Abstract
Human immunodeficiency virus type 1F12 (HIV-1F12) interferes with the replication of other strains of HIV. Its accessory protein, Nef, is sufficient for this phenotype, where the production and infectivity of HIV are impaired significantly. The analysis of three rare mutations in this Nef protein revealed that these effects could be separated genetically. Moreover, the defect in virus production correlated with the lack of processing of the p55Gag precursor in the presence of Nef from HIV-1F12. Importantly, the introduction of one of these mutations (E177G) into Nef from HIV-1NL4-3 also created a dominant-negative Nef protein. Effects of Nef from HIV-1F12 on virus production and Gag processing correlated with its altered subcellular distribution. Moreover, the association with two new cellular proteins with molecular masses of 74 and 75 kDa, which do not interact with other Nef proteins, correlated with the decreased virion infectivity. The identification of a dominant-negative protein for the production and infectivity of HIV suggests that Nef plays an active role at this stage of the viral replicative cycle.
The negative factor (Nef) of human immunodeficiency virus (HIV) and simian immunodeficiency virus plays a critical role in their pathogenicity. Viruses that lack the nef gene replicate to significantly lower levels than their wild-type counterparts and are generally considered nonpathogenic in vivo (8, 17, 18). However, the key role of this protein for lentiviral pathogenesis has not been elucidated fully. Identified functions of Nef include the removal of CD4 and major histocompatibility complex class I (MHC-I) molecules from the surface of the infected cell, which might facilitate the replication and immune evasion of HIV, respectively (5, 15, 19, 29, 31). Additionally, Nef activates signaling cascades, which increase levels of viral replication in infected cells (3, 4, 10, 11). Nef also plays a role in particle release (4, 9). Finally, Nef is packaged into virions and increases the infectivity of HIV (2, 27, 30, 32, 39). Contributions of these effects of Nef in vitro to its overall phenotype in vivo remain to be determined. Therefore, unrecognized functions of Nef could also contribute to its effects.
Nef provides the most significant growth advantage to HIV in quiescent primary cells (23, 33). However, functional studies on Nef have been complicated by the subtlety of some of its effects in transformed cell lines. One way to circumvent this problem was to use dominant-negative and antisense approaches that highlighted specific functions of Nef on cellular signaling and trafficking pathways (9, 10, 21, 22). Similarly, dominant-negative variants of viral proteins were instrumental in elucidating the processes of lentiviral morphogenesis and budding (34, 37). HIV-1F12 represents an example of a dominant-negative virus. Cells transfected with F12 proviral DNA do not produce virus particles despite the synthesis of all viral proteins. This effect also extends to the propagation of other HIV-1 isolates in these cells (13, 14). Functional studies on HIV-1F12 mapped these effects to the gag, vif, and nef genes (7). Subsequent studies suggested that Nef from HIV-1F12 (F12-Nef) alone could reconstitute this phenotype (24).
In this study, we demonstrate that F12-Nef, when expressed as a hybrid CD8-Nef protein, exerts a strong dominant-negative effect on the production and infectivity of HIV and interferes with the processing of the p55Gag precursor by the viral protease. These effects can be transferred to Nef from HIV-1NL4-3 (NL4-3–Nef) by a single point mutation. Whereas the effects on virus production and Gag processing correlated with a strictly perinuclear localization of dominant-negative Nef proteins, effects on virion infectivity could be mediated by its association with two new cellular proteins. These results suggest that Nef also plays an important role in viral morphogenesis and budding.
MATERIALS AND METHODS
Antibodies.
The following reagent was obtained through the AIDS Research and Reference Program, Division of AIDS Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health: antiserum to HIV-1 p25/24 Gag, from Kathelyn Steimer, Chiron Corporation (35). For the detection of Nef, the polyclonal rabbit serum pAKF3 (11) was used. The antibody against CD8 used for immunoprecipitation was from Pharmingen (San Diego, Calif.), and the fluorescein isothiocyanate (FITC)-conjugated anti-CD8 antibody used for immunofluorescence was purchased from Becton Dickinson (San Jose, Calif.).
Constructs.
Plasmid DNAs encoding replication-competent HIV proviruses were from the HIV-1 allele NL4-3 (1). The nef-deleted variant NL4-3pDs was generously provided by John Guatelli, University of California at San Diego. Expression plasmids for hybrid CD8-Nef proteins were generated by inserting the various nef genes into the EF promoter-driven expression plasmid for the extracellular and transmembrane portion of CD8 (CD8T) (22). In the construct encoding the CD8-F12-Nef protein, single point mutations encoding the respective amino acids in NL4-3–Nef were introduced by standard PCR mutagenesis methods.
Cells and transfections.
293T, Sx22-1, and NIH 3T3 cells were grown in Dulbecco modified minimal essential medium supplemented with 10% fetal calf serum and streptomycin-penicillin. Transfections were performed using Lipofectamine (Gibco BRL, Rockville, Md.) according to the manufacturer's instructions.
Virus production, infectivity, and Gag processing.
To assess the effects of Nef during virion production, 293T cells were transfected with proviral DNA and Nef expression plasmids at 1:1 molar ratio. At 48 h posttransfection, cells and cell culture supernatants were harvested. The cells were lysed in radioimmunoprecipitation assay (RIPA) buffer, and cleared supernatants were analyzed by Western blotting for Nef and Gag. Cell culture supernatants were cleared through a 45-μm-pore-size filter (Millipore, Bedford, Mass.) and stored at −80°C or used directly in infectivity assays. Measurements of the reverse transcriptase (RT) activity for quantification of virus production and the determination of the relative virion infectivity on Sx22-1 HeLa-CD4 indicator cells were performed as previously described (12).
Subcellular localization.
For the determination of the subcellular localization of hybrid CD8-Nef proteins, NIH 3T3 cells were plated on coverslips overnight and subsequently transfected with 1 μg of plasmid DNA. At 36 h posttransfection, cells were fixed in 3.7% formaldehyde for 5 min at room temperature, washed in phosphate-buffered saline (PBS), and then permeabilzed with 0.3% Triton X-100 in PBS for 3 min at room temperature. After the cells were washed in PBS, they were blocked in PBS–3% bovine serum albumin for 30 min at room temperature and then incubated with FITC-conjugated anti-CD8 antibody (Becton Dickinson) (1:20 dilution) for 1 h at room temperature. After extensive washing in PBS, the coverslips were mounted in Vectashield (Vector Laboratories, Burlingame, Calif.) and analyzed using a Leica DM IRBE confocal laser scanning microscope with a 63× oil objective. Pictures of individual optical sections were processed using Adobe PhotoShop software.
Analysis of F12-Nef-associated proteins.
At 30 h posttransfection, 293T cells transfected with 10 μg of plasmid DNA were labeled with 100 μCi of [35S]Met–[35S]Cys (Pro-Mix; Amersham Life Science, Arlington Heights, Ill.) for 10 h. Cells were then washed in PBS and lysed in RIPA buffer (150 mM NaCl, 50 mM Tris [pH 7.2], 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS]) for 30 min at 4°C, and cleared supernatants were immunoprecipitated with the anti-CD8 antibody. After 3 h of incubation, immunoprecipitates were washed extensively in RIPA buffer, separated by SDS–10% polyacrylamide gel electrophoresis (PAGE), and visualized by autoradiography.
RESULTS
F12-Nef interferes with production, infectivity, and Gag processing of HIV.
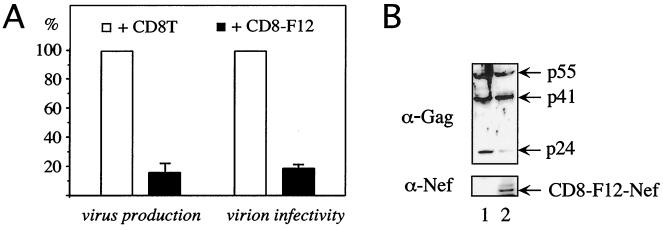
Previous studies suggested that the expression of F12-Nef could be sufficient to confer the phenotype of HIV-1F12 (7, 24). These effects depended on the myristoylation of the F12-Nef protein but, unlike with HIV-1F12, did not affect the processing of the p55Gag precursor (7, 13, 14, 24). Therefore, we decided to investigate the activity of a hybrid CD8-F12-Nef protein. This approach has been used widely to study the function of Nef (3, 9, 10, 22, 28, 41). To determine if this chimera reproduced the complete phenotype of HIV-1F12, the hybrid CD8-F12-Nef protein was coexpressed with the HIV-1NL4-3 provirus and virus production was monitored in 293T cells. As presented in Fig. 1A (virus production), the expression of the CD8-F12-Nef chimera resulted in a fivefold-reduced production of virus particles. This finding correlated with an almost complete loss of processing of the p55Gag precursor by the viral protease (Fig. 1B, compare lanes 1 and 2). The cleavage of the p55Gag precursor by the viral protease is a regulated multistep process (38). As indicated by the presence of the matrix-capsid (MA-CA) polyprotein intermediate (Fig. 1B, p41), the first cleavage step was not affected by F12-Nef. In sharp contrast, further processing of p41 into p17MA and p24CA was blocked (Fig. 1B, p24). Thus, the expression of the CD8-F12-Nef chimera alone recapitulated the phenotype of HIV-1F12. However, given the amount of virus particles produced even in the presence of F12-Nef, this finding could not explain the complete loss of replication of HIV-1F12 and HIV-1NL4-3/F12-Nef (24).
FIG. 1.
CD8-F12-Nef chimera acts as a dominant-negative Nef protein for the production and infectivity of HIV as well as Gag processing. (A) The hybrid CD8-F12-Nef protein inhibits the production and infectivity of HIV. Culture supernatants from 293T cells, which were cotransfected with the HIV-1NL4-3 proviral DNA and an expression plasmid for the truncated CD8 protein (CD8T) (+CD8T) or the CD8-F12-Nef chimera (+CD8-F12), were assayed for the amount of RT activity (virus production) and the relative infectivity of virions produced in a single round of replication (virion infectivity). Values are shown as the percentages of values produced in the absence of F12-Nef, with error bars showing the standard errors of the mean for three independent experiments performed in duplicate. (B) Western blotting of lysates from 293T cells used in panel A was performed with the indicated antibodies.
We also investigated the relative infectivity of virions produced in the presence of F12-Nef in a single round of replication (Fig. 1A, virion infectivity). Interestingly, F12-Nef also reduced strongly the infectivity of these particles and thus inhibited effects of proviral NL4-3–Nef. The expression of the extracellular and transmembrane portion of CD8 (CD8T) or of a CD8–NL4-3–Nef fusion protein had no effect on virus production, virion infectivity, or Gag processing of the full-length NL4-3 provirus in this experimental system (data not presented). We conclude that F12-Nef exerts a dominant-negative effect on the production and infectivity of HIV that blocks the replication of HIV-1F12 and HIV-1NL4-3/F12-Nef.
Effects of F12-Nef on production and infectivity of HIV are genetically separable.
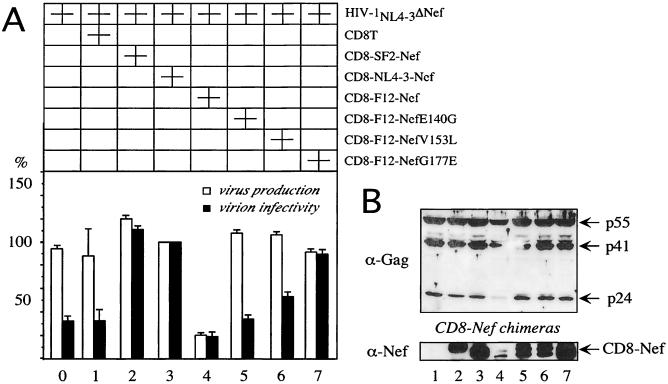
The sequence of F12-Nef is very similar to that of NL4-3–Nef. However, F12-Nef contains three point mutations (G140E, L153V, and E177G) that distinguish it from all other Nef proteins (7). To determine if these mutations were responsible for the phenotype of HIV-1F12, mutant hybrid CD8-F12-Nef proteins containing reversions of individual mutations were expressed and analyzed for their effects on the replication of HIV-1NL4-3 that lacked the nef gene (Fig. 2, HIV-1NL4-3ΔNef). Only the SF2-Nef and NL4-3–Nef proteins in the context of the CD8 chimera increased virion infectivity up to fourfold in this system (Fig. 2A, compare black bars 0, 1, 2, and 3). In sharp contrast, the hybrid CD8-F12-Nef protein suppressed the production (fivefold) and infectivity (up to twofold) of this provirus (Fig. 2A, bars 4). The E140G and L153V reversions in F12-Nef did not rescue virion infectivity (Fig. 2A, black bars 5 and 6). However, the reversion G177E resulted in the complete loss of the F12 phenotype (Fig. 2A, black bar 7). Of note, all three reversions abrogated the inhibition of Gag processing by F12-Nef (Fig. 2B, lanes 5, 6, and 7). Similar results were obtained in the presence of the wild-type HIV-1NL4-3 provirus (Fig. 1A and data not presented). Therefore, effects of F12-Nef on virus production and virion infectivity were separable genetically and are distinct. We conclude that the inhibition of Gag processing correlates with the decreased production but not infectivity of virus particles.
FIG. 2.
The negative effects of F12-Nef on virus production and virion infectivity are genetically separable. (A) Effects of F12-Nef and its single point revertant proteins on the production and infectivity of HIV. Culture supernatants from 293T cells, which were cotransfected with the HIV-1NL4-3ΔNef proviral DNA and the indicated Nef expression plasmids, were assayed for the amount of RT activity (virus production) and the relative infectivity of virions produced in a single round of replication (virion infectivity). Values are shown as the percentages of values produced in the absence of F12-Nef, with error bars showing the standard errors of the mean of three independent experiments performed in duplicate. (B) Western blotting of lysates of the 293T cells used in panel A was performed with the indicated antibodies.
A single point mutation converts NL4-3–Nef into a dominant-negative Nef protein.
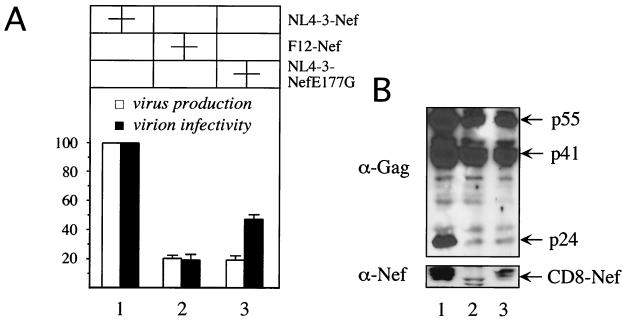
Our results indicated that the E177G change in F12-Nef was essential for its dominant-negative effects. Therefore, we wondered if the introduction of this mutation into a Nef protein with classical biological activities could result in a dominant-negative Nef protein. We constructed a plasmid that directed the synthesis of a mutant hybrid CD8–NL4-3–Nef protein that contained a glutamic acid instead of a glycine at position 177 (CD8–NL4-3–Nef E177G). Indeed, this mutant Nef protein inhibited the production and some of the infectivity of HIV-1NL4-3ΔNef similarly to F12-Nef (Fig. 3A, bars 2 and 3). As expected from our previous results, this reduction in virus production correlated with the block of processing of p55Gag (Fig. 3B, lanes 2 and 3). Interestingly, the infectivity of virions produced in the presence of the CD8–NL4-3–Nef E177G fusion protein was intermediate between that of F12-Nef and NL4-3–Nef (Fig. 3A, compare black bar 3 to black bars 1 and 2). We conclude that the E177G mutation confers many of the dominant-negative properties of F12-Nef to a Nef with classical biological activities.
FIG. 3.
One single point mutation confers the F12 phenotype onto NL4-3–Nef. (A) NL4-3–Nef E177G inhibits the production and infectivity of HIV. Culture supernatants from 293T cells, which were cotransfected with HIV-1NL4-3ΔNef proviral DNA and the indicated Nef expression plasmids, were assayed for the amount of RT activity (virus production) and the relative infectivity of virions produced in a single round of replication (virion infectivity). Values shown are the percentages of values produced in the absence of F12-Nef, with error bars showing the standard errors of the mean of three independent experiments performed in duplicate. (B) Western blotting of cell lysates of the 293T cells used in panel A were performed with the indicated antibodies.
F12-Nef displays atypical subcellular distribution.
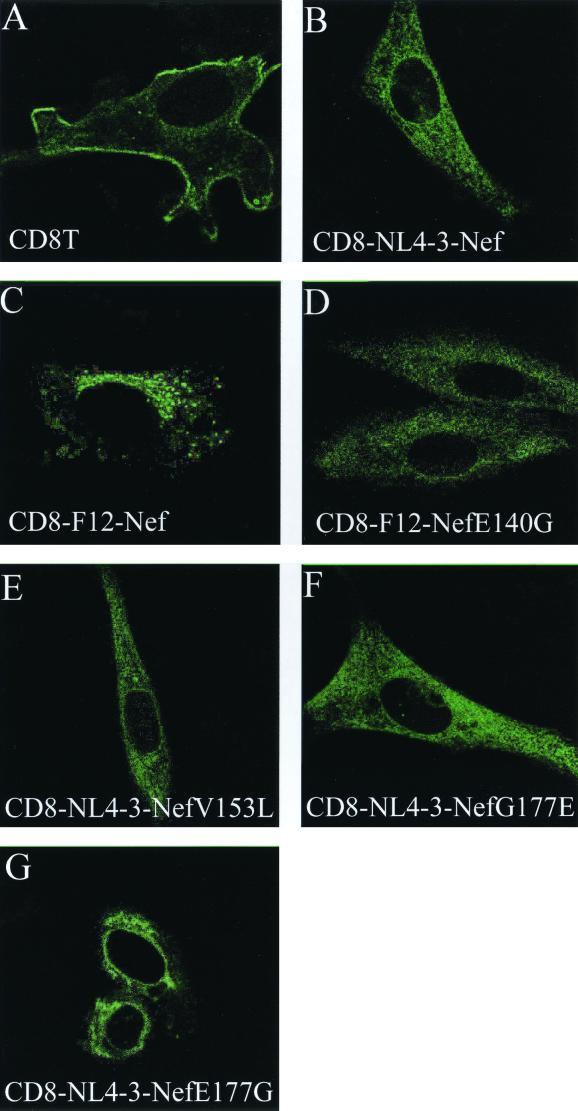
To gain insight into the mechanism of the dominant-negative effect of F12-Nef, we analyzed its subcellular distribution by confocal microscopy of NIH 3T3 cells. Similar to previous reports (16, 28), we found the hybrid CD8–NL4-3–Nef protein distributed throughout the cytoplasm and at the plasma membrane in a punctate pattern and in structures reminiscent of the endoplasmic reticulum and Golgi compartments (Fig. 4B). This staining was distinct from that observed with the extracellular and transmembrane portion of CD8 alone that was localized prominently at the plasma membrane (Fig. 4A, CD8T). In sharp contrast, the hybrid CD8-F12-Nef protein was found almost exclusively in large vesicles surrounding the nucleus and was not detected in other parts of the cytoplasm (Fig. 4C). This distribution was identical to that of the F12-Nef.GFP fusion proteins in lymphoid cells and fibroblasts (reference 24 and data not presented). The three F12-Nef mutants that lost the ability to interfere with virus production and Gag processing also did not display a strict perinuclear pattern and had a subcellular distribution that resembled that of the CD8–NL4-3–Nef (Fig. 4D, E, and F). Interestingly, the introduction of the E177G mutation into the hybrid CD8–NL4-3–Nef protein also resulted in its perinuclear accumulation with the relative exclusion from other areas of the cytoplasm (Fig. 4G). This restriction was less pronounced than that with the hybrid CD8-F12-Nef protein but was distinct from the hybrid CD8–NL4-3–Nef protein. Together, these results demonstrate that dominant-negative effects of F12-Nef on virus production and Gag processing correlate with its altered subcellular distribution and suggest that this localization plays an important role in its mechanism of action.
FIG. 4.
Dominant-negative Nef proteins are concentrated in the perinuclear area. Presented is an immunostaining of NIH 3T3 cells, which were transfected with the indicated expression plasmids. The subcellular distribution of the expressed proteins was visualized with a FITC-conjugated α-CD8 antibody by using confocal microscopy. Cells expressed the truncated CD8T protein (A), the hybrid CD8–NL4-3–Nef protein (B), the hybrid CD8-F12-Nef protein (C), the hybrid mutant CD8-F12-Nef E140G protein (D), the hybrid mutant CD8-F12-Nef V153L protein (E), the hybrid mutant CD8-F12-Nef G177E protein (F), and the hybrid mutant CD8–NL4-3–Nef E177G protein (G).
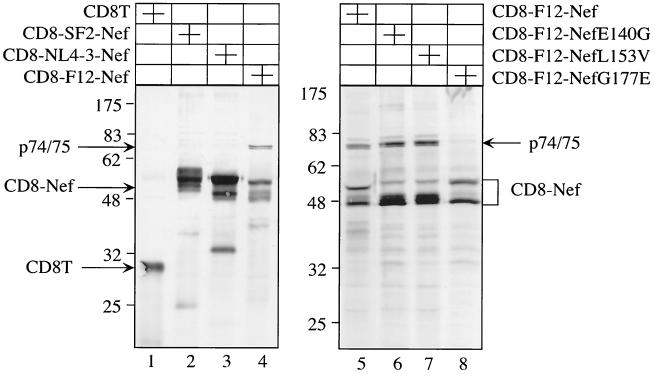
F12-Nef interacts with two cellular proteins with masses of 74 and 75 kDa.
Since Nef does not possess any known enzymatic activity and all of its known functions are mediated by interactions with cellular proteins, we wanted to determine if F12-Nef interacts with cellular partners different from those of NL4-3–Nef and SF2-Nef. To this end, 293T cells, which expressed our CD8-F12-Nef chimeras, were metabolically labeled and immune complexes precipitated with the anti-CD8 antibody. As presented in Fig. 5, under our relatively stringent conditions, few cellular proteins were associated with CD8T or CD8–NL4-3–Nef and CD8-SF2-Nef chimeras, respectively (Fig. 5, lanes 1 to 3). Given that most known interactions of Nef are weak and transient and are detected with more directed approaches, this finding was not surprising. In contrast, F12-Nef bound strongly to two cellular proteins with molecular masses of approximately 74 and 75 kDa (Fig. 5, lanes 4 and 5). Interestingly, these cellular proteins were also found to associate with the E140G and L153V but not with the G177E mutant F12-Nef proteins (Fig. 5, lanes 6 to 8). Thus, the association with p74/75 correlated with the ability of F12-Nef to reduce virion infectivity. Their identification should provide further insights into the mechanism of action of F12-Nef.
FIG. 5.
F12-Nef associates with cellular proteins with molecular masses of 74 and 75 kDa. 293T cells were transfected with the indicated expression plasmids and were metabolically labeled, and immune complexes were precipitated from cell lysates with the α-CD8 antibody. Presented is an autoradiograph of these immunoprecipitations after their separation by SDS–10% PAGE. Arrows give the positions of the truncated CD8T protein as well as the hybrid CD8-Nef and F12-Nef associated proteins.
DISCUSSION
This study demonstrates that the expression of Nef from HIV-1F12 as a CD8-fusion protein is sufficient to recapitulate the inhibitory effect of this provirus and results in the generation of a dominant-negative Nef protein. Both the production and the infectivity of HIV were significantly reduced in the presence of F12-Nef independently of the wild-type NL4-3–Nef. Importantly, a significant amount of the F12 phenotype could be transferred to Nef from HIV-1NL4-3 by a single amino acid substitution (E177G). Importantly, the relevance of this point mutation was confirmed in infection experiments (d'Aloja et al., unpublished data). Moreover, the activities of F12-Nef on the production and infectivity of HIV were mechanistically distinct. Whereas the inhibitory effect of F12-Nef on the processing of p55Gag correlated with the reduction of particle production and could be mediated by its strictly perinuclear subcellular localization, the reduction of virion infectivity by F12-Nef correlated with its binding to two cellular proteins with masses of 74 and 75 kDa. These results are summarized in Table 1.
TABLE 1.
Comparison of activities of F12-Nef and its revertant proteins
| Nef alleles | Virus production | Gag processing | Virion infectivity | Perinuclear concn | p74/75 binding |
|---|---|---|---|---|---|
| NL4-3–Nef | + | + | + | − | − |
| F12-Nef | − | − | − | + | + |
| F12-Nef E140G | + | + | − | − | + |
| F12-Nef L153V | + | + | − | − | + |
| F12-Nef G177E | + | + | + | − | − |
The use of CD8-Nef fusion proteins was instrumental for the reconstitution of the phenotype of HIV-1F12. Most likely, this chimera accounts for some of the discrepancy with the report by Olivetta et al. (24) that described subtler effects of the nonfusion F12-Nef, which did not inhibit the processing of p55Gag. The presence of the CD8 moiety, which increases the association of F12-Nef with cellular membranes that is essential for the antiviral effects of F12-Nef (7), might have exaggerated its phenotype. Indeed, the expression of a nonfusion F12-Nef also had lesser effects in our experimental system (data not presented). Moreover, the presence of epitope tags on the C terminus of F12-Nef could have lessened its biological effects (24). Similarly, we noticed that Nef-GFP fusion proteins, where the green fluorescence protein was fused to the C terminus of Nef, did not increase virion infectivity (data not presented). Alternatively, the expression of the nonfusion F12-Nef might have to occur in cis and/or with matched viral structural proteins to be fully functional. Thus, only the expression of the hybrid CD8-Nef-F12 protein revealed the full dominant-negative potential of F12-Nef.
Whereas three changed residues (E140, L153, G177) in F12-Nef appear to be critical for its activity, the introduction of only G177 into NL4-3–Nef conferred upon it most of the dominant-negative properties. In turn, the presence of G177 was required but not sufficient for the inhibitory action of F12-Nef. This paradox might reflect slight variations in the conformation of these Nef proteins, which differ in more than the three analyzed F12 residues. Since the structure of the flexible loop is unknown, it is possible that E177 regulates its accessibility or that of the entire C terminus for protein-protein interactions (such as those with the cellular p74/75) that mediate effects of the dominant-negative Nef proteins. Moreover, the conformation of NL4-3–Nef might be more susceptible to changes at this critical residue than that of F12-Nef. Finally, since the E177G mutation in NL4-3–Nef did not result in the complete phenotype of F12-Nef, we are currently investigating whether the introduction of additional mutations at positions G140 and V153 will increase its dominant-negative activity.
It seems likely that this inhibition by F12-Nef reveals an important property of the wild-type protein. Indeed, positive effects on the production and infectivity of HIV are well-known functions of Nef (2, 4, 9, 32). However, specific mutations in the nef gene resulted previously in Nef-negative but not dominant-negative phenotypes. Thus, effects of F12-Nef have to be based on an active principle. Of note, an involvement of Nef in the processing of viral polyprotein precursors had not been reported previously. Thus, F12-Nef might have revealed a new function of Nef that would have been difficult to detect in the absence of its dominant-negative variants. This new mechanism is supported by other biological activities of F12-Nef. It fails to remove CD4 and MHC-I molecules from the plasma membrane, and it does not activate p21-activated kinase (PAK) (7, 24; d'Aloja et al., unpublished data). However, since mutant Nef proteins that are defective in these functions do not exert an F12-Nef phenotype, the loss of these activities is not sufficient to explain the F12 phenomenon.
So, what could be the molecular mechanisms of suppressed production and infectivity of HIV by F12-Nef? Although the enhancement of virion infectivity is an established function of Nef, relatively little is known about its mechanism. Nef increases virion infectivity in the presence (12, 19) and absence (2, 26) of CD4. This effect can be only partially attributed to cellular activation by Nef (9, 36, 40) but appears to require the integrity of the flexible loop at the C terminus of Nef (6). Of note, the dominant-negative effect of F12-Nef on virion infectivity correlated with the binding of p74/75, which required the glycine at position 177. Thus, the identification of p74/75 might reveal the mechanism of decreased virion infectivity by Nef.
Effects of Nef on virus production are mediated at two levels. First, cellular activation by Nef increases transcription of the viral genome (4, 9, 10, 21). Additionally, Nef plays a poorly characterized role in particle release (4, 9). F12-Nef interferes at the latter step and may thus prove instrumental for our understanding of the role of Nef in the replicative cycle of HIV. The block of p55Gag processing and virus production by F12-Nef is reminiscent of the phenotype observed with Gag proteins that are not properly targeted to the plasma membrane (20). Thus, F12-Nef could act via a similar mechanism. One possible scenario is that Nef travels with Gag molecules during viral morphogenesis and budding and enhances the processing of Gag. This scenario predicts that Nef has a viral target, such as the Gag or GagPol precursors, with which it displays a genetic functional synergy (25, 30). Since the interaction with its target probably mediates the incorporation of Nef into virus particles, the low copy number of Nef in virions argues for the GagPol precursor as the binding partner for Nef (27, 39). As suggested by its perinuclear localization, inhibition of p55Gag processing, and virus production, the dominant-negative effects of F12-Nef could result from the misrouting of its target away from the plasma membrane. In this scenario, the viral protease would not be activated, which resembles the situation with unmyristoylated Gag proteins (20). Of note, steady-state levels of our hybrid CD8-F12-Nef protein were very low in cells (Fig. 1 to 3), suggesting that F12-Nef and its target are degraded rapidly. While the individual dominant-negative effects of F12-Nef on virion infectivity and virus production were not absolute, they could synergize with each other, leading to a more complete inhibition of viral replication, which was observed previously with the HIV-1F12 provirus (24). With the example of HIV-1F12, Nef joins the growing list of HIV proteins whose dominant-negative versions interfere with the viral replicative cycle. As cells expressing F12-Nef do not support viral replication yet synthesize sufficient viral proteins to elicit a vigorous immune response, the incorporation of dominant-negative Nef proteins into antiviral strategies should be considered.
ACKNOWLEDGMENTS
We are grateful to John Guatelli for providing Δnef proviral DNA and to Nikolaus Müller-Lantzsch for the polyclonal anti-Nef antibody. We thank Björn Schwer for generous help with confocal microscopy and Matthias Geyer for fruitful discussions.
O.T.F. acknowledges support from the Deutsche Forschungsgemeinschaft. This research was funded by grants from AIDS Project of the Ministry of Health, Rome, Italy; National Institutes of Health (1RO1AI38532–01); and Howard Hughes Medical Institute.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 4.Collette Y, Olive D. The primate lentivirus-encoded Nef protein can regulate several steps of the viral replication cycle. Virology. 1999;265:173–177. doi: 10.1006/viro.1999.0053. [DOI] [PubMed] [Google Scholar]
- 5.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 6.Craig H M, Pandori M W, Guatelli J C. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95:11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Aloja P, Olivetta E, Bona R, Nappi F, Pedacchia D, Pugliese K, Ferrari G, Verani P, Federico M. gag, vif, and nef genes contribute to the homologous viral interference induced by a nonproducer human immunodeficiency virus type 1 (HIV-1) variant: identification of novel HIV-1-inhibiting viral protein mutants. J Virol. 1998;72:4308–4319. doi: 10.1128/jvi.72.5.4308-4319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, Lawson V A, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan J S, Cunningham A D D, Dowton D, Mills J. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 9.Fackler O T, Lu X, Frost J A, Geyer M, Jiang B, Luo W, Abo A, Alberts A S, Peterlin B M. p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol Cell Biol. 2000;20:2619–2627. doi: 10.1128/mcb.20.7.2619-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fackler O T, Luo W, Geyer M, Alberts A S, Peterlin B M. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol Cell. 1999;3:729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 11.Fackler O T, Kienzle N, Kremmer E, Boese A, Schramm B, Klimkait T, Kucherer C, Mueller-Lantzsch N. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur J Biochem. 1997;247:843–851. doi: 10.1111/j.1432-1033.1997.00843.x. [DOI] [PubMed] [Google Scholar]
- 12.Fackler O T, Peterlin B M. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- 13.Federico M, Nappi F, Ferrari G, Chelucci C, Mavilio F, Verani P. A nonproducer, interfering human immunodeficiency virus (HIV) type 1 provirus can be transduced through a murine leukemia virus-based retroviral vector: recovery of an anti-HIV mouse/human pseudotype retrovirus. J Virol. 1995;69:6618–6626. doi: 10.1128/jvi.69.11.6618-6626.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federico M, Nappi F, Bona R, D'Aloja P, Verani P, Rossi G B. Full expression of transfected nonproducer interfering HIV-1 proviral DNA abrogates susceptibility of human He-La CD4+ cells to HIV. Virology. 1995;206:76–84. doi: 10.1016/s0042-6822(95)80021-2. [DOI] [PubMed] [Google Scholar]
- 15.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R D. Brief report: absence of intact nef sequences in a long-term nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 19.Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999;9:622–631. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y M, Tian C J, Yu X F. A bipartite membrane-binding signal in the human immunodeficiency virus type 1 matrix protein is required for the proteolytic processing of Gag precursors in a cell type-dependent manner. J Virol. 1998;72:9061–9068. doi: 10.1128/jvi.72.11.9061-9068.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X, Wu X, Plemenitas A, Yu H, Sawai E T, Abo A, Peterlin B M. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr Biol. 1996;6:1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 22.Lu X, Yu H, Liu S H, Brodsky F M, Peterlin B M. Interactions between HIV-1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 23.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivetta E, Pugliese K, Bona R, D'Aloja P, Ferrantelli F, Santarcangelo A C, Mattia G, Verani P, Federico M. cis expression of the F12 human immunodeficiency virus (HIV) Nef allele transforms the highly productive NL4–3 HIV type 1 to a replication-defective strain: involvement of both Env gp41 and CD4 intracytoplasmic tails. J Virol. 2000;74:483–492. doi: 10.1128/jvi.74.1.483-492.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono T, Iwatani Y, Nishimura A, Ishimoto A, Sakai H. Functional association between the nef gene product and gag-pol region of HIV-1. FEBS Lett. 2000;466:233–238. doi: 10.1016/s0014-5793(99)01791-3. [DOI] [PubMed] [Google Scholar]
- 26.Pandori M, Craig H, Moutouh L, Corbeil J, Guatelli J. Virological importance of the protease-cleavage site in human immunodeficiency virus type 1 Nef is independent of both intravirion processing and CD4 down-regulation. Virology. 1998;251:302–316. doi: 10.1006/viro.1998.9407. [DOI] [PubMed] [Google Scholar]
- 27.Pandori M W, Fitch N J, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piguet V, Wan L, Borel C, Mangasarian A, Demaurex N, Thomas G, Trono D. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat Cell Biol. 2000;2:163–167. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross T M, Oran A E, Cullen B R. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol. 1999;9:613–621. doi: 10.1016/s0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- 30.Schorr J, Kellner R, Fackler O, Freund J, Konvalinka J, Kienzle N, Krausslich H G, Mueller-Lantzsch N, Kalbitzer H R. Specific cleavage sites of Nef proteins from human immunodeficiency virus types 1 and 2 for the viral proteases. J Virol. 1996;70:9051–9054. doi: 10.1128/jvi.70.12.9051-9054.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz O, Marechal V, Danos O, Heard J M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimano R, Inubushi R, Oshima Y, Adachi A. Inhibition of HIV/SIV replication by dominant negative Gag mutants. Virus Genes. 1999;18:197–201. doi: 10.1023/a:1008054111697. [DOI] [PubMed] [Google Scholar]
- 35.Steimer K S, Puma J P, Powers M D, George-Nascimento C, Stephans J C, Levy J A, Sanchez-Pescador R, Luciw P, Barr P J, Hallewell R A. Differential antibody responses of individuals infected with AIDS-associated retrovirus surveyed using the viral core antigen p25 gag expressed in bacteria. Virology. 1986;150:283–290. doi: 10.1016/0042-6822(86)90289-8. [DOI] [PubMed] [Google Scholar]
- 36.Tokunaga K, Ikuta K, Adachi A, Matsuda M, Kurata T, Kojima A. The cellular kinase binding motifs (PxxP and RR) in human immunodeficiency virus type 1 Nef protein are dispensable for producer-cell-dependent enhancement of viral entry. Virology. 1999;257:285–289. doi: 10.1006/viro.1999.9682. [DOI] [PubMed] [Google Scholar]
- 37.Trono D, Feinberg M B, Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989;59:113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- 38.Vogt V M. Proteolytic processing and particle maturation. Curr Top Microbiol Immunol. 1996;214:95–131. doi: 10.1007/978-3-642-80145-7_4. [DOI] [PubMed] [Google Scholar]
- 39.Welker R, Kottler H, Kalbitzer H R, Krausslich H G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 40.Wiskerchen M, Cheng-Mayer C. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology. 1996;224:292–301. doi: 10.1006/viro.1996.0531. [DOI] [PubMed] [Google Scholar]
- 41.Xu X N, Laffert B, Screaton G R, Kraft M, Wolf D, Kolanus W, Mongkolsapay J, McMichael A J, Baur A S. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J Exp Med. 1999;189:1489–1496. doi: 10.1084/jem.189.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]