Neurotrophic factors as a therapeutic approach in neurodegenerative diseases: A major unmet need in the field of central nervous system diseases is disease-modifying treatments. While for decades there have been various symptomatic treatments available to alleviate the symptoms of the disease, disease-modification, i.e. treatments that stop, significantly delay, or reverse the progression of the disease, has been turned out to a difficult goal to achieve.
Due to their neuroprotective and neurorestorative properties, there has been a long-standing interest in using neurotrophic factors (NTF) as therapeutics in neurodegenerative diseases. As NTFs are proteins, a major challenge in their therapeutic use has been drug delivery. How to administer NTFs in a non-invasive and patient-friendly way while allowing sufficient brain penetration? Four growth factors, glial cell-line derived neurotrophic factor (GDNF; Kirkeby and Barker, 2019), neurturin (Marks et al., 2010), platelet-derived growth factor BB (Paul et al., 2015) and cerebral dopamine neurotrophic factor (CDNF) (Huttunen et al., 2023) have been tested in clinical trials in Parkinson’s disease during the past 25 years with various intracerebral delivery approaches with encouraging but varying outcomes. Both protein and gene transfer–based approaches used in clinical trials require a neurosurgical procedure and local intracranial delivery methods to allow sufficient bioavailability in the target brain region, which has been a major practical limitation for the broader use of NTF-based therapies. Moreover, therapies requiring invasive procedures are typically considered a treatment option only for patients with a moderately advanced disease. This is particularly important, as for NTF-based therapies in neurodegenerative disorders, better efficacy can be expected in earlier-stage patients with more neurons left to be protected and revived. Therefore, there has been significant interest in developing NTF-based treatment approaches with peripheral, patient-friendly routes of administration, such as small molecule NTF receptor agonists and other types of NTF mimetics that could pass the blood–brain barrier (BBB).
CDNF is an unconventional neurotrophic factor with therapeutic potential in multiple diseases: CDNF was originally described as a novel neurotrophic factor for dopamine neurons (Lindholm et al., 2007). Studies in CDNF knockout mice revealed an intriguing phenotype with loss of enteric dopamine neurons without significant degeneration in the central nervous system dopamine pathways (Lõhelaid et al., 2024). Notably, CDNF and its closest human relative MANF protect cells via their actions by modulating the endoplasmic reticulum (ER) stress response and unfolded protein response pathway (UPR), which is distinct from conventional NTFs, such as nerve growth factor, brain-derived neurotrophic factor, and glial cell-line derived neurotrophic factor. CDNF interacts with the master regulator of the UPR pathway GRP78 and modulates UPR signaling to promote neuronal survival and functional recovery (Figure 1; Lõhelaid et al., 2024). CDNF modulates the activity of the UPR system via a common ligand of the three UPR receptors and provides non-selectivity which could be a benefit in terms of therapeutic efficacy but should also be considered from the safety perspective. Most other pharmacological approaches for targeting the UPR are based on the inhibition or activation of a single arm/receptor of the UPR system (IRE1, PERK, or ATF6) (Hetz et al, 2019).
Figure 1.
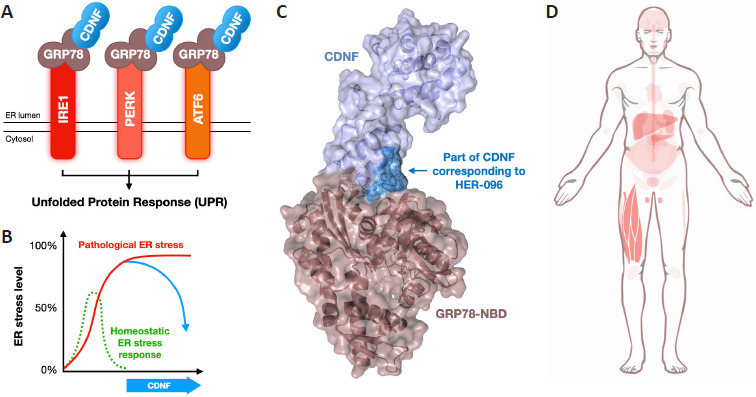
CDNF protects and promotes functional recovery of neurons via modulation of the unfolded protein response pathway.
(A) The UPR pathway is composed of three parallel signaling receptors, IRE1, PERK and ATF6, which share a common ligand and master regulator GRP78. Dissociation of GRP78 from the UPR receptors allows activation of downstream signaling. CDNF binds to the regulatory domain of GRP78 and via this interaction helps the cells to cope under excessive or prolonged ER stress conditions (B). Created with Keynote (Apple). (C) HER-096 was developed based on the binding interface (dark blue) of CDNF (light violet) and the nucleotide-binding domain GRP78 (GRP78-NBD; brown). HER-096 is a ~1000 Da peptidomimetic engineered to resist proteolysis and penetrate the blood-brain barrier. The CDNF-GRP78 complex structure was solved by SAXS (Unpublished data). Created with Pymol (Schrodinger Inc). (D) In addition to the nervous system, CDNF is also expressed in several peripheral tissues, such as skeletal muscle, heart, liver, and testis. Source: https://www.proteinatlas.org/ENSG00000185267-CDNF/tissue. Image credit: Human Protein Atlas (Uhlén et al, 2015, https://v23.proteinatlas.org/ENSG00000185267-CDNF/tissue; panel D). Cited according to the license/citation instructions provided on the website (https://www.proteinatlas.org/about/licence). ATF6: Activating transcription factor 6; CDNF: cerebral dopamine neurotrophic factor; ER: endoplasmic reticulum; GRP78: glucose-regulated protein 78; IRE1: inositol-requiring enzyme 1; NBD: nucleotide-binding domain; PERK: protein kinase R-like endoplasmic reticulum kinase; SAXS: small-angle X-ray scattering; UPR: unfolded protein response.
The UPR pathway is a homeostatic system that responds to various disturbances in cellular homeostasis, and in particular within the ER, including protein misfolding and calcium dyshomeostasis. The UPR pathway is also engaged in active crosstalk with inflammation-related processes and mitochondrial bioenergetics (Hetz et al., 2020). Interestingly, CDNF has also been reported to directly interact with α-synuclein (Lõhelaid et al., 2024), which is the main misfolded and aggregated protein component of Lewy bodies, the neuropathological hallmark of Parkinson’s disease.
The UPR system is ubiquitously expressed and important for cellular viability and functionality. As also CDNF is rather broadly expressed in mammalian tissues, despite the rather specific knockout mouse phenotype its functions likely go beyond the dopamine neurons. Accordingly, therapeutic effects of CDNF protein have been described in animal models of Parkinson’s disease, amyotrophic lateral sclerosis, Huntington’s disease, and ischemic and hemorrhagic stroke (Lõhelaid et al., 2024). The related protein MANF has been shown to be a critical factor for the survival of pancreatic beta cells and also serves as a cardiomyokine (Liu et al., 2022). Thus, both CDNF and MANF hold great therapeutic potential in diseases whose pathobiology involves abnormal UPR signaling, such as various neurodegenerative diseases. Importantly for the disease-modification goals, CDNF has not only shown neuroprotective effects but also the capacity to promote neurorestoration in preclinical studies (Lõhelaid et al., 2024).
Several preclinical studies in degenerative central nervous system disease models have shown that a single administration of CDNF has long-lasting therapeutic effects (days to weeks) despite the fact the brain half-life of exogenously administered CDNF protein is only a few hours (Lõhelaid et al., 2024). This suggests a hit-and-run mechanism and that therapeutic modulation of the UPR pathway does not require the constant presence of CDNF-like activity in the brain. This is an important consideration for pharmacokinetic-pharmacodynamic relationships and dosing regimens in future clinical trials.
Development of BBB-penetrating CDNF mimetics: Various approaches have been taken to mimic the pharmacological activity of NTFs by e.g. small molecules and peptides that engage with receptors and target pathways of NTFs (Atkinson and Dickman, 2023). For CDNF, it was found that the C-terminal domain of the protein holds the activity that is key to ER stress modulation and neuroprotection (Saarma et al., 2018). Intriguingly, shorter peptides generated from this area of CDNF showed the ability to cross cell membranes and also the BBB.
Following these key findings, we generated a small library of peptides and peptidomimetic compounds to identify compounds that (1) mimic the neuroprotective activity of CDNF, (2) can be transported across the BBB, and (3) have drug-like properties including sufficient metabolic stability. A small number of compounds were then taken through lead optimization after which a compound called HER-096 was selected as the preclinical lead compound. HER-096 is a ~1000 Da retro-inverso-isomerized peptide with high potency in neuroprotection, good metabolic stability, and the ability to cross the BBB in preclinical studies (Kulesskaya et al., 2023).
Subcutaneous administration of HER-096 in rats provided a pharmacokinetic profile both in plasma and cerebrospinal fluid (CSF) that supported the intended therapeutic dosing. Preclinical data suggest that HER-096, similar to the parent protein CDNF, operates via a hit-and-run mechanism, and therefore steady-state brain levels are not required for a therapeutic effect. In rats, subcutaneously administered HER-096 has a plasma half-life of ~30 minutes but allows brain exposure equivalent of approximately 20% CSF (or striatal interstitial fluid) to plasma ratio (Kulesskaya et al, 2023). Importantly, the CSF pharmacokinetic profile resembles the interstitial fluid pharmacokinetic profile (half-lives of 1.3 hours and 1.9 hours and dose-normalized AUCinf 35 h × ng/mL and 51 h × ng/mL, respectively) that allows to consider the CSF exposure as a valid surrogate for assessment of brain exposure level in further preclinical and clinical studies.
In vivo, therapeutic effects of HER-096 were studied in an aged mouse model of synucleinopathy, based on bilateral nigral injection of preformed α-synuclein oligomers and chronic glucocerebrosidase inhibition in aged animals (Kulesskaya et al., 2023). This animal model displays progressive loss of nigral dopamine neurons together with a robust induction of microgliosis and ER stress. HER-096 subcutaneously administered three times a week for 4–6 weeks showed significant (50%–60%) protection of dopamine neurons in the substantia nigra which was associated with a robust reduction in nigral α-synuclein aggregate levels and microgliosis. Importantly, subcutaneously administered HER-096 strongly reduced the levels of serine-724 phosphorylated IRE1 (active form) and activated, nucleus-localized ATF6 in dopamine neurons indicating engagement with the target pathway of CDNF.
The ubiquitous expression of the target pathway components together with systemic exposure of HER-096 could result in adverse effects. On the other hand, the short terminal half-life of the compound with a hit-and-run mechanism of action allows intermittent dosing which could be expected to limit potential adverse events. One concern in chronic dosing, particularly in the elderly population, relates to potential oncogenic effects as GRP78 and the UPR pathway are associated with the regulation of cell survival and have been found to be deregulated in cancer cells (Akinyemi et al, 2023). In 28-day daily repeated dosing GLP toxicology studies in rats and Beagle dogs, there were no systemic adverse events related to HER-096 (our unpublished results). Toxicology studies with chronic dosing have not been completed yet.
The safety and tolerability of HER-096 were recently tested in a Phase 1a study in healthy human volunteers. Single subcutaneous doses of HER-096 up to 300 mg were tested, and were shown to be safe and well tolerated (Herantis Pharma Plc, 2023). Importantly, this first-in-human study also demonstrated CSF presence of HER-096 after subcutaneous dosing showing that HER-096 can pass the BBB in humans.
Conclusive remarks: In summary, HER-096 is the first CDNF-mimetic to enter clinical trials. A more patient-friendly subcutaneous route of administration is a major improvement compared to the previous intracranial CDNF infusion approach as it allows access to earlier-stage patients. The potential for disease modification and the ability to penetrate the BBB makes HER-096 a highly interesting drug candidate for Parkinson’s disease. HER-096 differs from most other pharmacological approaches targeting the UPR pathway with its ability to modulate signaling of all three arms of the UPR pathway, which could be an advantage. Next, safety, tolerability, and pharmacokinetics of multiple doses of HER-096 will be assessed in Parkinson’s disease patients in a Phase 1b study that is planned to start in late 2024. The first assessment of the clinical efficacy of a CDNF-based therapeutic is then expected to follow in a couple of years. Disease modification in Parkinson’s disease is a major challenge for clinical development, and it will be important to develop and advance multimodal biomarkers to support the next clinical phases. Finally, exploration of indications beyond Parkinson’s disease will be an exciting extension of the currently ongoing work.
This work has been supported by Herantis Pharma Plc and the European Innovation Council (grant acronym ReTreatPD).
NK, KMH, and HJH are employees and share/option right holders of Herantis Pharma Plc, and inventors in a patent application related to HER-096. No conflicts of interest exist between Herantis Pharma Plc and the publication of the article.
Additional file: Open peer review report 1 (77.5KB, pdf) .
Footnotes
Open peer reviewer: Giulia Di Lazzaro, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Italy.
P-Reviewer: Lazzaro GD; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Akinyemi AO, Simpson KE, Oyelere SF, Nur M, Ngule CM, Owoyemi BCD, Ayarick VA, Oyelami FF, Obaleye O, Esoe DP, Liu X, Li Z. Unveiling the dark side of glucose-regulated protein 78 (GRP78) in cancers and other human pathology: a systematic review. Mol Med. 2023;21(29):112. doi: 10.1186/s10020-023-00706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson E, Dickman R. Growth factors and their peptide mimetics for treatment of traumatic brain injury. Bioorg Med Chem. 2023;90:117368. doi: 10.1016/j.bmc.2023.117368. [DOI] [PubMed] [Google Scholar]
- Herantis Pharma Plc Inside information: Herantis Pharma announces positive topline data from the Phase 1a clinical trial of HER-096, a disease modifying therapeutic developed for Parkinson’s disease. 2023 Available at: https://herantis.com/mfn_news/inside-information-herantis-pharma-announces-positive-topline-data-from-the-phase-1a-clinical-trial-of-her-096-a-disease-modifying-therapeutic-developed-for-parkinsons-disease/ Accessed April 13, 2024. [Google Scholar]
- Hetz C, Axten JM, Patterson JB. Pharmacological targeting of the unfolded protein response for disease intervention. Nat Chem Biol. 2019;15:764–775. doi: 10.1038/s41589-019-0326-2. [DOI] [PubMed] [Google Scholar]
- Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21:421–438. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen HJ, et al. Intraputamenal cerebral dopamine neurotrophic factor in Parkinson’s disease: a randomized, double-blind, multicenter phase 1 trial. Mov Disord. 2023;38:1209–1222. doi: 10.1002/mds.29426. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Barker RA. Parkinson disease and growth factors - is GDNF good enough? Nat Rev Neurol. 2019;15:312–314. doi: 10.1038/s41582-019-0180-6. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N, Bhattacharjee A, Holmström KM, Vuorio P, Henriques A, Callizot N, Huttunen HJ. HER-096 is a CDNF-derived brain-penetrating peptidomimetic that protects dopaminergic neurons in a mouse synucleinopathy model of Parkinson’s disease. Cell Chem Biol. 2023;31:593–606. doi: 10.1016/j.chembiol.2023.11.005. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Voutilainen MH, Laurén J, Peränen J, Leppänen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, Tuominen RK, Saarma M. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature. 2007;448:73–77. doi: 10.1038/nature05957. [DOI] [PubMed] [Google Scholar]
- Liu YY, Huo D, Zeng LT, Fan GQ, Shen T, Zhang TM, Cai JP, Cui J. Mesencephalic astrocyte-derived neurotrophic factor (MANF): Structure, functions and therapeutic potential. Ageing Res Rev. 2022;82:101763. doi: 10.1016/j.arr.2022.101763. [DOI] [PubMed] [Google Scholar]
- Lõhelaid H, Saarma M, Airavaara M. CDNF and ER stress: pharmacology and therapeutic possibilities. Pharmacol Ther. 2024;254:108594. doi: 10.1016/j.pharmthera.2024.108594. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Paul G, Zachrisson O, Varrone A, Almqvist P, Jerling M, Lind G, Rehncrona S, Linderoth B, Bjartmarz H, Shafer LL, Coffey R, Svensson M, Mercer KJ, Forsberg A, Halldin C, Svenningsson P, Widner H, Frisén J, Pålhagen S, Haegerstrand A. Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson’s disease patients. J Clin Invest. 2015;125:1339–1346. doi: 10.1172/JCI79635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarma M, Airavaara M, Voutilainen MH, Yu LY, Lindahl M. C-terminal cdnf and manf fragments, pharmaceutical compositions comprising same and uses thereof. 2018 Patent WO2018202957A1. Available at: https://pubchem.ncbi.nlm.nih.gov/patent/WO-2018202957-A1. Accessed April 13, 2024. [Google Scholar]
- Uhlén M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


