Abstract
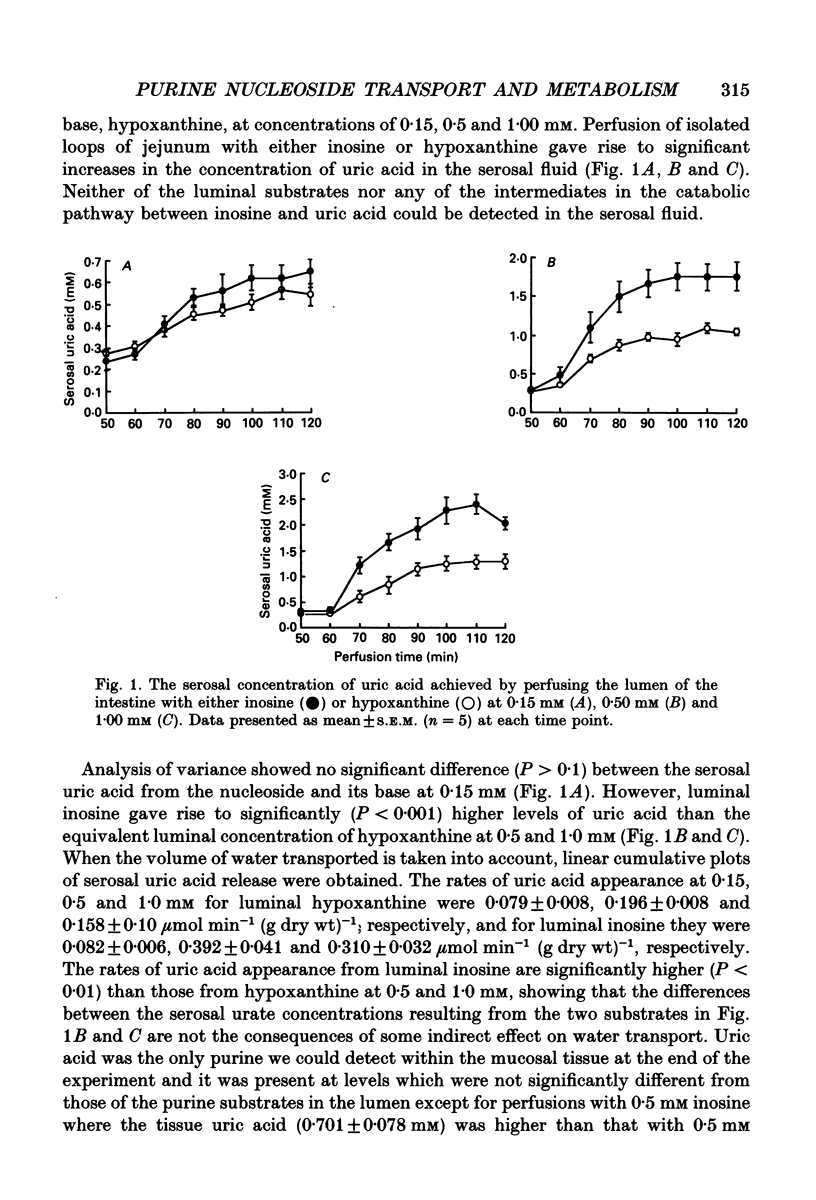
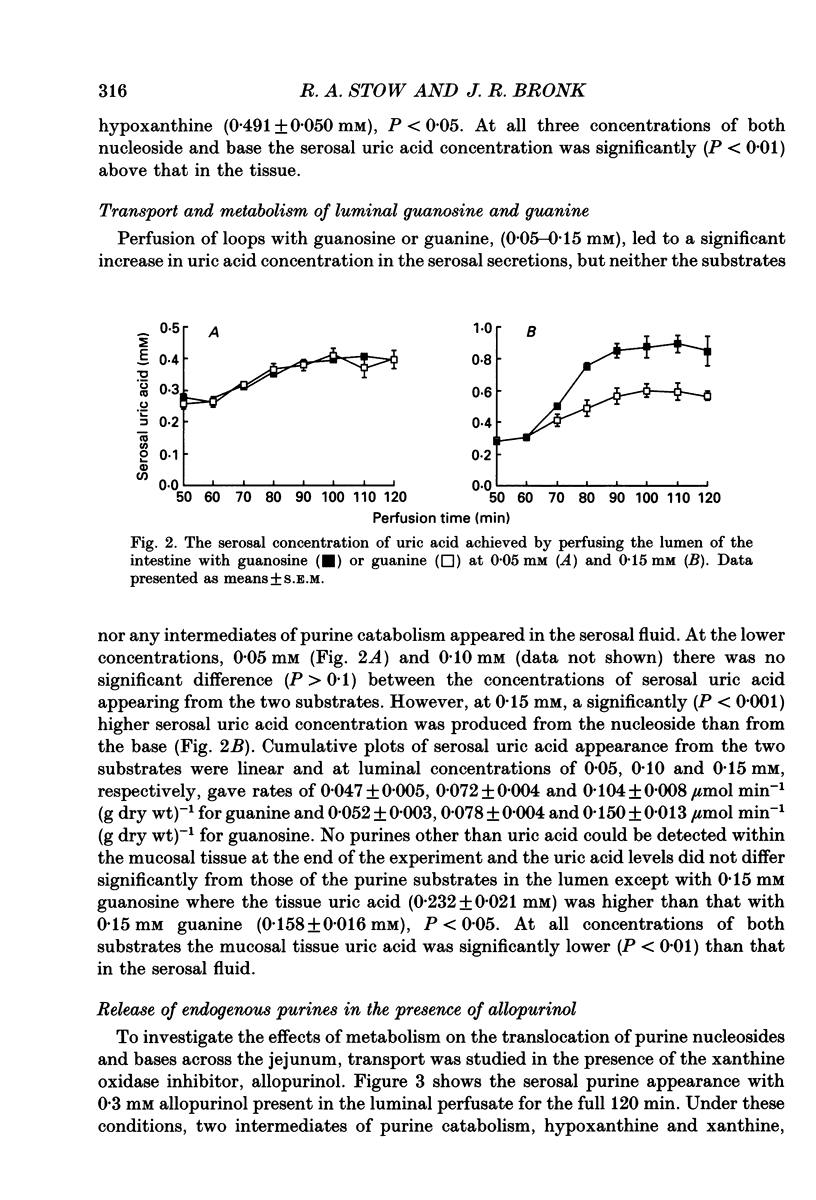
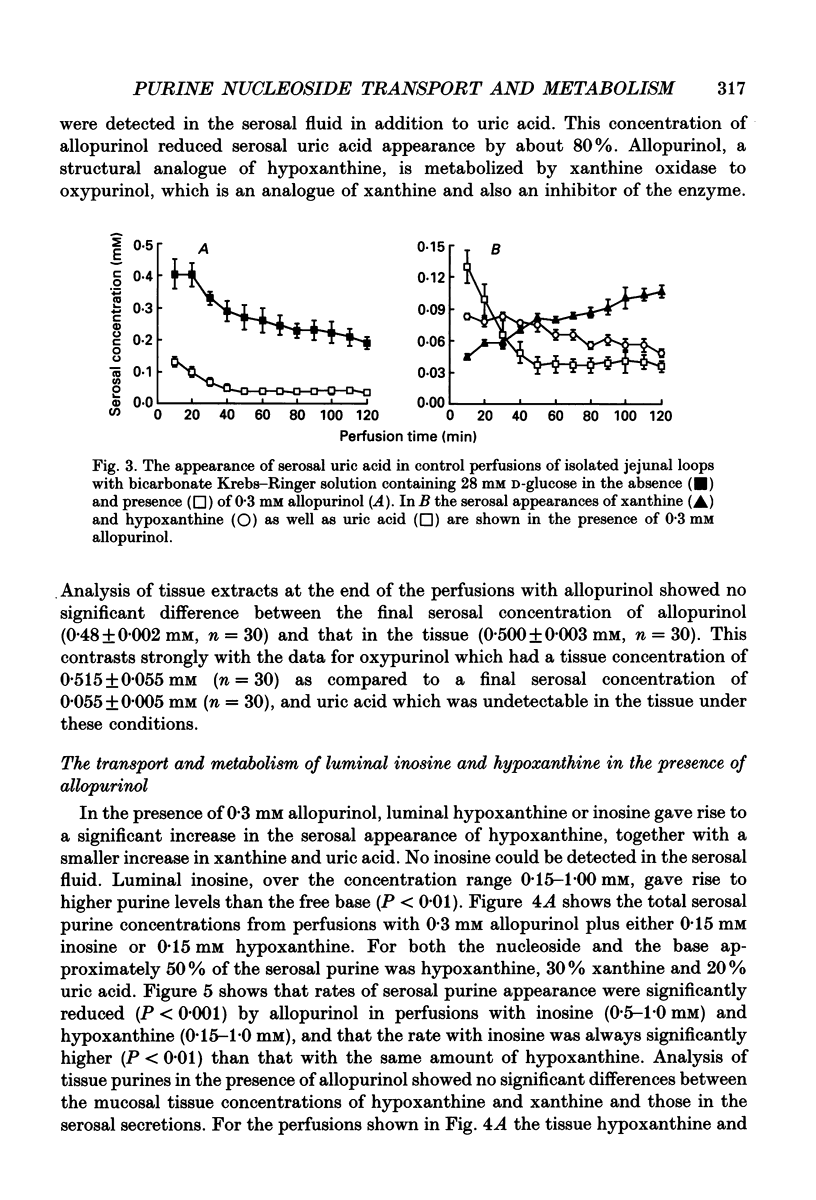
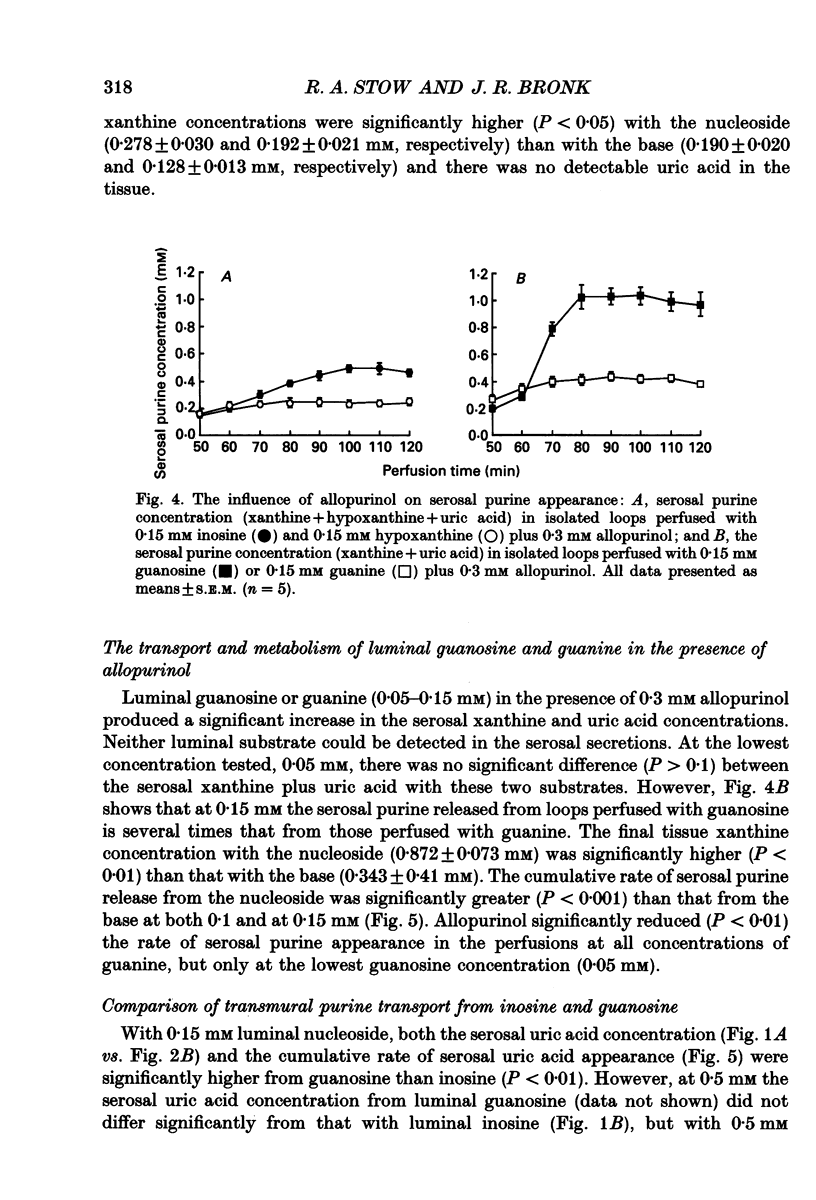
1. The absorption and metabolism of purine nucleosides and their constituent bases has been investigated by perfusion through the lumen of isolated loops of rat jejunum. In control perfusions and those with luminal purines or purine nucleosides, high-performance liquid chromatography (HPLC) revealed uric acid as the only detectable purine in the mucosal epithelial layer and the serosal secretions unless the xanthine oxidase inhibitor allopurinol was present. 2. Adenosine (0.5 mM) was quantitatively deaminated to inosine in the lumen after perfusion for 30 min. 3. Luminal inosine and hypoxanthine (0.15-1.0 mM) increased the serosal uric acid concentration significantly (P < 0.001); at 0.5 and 1.0 mM the nucleoside gave a significantly greater (P < 0.01) rate of serosal uric acid appearance than the base. 4. Luminal guanosine (0.05-0.50 mM) and guanine (0.05-0.15 mM) increased the serosal uric acid concentration significantly (P < 0.001); with 0.15 mM nucleoside the serosal uric acid appeared significantly faster (P < 0.01) than it did from the base. 5. Luminal allopurinol (0.3 mM) inhibited xanthine oxidase by 80% and reduced serosal purine appearance significantly (P < 0.01) from luminal guanine, hypoxanthine and inosine. With allopurinol, guanosine (0.1 and 0.15 mM) and inosine (0.1-1.0 mM) gave significantly higher (P < 0.01) total serosal purine concentrations than their respective bases. 6. Inosine and guanosine were cleaved to their respective bases plus ribose phosphate by the action of a cytoplasmic nucleoside phosphorylase, which was found to have widely different Michaelis constants (Km; 318 +/- 45 and 41.4 +/- 3.6 microM for inosine and guanosine, respectively) and maximum velocities (Vmax; 79.3 +/- 4.0 and 20.5 +/- 0.05 mumol min-1 (mg protein)-1 for inosine and guanosine, respectively). 7. We conclude that hypoxanthine and guanine absorbed by rat small intestine are oxidized to uric acid which is released in the serosa. The corresponding nucleosides are split by phosphorolysis after absorption and the resulting purine bases are converted to uric acid which appears on the serosal side with similar quantities of ribose phosphate.
Full text
PDF



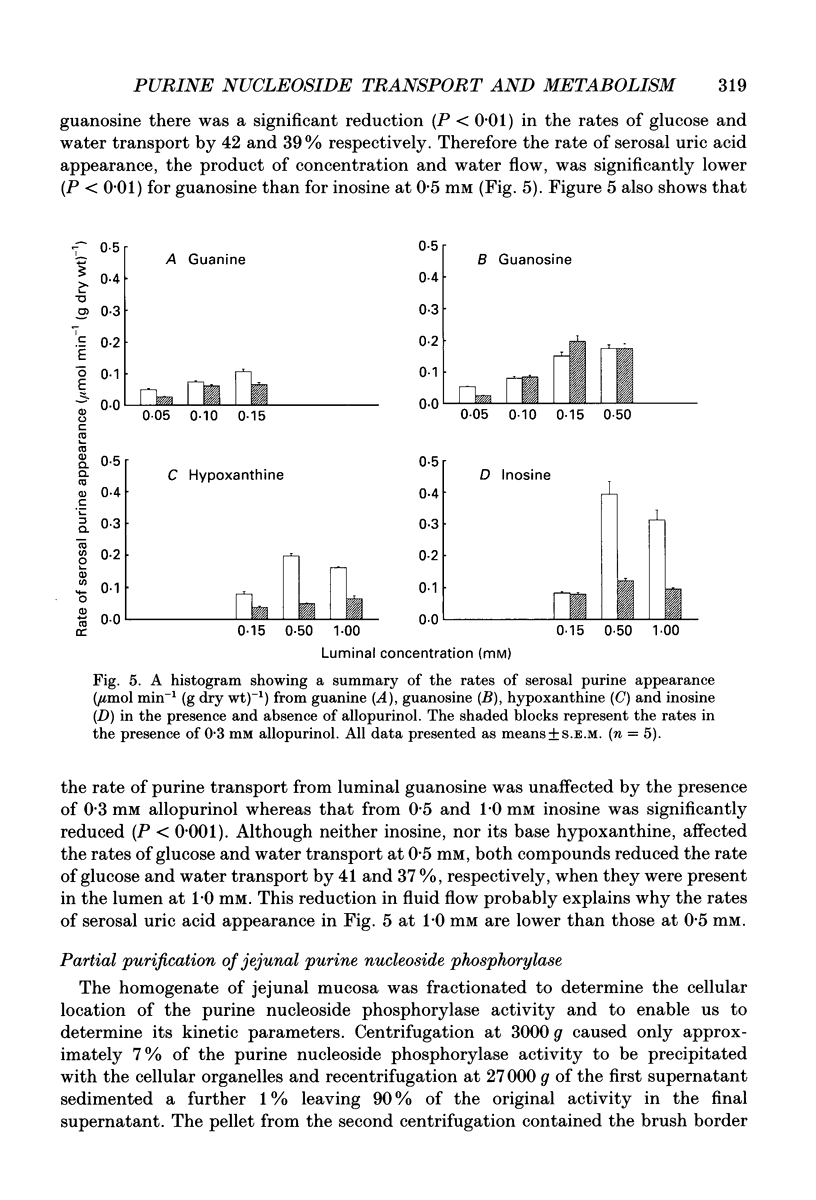




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
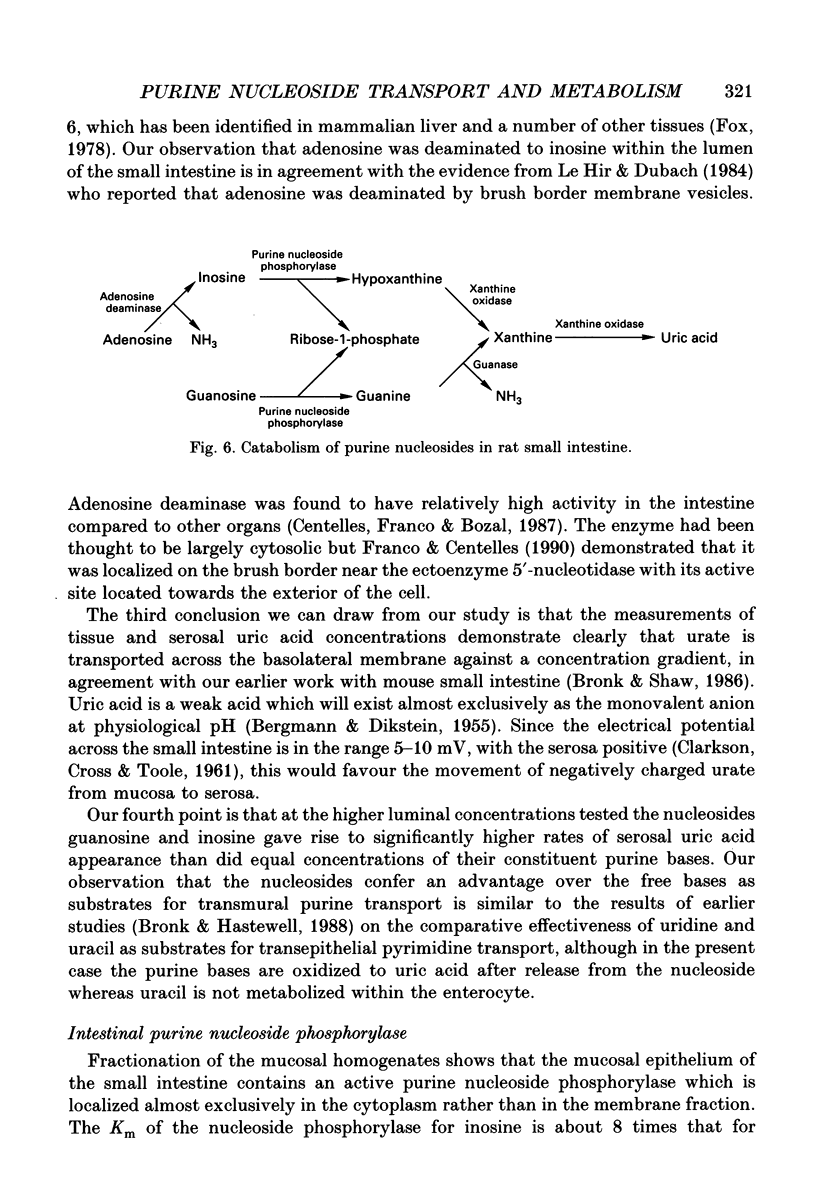
- Berlin R. D., Hawkins R. A. Secretion of purines by the small intestine: general characteristics. Am J Physiol. 1968 Oct;215(4):932–941. doi: 10.1152/ajplegacy.1968.215.4.932. [DOI] [PubMed] [Google Scholar]
- Bronk J. R., Hastewell J. G. The specificity of pyrimidine nucleoside transport and metabolism by rat jejunum in vitro. J Physiol. 1989 Jan;408:405–411. doi: 10.1113/jphysiol.1989.sp017466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Hastewell J. G. The transport and metabolism of naturally occurring pyrimidine nucleosides by isolated rat jejunum. J Physiol. 1988 Jan;395:349–361. doi: 10.1113/jphysiol.1988.sp016923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Hastewell J. G. The transport and metabolism of the uridine mononucleotides by rat jejunum in vitro. J Physiol. 1989 Jan;408:129–135. doi: 10.1113/jphysiol.1989.sp017451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Hastewell J. G. The transport of pyrimidines into tissue rings cut from rat small intestine. J Physiol. 1987 Jan;382:475–488. doi: 10.1113/jphysiol.1987.sp016379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Shaw M. I. The transport of uric acid across mouse small intestine in vitro. J Physiol. 1986 Sep;378:229–239. doi: 10.1113/jphysiol.1986.sp016216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKSON T. W., CROSS A. C., TOOLE S. R. Electrical potentials across isolated small intestine of the rat. Am J Physiol. 1961 Jun;200:1233–1235. doi: 10.1152/ajplegacy.1961.200.6.1233. [DOI] [PubMed] [Google Scholar]
- CSAKY T. Z. Significance of sodium ions in active intestinal transport of nonelectrolytes. Am J Physiol. 1961 Dec;201:999–1001. doi: 10.1152/ajplegacy.1961.201.6.999. [DOI] [PubMed] [Google Scholar]
- Centelles J. J., Franco R., Bozal J. Distribution of adenosine deaminase in some rat tissues. Inhibition by ethanol and dimethyl sulfoxide. Comp Biochem Physiol B. 1987;86(1):95–98. doi: 10.1016/0305-0491(87)90180-5. [DOI] [PubMed] [Google Scholar]
- Fisher R. B., Gardner M. L. A kinetic approach to the study of absorption of solutes by isolated perfused small intestine. J Physiol. 1974 Aug;241(1):211–234. doi: 10.1113/jphysiol.1974.sp010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R., Centelles J. J. Release of adenosine deaminase from rat intestinal mucosa. Biochem Soc Trans. 1990 Aug;18(4):641–642. doi: 10.1042/bst0180641. [DOI] [PubMed] [Google Scholar]
- HOLT J. H., MILLER D. The localization of phosphomonoesterase and aminopeptidase in brush borders isolated from intestinal epithelial cells. Biochim Biophys Acta. 1962 Apr 9;58:239–243. doi: 10.1016/0006-3002(62)91004-1. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. The utilization of glucose and production of lactate by in vitro preparations of rat small intestine: effects of vascular perfusion. J Physiol. 1976 Mar;255(3):775–795. doi: 10.1113/jphysiol.1976.sp011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms V., Stirling C. E. Transport of purine nucleotides and nucleosides by in vitro rabbit ileum. Am J Physiol. 1977 Jul;233(1):E47–E55. doi: 10.1152/ajpendo.1977.233.1.E47. [DOI] [PubMed] [Google Scholar]
- Khan A. H., Wilson S., Crawhall J. C. The influx of uric acid and other purines into everted jejunal sacs of the rat and hamster. Can J Physiol Pharmacol. 1975 Feb;53(1):113–119. doi: 10.1139/y75-015. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Dubach U. C. Sodium gradient-energized concentrative transport of adenosine in renal brush border vesicles. Pflugers Arch. 1984 May;401(1):58–63. doi: 10.1007/BF00581533. [DOI] [PubMed] [Google Scholar]
- Oh J. H., Dossetor J. B., Beck I. T. Kinetics of uric acid transport and its production in rat small intestine. Can J Physiol Pharmacol. 1967 Jan;45(1):121–127. doi: 10.1139/y67-013. [DOI] [PubMed] [Google Scholar]
- Parsons D. S., Shaw M. I. Application of high performance liquid chromatography to study transport and metabolism of nucleic acid derivatives by rat jejunum in vitro: endogenous washout. Q J Exp Physiol. 1983 Jan;68(1):39–51. doi: 10.1113/expphysiol.1983.sp002701. [DOI] [PubMed] [Google Scholar]
- Parsons D. S., Shaw M. I. Use of high performance liquid chromatography to study absorption and metabolism of purines by rat jejunum in vitro. Q J Exp Physiol. 1983 Jan;68(1):53–67. doi: 10.1113/expphysiol.1983.sp002702. [DOI] [PubMed] [Google Scholar]
- Reiter S., Simmonds H. A., Webster D. R., Watson A. R. On the metabolism of allopurinol. Formation of allopurinol-1-riboside in purine nucleoside phosphorylase deficiency. Biochem Pharmacol. 1983 Jul 15;32(14):2167–2174. doi: 10.1016/0006-2952(83)90222-8. [DOI] [PubMed] [Google Scholar]
- SCHANKER L. S., JEFFREY J. J., TOCCO D. J. INTERACTION OF PURINES WITH THE PYRIMIDINE TRANSPORT PROCESS OF THE SMALL INTESTINE. Biochem Pharmacol. 1963 Sep;12:1047–1053. doi: 10.1016/0006-2952(63)90028-5. [DOI] [PubMed] [Google Scholar]
- SCHANKER L. S., TOCCO D. J. Active transport of some pyrimidines across the rat intestinal epithelium. J Pharmacol Exp Ther. 1960 Feb;128:115–121. [PubMed] [Google Scholar]
- SCHANKER L. S., TOCCO D. J. Some characteristics of the pyrimidine transport process of the small intestine. Biochim Biophys Acta. 1962 Jan 29;56:469–473. doi: 10.1016/0006-3002(62)90598-x. [DOI] [PubMed] [Google Scholar]
- Simmonds H. A., Cadenhead A., Jones A. S., Hatfield P. J., Cameron J. S. The effect of allopurinol on oral purine absorption and excretion in the pig. Adv Exp Med Biol. 1974;41:653–655. doi: 10.1007/978-1-4757-1433-3_35. [DOI] [PubMed] [Google Scholar]
- Simmonds H. A., Cameron J. S., Morris G. S., Davies P. M. Allopurinol in renal failure and the tumour lysis syndrome. Clin Chim Acta. 1986 Oct 31;160(2):189–195. doi: 10.1016/0009-8981(86)90141-5. [DOI] [PubMed] [Google Scholar]
- WILSON D. W., WILSON H. C. Studies in vitro of the digestion and absorption of purine ribonucleotides by the intestine. J Biol Chem. 1962 May;237:1643–1647. [PubMed] [Google Scholar]
- Wheeler C. P., Yudilevich D. L. Transport and metabolism of adenosine in the perfused guinea-pig placenta. J Physiol. 1988 Nov;405:511–526. doi: 10.1113/jphysiol.1988.sp017345. [DOI] [PMC free article] [PubMed] [Google Scholar]


