Abstract
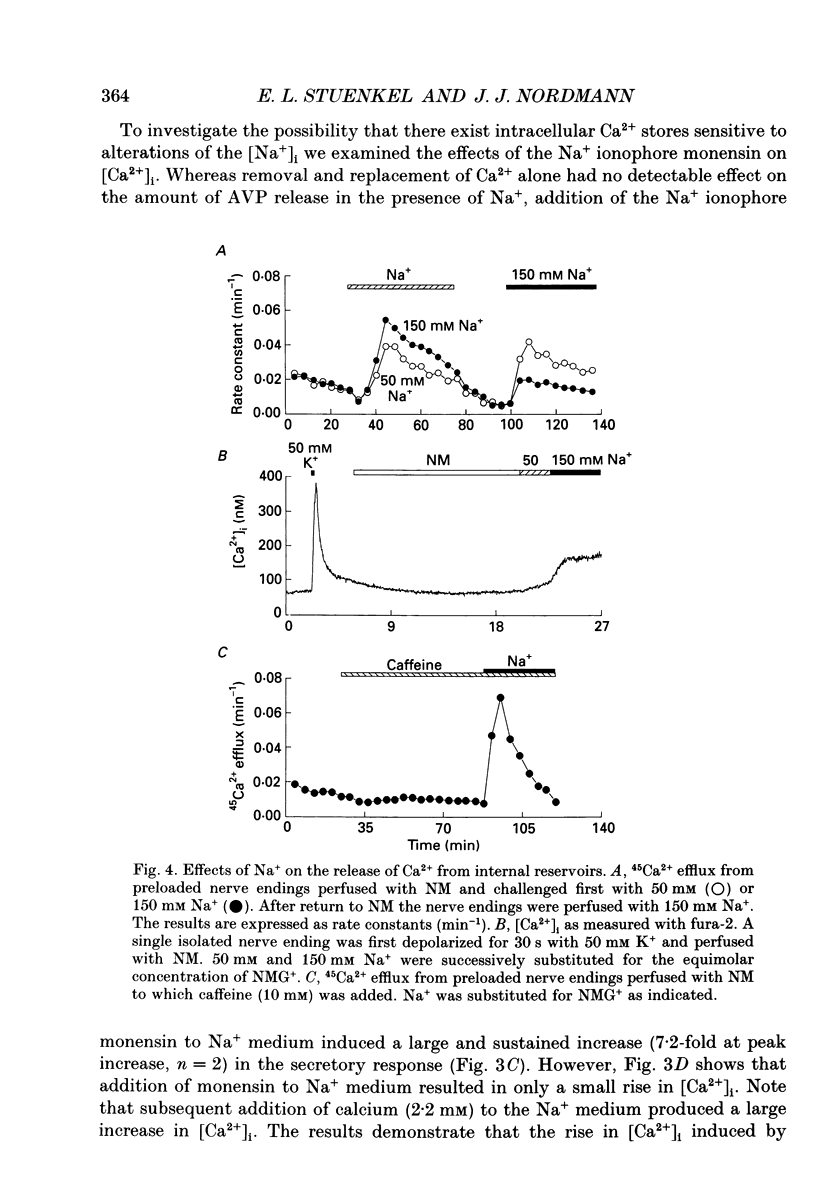
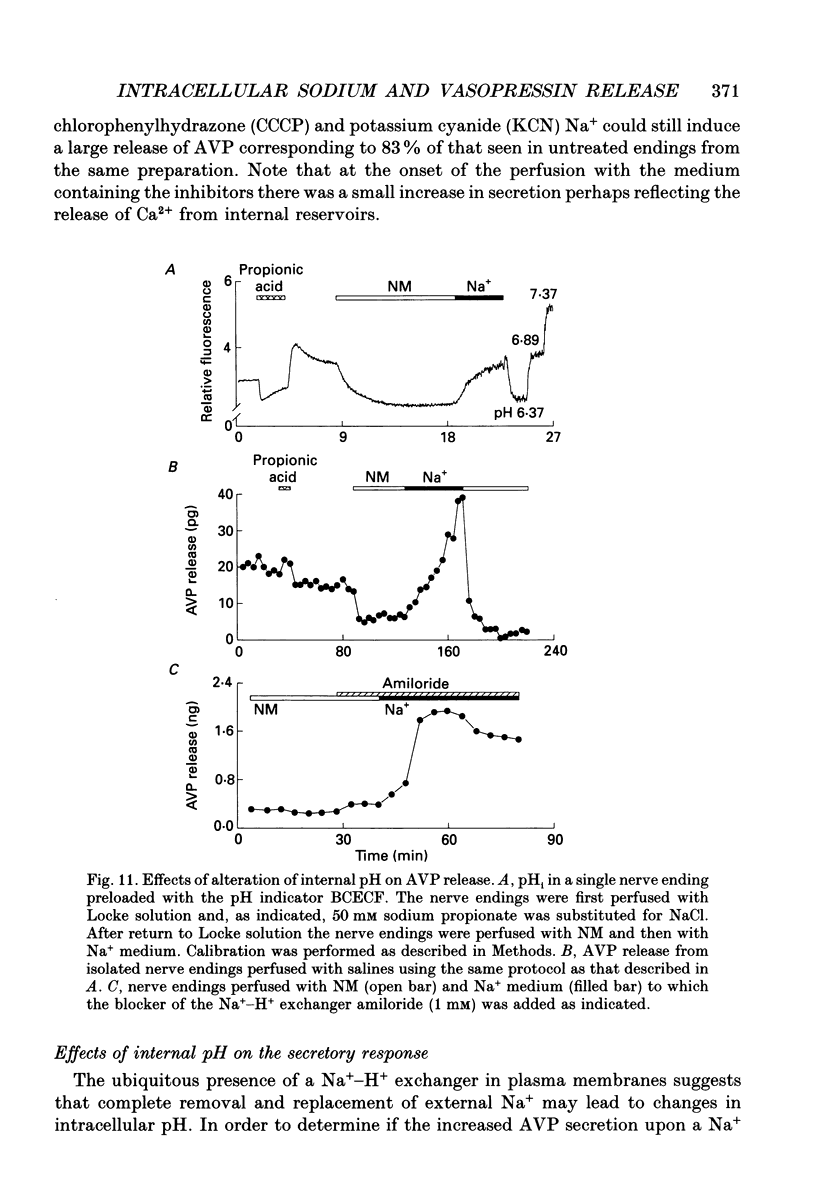
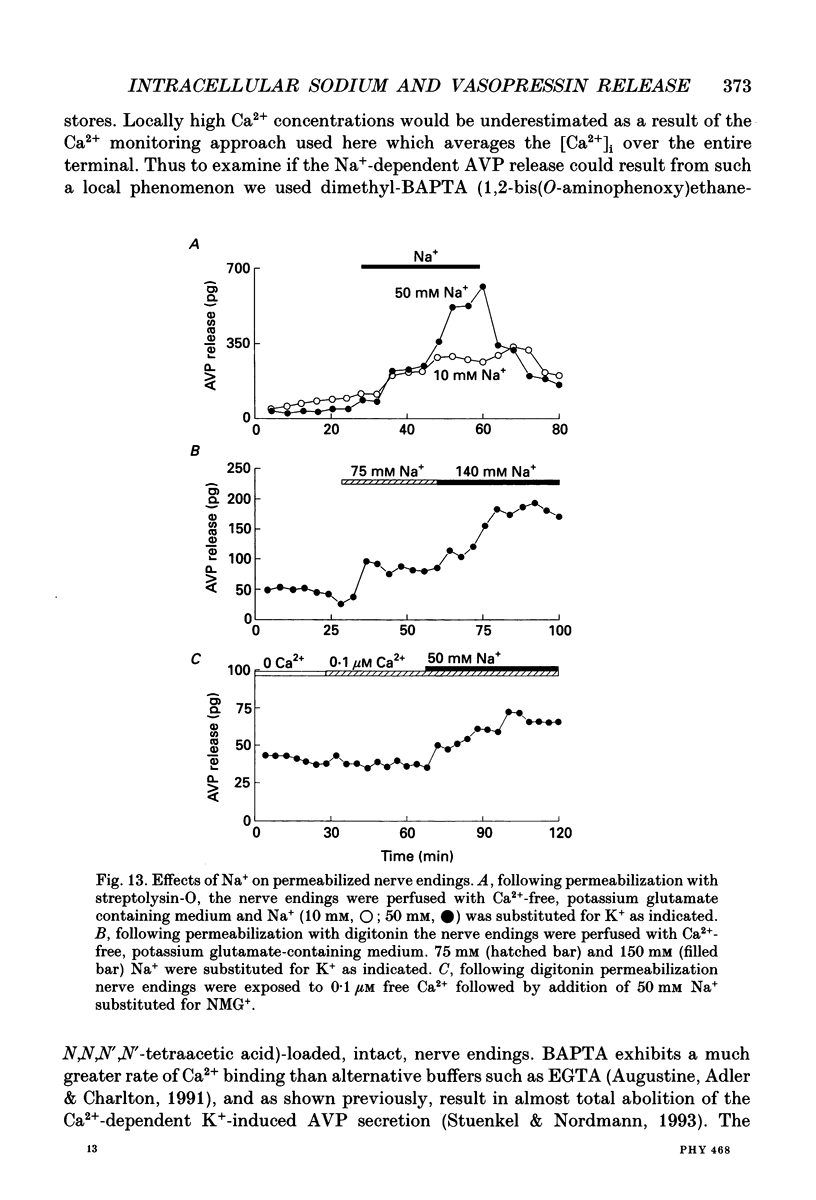
1. The effects of Na+ on vasopressin release and on redistribution of Ca2+, Na+ and H+ in isolated rat neurohypophysial nerve endings have been studied. 2. Substituting Na+ for a non-permanent cation produced a pronounced and sustained release of vasopressin. This increase occurred in the absence of external Ca2+ and in nerve endings loaded with the Ca2+ chelator dimethyl-BAPTA (1,2-bis-(O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid). 3. The effect of Na+ was independent of a rise in intracellular Ca2+ as judged by the measurement of [Ca2+]i using the indicator fura-2 and 45Ca2+ efflux studies. Although Na+ could release Ca2+ from internal reservoirs the small elevation in [Ca2+]i induced by Na+ could not explain the large and sustained increase in vasopressin secretion. 4. The channel blockers TTX (tetrodotoxin), D888 (desmethyoxyverapamil), N144 (5-nitro-2-(phenylpropylamino)-benzoic acid) or SITS (4-acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid) could not prevent the Na(+)-dependent increase in vasopressin release. Similarly this increase was not affected by metabolic inhibitors (Ruthenium Red and KCN) nor by CCCP (carbonyl cyanide m-chlorophenylhydrazone), an uncoupler of oxidative phosphorylation. 5. Selectivity among monovalent cations to promote secretion was found with the largest effect on the secretory response being produced by Na+. Similarly Cl- was found to be the most potent anion studied for inducing, in the presence of Na+, an increase in neurohormone release. 6. Measuring [Na+]i by means of the Na+ indicator SBFI showed that the extent of the secretory response was correlated with the intraterminal Na+ concentration. 7. The Na(+)-induced, Ca(2+)-independent release of vasopressin occurred by exocytosis as judged (i) by the linear relationship between the amount of vasopressin secreted and that of the co-localized neurophysin and (ii) by the demonstration that the extracellular marker horseradish peroxidase was only found in endocytotic vacuoles and not in the cytoplasm of the stimulated nerve endings. 8. The Na(+)-dependent secretory response found on addition of extracellular Na+ was not the result of the change in internal pH as measured with the indicator BCECF and as mimicked by addition of propionic acid. 9. Addition of Na+ to digitonin- or streptolysin-O-permeabilized nerve endings in the presence or absence of Ca2+ also gave rise to an increase in vasopressin secretion. 10. It is concluded that an increase in internal Na+ per se can promote, in the absence of a rise in intracellular Ca2+, an increase in neuropeptide secretion.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam-Vizi V. External Ca(2+)-independent release of neurotransmitters. J Neurochem. 1992 Feb;58(2):395–405. doi: 10.1111/j.1471-4159.1992.tb09736.x. [DOI] [PubMed] [Google Scholar]
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood H. L., Swenarchuk L. E., Gruenwald C. R. Long-term synaptic facilitation during sodium accumulation in nerve terminals. Brain Res. 1975 Dec 12;100(1):198–202. doi: 10.1016/0006-8993(75)90260-7. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Adler E. M., Charlton M. P. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernath S. Calcium-independent release of amino acid neurotransmitters: fact or artifact? Prog Neurobiol. 1992;38(1):57–91. doi: 10.1016/0301-0082(92)90035-d. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol. 1987 Sep;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. Requirements for hormone release from permeabilized nerve endings isolated from the rat neurohypophysis. J Physiol. 1987 Sep;390:71–91. doi: 10.1113/jphysiol.1987.sp016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985 Dec;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Atwood H. L. Modulation of transmitter release by intracellular sodium in squid giant synapse. Brain Res. 1977 Oct 7;134(2):367–371. doi: 10.1016/0006-8993(77)91081-2. [DOI] [PubMed] [Google Scholar]
- Charlton M. P., Thompson C. S., Atwood H. L., Farnell B. Synaptic transmission and intracellular sodium: ionophore induced sodium loading of nerve terminals. Neurosci Lett. 1980 Feb;16(2):193–196. doi: 10.1016/0304-3940(80)90343-2. [DOI] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- Dayanithi G., Nordmann J. J. Chloride and magnesium dependence of vasopressin release from rat permeabilized neurohypophysial nerve endings. Neurosci Lett. 1989 Dec 4;106(3):305–309. doi: 10.1016/0304-3940(89)90181-x. [DOI] [PubMed] [Google Scholar]
- Fill M., Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988 Oct;11(10):453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Influence of sodium ions on transmitter release. Nature. 1965 Jun 5;206(988):1047–1048. doi: 10.1038/2061047a0. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of tetanic and post-tetanic potentiation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):353–371. doi: 10.1113/jphysiol.1973.sp010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of stimulation-induced changes in transmitter release at the frog neuromuscular junction. J Gen Physiol. 1982 Oct;80(4):613–638. doi: 10.1085/jgp.80.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchnik S., Venosa R. A. Role of sodium ions in the response of the frequency of miniature end-plate potentials to osmotic changes in the neuromuscular junction. Nature. 1969 Apr 12;222(5189):169–171. doi: 10.1038/222169a0. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Stuenkel E. L. Ca(2+)-independent regulation of neurosecretion by intracellular Na+. FEBS Lett. 1991 Nov 4;292(1-2):37–41. doi: 10.1016/0014-5793(91)80828-q. [DOI] [PubMed] [Google Scholar]
- Nussinovitch I., Rahamimoff R. Ionic basis of tetanic and post-tetanic potentiation at a mammalian neuromuscular junction. J Physiol. 1988 Feb;396:435–455. doi: 10.1113/jphysiol.1988.sp016971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Mine K., Fujiwara M. The Na(+)-dependent release of endogenous dopamine and noradrenaline from rat brain synaptosomes. J Pharmacol Exp Ther. 1990 Mar;252(3):1283–1288. [PubMed] [Google Scholar]
- Ortiz C. L. Intracellular sodium accumulation and transmitter release at crustacean neuromuscular junctions. Fed Proc. 1980 Apr;39(5):1524–1526. [PubMed] [Google Scholar]
- Pollard H. B., Tack-Goldman K., Pazoles C. J., Creutz C. E., Shulman N. R. Evidence for control of serotonin secretion from human platelets by hydroxyl ion transport and osmotic lysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5295–5299. doi: 10.1073/pnas.74.12.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Lev-Tov A., Meiri H. Primary and secondary regulation of quantal transmitter release: calcium and sodium. J Exp Biol. 1980 Dec;89:5–18. doi: 10.1242/jeb.89.1.5. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Meiri H., Erulkar S. D., Barenholz Y. Changes in transmitter release induced by ion-containing liposomes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5214–5216. doi: 10.1073/pnas.75.10.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh E., Nakazato Y. Effects of monensin and veratridine on acetylcholine release and cytosolic free Ca2+ levels in cerebrocortical synaptosomes of rats. J Neurochem. 1991 Oct;57(4):1270–1275. doi: 10.1111/j.1471-4159.1991.tb08289.x. [DOI] [PubMed] [Google Scholar]
- Sherman R. G., Atwood H. L. Synaptic facilitation: long-term neuromuscular facilitation in crustaceans. Science. 1971 Mar 26;171(3977):1248–1250. doi: 10.1126/science.171.3977.1248. [DOI] [PubMed] [Google Scholar]
- Statham H. E., Duncan C. J. The effect of sodium ions of MEPP frequency at the frog neuromuscular junction. Life Sci. 1977 Jun 1;20(11):1839–1845. doi: 10.1016/0024-3205(77)90219-3. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L. Effects of membrane depolarization on intracellular calcium in single nerve terminals. Brain Res. 1990 Oct 8;529(1-2):96–101. doi: 10.1016/0006-8993(90)90815-s. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L., Ernst S. A. Multiple calcium mobilization pathways in single avian salt gland cells. Am J Physiol. 1990 Feb;258(2 Pt 1):C289–C298. doi: 10.1152/ajpcell.1990.258.2.C289. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L., Machen T. E., Williams J. A. pH regulatory mechanisms in rat pancreatic ductal cells. Am J Physiol. 1988 Jun;254(6 Pt 1):G925–G930. doi: 10.1152/ajpgi.1988.254.6.G925. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L., Nordmann J. J. Intracellular calcium and vasopressin release of rat isolated neurohypophysial nerve endings. J Physiol. 1993 Aug;468:335–355. doi: 10.1113/jphysiol.1993.sp019775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchard S. J., Lattanzio F. A., Jr, Rubin R. W., Pressman B. C. Stimulation of catecholamine secretion from cultured chromaffin cells by an ionophore-mediated rise in intracellular sodium. J Cell Biol. 1982 Sep;94(3):531–539. doi: 10.1083/jcb.94.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenarchuk L. E., Atwood H. L. Long-term synaptic facilitation with minimal calcium entry. Brain Res. 1975 Dec 12;100(1):205–208. doi: 10.1016/0006-8993(75)90261-9. [DOI] [PubMed] [Google Scholar]
- Toescu E. C., Nordmann J. J. Effect of sodium and calcium on basal secretory activity of rat neurohypophysial peptidergic nerve terminals. J Physiol. 1991 Feb;433:127–144. doi: 10.1113/jphysiol.1991.sp018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. J., Chandler L. J., Leslie S. W. Calcium-dependent and -independent release of endogenous dopamine from rat striatal synaptosomes. Brain Res. 1988 Nov 8;473(1):91–98. doi: 10.1016/0006-8993(88)90319-8. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Augmentation and facilitation of transmitter release. A quantitative description at the frog neuromuscular junction. J Gen Physiol. 1982 Oct;80(4):583–611. doi: 10.1085/jgp.80.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Curran M., Cohen F. S., Brodwick M. Simultaneous electrical and optical measurements show that membrane fusion precedes secretory granule swelling during exocytosis of beige mouse mast cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1585–1589. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]



