specific variants or classes of variants (i.e. loss of function variants) in a candidate gene [56]. Several databases with this functionality exist today (i.e. VariantMatcher, Franklin, Geno2MP), but they are not yet connected through the MME, and the amount of data currently queryable in these systems is limited.
Meaningful data sharing requires adhering to the FAIR principles [57]. Data should be shared in databases that are well known in the RD clinical and research communities, be accessible through controlled access mechanisms that protect research participants, be well organized and structured so those who access the data understand it, with a sufficient amount of information (i.e., detailed phenotype) to support independent analysis. Federated systems such as MME provide a good model where the data can be locally hosted while providing global access to allow the use of the data to improve gene discovery and diagnosis. Cloud-based research environments like AnVIL [58] and Genomics England provide secure environments where the tools can be brought to the data to support research.
RNA sequencing and transcriptome datasets
RNA sequencing has increasingly emerged as a tool for RD diagnosis [59]. It enables the detection of aberrant gene expression, splicing, or allelic expression that can be paired with NGS of DNA to focus analysis onto genes with altered transcription, or it can be used to aid in interpreting VUSs identified through ES/GS analysis [60–62]. Studies have reported diagnostic yields ranging from 7 to 36% [63, 64]. Challenges with this approach which need further work relate to the temporal (developmental stage) and spatial (tissue type) variability in gene expression and limited analytical pipelines.
Reduced sequencing costs have driven the creation of comprehensive functional genomic atlases, including FANTOM [65], ENCODE [66], GTEx [67], and the Human Cell Atlas [68], which catalogue genes and their expression levels across different tissues and organs. The rapid progress made in identifying RD variants now enables the systematic exploration of associations between RD phenotypes and tissue and cell types. Shared patterns of gene expression linked to phenotypes can be used to evaluate the impact of genetic variants while furthering our understanding of disease etiology by defining the affected cell types and developmental time windows.
Additional OMICS approaches
Several additional omics strategies are developed and, to varying extents, implemented in clinical practice to pursue optimal diagnostic coverage for patients with RD. These include ATAC-sequencing, metabolomics, and lipidomics. Since the individual-omics platforms address different aspects of (patho)physiology, they may increase the diagnostic yield. Multiple diagnostic technologies may be applied simultaneously or sequentially (e.g. metabolomics to follow up on a VUS identified by sequencing) [1].
ATAC-seq
Gene expression is directly related to chromatin accessibility. An increasing number of RDs involve genes that impact chromatin accessibility [69, 70]. Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) assesses chromatin accessibility and, as such, identifies active (i.e. euchromatin) and inactive (i.e. heterochromatin) regions of the genome. Currently, the diagnostic application of ATAC-seq is limited as it is mostly applied in research settings [71]. Ongoing efforts are directed towards methodological improvements of ATAC-seq for multiple cell types and tissues, with the goal of diagnostic utilization [72].
Metabolomics
Metabolites, small organic molecules, are intermediates or end products of enzymatic processes and, as such, faithfully reflect ongoing (patho)physiological processes. Their levels in biological fluids such as blood or urine may vary based on gene function, disease processes, and exogenous factors (diet, environment, medication). Traditionally, metabolic diagnostics are performed in a targeted manner, assessing levels of specific metabolites, and guided by phenotypic or molecular findings. Recent technological advances have led to the introduction of untargeted metabolomics, a considerably more comprehensive test acquiring a near-complete view of the metabolome, in diagnostic practice [73]. As expected, the diagnostic yield of untargeted metabolomics is significantly increased when compared to conventional metabolic screening [74]. However, the increased diagnostic yield for patients seeking a diagnosis after the initial evaluation is more limited [75]. Although not accessible yet in the large majority of clinical centers, untargeted metabolomics is now positioned in the early clinical stages of the diagnostic arena and is becoming the standard of care in some centers.
Lipidomics
Compared to metabolomics, the diagnostic implementation of untargeted analysis of lipids, i.e. lipidomics, is in its infancy due to the biochemical complexity of this class of molecules and the relatively limited clinical evidence. Parallel to metabolomics, however, technological developments in mass spectrometry have significantly advanced the possibilities of lipidomic studies in biological fluids and patients’ cells. Lipidomics is currently not applied in RD diagnostics of individual patients, but there are ongoing research efforts in developing reference datasets in patient cohorts and controls. There have been advancements involving lipid biomarkers and understanding the underlying pathophysiology of lipid metabolism disorders, such as peroxisomal disorders [76], which may enable clinical adoption in the coming years.
High throughput functional studies
Multiplexed assays for variant effects (MAVEs) provide new opportunities to test and prioritize rare genetic variants en masse [77–79]. A notable example of this approach has enabled profiling all possible SNVs, including missense variants for functional effects in critical regions of BRCA1 [80]. Comparable applications have since been extended to map functional effects for missense variants for genes such as MSH2 in Lynch Syndrome [81], KCNH2 in long QT syndrome [82], and NPC1 in Niemann-Pick disease type C [83]. In addition, several massively parallel assays enable more routine testing of the functional effects of variants in non-coding regions, including regulatory regions, splice junctions, and UTR sequences [84–86]. Albeit limited to the specific genes, these data are improving computational approaches for classifying rare variants for pathogenicity [87, 88] and in the future, may facilitate RD diagnosis through the accessibility of an Atlas of Variant Effects (AVE) for many RD-relevant genes and regulatory regions [89]. Caution is warranted in the interpretation of non-coding variation without functional validation. Data sharing and collaborative efforts are required for expanding our understanding and assigning correct diagnoses in such clinical situations and phenotypes.
Multi-OMICS
Several studies have shown complementarity and synergism in combining multiple omics modalities. For example, combining GS with ATAC-seq has led to novel associations of genes with disease [90]. In addition, integrative analyses of RNA-seq with ATAC-seq have revealed a novel marker in breast cancer [91]. A clear association between genetic variants and levels of metabolites in individual patients was recently described in a large study combining genomics and metabolomics [91], highlighting the metabolic and the underlying genetic diversity of humans and pinpointing genetic changes at and near gene loci that cause inherited metabolic disorders. The overall diagnostic efficiency of metabolomics and its specific benefits of genetic variants’ prioritization tool are currently under study; until then metabolomics has yet to be part of standard clinical care [92, 93].
Model systems and organisms
Model systems have proven instrumental for understanding pathophysiology, confirming causal genotype–phenotype associations, and developing therapies for RD since studying the natural course of the disease in humans is limited due to low disease prevalence [94]. Model systems provide a powerful tool to understand the impact of genetic variation on phenotype and may include cultured cells (including primary, immortalized, or reprogrammed stem cell lines), organoids, yeast, worms, flies, fish, mice, or larger animals. Collaborative efforts are well underway to characterize knockout mice lines for every gene through the International Mouse Phenotyping Consortium [95]. Recapitulation of disease features and course—both clinical and biochemical—remains challenging. For example, Montoro and colleagues reviewed existing model systems for the neurometabolic disorder X-linked adrenoleukodystrophy (ALD) [96]. Model systems, ranging from cultured cells to plants to chimpanzees, share the genetic defect and biochemical aberrations associated with ALD, but each failed to fully recapitulate the disease, highlighting the challenge of choosing or creating an appropriate (human) disease model.
Using animal models as a diagnostic platform has evolved to a lesser extent than patient-derived material. Variants can be assessed in models by whole gene replacement or generation of specific variants. Each model system has both advantages and pitfalls, the choice of a model will depend on accessibility, ease of use, and, importantly, the presence of a valid readout for pathogenicity. While some genes may specifically affect a single cell type, others may cause systemic disease and may benefit from a whole animal model system. An additional challenge is mapping the human variation correctly into the genome of another organism. Recent advances in genome editing will aid in confirming diagnoses and developing model systems for RD.
Rapid evaluation of pathogenicity is essential for clinical utilization, and generating animal models with patient-specific variants is time-consuming. An alternate approach to changing the genome of the model system by introducing the variant of interest involves (over)expression of the patients’ gene in a knock-out rescue model system. Depending on the gene, model, and readout, these complementation assays may be done in transiently and relatively rapidly, enabling use in clinical diagnostics [97]. Recently, C. elegans whole gene humanized animal models were developed [98]. Increasing the availability of models will allow assessments of the functional consequences of many variants and will enhance the application of model systems in routine diagnostic care.
Combining gene editing of induced pluripotent stem cells (iPSCs) and cellular differentiation with transcriptomics is a powerful tool for studying RDs. iPSCs can be generated from easily accessible somatic cells like skin or blood and used to model the disease in vitro. Alternatively, CRISPR/Cas9 gene-editing technology allows the insertion of candidate variants into healthy iPSCs to create patient-specific disease models, whose effect can be assessed by RNA sequencing in the specific differentiated cell types. Bioinformatics tools, such as gene set enrichment analysis (GSEA), reveal perturbed pathways, known disease genes, and genes associated with particular HPO terms and allow for direct comparison to the patient’s phenotype [64]. Once established, these cellular disease models allow for further studies using molecular assays to understand disease etiology and develop new therapies.
Artificial intelligence (AI)
Large-scale and heterogeneous data, from omics to medical records to images, are generated at an increasing pace on patients with RD. It can be difficult or impossible to manually interpret and integrate the data or develop rules for predicting the diagnosis or the response to treatment. AI approaches are particularly attractive in this context [99]. AI is an umbrella term encompassing both symbolic approaches, which explicitly represent and interrogate expert knowledge with rules, and numeric approaches (usually referred to as Machine Learning), which use algorithms to extract information from data automatically. Challenges include the lack of data for many RDs, the high degree of clinical variability within and across RDs, batch effects, and the many different data sources (DNA sequencing, clinical features, imaging, metabolic analysis, etc.), each of which requires different computational processing.
AI has been widely used for image analysis. Approximately 1 in 3 RDs have a facial phenotype, and these phenotypes are increasingly being refined in a 3D space due to advances in computer science and increasing accessibility of 3D imaging devices at reducing cost. 3D imaging overcomes the inherent limitations of 2D imaging. Also, 3D facial phenotypes can be converted to standard and computer-readable text outputs for integration with other text-based results and various omics technologies [100]. 3D imaging can also be used to monitor treatment and clinical trial response—demonstrating it as a technology that can bridge diagnosis to therapy [101].
AI approaches are also widely used for the analysis of omics data in general, the interpretation of genomic variants, and the integration of various types of multiomics and even multimodal data. Importantly, analysis and interpretation of biomedical data using AI approaches benefit from expert/prior knowledge. Symbolic AI is based on this principle and leverages Knowledge Representation (KR), representing information about a domain in a form that computer algorithms can use to solve complex tasks. Ontologies are an approach to KR widely used in RD research and clinical care. The previously described HPO, together with HPO-based computational models of over 8500 diseases, can enable specific weighted fuzzy matching between patient features and the disease models to enable clinical decision support [102, 103]. Approaches that extend this to prioritize genes and variants identified by sequencing have been shown to improve diagnostic pipelines by many projects, including the 100,000 Genomes project [104]. Aside from potential benefits, there are also risks in the application of AI. For example, when used by clinicians not exhaustively formed in molecular genetics, misdiagnoses might be made.
How can these innovative technologies be applied efficiently to obtain a diagnosis & treatment and care?
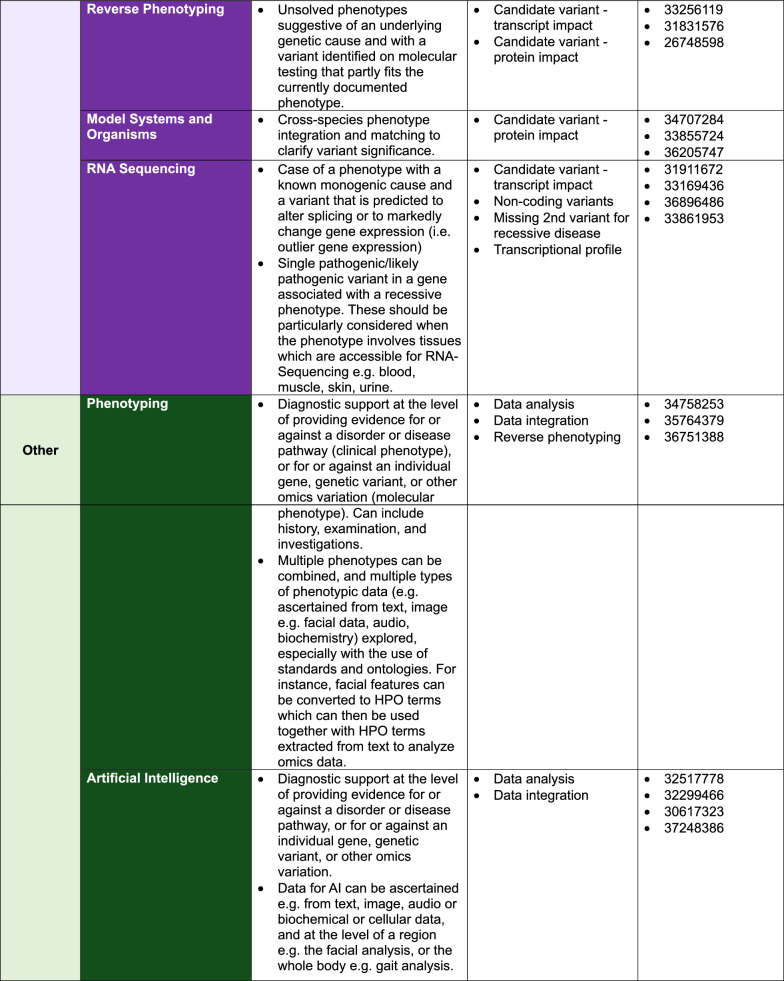
Broad application of novel technologies in clinical diagnostics commonly involves a progression through three stages: ‘pre-clinical’ (basic research), ‘early clinical’ (translational research, and ‘standard of care’ (clinical adoption) as illustrated in Fig. 1. The basic research stage normally involves the primary discovery and development of fundamental principles related to the technology. This typically happens in the basic academic laboratory setting and may provide some general insights into the potential application of the technology in the clinical setting, leading to the second stage of implementation involving translational research. At this stage, technology is systematically tested in relation to a particular clinical application. This involves typically larger clinical cohorts, consented through research protocols, and often involves clinical in addition to basic research laboratories. These studies can sometimes be national or international in scale and often aim to collect information about overall health systems impact in addition to validating the clinical utility of the technology. The final stage of clinical implementation of technologies that are ultimately proven valuable in the first two stages involves health systems implementation and clinical guidelines development. This stage can be highly variable globally, as it often depends on the jurisdictional health regulatory and funding implications. Jurisdictional health regulation is often national but can also be highly specific at the state/provincial level. Funding can also involve national or lesser jurisdictions such as health ministries and national health insurance, private health insurance, and patient pay systems, all adding layers of complexity. This final stage of implementation is often the longest and most challenging as it involves a much broader range of stakeholders, and it is often where promising technologies fail clinical adoption and implementation.
Fig. 1.
Innovative technologies enabling RD diagnosis and their current state of development
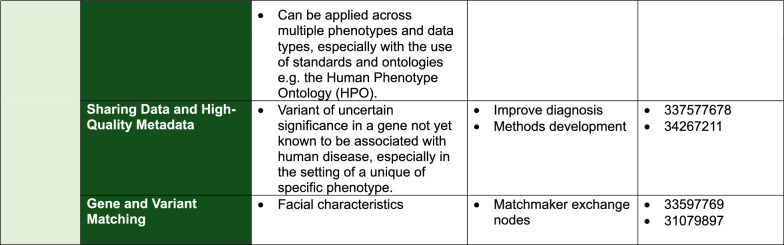
Phenotypes and known/suspected molecular mechanisms can help guide the use of existing and novel technologies [29]. We provide expert recommendations via the decision matrix in Fig. 2 with factors to be considered and with reference to published case vignettes.
Fig. 2.
Matrix of innovative technologies and related phenotypic and molecular categories, including PMIDs of case vignettes. *Read depth: The number of times each individual base has been sequenced
From diagnosis to personalized therapy and care
A confirmed diagnosis is essential for closure, proper disease and prognostic information for the individual and family members, including genetic counselling for other family members at risk and future offspring, access to services in the community, and increasingly for therapeutic and preventive interventions. Knowing the cause of disease is a stepping stone for P4 medicine: Personalized, Predictive, Participatory, and Preventive [106].
Ideally, early diagnosis allows disease-modifying therapies to exert their effect in the crucial “neurodevelopmental time window,” potentially preventing (progression of) RD phenotypes [107] as well as somatic complications. Therapies targeting the underlying cause of disease include medical diet, nutritional supplements, (repurposed) pharmacologic medication, organ or stem cell transplantation, and increasingly regenerative and RNA/gene therapies. Supportive interventions such as physio- and speech therapy, and special aid at school are also essential for optimizing patient outcomes. Customized preventive measures, such as screening for malignancies or sick day protocols for inherited metabolic disorders, can also be taken once the diagnosis is known. All in all, management changes based on ES/GS diagnoses vary from 15 to 40% in the literature [108].
Care Pathways (CPWs) are required to structure and harmonize care processes and continuously improve them within the patient-centered care concept [109]. CPWs aim to have “the right person, in the right place, doing the right thing, at the right time, with the right outcome, and all with attention to the patient experience.” Evidence-based resources and CPWs may be developed for single RDs (e.g., Huntington’s disease [110]) or disorders encompassing multiple related etiologies (e.g., hereditary ataxias [111]). Steps or constituents of every CPW include various combinations of diagnostic procedures, specific or symptomatic treatments, long-term care, surveillance or monitoring, rehabilitation, palliative services, self-management, etc. Whereas clinical guidelines provide generic standards and recommendations, CPWs consider the local organization of services, available competencies and resources, healthcare provider structures, and care systems [112]. The application of every step of a CPW in any given patient may also depend on their disease presentation, severity, and psychosocial circumstances.
Future perspectives
In this post-genome era, where we can generate huge amounts of molecular and other health data, we are rapidly entering the stage of scalable data interpretation of “-omic” technologies. To capture the power and promise of this health data revolution, we will rely exceedingly on algorithms to help us decipher the “data patterns” and identify useful ones as health biomarkers. We will further need to harmonize across-omics beyond genomes, extending from matchmaking carriership of rare genetic variants in patients with similar diseases to broadly assessing the sharing of multiple layers of -omics signatures. This omics and the broader health information revolution is inevitable as it follows similar data-driven transformation in other aspects of society, including communications, financial, transportation, social, and marketing industries, to name a few. With greater power comes greater responsibility; hence, it is critical to continue in parallel to develop the regulatory, ethical, and other societal standards to increase benefits and minimize risk and harm from these technologies.
There is an opportunity to build on existing technologies and informatic approaches that have been largely or frequently applied to diagnostics and extend these for treatment monitoring, be it through metabolic biomarkers, digital (e.g. imaging biomarkers), or epigenetic signatures of various stages of disease. To pick one example, phenotypic terminologies and ontologies have largely supported static cross-sectional implementations of individual terms. A key advance would be to systematically connect these terms into standardized longitudinal disease profiles that not only better support current, e.g. diagnostic use cases but can also support more precise and objective, personalized, predictive, preventative, and treatment measures.
There are multiple factors to address the need for better diversity and equity in diagnostics, and a detailed discussion of these is out of the scope of this review and has been discussed elsewhere [113]. One critical area to highlight is the emergence of the CARE Principles of Indigenous Data Governance, which provide an international framework for the ethical use of Indigenous data [114]. CARE Principles reflect the crucial role of culturally safe and responsive approaches supporting appropriate data acquisition, data use, and sharing, and complement the existing FAIR principles [115] as a key principle guiding equity and ethics in open data movements.
Once a diagnosis is established, the patient’s journey continues. And it must do so towards personalized care and therapeutic interventions to prevent morbidity and disability. Given the ever-growing number of RD interventions and ongoing trials, this can be a daunting task for the clinician. Indeed, several resources have been created to support the patient, family, and clinicians to get the therapies on the radar. Firstly, the ‘Treatabolome’, defined as a database of RD-specific treatments directly linked to the gene and variant level, will allow the flagging of already available therapies at the time of diagnosis [116]. Initially developed for neurometabolic (Treatable Intellectual disability app [2] and neurogenetic diseases (neuromuscular, epilepsy), this is now branching out to all phenotypes and conditions. Secondly, the UTOPIA (Unlocking Treatment Options, Personalized In-Time Access) RDs knowledge management platform, deployed at the Rare Care Centre at Perth Children’s Hospital, uses an individual’s phenotypic and (molecular) diagnostic information in combination with externally curated data sets (e.g. Orphanet, Clinicaltrials.gov, etc.) and AI. It delivers personalized healthcare summaries and pathways, and individualized flagging of relevant clinical trials, research, and non-health services (e.g. in education, community, and disability sectors). Ideally, treatment options are flagged in the ES/GS report for the clinician to consider lower thresholds and avoid delays.
New targets for treatment are identified at the genomic, epigenomic-transcriptomic, and metabolomic levels using model systems, such as (differentiated) iPSCs, organoids, and organisms. Deep phenotyping of these models allows for biomarker (e.g. metabolic, radiologic, epigenetic) identification. Potentially treatable manifestations can be identified via radiological, electrophysiological, hematological, somatic, neuropsychiatric, and contextual characterization. Reliable and relevant outcome measures are essential for adequately evaluating treatment safety and efficacy of novel interventions or repurposed drugs for RDs. Clinical heterogeneity and small patient numbers require special trial designs (e.g. N-of-1) and biostatistics. Personalized outcome measures patient & family participation with proper ethical and legal considerations are central in the process.
Technological advances drive the need for better education and workforce capacity building to ensure efficient, equitable, culturally appropriate, and value-adding deployment of existing and emerging diagnostics. This necessitates novel educational approaches that complement existing training paradigms that build capacity within primary care and specialist care, enable communities of practice between primary and specialist care (e.g. Project ECHO®), and are accessible and tailored to a diverse range of stakeholders and healthcare practitioners be they doctors, nurses, allied health, pharmacists or others.
Conclusion
Adopting genomic testing technologies such as genome sequencing, transcriptomics, epigenomics, and functional genomic technologies such as metabolomics for RDs is expected to impact healthcare systems significantly. Genomic testing technologies offer several benefits, such as improved accuracy in diagnosis, personalized treatment plans, and the potential to develop new therapies. This information can then be used to develop personalized patient treatment plans, such as gene or targeted drug therapies. Furthermore, bioinformatics and AI can help healthcare professionals analyze large genomic datasets and identify patterns that may be difficult to detect through manual analysis. This can help identify new treatment targets and improve the efficiency and accuracy of RD diagnosis and treatment. The developments in genomic testing technologies, bioinformatics, and AI for healthcare are part of a broader trend toward using big data and AI in many areas of our society. In recent years, big data and AI have transformed many industries, including finance, transportation, retail, and entertainment.
Despite these benefits, implementing genomic testing technologies into healthcare systems presents several challenges. One significant challenge is the high cost of testing, which can limit accessibility for patients, particularly in low-income or resource-limited settings. For example, the cost of GS can range from a few hundred to several thousand dollars, making it difficult for some patients to afford [117–119]. To address this challenge, efforts are being made to develop more affordable and scalable genomic testing technologies. Recognition and classification of pathogenic genetic variation is incomplete but improving using technologies described here. Another challenge is the interpretation of genomic data, which requires specialized knowledge and expertise that may only be readily available in some healthcare settings. Training programs are being developed to address this challenge and increase the number of healthcare professionals with expertise in genomic testing interpretation.
Finally, implementing genomic testing technologies into healthcare systems will require changes in how healthcare is delivered, policies, and guidelines. For example, health systems will need to develop policies and guidelines to address ethical and legal issues related to genomic testing, such as patient confidentiality and genetic discrimination. Furthermore, healthcare providers will need to be educated about the benefits and limitations of genomic testing to ensure appropriate use and interpretation of the data.
In conclusion, adopting genomic testing technologies for RDs has the potential to transform healthcare systems. Despite the challenges associated with implementing these technologies, efforts are underway to develop more affordable and scalable genomic testing technologies, increase healthcare professionals’ expertise in genomic testing interpretation, and develop policies and guidelines to ensure ethical and appropriate use of genomic data. With continued efforts to overcome these challenges, integrating genomic testing into healthcare systems can revolutionize the diagnosis and treatment of RDs, benefiting patients and their families.
In relation to diagnosis, every time we make a diagnosis, we learn something new about the function of the genome that can contribute to developing therapies in the long term. Technological innovation to create effective and accessible diagnostic tools creates an important avenue to a healthier future for individuals living with RDs.
Acknowledgements
The authors gratefully acknowledge Dr. Sarah Bowdin for her timportant early contribution and leadership as former Co-Chair of our Task Force, and Ms Alexandra Tataru (IRDiRC) for her administrative support. This manuscript was prepared by the authors in their personal capacity. The views and opinions expressed here are those of the authors and do not necessarily reflect the views, opinions, or position of their employers, any subsidiary companies, or any government agency.
Abbreviations
- 2D
2-dimensional
- 3D
3-dimensional
- AI
Artificial intelligence
- ALD
Adrenoleukodystrophy
- ATAC-seq
Assay for transposase-accessible chromatin using sequencing
- AVE
Atlas of variant effects
- CARE
Collective benefit, authority to control, responsibility, and ethics
- ClinGen
Clinical genome resource
- CPWs
Care pathways
- DNA
Deoxyribonucleic acid
- DNAm
DNA methylation
- ES
Exome sequencing
- FAIR
Findability, accessibility, interoperability, and reusability
- GA4GH
Global Alliance for Genomics and Health
- GC
Gene curation
- GDR
Gene-disease relationships
- GenCC
Gene curation coalition
- GS
Genome sequencing
- GSEA
Gene set enrichment analysis
- HPO
Human Phenotype Ontology
- iPSCs
Induced pluripotent stem cells
- IRDiRC
International Rare Diseases Research Consortium
- KR
Knowledge representation
- MAVEs
Multiplexed assays for variant effects
- MME
Matchmaker exchange
- NGS
Next-generation sequencing
- OMIM
Online inheritance in man
- RD
Rare diseases(s)
- RNA
Ribonucleic acid
- ROI
Return on investment
- SNP
Single nucleotide polymorphism
- SNV
Single nucleotide variant
- UMLS
Unified medical language system
- UTOPIA
Unlocking treatment options, personalised in-time access
- UTR
UTR sequences
- VUS
Variant of uncertain significance
- WGS
Whole genome sequencing
Author contributions
CVK and GB proposed and developed the related IRDiRC Task Force. CVK, AODL, GB, and BS led the related IRDiRC Task Force, wrote multiple sections, and performed overall review and editing of the manuscript. MCVL participated in the related IRDiRC Task Force and contributed to the coordination of the Task Force and writing of the manuscript, including creation and design of the illustrations of the paper. AB, PD, TG, JJ, KK, TL, SM, PNR, SS, RS, and CS are members of the related IRDiRC Task Force and contributed to the overall structuring and writing of the paper. All authors read and approved the final manuscript.
Funding
The IRDiRC Task Force on Integrating New Technologies for the Diagnosis of Rare Disease was supported by the Scientific Secretariat of IRDiRC, funded by the European Union through the European Joint Programme on Rare Disease (EJP RD) under the European Union’s Horizon 2020 Research and Innovation Programme Grant Agreement N°825575. The Scientific Secretariat is hosted at the French National Institute of Health and Medical Research (INSERM) in Paris, France. TL is supported by the Feilman Foundation, the Stan Perron Charitable Foundation, and the McCusker Charitable Foundation via Channel 7 Telethon Trust. SM is supported by U01HG011762 and AODL by U01HG011755 from NHGRI.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
The study does not involve human data or human tissue. No ethics approval was required.
Consent for publication
The study does not contain any individual person’s data.
Competing interests
CVK, AB, PD, TG, JJ, KK, TL, MCVL, PNR, RS, and GB declare no competing interests. BS is a cofounder of EpiSign Inc., a software biotech company. AODL is a scientific advisor for Tome Biosciences and Congenica Inc. SBM is an advisor for BioMarin, MyOme and Tenaya Therapeutics. SS is an employee of Sanofi. CS is an employee of Genomics England.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zurynski Y, et al. Australian children living with rare diseases: experiences of diagnosis and perceived consequences of diagnostic delays. Orphanet J Rare Dis. 2017;12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoytema van Konijnenburg EMM, et al. Treatable inherited metabolic disorders causing intellectual disability: 2021 review and digital app. Orphanet J Rare Dis. 2021;16:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graessner H, Zurek B, Hoischen A, Beltran S. Solving the unsolved rare diseases in Europe. Eur J Hum Genet. 2021;29:1319–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lübbeke A, Carr AJ, Hoffmeyer P. Registry stakeholders. EFORT Open Rev. 2019;4:330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day S, Rennie S, Luo D, Tucker JD. Open to the public: paywalls and the public rationale for open access medical research publishing. Res Involv Engagem. 2020;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vreman RA, et al. Assessment of significant benefit for orphan medicinal products by European regulators may support subsequent relative effectiveness assessments by health technology assessment organizations. Drug Discov Today. 2020;25:1223–31. [DOI] [PubMed] [Google Scholar]
- 7.Thorogood A, et al. International federation of genomic medicine databases using GA4GH standards. Cell Genom. 2021;1:100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson K. High-profile autism genetics project paused amid backlash. Nature. 2021;598:17–8. [DOI] [PubMed] [Google Scholar]
- 9.Hastings Ward J, et al. Research participants: critical friends, agents for change. Eur J Hum Genet. 2022;30:1309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Köhler S, et al. Expansion of the Human Phenotype Ontology (HPO) knowledge base and resources. Nucleic Acids Res. 2019;47:D1018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang H, et al. Whole genome sequencing of one complex pedigree illustrates challenges with genomic medicine. BMC Med Genom. 2017;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smedley D, et al. A whole-genome analysis framework for effective identification of pathogenic regulatory variants in mendelian disease. Am J Hum Genet. 2016;99:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baynam G, et al. 3-dimensional facial analysis-facing precision public health. Front Public Health. 2017;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shefchek KA, et al. The Monarch Initiative in 2019: an integrative data and analytic platform connecting phenotypes to genotypes across species. Nucleic Acids Res. 2020;48:D704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasilevsky NA, et al. Plain-language medical vocabulary for precision diagnosis. Nat Genet. 2018;50:474–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastarache L, et al. Phenotype risk scores identify patients with unrecognized Mendelian disease patterns. Science. 2018;359:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son JH, et al. Deep phenotyping on electronic health records facilitates genetic diagnosis by clinical exomes. Am J Hum Genet. 2018;103:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen JOB, et al. The GA4GH phenopacket schema defines a computable representation of clinical data. Nat Biotechnol. 2022;40:817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swietlik EM, et al. ‘There and back again’-forward genetics and reverse phenotyping in pulmonary arterial hypertension. Genes (Basel). 2020;11:1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landini S, et al. Reverse phenotyping after whole-exome sequencing in steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2020;15:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amberger JS, Bocchini CA, Scott AF, Hamosh A. OMIM.org: leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019;47:D1038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiStefano MT, et al. The gene curation coalition: a global effort to harmonize gene-disease evidence resources. Genet Med. 2022;24:1732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez-Luquez KY, Carpena MX, Karam SM, Tovo-Rodrigues L. The contribution of whole-exome sequencing to intellectual disability diagnosis and knowledge of underlying molecular mechanisms: a systematic review and meta-analysis. Mutat Res Rev Mutat Res. 2022;790:108428. [DOI] [PubMed] [Google Scholar]
- 24.Similuk MN, et al. Clinical exome sequencing of 1000 families with complex immune phenotypes: toward comprehensive genomic evaluations. J Allergy Clin Immunol. 2022;150:947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarailo-Graovac M, Wasserman WW, Van Karnebeek CDM. Impact of next-generation sequencing on diagnosis and management of neurometabolic disorders: current advances and future perspectives. Expert Rev Mol Diagn. 2017;17:307–9. [DOI] [PubMed] [Google Scholar]
- 26.Schofield D, et al. Cost-effectiveness of massively parallel sequencing for diagnosis of paediatric muscle diseases. NPJ Genom Med. 2017;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Britten-Jones AC, et al. The diagnostic yield of next generation sequencing in inherited retinal diseases: a systematic review and meta-analysis. Am J Ophthalmol. 2023;249:57–73. [DOI] [PubMed] [Google Scholar]
- 28.Menke J. Early detection of intestinal cancer–a task for the public health office? Offentl Gesundheitswes. 1987;49:282–4. [PubMed] [Google Scholar]
- 29.Wojcik MH, et al. Beyond the exome: what’s next in diagnostic testing for Mendelian conditions. Am J Hum Genet. 2023;110:1229–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wortmann SB, et al. How to proceed after ‘negative’ exome: a review on genetic diagnostics, limitations, challenges, and emerging new multiomics techniques. J Inherit Metab Dis. 2022;45:663–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavan S, et al. Clinical practice guidelines for rare diseases: the orphanet database. PLoS ONE. 2017;12:e0170365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodenreider O. The unified medical language system (UMLS): integrating biomedical terminology. Nucleic Acids Res. 2004;32:D267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haendel M, et al. How many rare diseases are there? Nat Rev Drug Discov. 2020;19:77–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrhart F, et al. A resource to explore the discovery of rare diseases and their causative genes. Sci Data. 2021;8:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umlai U-KI, Bangarusamy DK, Estivill X, Jithesh PV. Genome sequencing data analysis for rare disease gene discovery. Brief Bioinform. 2022;23:bbab363. [DOI] [PubMed] [Google Scholar]
- 36.Macken WL, et al. Specialist multidisciplinary input maximises rare disease diagnoses from whole genome sequencing. Nat Commun. 2022;13:6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logsdon GA, Vollger MR, Eichler EE. Long-read human genome sequencing and its applications. Nat Rev Genet. 2020;21:597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruels CC, et al. Diagnostic capabilities of nanopore long-read sequencing in muscular dystrophy. Ann Clin Transl Neurol. 2022;9:1302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen ASA, et al. Genomic answers for children: dynamic analyses of >1000 pediatric rare disease genomes. Genet Med. 2022;24:1336–48. [DOI] [PubMed] [Google Scholar]
- 40.Pauper M, et al. Correction: long-read trio sequencing of individuals with unsolved intellectual disability. Eur J Hum Genet. 2021;29:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamichi K, Stacey A, Mustafi D. Targeted long-read sequencing allows for rapid identification of pathogenic disease-causing variants in retinoblastoma. Ophthalmic Genet. 2022;43:762–70. [DOI] [PubMed] [Google Scholar]
- 42.Glinos DA, et al. Transcriptome variation in human tissues revealed by long-read sequencing. Nature. 2022;608:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadikovic B, Levy MA, Aref-Eshghi E. Functional annotation of genomic variation: DNA methylation episignatures in neurodevelopmental Mendelian disorders. Hum Mol Genet. 2020;29:R27–32. [DOI] [PubMed] [Google Scholar]
- 45.Sadikovic B, et al. Clinical epigenomics: genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet Med. 2021;23:1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy MA, et al. Novel diagnostic DNA methylation episignatures expand and refine the epigenetic landscapes of Mendelian disorders. HGG Adv. 2022;3:100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aref-Eshghi E, et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 Mendelian neurodevelopmental disorders. Am J Hum Genet. 2021;108:1161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hildonen M, et al. DNA methylation signature classification of rare disorders using publicly available methylation data. Clin Genet. 2023;103:688–92. [DOI] [PubMed] [Google Scholar]
- 49.Landrum MJ, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bamshad MJ, Nickerson DA, Chong JX. Mendelian gene discovery: fast and furious with no end in sight. Am J Hum Genet. 2019;105:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boycott KM, Azzariti DR, Hamosh A, Rehm HL. Seven years since the launch of the matchmaker exchange: the evolution of genomic matchmaking. Hum Mutat. 2022;43:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McWalter K, Torti E, Morrow M, Juusola J, Retterer K. Discovery of over 200 new and expanded genetic conditions using GeneMatcher. Hum Mutat. 2022;43:760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laurie S, et al. The RD-connect genome-phenome analysis platform: accelerating diagnosis, research, and gene discovery for rare diseases. Hum Mutat. 2022;43:717–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pais LS, et al. seqr: a web-based analysis and collaboration tool for rare disease genomics. Hum Mutat. 2022;43:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osmond M, et al. PhenomeCentral: 7 years of rare disease matchmaking. Hum Mutat. 2022;43:674–81. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigues EDS, et al. Variant-level matching for diagnosis and discovery: challenges and opportunities. Hum Mutat. 2022;43:782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belien JAM, Kip AE, Swertz MA. Road to FAIR genomes: a gap analysis of NGS data generation and sharing in the Netherlands. BMJ Open Sci. 2022;6:e100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schatz MC, et al. Inverting the model of genomics data sharing with the NHGRI genomic data science analysis, visualization, and informatics lab-space. Cell Genom. 2022;2:100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montgomery SB, Bernstein JA, Wheeler MT. Toward transcriptomics as a primary tool for rare disease investigation. Cold Spring Harb Mol Case Stud. 2022;8:a006198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cummings BB, et al. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med. 2017;9:eaal5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kremer LS, et al. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat Commun. 2017;8:15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frésard L, et al. Identification of rare-disease genes using blood transcriptome sequencing and large control cohorts. Nat Med. 2019;25:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yépez VA, et al. Clinical implementation of RNA sequencing for Mendelian disease diagnostics. Genome Med. 2022;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fear VS, et al. CRISPR single base editing, neuronal disease modelling and functional genomics for genetic variant analysis: pipeline validation using Kleefstra syndrome EHMT1 haploinsufficiency. Stem Cell Res Ther. 2022;13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.FANTOM. https://fantom.gsc.riken.jp/.
- 66.ENCODE. https://www.encodeproject.org/.
- 67.GTEx Portal. https://gtexportal.org/home/.
- 68.Towards a Human Cell Atlas: Taking notes from the past. https://www.humancellatlas.org/.
- 69.Larizza L, Finelli P. Developmental disorders with intellectual disability driven by chromatin dysregulation: clinical overlaps and molecular mechanisms. Clin Genet. 2019;95:231–40. [DOI] [PubMed] [Google Scholar]
- 70.Latypova X, et al. Haploinsufficiency of the Sin3/HDAC corepressor complex member SIN3B causes a syndromic intellectual disability/autism spectrum disorder. Am J Hum Genet. 2021;108:929–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, et al. ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat Commun. 2018;9:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corces MR, et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods. 2017;14:959–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haijes HA, et al. Direct infusion based metabolomics identifies metabolic disease in patients’ dried blood spots and plasma. Metabolites. 2019;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller MJ, et al. Untargeted metabolomic analysis for the clinical screening of inborn errors of metabolism. J Inherit Metab Dis. 2015;38:1029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Almontashiri NAM, et al. Clinical validation of targeted and untargeted metabolomics testing for genetic disorders: a 3 year comparative study. Sci Rep. 2020;10:9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herzog K, et al. Plasma lipidomics as a diagnostic tool for peroxisomal disorders. J Inherit Metab Dis. 2018;41:489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gasperini M, Starita L, Shendure J. The power of multiplexed functional analysis of genetic variants. Nat Protoc. 2016;11:1782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weile J, Roth FP. Multiplexed assays of variant effects contribute to a growing genotype-phenotype atlas. Hum Genet. 2018;137:665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Starita LM, et al. Variant Interpretation: functional assays to the rescue. Am J Hum Genet. 2017;101:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Findlay GM, et al. Accurate classification of BRCA1 variants with saturation genome editing. Nature. 2018;562:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott A, et al. Saturation-scale functional evidence supports clinical variant interpretation in Lynch syndrome. Genome Biol. 2022;23:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ng C-A, et al. A massively parallel assay accurately discriminates between functionally normal and abnormal variants in a hotspot domain of KCNH2. Am J Hum Genet. 2022;109:1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erwood S, et al. Saturation variant interpretation using CRISPR prime editing. Nat Biotechnol. 2022;40:885–95. [DOI] [PubMed] [Google Scholar]
- 84.Tewhey R, et al. Direct identification of hundreds of expression-modulating variants using a multiplexed reporter assay. Cell. 2016;165:1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Griesemer D, et al. Genome-wide functional screen of 3’UTR variants uncovers causal variants for human disease and evolution. Cell. 2021;184:5247-5260.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenberg AB, Patwardhan RP, Shendure J, Seelig G. Learning the sequence determinants of alternative splicing from millions of random sequences. Cell. 2015;163:698–711. [DOI] [PubMed] [Google Scholar]
- 87.Luppino F, Adzhubei IA, Cassa CA, Toth-Petroczy A. DeMAG predicts the effects of variants in clinically actionable genes by integrating structural and evolutionary epistatic features. Nat Commun. 2023;14:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Livesey BJ, Marsh JA. Using deep mutational scanning to benchmark variant effect predictors and identify disease mutations. Mol Syst Biol. 2020;16:e9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fowler DM, et al. An atlas of variant effects to understand the genome at nucleotide resolution. Genome Biol. 2023;24:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vadgama N, et al. De novo and inherited variants in coding and regulatory regions in genetic cardiomyopathies. Hum Genom. 2022;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu K, et al. Integrative analyses of scRNA-seq and scATAC-seq reveal CXCL14 as a key regulator of lymph node metastasis in breast cancer. Hum Mol Genet. 2021;30:370–80. [DOI] [PubMed] [Google Scholar]
- 92.Graham E, et al. Integration of genomics and metabolomics for prioritization of rare disease variants: a 2018 literature review. J Inherit Metab Dis. 2018;41:435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kerkhofs MHPM, et al. Cross-omics: integrating genomics with metabolomics in clinical diagnostics. Metabolites. 2020;10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hmeljak J, Justice MJ. From gene to treatment: supporting rare disease translational research through model systems. Dis Model Mech. 2019;12:dmm039271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Birling M-C, et al. A resource of targeted mutant mouse lines for 5,061 genes. Nat Genet. 2021;53:416–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Montoro R, Heine VM, Kemp S, Engelen M. Evolution of adrenoleukodystrophy model systems. J Inherit Metab Dis. 2021;44:544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Segers K, et al. Rapid prenatal diagnosis of fetal Zellweger syndrome by biochemical tests, complementation studies, and molecular analyses. Prenat Diagn. 2013;33:201–3. [DOI] [PubMed] [Google Scholar]
- 98.Hopkins CE, Brock T, Caulfield TR, Bainbridge M. Phenotypic screening models for rapid diagnosis of genetic variants and discovery of personalized therapeutics. Mol Aspects Med. 2023;91:101153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347–58. [DOI] [PubMed] [Google Scholar]
- 100.Cliniface. Cliniface. https://cliniface.org.
- 101.Jamuar S, et al. 3D facial analysis for rare disease diagnosis and treatment monitoring: proof-of-concept plan for hereditary angioedema. PLOS Digit Health. 2023;2:e0000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robinson PN, et al. The Human Phenotype Ontology: a tool for annotating and analyzing human hereditary disease. Am J Hum Genet. 2008;83:610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Köhler S, et al. Clinical diagnostics in human genetics with semantic similarity searches in ontologies. Am J Hum Genet. 2009;85:457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.100,000 Genomes Project Pilot Investigators et al. 100,000 genomes pilot on rare-disease diagnosis in health care-preliminary report. N Engl J Med385, 1868–1880 (2021). [DOI] [PMC free article] [PubMed]
- 105.Wojcik MH, et al. Unique capabilities of genome sequencing for rare disease diagnosis. BMJ Yale. 2023. 10.1101/2023.08.08.23293829v1. [Google Scholar]
- 106.van Eeghen AM, et al. Personalized medicine for rare neurogenetic disorders: can we make it happen? Cold Spring Harb Mol Case Stud. 2022;8:a006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kotulska K, et al. Prevention of epilepsy in infants with tuberous sclerosis complex in the EPISTOP trial. Ann Neurol. 2021;89:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tarailo-Graovac M, et al. Exome sequencing and the management of neurometabolic disorders. N Engl J Med. 2016;374:2246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tumiene B, Graessner H. Rare disease care pathways in the EU: from odysseys and labyrinths towards highways. J Community Genet. 2021;12:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bachoud-Lévi A-C, et al. International guidelines for the treatment of huntington’s disease. Front Neurol. 2019;10:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Silva RN, et al. Diagnosis and management of progressive ataxia in adults. Pract Neurol. 2019;19:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rotter T, et al. The effects of clinical pathways on professional practice, patient outcomes, length of stay, and hospital costs: cochrane systematic review and meta-analysis. Eval Health Prof. 2012;35:3–27. [DOI] [PubMed] [Google Scholar]
- 113.Adachi T, et al. Enhancing equitable access to rare disease diagnosis and treatment around the world: a review of evidence, policies, and challenges. Int J Environ Res Public Health. 2023;20:4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Global Indigenous Data Alliance. Global Indigenous Data Alliance. https://www.gida-global.org (2023).
- 115.D’Angelo CS, et al. Barriers and considerations for diagnosing rare diseases in indigenous populations. Front Pediatr. 2020;8:579924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bonne G. The treatabolome, an emerging concept. J Neuromuscul Dis. 2021;8:337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Incerti D, et al. Cost-effectiveness of genome sequencing for diagnosing patients with undiagnosed rare genetic diseases. Genet Med. 2022;24:109–18. [DOI] [PubMed] [Google Scholar]
- 118.Soilly AL, et al. Cost of exome analysis in patients with intellectual disability: a micro-costing study in a French setting. BMC Health Serv Res. 2023;23:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yeung A, et al. A cost-effectiveness analysis of genomic sequencing in a prospective versus historical cohort of complex pediatric patients. Genet Med. 2020;22:1986–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.