Abstract
1. In previous work, we identified a prolonged and intense hyperaemic response of rat sciatic endoneurial vasa nervorum produced by epineurial application of capsaicin. We postulated that this response, which was blocked by substance P (SP) or calcitonin gene-related peptide (CGRP) antagonists, was a result of local release of neuropeptides on the 'feeding' epineurial vascular plexus. 2. In the present study, we evaluated factors that might influence capsaicin-induced hyperaemia of the rat sciatic endoneurium as measured by hydrogen clearance: central afferent connections, the epineurial vascular plexus, the release of histamine and administration of opiates. 3. Interruption of central afferent connections by proximal nerve section or removal of the epineurial vascular plexus did not influence baseline endoneurial perfusion. Plexus removal, but not proximal section, prevented capsaicin hyperaemia. 4. The epineurial vascular plexus was desensitized to the effect of capsaicin by prior application of capsaicin. Capsaicin hyperaemia was also prevented by: topical treatment with Spantide II ((D-NicLys1,3-Pal3,D-Cl2Phe5,Asn6,D-Trp7,9,Nl e11) substance P) an SP antagonist, systemic pretreatment with a combination of H1 and H2 histamine receptor antagonists, systemic pretreatment with cromolyn sodium or systemic pretreatment with morphine. None of these pretreatments influenced baseline perfusion. When systemic morphine was given together with systemic naloxone, an opiate antagonist, capsaicin-induced hyperaemia was restored. 5. These findings indicate that the capsaicin hyperaemia of vasa nervorum is locally mediated, is independent of central afferent connections and is sensitive to a variety of interventions. It requires an intact epineurial plexus that 'feeds' endoneurial microvessels and the release of histamine by mast cells. Its inhibition by morphine suggests that there are local opiate receptors on epineurial perivascular peptidergic fibres.
Full text
PDF



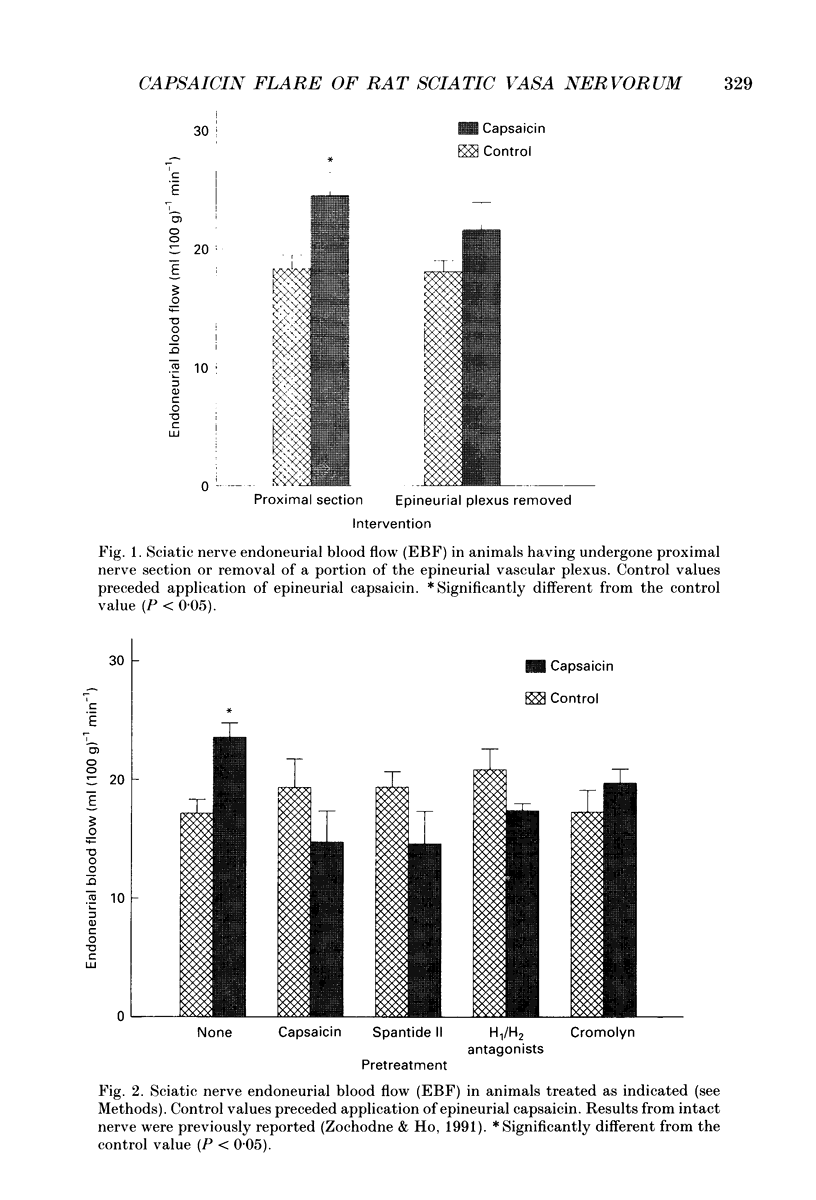
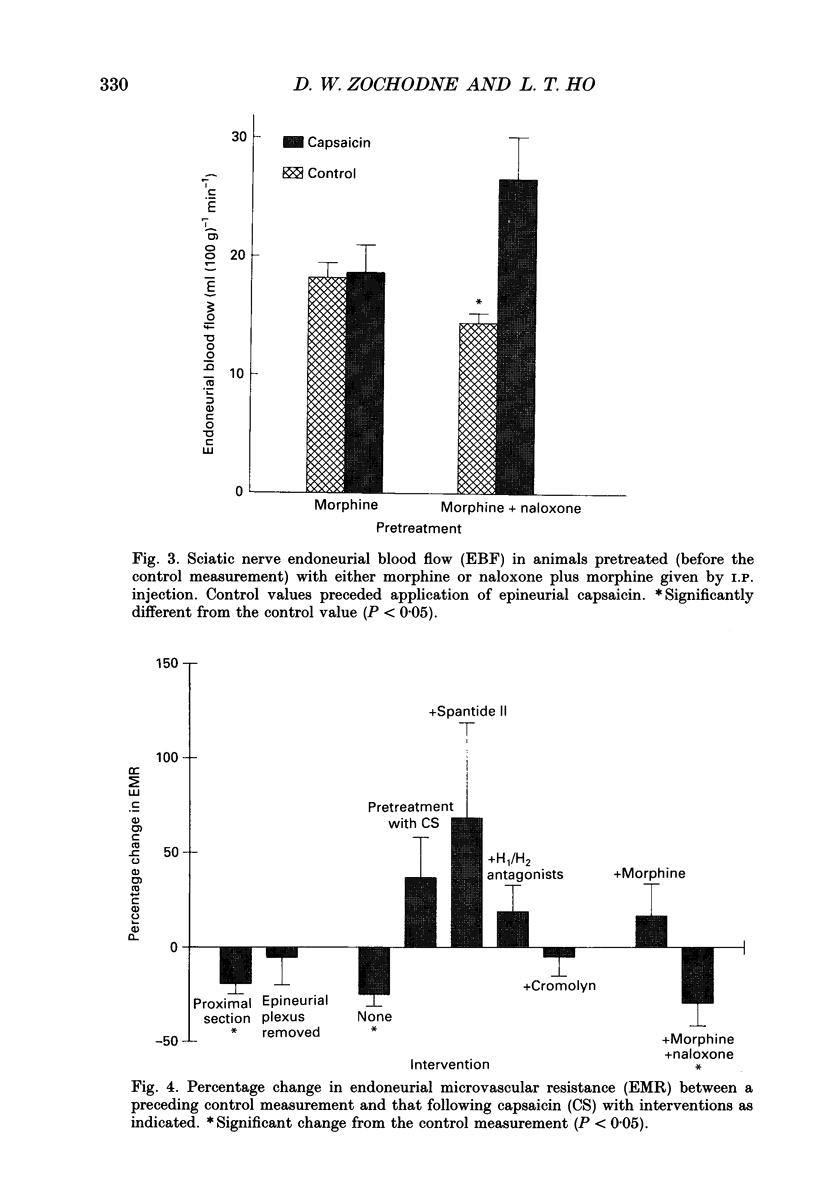



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell M. A., Weddell A. G. A descriptive study of the blood vessels of the sciatic nerve in the rat, man and other mammals. Brain. 1984 Sep;107(Pt 3):871–898. doi: 10.1093/brain/107.3.871. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., MacDonald S. G., Kage R., Luber-Narod J., Leeman S. E. Substance P receptor. Biochemical characterization and interactions with G proteins. Ann N Y Acad Sci. 1991;632:79–93. doi: 10.1111/j.1749-6632.1991.tb33096.x. [DOI] [PubMed] [Google Scholar]
- Brodin E., Gazelius B., Panopoulos P., Olgart L. Morphine inhibits substance P release from peripheral sensory nerve endings. Acta Physiol Scand. 1983 Apr;117(4):567–570. doi: 10.1111/j.1748-1716.1983.tb07228.x. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Carpenter S. E., Lynn B. Vascular and sensory responses of human skin to mild injury after topical treatment with capsaicin. Br J Pharmacol. 1981 Jul;73(3):755–758. doi: 10.1111/j.1476-5381.1981.tb16812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. J., Lagerlund T. D., Low P. A. Analysis of H2 clearance curves used to measure blood flow in rat sciatic nerve. J Physiol. 1989 Jul;414:35–54. doi: 10.1113/jphysiol.1989.sp017675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. L., Emson P. C., Leigh B. K., Gilbert R. F., Iversen L. L. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980 Mar 27;284(5754):351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Jordan C. C., Oehme P., Renner H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol. 1983 Feb;335:449–465. doi: 10.1113/jphysiol.1983.sp014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J., Hökfelt T., Post C., Brodin E., Sundström E., Jonsson G., Terenius L., Leander S., Fischer J. A., Verhofstad A. Immunohistochemical and behavioral analysis of spinal lesions induced by a substance P antagonist and protection by thyrotropin releasing hormone. Exp Brain Res. 1989;74(2):279–292. doi: 10.1007/BF00248861. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143–201. [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. A pharmacological and histological investigation of the involvement of mast cells in cutaneous axon reflex vasodilatation. Q J Exp Physiol Cogn Med Sci. 1975 Apr;60(2):123–130. doi: 10.1113/expphysiol.1975.sp002298. [DOI] [PubMed] [Google Scholar]
- Low P. A., Tuck R. R. Effects of changes of blood pressure, respiratory acidosis and hypoxia on blood flow in the sciatic nerve of the rat. J Physiol. 1984 Feb;347:513–524. doi: 10.1113/jphysiol.1984.sp015079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A. Capsaicin and primary afferent neurons: from basic science to human therapy? J Auton Nerv Syst. 1991 Apr;33(1):1–14. doi: 10.1016/0165-1838(91)90013-s. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Mast cells in human peripheral nerve. Acta Neurol Scand. 1971;47(3):357–368. doi: 10.1111/j.1600-0404.1971.tb07490.x. [DOI] [PubMed] [Google Scholar]
- PORSZASZ J., JANCSO N. Studies on the action potentials of sensory nerves in animals desensitized with capsaicine. Acta Physiol Acad Sci Hung. 1959;16:299–306. [PubMed] [Google Scholar]
- Piotrowski W., Foreman J. C. Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br J Dermatol. 1986 Jan;114(1):37–46. doi: 10.1111/j.1365-2133.1986.tb02777.x. [DOI] [PubMed] [Google Scholar]
- Rechthand E., Hervonen A., Sato S., Rapoport S. I. Distribution of adrenergic innervation of blood vessels in peripheral nerve. Brain Res. 1986 May 21;374(1):185–189. doi: 10.1016/0006-8993(86)90409-9. [DOI] [PubMed] [Google Scholar]
- Reid M. S., Hökfelt T., Herrera-Marschitz M., Håkanson R., Feng D. M., Folkers K., Goldstein M., Ungerstedt U. Intranigral substance P stimulation of striatal dopamine release is inhibited by spantide II: a new tachykinin antagonist without apparent neurotoxicity. Brain Res. 1990 Nov 5;532(1-2):175–181. doi: 10.1016/0006-8993(90)91757-8. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Savitt J. M., Jacobowitz D. M. Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry. 1985;82(1):5–8. doi: 10.1007/BF00502084. [DOI] [PubMed] [Google Scholar]
- Stein C., Comisel K., Haimerl E., Yassouridis A., Lehrberger K., Herz A., Peter K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991 Oct 17;325(16):1123–1126. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- Zochodne D. W., Ho L. T. Influence of perivascular peptides on endoneurial blood flow and microvascular resistance in the sciatic nerve of the rat. J Physiol. 1991 Dec;444:615–630. doi: 10.1113/jphysiol.1991.sp018897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne D. W., Huang Z. X., Ward K. K., Low P. A. Guanethidine-induced adrenergic sympathectomy augments endoneurial perfusion and lowers endoneurial microvascular resistance. Brain Res. 1990 Jun 11;519(1-2):112–117. doi: 10.1016/0006-8993(90)90067-l. [DOI] [PubMed] [Google Scholar]
- Zochodne D. W., Low P. A. Adrenergic control of nerve blood flow. Exp Neurol. 1990 Sep;109(3):300–307. doi: 10.1016/s0014-4886(05)80021-4. [DOI] [PubMed] [Google Scholar]


