Abstract
Background
Cell and gene therapy products (CGTPs) often receive accelerated approvals, lacking comprehensive long-term safety and efficacy data, which can raise significant safety concerns. This research aims to study the post-marketing surveillance (PMS) of CGTPs in the European Union (EU), the United States (US), Japan, South Korea, and China, to offer insights for the development of a secure and standardized post-marketing regulatory framework for CGTPs.
Methods
Related regulations and the implementation effect of PMS for approved CGTPs were studied searching PubMed, CNKI, and the official websites of the European Medicines Agency, the US Food and Drug Administration, Japan’s Pharmaceuticals and Medical Device Agency, South Korea’s Ministry of Food and Drug Safety, and the National Medical Products Administration of China.
Results
Compared to those in China, the guidelines of PMS for CGTPs in the EU, the US, Japan, and South Korea was more comprehensive. Notably, the EU had dedicated regulations and supporting guidelines of PMS. Of the 26 CGTPs approved in the EU, 88% were under additional monitoring, 38% received conditional marketing authorization, and 12% were authorized under exceptional circumstances, with 77% designated as orphan drugs. The US had released 34 guidelines specifically for CGTPs which, forming the foundation of post-marketing risk management. Among the 27 CGTPs approved in the US, 22% were required to perform risk evaluation and mitigation strategies, 37% added black box warnings in the package inserts, 63% mandated to post-marketing requirements, and 15% subject to post-marketing commitments. In Japan, stringent supervision measures encompassing all-case surveillance (79%) and re-examination (53%) were applied to the 19 approved CGTPs, with 21% approved through conditional and time-limited approval. The PMS for CGTPs in South Korea, mainly included PSUR, re-examination, and re-evaluation. China had introduced several relevant regulations, which consisted of general statements and lacked detailed guidance.
Conclusions
This study demonstrates that the regulatory policies of PMS for CGTPs in the EU, the US, Japan, and South Korea were comprehensive. The implementation of PMS for CGTPs in the EU, the US, and Japan was well developed. This knowledge holds valuable insights for China’s future learning and development in this field.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03637-z.
Keywords: Cell and gene therapy products, Post-marketing surveillance, Regulations, Advanced therapy medicinal products
Background
Cell and gene therapy products (CGTPs), including cell therapy products (CTPs) and gene therapy products (GTPs), belong to advanced therapy medicinal products (ATMPs), which have shown great significance to the treatment of serious diseases [1–5]. CTPs include cellular immunotherapy, cancer vaccines, and other types of autologous and allogeneic cells for certain indications, including hematopoietic stem cells and adult and embryonic stem cells. GTPs attempt to modify or manipulate gene expression or to alter the biological characteristics of living cells to treat human diseases [6–9]. Due to their unique mechanism of action and characteristics, CGTPs face many challenges that cover manufacturing, non-clinical development, clinical trial, and PMS [10].
In most cases, the CGTPs for rare diseases are developed with challenges including limited knowledge of the disease and small sample sizes in clinical trials. Therefore, a controlled and randomized clinical trial may not be feasible or ethically justified in those cases [3]. Moreover, due to the great interest for public health, many CGTPs are considered promising in the treatment of patients in conditions with no therapeutic alternative achieved approval through flexible regulatory programs, involving conditional approval and exceptional approval in the EU, accelerated approval in the US, conditional and time-limited approval in Japan, fast track approval in South Korea, and conditional approval and exceptional approval in China.
Until June 2024, there are 26 CGTPs, 27 CGTPs, 19 CGTPs, 17 CGTPs, and 7 CGTPs granted marketing authorization by the European Medicines Agency (EMA) of European Union, the Food and Drug Administration (FDA) of United States, the Pharmaceuticals and Medical Device Agency (PMDA) of Japan, South Korea’s Ministry of Food and Drug Safety (MFDS), and the National Medical Products Administration (NMPA) of China respectively [11–15]. How do these regulatory agencies implement and conduct PMS for CGTPs? What are the differences of the PMS framework for CGTPs in these counties? For this purpose, we investigated the PMS regulative guidelines laid down in the EU, the US, Japan, South Korea, and China subject to CGTPs. The requirements and implementation of PMS for CGTPs in these regions were analyzed as well, which will provide a reference for proposed a risk-based post-marketing framework for regulating CGTPs.
Methods
To analyze related regulations and the implementation effect of PMS for CGTPs, a descriptive research was carried out from PubMed and CNKI using keywords including “cell therapy,” “gene therapy,” “advanced therapy,” “regenerative medicine,” “post-marketing,” “regulation,” “supervision,” “post-marketing supervision,” “regulatory strategy,” “regulatory framework,” “post-marketing study,” “risk management,” and “pharmacovigilance.”
The guidelines and public available data related to CGTPs were collected on the official websites of EMA, FDA, PMDA, MFDS and NMPA, including type of CGTPs (CTPs and GTPs) that granted marketing authorization between October 5, 2009, and June 30, 2024. The particular methods used for obtaining the lists of marketed CGTPs in the EU, the US, Japan, South Korea, and China are described in Additional file 1: Table S1 [11–15]. Four key points were included: priority review/accelerated assessment, special designation (such as PRIME program in the EU, breakthrough therapy designation/fast track designation/regenerative medicine advanced therapy designation in the US, and Sakigake in Japan), approval types (i.e., conditional approval and exceptional circumstances approval in the EU, accelerated approval in the US, conditional and time-limited approval in Japan), and orphan drug designation.
Furthermore, the implementation effect of PMS for the authorized CGTPs in different regions were analyzed, except those in South Korea and China since limited data available on the official website. The PMS measures for CGTPs with the key elements of PMS in the EU, the US, and Japan were outlined as follows.
In the EU, PMS mainly involves post-marketing risk management, including safety concerns (important identified risks, important potential risks, missing information), routine/additional risk minimization measures (RMM), routine/additional pharmacovigilance (PV) activities, and additional monitoring. The relevant information was found in the European Public Assessment Reports for the respective products. In the US, PMS primarily refers to risk evaluation and mitigation strategies (REMS), black box warnings, post-marketing requirements (PMR), and post-marketing commitments (PMC, excluding chemistry, manufacturing, and control). Information on REMS was obtained from the review reports. Black box warnings were found in the package inserts, and PMR and PMC are obtained from the “Post-Marketing Requirements and Commitments” database [16]. PMS in Japan principally encompasses all-case surveillance, re-examination periods, requirements for doctors and medical institutions, and dissemination of guidelines for proper use. The relevant information was found in the review reports.
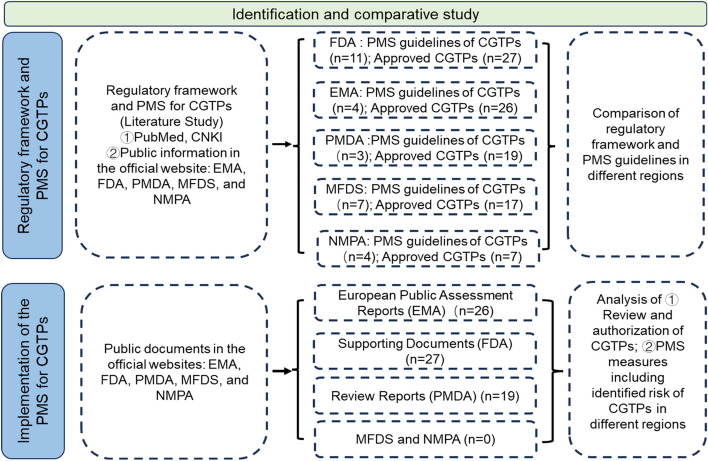
The following specific analysis was conducted by 2 reviewers independently. Firstly, the public documents including regulatory framework to CGTPs were collected with NoteExpress. Secondly, unrelated documents were excluded manually after review of the titles, abstract, claims, and full text. Finally, the information and data were collected from the remaining documents the data were analyzed using the software SPSS 26. The research flow was shown in Fig. 1.
Fig. 1.
Research flow
Results
The regulations of PMS of CGTPs in different regions
Guidelines and requirements laid down in the regulations of PMS of CGTPs in EU, US, Japan, South Korea, and China are analyzed as follows.
The PMS of CGTPs in the EU
CGTPs in the EU are regulated as ATMPs, which are innovative medicinal products for human use that are based on genes, tissues, or cells, including CGTPs and tissue engineering products. The Committee for Advanced Therapies (CAT) of the EMA is responsible for the evaluation and drafts opinions on the marketing authorization application of ATMPs. The opinions are further discussed by the Committee for Medicinal Products for Human Use (CHMP) to generate the final opinion [17].
To target an unmet medical need or area of major public health interest, the EMA applies support mechanisms to expedite the marketing of ATMPs, including the priority medicines (PRIME) program, accelerated assessment, conditional marketing authorization, and exceptional circumstances authorization [18–20].
The EU released regulations for PMS of medicinal products, which aim to collect and provide data to allow assessment of the safety or efficacy of drugs in the post-approval scenario. Legislation, guidelines on PV, and regulatory guidance on post-marketing activities for other medicinal products for human use are also well adapted to ATMPs [3]. Due to the special features of ATMPs, specific guidelines for ATMPs are issued (Table 1). In 2008, the EU published Guidelines on Safety and Efficacy Follow-Up-Risk Management of Advanced Therapy Medicinal Products [21], providing detailed and specific rules for post-authorization PV, the risk management system, and safety and efficacy follow-up of ATMPs. Furthermore, the EMA launched a public consultation on the revised guideline on clinical evaluation in 2018, which considered the early detection of risks during the product development phase and the design of adequate post-authorization studies to monitor the safety and efficacy of ATMPs [22]. This guideline provides key requirements for ATMPs encompassing PV systems, post-authorization safety study (PASS), risk identification, post-authorization efficacy studies (PAES), adverse reaction management and reporting, the effectiveness evaluation of risk management systems, and the application of corresponding Good Pharmacovigilance Practices (GVP) modules. Moreover, a series of supporting guidelines have been released to further guide a series of measures such as PASS, PAES, studies on small populations, and SmPC for ATMPs [23, 24]. Above all, EU published a regulation and 11 supporting guidelines of PMS for ATMPs, which related to PV system, safety concerns, RMM, and post-authorization measures for ATMPs (Table 1).
Table 1.
The guidelines of PMS for ATMPs in the EU
| No | Guidelines | Main topics |
|---|---|---|
| 1 |
Guideline on safety and efficacy follow-up-risk management of advanced therapy medicinal products [21] Guideline on safety and efficacy follow-up and risk management of advanced therapy medicinal products draft [22] |
Safety and efficacy follow-up risk management |
| 2 | Guideline on scientific requirements for the environmental risk assessment of gene therapy medicinal products [25] | Environmental risk assessment |
| 3 | Reflection paper on management of clinical risks deriving from insertional mutagenesis [26] | Insertional mutagenesis |
| 4 | Guideline on follow-up of patients administered with gene therapy medicinal products [27] | Follow-up of patients administered with gene therapy medicinal products |
| 5 | Guideline on potency testing of cell-based immunotherapy medicinal products for the treatment of cancer [28] | Potency testing |
| 6 | Guideline on good pharmacovigilance practices (GVP) Module VIII—Post-authorization safety studies (Rev 3) [29] | PASS |
| 7 | Scientific guidance on post-authorization efficacy studies | PAES |
| 8 | Guideline on good pharmacovigilance practices (GVP) Module V—Risk management systems (Rev 2) [30] | Introduction of risk management system |
| 9 | Guideline on good pharmacovigilance practices (GVP) Module XVI—Risk minimization measures: selection of tools and effectiveness indicators (Rev 2) [31] | Selection of tools and effectiveness indicators for RMM |
| 10 | Draft guideline on core SmPC, labeling and package leaflet for advanced therapy medicinal products (ATMPs) containing genetically modified cells [32]a | Core SmPC, labeling and package leaflet |
| 11 | Clinical trials in small populations [23]b | Small populations studies |
aRoutine risk minimization measures are one of the major elements of safety and efficacy follow-up and risk management, which refer to the management of risks as explained and minimized in the SmPC, the package leaflet, the labeling, the pack size and design, and the legal (prescription) status of the product
bFor post-marketing clinical trials that there are a limited number of patients (most of ATMPs is used for treating rare diseases), the principles described in the guideline on clinical trials in small populations should be carefully considered
RMP is one of the most important documents for PMS, which aims that the risks of medicinal products are evaluated at regular intervals or in response to the progress of PMS and a set of PV activities to minimize the risks of drugs [30]. To this end, the RMP contains the following key requirements: safety concerns, PV plan, and risk minimization plan [30]. RMP became a mandatory requirement for new drugs in the EU in 2005. After the promulgation of the GVP in 2012, the EMA adopted the GVP module V-Risk Management System, and the reversion was released in 2017. To give marketing authorization holders (MAH) further advice on RMP in the post authorization phase of products, the EMA has published Risk management plans (RMP) in post-authorization phase: questions and answers and related supporting guidelines [33, 34]. The RMP for ATMPs considers the important identified or potential risks of ATMPs. All the guidelines related to RMP in the EU were listed in Additional file 1: Table S2 [29–31, 33–42].
To ensure that the medicinal product is used safely, the EMA set up PVP and RMM, including routine and additional measures, respectively. CGTPs that are being monitored particularly closely by regulatory authorities are under “Additional Monitoring.”
PV activities are intended to characterize and quantify clinically relevant risks, and to identify new adverse reactions, which includes routine PV activities and additional PV. RMM denote interventions that intend to prevent or reduce the occurrence of adverse reactions associated with the exposure to a medicine, or to reduce their severity or impact on the patient should adverse reactions occur, which are divided into routine RMM and additional RMM. Routine PV activities and routine RMM are mandatory for all medicine products.
Periodic safety update report (PSUR) is a PV document, and the EMA assess information in PSURs to determine if there are new risks identified for a medicine and/or if its risk–benefit balance has changed. If there is a concern about a risk affecting the benefit-risk balance of a medicinal product, additional PV activities or additional RMM may be required. For example, on February 20, 2023, Etranacogene Dezaparvovec was approved in the EU, and an additional RMM (Health care professional guide, patient guide and patient card) is required for the important identified risk (hepatotoxicity) [43]. The definitions of key elements related to RMP are introduced in Additional file 1: Table S3 [29–31, 35, 44–53].
In addition, a PASS may be conducted to obtain further information on drug safety or measure the effectiveness of RMM, which can be divided into mandatory and voluntary categories [29, 54]. A series of guidelines for PASS have been issued in the EU [55–57]. Moreover, MAH may be required to conduct PAES within the authorized indications to supplement existing efficacy data. Guidelines have been developed for PAES to offer scientific guidance on study design and study principles [48].
The PMS of CGTPs in the US
CGTPs are classified as biologics in the US, which are supervised by the Center for Biological Products Evaluation and Research (CBER) [58, 59]. The Office of Tissue and Advanced Therapies is responsible for review of CGTPs. CGTPs could be authorized for marketing in the US by expedited programs, including fast track designation, breakthrough therapy designation, accelerated approval, priority review designation, and regenerative medicine advanced therapy designation [60, 61]. In addition, orphan drugs designation can also promote and approval of some CGTPs as well.
The FDA has successively released 34 guidelines for CGTPs, ranging from nonclinical and clinical studies to post-marketing studies [62–66]. In 2020, the FDA issued the latest revision of “Long Term Follow-Up After Administration of Human Gene Therapy Products, Guidance for Industry” specific for CGTPs [67]. All the main regulations and relevant supporting regulations of the PMS for CGTPs in the US are shown in Table 2.
Table 2.
The guidelines of PMS for CGTPs in the US
| No | Guidelines | Main topics |
|---|---|---|
| 1 | Guidance for industry: long term follow-up after administration of human gene therapy products [68] | Long term follow-up risk management |
| 2 | Guidance for industry and health: testing of retroviral vector-based human gene therapy products for replication competent retrovirus during product manufacture and patient follow-up [69] | Follow-up and risk management |
| 3 | Guidance for industry: post-marketing studies and clinical trials —implementation of Sect. 505(o)(3) of the Federal Food, Drug, and Cosmetic Act [70] | Post-marketing studies and clinical trials |
| 4 | Draft guidance: post-marketing studies and clinical trials—implementation of Sect. 505(o)(3) of the Federal Food, Drug, and Cosmetic Act Guidance for Industry [71] | Post-marketing studies and clinical trials |
CGTPs are subject to the PMS for biological products, including REMS, post-marketing reporting of adverse experiences, pediatrics, PMR, and PMC [3]. REMS may require the MAH to communicate directly to health care providers, pharmacists, nurses, and other participants involved in the delivery of health care or medications, which may provide information about a specific serious risk associated with that medication and steps to take to reduce the risk [72]. Moreover, to evaluate whether a REMS has achieved the goal of risk mitigation, REMS assessment is required to be submitted. The FDA has issued a draft guidance for REMS assessment, providing suggestions for reporting REMS assessment. Additionally, a series of REMS related guidelines or Q&A were published by FDA to better guide sponsors in conducting risk management [73–83] (Additional file 1: Table S4).
A PV plan in the US may be appropriate for products for which (1) serious safety risks have been identified pre- or post-approval or (2) at-risk populations have not been adequately studied. Sponsors may discuss the nature of the safety concerns posed by such a product with the agency and the determination whether a PVP is appropriate.
The FDA requires MAH to submit a post-marketing periodic safety reports in the form of periodic adverse drug experience report (PADER) or periodic adverse experience report (PAER) or PSUR after authorization [84, 85]. Applicants can also use an alternative reporting format, the International Council for Harmonisation (ICH)3 E2C(R2) Periodic Benefit-Risk Evaluation Report (PBRER) in place to satisfy the periodic post-marketing safety reporting requirements [86].
PMR and PMC refer to studies and clinical trials that sponsors conduct after approval to gather additional information about a product’s safety, efficacy, or optimal use. If safety issues are detected in post-marketing studies and clinical trials or post-marketing real-world use, the FDA will convene an interdisciplinary team to make decisions on continued monitoring, changes to product labeling, or drug recalls. For example, the FDA announced to investigate serious risk of T cell malignancy following BCMA-directed or CD19-directed autologous chimeric antigen receptor (CAR) T cell immunotherapies based on the reports of T cell malignancies that the FDA received [87]. Adding a black box warning to the label is the most powerful action other than recalling the drug.
GTPs are designed to achieve therapeutic effect through permanent or long-acting changes in the human body. As a result of long-term exposure to an investigational GTPs, study subjects may be at increased risk of undesirable and unpredictable outcomes that may present as delayed adverse events. To understand and mitigate the risk of a delayed adverse event, subjects in gene therapy trials may be monitored for an extended period of time, which is commonly referred to as the “long-term follow-up” (LTFU) period (of a clinical study). LTFU observations are extended assessments that continue some of the scheduled observations of a clinical trial past the active follow-up period and are an integral portion of the study of some investigational GTPs. Not all GTPs will be required LTFU observations and a risk assessment should be performed by a sponsor based on several factors as outlined in Long Term Follow-Up After Administration of Human Gene Therapy Products, Guidance for Industry [68], which was released providing recommendations for the study design of LTFU observations, with specific considerations for GTPs.
The PMS of CGTPs in Japan
CGTPs were categorized as regenerative medical products by the Ministry of Health, Labor and Welfare (MHLW) in Japan [88, 89]. As an affiliate of MHLW, PMDA is responsible for scientific review of applications for marketing approval of drugs, medical devices, etc., monitoring their safety after they are marketed. The regenerative medical products are evaluated by PMDA’s Office of Cellular and Tissue-based Product [90]. To encourage innovation of regenerative medical product, dual-track regulation is implemented to regulate an investigational regenerative medical product in Japan [91]. Japan has initiated an expedited approval system under the PMD Act, including conditional and time-limited approvals, orphan drugs designation, Sakigake, and priority review [92, 93]. For example, beperminogene perplasmid was assessed through priority review, and conditional and time-limited approval was obtained only 18 months after submitting the marketing authorization application.
In general, the regenerative medical products including CGTPs in Japan are subject to that PMS of conventional pharmaceutical products (Table 3). As soon as the regenerative medicine product receives marketing authorization, Good Quality Practice and Good Vigilance Practice should be followed [94].
Table 3.
The guidelines of PMS for CGTPs in Japan
RMP Guidance in Japan was issued in April, 2012. Applicants have been required to implement RMP for all drugs including regenerative medical products since 2013. Similar to that in EU, a RMP includes three key elements: (1) safety specification, (2) routine/additional PV activities, (3) routine/additional risk minimization activities. In particular, additional PV activities such as “Early post-marketing Phase Vigilance (EPPV)” of new drugs or “use-results surveys,” “post-marketing clinical studies,” and “distributing of materials to ensure the proper use of drugs that require caution” are conducted for re-examination/re-evaluation application [98]. Usually, EPPV is conducted during the first 6 months from the time of initial marketing to ensure understanding of the proper use of the drug and minimize the harm of ADRs, etc., by collecting information on serious ADRs, etc., and taking necessary safety measures [96].
The execution of RMP is stated in PSUR, which is submitted every 6 months for the first 2 years after the drug is initially marketed, and once a year thereafter. To assess safety and efficacy, long-term follow-up is needed for the regenerative medical products including CGTPs with high potential risk, or the products with conditional and time-limited approval [99]. However, how to conduct long-term follow-up is one of the challenging issues with CGTPs.
The PMS of CGTP in South Korea
CGTPs are regulated as biological products by the MFDS in South Korea. The cell and gene therapy products division in the National Institute for Food and Drug Safety Assessment (NIFDS) under MFDS is responsible for reviewing and evaluating the quality, safety, and efficacy of CGTPs [100]. The Advanced Therapy Products Research Division is responsible for product testing and research related to the regulatory activities of CGTPs. The Biopharmaceuticals Policy Division and Quality Management Division in MFDS headquarters hold primary responsibility over policy development, safety evaluation, and post-marketing safety management [100]. Many CGTPs follow the route of an expanded access program, fast-track approval, or pre-review system [100].
CGTPs, as with other drug products, are regulated under the Pharmaceutical Affairs Act (PAA) [101]. The PMS of CGTPs in South Korea mainly includes RMP, re-examination, and re-evaluation [102, 103]. A RMP in South Korea usually includes the following items: (1) safety-focused review items, (2) efficacy-focused review items, (3) pharmaceutical surveillance plans, (4) risk mitigation measures [104].
PSUR are required to be submitted every 6 months in the first 2 years from the approval date, once a year thereafter, and the final report should be submitted within 2 months after the expiration of the surveillance period [105]. In addition to PSUR, product license renewal, periodic Good Manufacture Practice inspection, and advertising monitoring, the PMS of CGTPs in South Korea also includes two specific PV systems, namely re-examination and re-evaluation [102, 103].
Re-examination and re-evaluation are mandatory PV systems in South Korea. The re-examination process includes identifying re-examination subjects, submitting and reviewing re-examination applications, PMS, announcing re-examination results, and subsequent actions. The re-examination subjects are determined by the MFDS. After marketing authorization, MAH submits a re-examination plan to the MFDS and identifies any adverse events under routine medical treatment for a certain period of time. For new medicinal products, 3000 cases have to be investigated within 6 years. However, for CTPs, only 600 patients for investigation in 6 years are needed in re-examination plans. Re-examination results, along with the safety data collected, are comprehensively reviewed and reflected in the product label [105]. The safety and efficacy of marketed products will be re-evaluated periodically according to the label of comparable products as well as the latest literature information. This information is also reflected in the label [53].
The MAH are required to submit a review to MFDS every 4 or 6 years after marketing, except for orphan drugs. For CGTPs, the MAH also needs to submit a product safety management plan with RMP, focusing on risk mitigation and controls, including medication guides for patients and measures to ensure safe use, so as to minimize adverse events arising from the use of marketed products [102]. For products subject to re-examination, their product approvals have to be renewed at 5-year intervals on the basis of re-evaluation results from the date when re-examination is completed [101]. The regulations and guidelines of PMS for CGTPs in South Korea are shown in Table 4.
Table 4.
The regulations/guidelines of PMS for CGTPs in South Korea
| Regulations/guidelines | Main topics | |
|---|---|---|
| 1 | Pharmaceutical Affairs Act[101] | General regulation |
| 2 | Regulation for pharmaceutical approvals, notifications and reviews [106] | Approval, notification and review |
| 3 | Regulation on the execution of re-evaluation of pharmaceuticals [53] | Re-evaluation |
| 4 | Standard for re-examination of new drugs [105] | Re-examination |
| 5 | Regulation on safety information control of medicinal products [107] | Collection of safety information |
| 6 | Enforcement Decree of the Pharmaceutical Affairs Act [108] | Distribution, exaggerated advertising and collection and testing |
| 7 | Guideline on re-examination affairs of new drug, etc. [51] | Re-examination |
The PMS of CGTPs in China
In China, CGTPs are classified as biological products by the NMPA, which are subject to the PMS regulation for biological products [109]. The Center for Drug Evaluation (CDE) under NMPA is responsible for reviewing and evaluating quality, safety, and efficacy of CGTPs for marketing authorization. CDE is also in charge of part of PMS of medicinal products including CGTPs [110].
In order to provide patients who suffer from life-threatening diseases or diseases that do not have effective treatment strategy with the opportunity to receive treatment as soon as possible, China accelerates review and approval of drugs through expedited programs, including breakthrough therapy designation, priority review pathway, conditional approval pathway, and exceptional approval [111]. These expedited programs are commonly used in CGTPs regulation [112].
All the medical products including CGTPs are regulated under the Drug Administration Law of the People’s Republic of China (2019 edition). According to this law, the MAH should develop a post-marketing RMP for pharmaceutical products, actively carry out the agreed post-marketing studies, and further confirm their safety, efficacy, and quality reproducibility. It requires that research on drugs under conditional approval be completed within various specified time (in principle, it should not exceed 4 years [113]) and MAH should collect and analyze adverse events and conduct post-marketing adverse reaction monitoring [114]. The NMPA issued the Good Pharmacovigilance Practice in May 2021 [115]. According to this document, the MAH needs to conduct post-marketing safety studies, and the scope, type, initiation, purposes, and methods of post-marketing safety studies is established. It proposes that, for innovative drugs, PSURs should be submitted once a year before first re-registration, and once every 5 years thereafter. However, only principled technical requirements are included, and more detailed technical guidelines are further needed.
In December 2021, NMPA issued “Technical Guidelines for Long-term Follow-up Clinical Studies of Gene Products” [116], which is applicable to the long-term follow-up study of GTPs, making sure of timely collection of signals of delayed adverse reactions, identifying and reducing such risks, and obtaining information on the long-term safety and effectiveness of GTPs. In this document, the requirements for the purposes, considerations, design, and implementation of long-term follow-up clinical studies for GTPs, and some special considerations for different GTPs are also provided. This guideline applies primarily to clinical studies with long-term follow-up, including those that may need to be continued by sponsors after marketing approval. Principled recommendations on long-term post-marketing monitoring are also supplied in the guidelines, while detailed introduction to safety, efficacy, and research methods is limited. The supporting guidance for the research protocol as well as the study report of PASS and PAES are further needed.
In January 2022, NMPA published “Technical Guidelines for Clinical RMP of Chimeric Antigen Receptor T cell (CAR-T) Therapeutic Products” [117], guiding RMP study in post-marketing clinical trials of CAR-T cell therapeutic products. Another guideline, “Guidance for Writing a Clinical Risk Management Plan (for Trial Implementation),” was released in January 2022 by NMPA [118], which provides principled provisions for the RMP format in marketing approval applications. The content and format of RMP template in this guideline mainly refers to those of EU. This guideline proposed that the post-marketing RMPs should be developed with full reference to the “clinical RMP” confirmed by the CDE at the time of approval of the marketing application.
Up to June 2024, a total of 7 CGTPs, equecabtagene autoleucel, axicabtagene ciloleucele, relmacabtagene autoleucel, E1B/E3 deficient adenovirus, recombinant p53 gene, inaticabtagene autoleucel, and zevorcabtagene autoleucel have obtained approval in China, and most of them are CAR-T products. Moreover, lots of CGTPs are currently in the preclinical or clinical trial stage. Therefore, guidelines specific to post-marketing efficacy and safety studies for CAR-T products should be issued, covering aspects such as study design, primary endpoints and indicators, data collection, and analysis, to align with the current rapidly progressing state of CGTPs research in China. Additionally, more details on the updated requirements, submission, and effectiveness evaluation of post-marketing RMP are needed. Systematic post-marketing regulatory activities in China, including RMP and PVP, should also be improved. The current guidelines of PMS for CGTPs in China are summarized in Table 5.
Table 5.
The guidelines of PMS for CGTPs in China
| Guideline | Main content | |
|---|---|---|
| 1 | Technical guidelines for long-term follow-up clinical studies of gene therapy products [116] | Long term follow-up |
| 2 | Technical guidelines for clinical risk management plan of chimeric antigen receptor T cell (CAR-T) therapeutic products [117] | Risk management |
| 3 | Guidance for writing a clinical risk management plan (for Trial Implementation) [118] | Risk management |
| 4 | Guideline for pharmacovigilance examination [119] | PV |
Comparison of PMS for CGTPs in different regions
The EU, the US, Japan, and South Korea have established specialized committees or divisions to regulate CGTPs, respectively. There are no special committees/divisions established for CGTPs regulation in China. All regions issued support mechanisms to accelerate the marketing of CGTPs and established relatively complete legal and regulatory systems for the PMS of CGTPs as well. The regulatory frameworks of CGTPs in these regions are summarized in Additional file 1: Table S5.
Among these regions, the EU has the most detailed risk management system, providing comprehensive guidance on the classification of RMP changes, submission requirements, and the assessment of effectiveness of RMM. The US also has specified requirements for the RMM assessment of effectiveness. While detailed reports on post-marketing RMP changes, submission requirements, and RMM effectiveness evaluation in China are not readily available.
The guideline, Pharmacovigilance Planning, released by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been adopted by the EU, the US, Japan, and China. Therefore, these regions have a similar PV system. In general, PV plans in each region are primarily designed to address safety concerns of pharmaceuticals, with efficacy studies when necessary. Despite this, there are still slight variations in the requirements for PV across different regions. Unlike the EU, in the US, there is no requirement for or equivalent of Pharmacovigilance System Master File (PSMF); however, the MAH must be able to demonstrate that they have robust PV systems and written procedures in place, including periodic safety report requirements and REMS. In addition, Japan and South Korea conduct re-examination and re-evaluation for drugs after they have been marketed, whereas the EU, the US, and China do not mandate this process [120]. Moreover, the frequency of submission for PSURs varies across regions. The variations in the PV requirements and the submission frequency of PSUR among the regions are listed in Table 6.
Table 6.
The parameters of PMS for CGTPs in the EU, the US, Japan, South Korea, and China
| Framework | the EU | the US | Japan | South Korea | China |
|---|---|---|---|---|---|
| Main guidelines for the PMS of CGTPs |
1) Guideline on safety and efficacy follow-up-risk management of advanced therapy medicinal products 2) Guideline on safety and efficacy follow-up and risk management of advanced therapy medicinal products draft |
1) Guidance for industry: post-marketing studies and clinical trials —implementation of Sect. 505(o)(3) of the Federal Food, Drug, and Cosmetic Act 2) REMS: FDA’s application of statutory factors in determining when a REMS is necessary 3) Guidance for industry long term follow-up after administration of human gene therapy products guidance for industry |
Risk management plan guidance Procedures for developing post-marketing study plan |
Guideline on re-examination affairs of new drugs etc |
1) Technical guidelines for long-term follow-up clinical studies of gene products 2) Technical guidelines for clinical risk management plan of chimeric antigen receptor T cell (CAR-T) therapeutic products 3) Guidance for writing a clinical risk management plan (for Trial Implementation) 4) Guideline for pharmacovigilance examination |
| Tools for PMS |
RMP PSMF PSUR |
REMS Reporting of adverse reactions Product labeling PMR and PMC PSUR |
RMP GVP Good post-marketing study practice PSUR |
RMP GMP inspection Product license renewal PSUR |
RMP PV system PSUR |
| Submission of PSUR | Every 6 months for the first 2 years after initial marketing, once a year for the next 2 years, and every 3 years thereafter | Every quarter within 3 years after drug approval, and once a year thereafter | Every 6 months for the first 2 years after the drug is initially marketed, and once a year thereafter | Every 6 months in the first 2 years from the approval date, once a year thereafter, and the final report should be submitted within 2 months after the expiration of the surveillance period | Once a year before first re-registration, and once every 5 years thereafter |
| Mandatory PV requirements |
Routine and additional PV activities Routine and additional RMM Serious ADR reports |
Adverse event monitoring Routine and additional PV PMR Spontaneous reports |
Routine and additional PV activities Routine and additional risk minimization activities Re-examination Reporting of adverse drug reactions |
Re-examination Re-evaluation |
Routine PVP Mandatory additional PVP Routine RMM |
| Optional PV requirements | Voluntary PV activities (i.e., voluntary PASS) |
PMC Risk minimization action plan |
Voluntary surveillance and studies | Spontaneous ADR monitoring |
Voluntary additional PVP Spontaneous ADR reporting |
There are certain challenges in PMS of CGTPs among these regions. Within the EU, each member state conducts PV activities based on the framework of the EU’s PV system. However, each country has established its own safety management and technical agencies responsible for PV. Variations in regulatory approaches among different EU member states may present challenges in achieving harmonization. Additionally, due to the unique nature of CGTPs, extended patient follow-up is typically necessary after marketing authorization, with follow-up periods generally longer than those for conventional drugs. The frequent movement of individuals between different European countries complicates long-term follow-up efforts. The PV plan is a key component of the RMP. Therefore, establish RMP guidelines specific to CGTPs in Japan, South Korea, and China may promote adequate monitoring of the safety of CGTPs. The lack of publicly available national-level adverse reaction monitoring data in China also constitutes a critical issue that requires urgent attention and redressal efforts. Additionally, detailed post-marketing study guidelines should be issued in China as well.
A comparative overview of the PMS of CGTPs in the EU, the US, Japan, South Korea, and China is shown in Table 6.
Current status of implementation of the PMS for CGTPs in different regions
List of the CGTPs approved in different regions
The public available data related to CGTPs were collected on the official websites that granted marketing authorization between October 5, 2009, and June 30, 2024. As a result, 96 CGTPs were finally included, of which 26 products were approved in the EU (7 products were withdrawn), 27 in the US, 19 in Japan, 17 in South Korea, and 7 in China (Additional file 1: Table S6).
The implementation of PMS for the CGTPs approved in the EU
The support mechanisms for the accelerated marketing of CGTPs in the EU were shown in Additional file 1: Table S7. As shown in Additional file 1: Table S7 [43, 121–144], 8 CTPs and 18 GTPs have been approved by the EMA, among which 14 products (54%) were granted to eligibility to PRIME, 10 products (38%) received conditional marketing authorization, 3 products (12%) were authorized under exceptional circumstances, 20 products (77%) were designated orphan medicines, and 12 product (46%) accessed accelerated assessment.
Table 7 shows the implementation of PMS for the CGTPs approved in the EU. As shown in Table 7, PSURs, routine PVP, and routine RMM are required for all 25 products (100%). Twenty-three products (88%) are under additional monitoring. On average, 13 points of safety concern need to be considered for each product. Seventeen points of safety concerns of alipogene tiparvovec are found in the current edition of RMP summary, which are not categorized into three types of risks (identified risks, important potential risks, and missing information). Therefore, this product is included for further discussion. Among the other 25 products, only darvadstrocel (4%) does not have important identified risks, for which important potential risks and missing information need to be considered; the other 24 (96%) products have all three types of safety concerns. N. A. indicates not applicable If there are 6 important identified risks, and relative measures are taken for three of them, the proportion in the table will be 50%
Table 7.
The implementation of PMS for the CGTPs approved in the EU
| Name | Routine RMM | Additional RMM | Additional PVP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aa | Bb | Cc | Aa | Bb | Cc | Aa | Bb | Cc | ||||
| Exagamglogene autotemcel [145] | 100 | 100 | 100 | 100 | 100 | 33 | 100 | 100 | 100 | |||
| Etranacogene dezaparvovec [43] | 100 | 100 | 100 | 50 | 83 | 33 | 100 | 100 | 100 | |||
| Tabelecleucel [121] | 100 | 100 | 75 | 0 | 0 | 0 | 100 | 100 | 100 | |||
| Lisocabtagene maraleucel [122] | 100 | 75 | 50 | 29 | 13 | 0 | 100 | 63 | 100 | |||
| Valoctocogene roxaparvovec [123] | 100 | 100 | 100 | 50 | 100 | 33 | 100 | 100 | 67 | |||
| Eladocagene exuparvovec [124] | 100 | 100 | 100 | 100 | 33 | 0 | 100 | 100 | 100 | |||
| Ciltacabtagene autoleucel [125] | 100 | 100 | 83 | 40 | 20 | 0 | 100 | 100 | 100 | |||
| Idecabtagene vicleucel [126] | 100 | 80 | 100 | 60 | 0 | 0 | 100 | 80 | 67 | |||
| Elivaldogene autotemcel [127] | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | |||
| Autologous CD34 + cells encoding ARSA gene [128] | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||
| Brexucabtagene autoleucel [129] | 100 | 100 | 100 | 40 | 0 | 0 | 100 | 100 | 100 | |||
| Onasemnogene abeparvovec [130] | 100 | 100 | 50 | 0 | 0 | 0 | 100 | 100 | 100 | |||
| Betibeglogene autotemcel [131] | 100 | 100 | 100 | 100 | 100 | 50 | 100 | 100 | 50 | |||
| Voretigene neparvovec [132] | 100 | 100 | 100 | 100 | 33 | 0 | 100 | 100 | 100 | |||
| Axicabtagene ciloleucel [133] | 100 | 100 | 50 | 40 | 0 | 0 | 100 | 71 | 100 | |||
| Tisagenlecleucel [134] | 100 | 63 | 80 | 33 | 13 | 0 | 100 | 100 | 100 | |||
| Autologous CD34 + cells encoding ARSA gene [128] | N.A | 100 | 100 | N.A | 29 | 0 | N.A | 100 | 100 | |||
| Spheroids of human autologous matrix-associated chondrocytes[136] | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||
| ACD34 + ECF [137] | 100 | 80 | 100 | 50 | 20 | 0 | 75 | 100 | 100 | |||
| gmATC [138] | 100 | 100 | 100 | 33 | 10 | 0 | 100 | 100 | 100 | |||
| Talimogene laherparepvec [139] | 100 | 73 | 40 | 29 | 55 | 20 | 29 | 55 | 60 | |||
| Ex vivo expanded autologous human corneal epithelial cells containing stem cells [140] | 100 | 83 | 60 | 100 | 83 | 0 | 100 | 100 | 100 | |||
| Sipuleucel-T [141] | 100 | 71 | 50 | 100 | 100 | 100 | 25 | 43 | 17 | |||
| Matrix-applied characterized autologous cultured chondrocytes [142] | 100 | 100 | 50 | 100 | 100 | 0 | 0 | 25 | 33 | |||
| Characterized viable autologous cartilage cells expanded ex vivo expressing specific marker proteins [144] | 100 | 100 | 100 | 100 | 100 | 100 | 17 | 0 | 100 | |||
Abbreviations: ACD34 + ECF, autologous CD34 + enriched cell fraction that contains CD34 + cells transduced with retroviral vector that encodes for the human ADA cDNA sequence; gmATC, allogeneic T cells genetically modified with a retroviral vector encoding for a truncated form of the human low affinity nerve growth factor receptor and the herpes simplex I virus thymidine kinase
N. A. indicates not applicable
If there are 6 important identified risks, and relative measures are taken for three of them, the proportion in the table will be 50%
aThe proportion of important identified risks, %
bThe proportion of important potential risks, %
cThe proportion of missing information
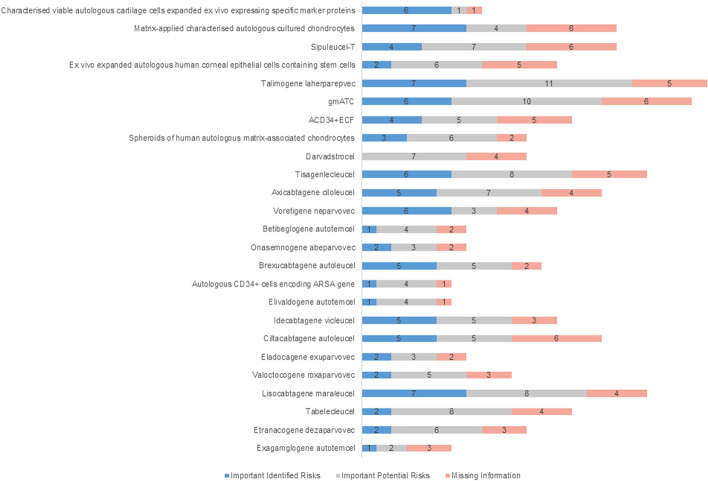
For each important identified risk, the percentage of additional RMM and additional PV averages are 59% and 78% respectively. The percentage of additional RMM and additional PV averages are 47% and 88%, respectively, for each product with important potential risk. For each product with missing information, the percentage of additional RMM and additional PV averages are 18% and 85%, respectively (Fig. 2 [43, 121–142, 144, 145] and Table 7). Moreover, the missing information has been analyzed, including long-term safety (listed under “missing information” in the RMP for 17 of 25 products, 68%), long-term efficacy (44%), use in pregnant or lactating women (48%), use in pediatric patients or adolescents (32%), use in the elderly (24%), use in patients in other age groups (8%), use in patients with other diseases or treatments (28%), use in populations not studied in clinical trials (patients with different subtypes, severity and race, 16%), repeat or long-term use (4%), immunogenicity (24%), reproductive toxicity and other adverse reactions (4%), and lack of specific clinical data and off-label (24%).
Fig. 2.
The identified risks of the CGTPs approved in the EU. Abbreviations: ACD34 + ECF, autologous CD34 + enriched cell fraction that contains CD34 + cells transduced with retroviral vector that encodes for the human ADA cDNA sequence; gmATC, allogeneic T cells genetically modified with a retroviral vector encoding for a truncated form of the human low affinity nerve growth factor receptor and the herpes simplex I virus thymidine kinase
The implementation of PMS for the CGTPs approved in the US
The FDA promotes and accelerates the approval of CGTPs through expedited programs, etc., including regenerative medicine advanced therapy designation, fast track designation, breakthrough therapy designation, accelerated approval, priority review designation, and orphan drugs designation. Seven CTPs and 20 GTPs have been approved in the US, among which 7 products (26%), 7 products (26%), and 14 products (52%) were granted to regenerative medicine advanced therapy designation, fast track designation, and breakthrough therapy designation, respectively. No product (0%) was authorized through accelerated approval, 21 products (78%) obtained priority review, and 15 products (56%) were designated orphan medicines (Additional file 1: Table S8) [146–167].
PMS measures for these products include REMS, PMR, and PMC. Six products (tisagenlecleucel, axicabtagene ciloleucel, brexucabtagene autoleucel, ciltacabtagene autoleucel, lisocabtagene maraleucel, and idecabtagene vicleucel) are required REMS (22%), but not for the remaining 21 products (78%), as based on the submitted safety data at the time of license application. In the approved 27 products, 10 of them were added black box warnings in their package inserts, accounting for 37%; 17 products necessitate PMR, and elivaldogene autotemcel has the highest rate with 5 PMR; only 4 of the applicants have made post-marketing commitments (Table 8).
Table 8.
The implementation of PMS for the CGTPs approved in the US
| Name | REMS | Black box warning | PMR | PMC |
|---|---|---|---|---|
| Fidanacogene elaparvovec-dzkt [168] | No | No | 0 | 0 |
| Atidarsagene autotemcel [169] | No | No | 2 | 0 |
| Lifileucel [170] | No | Yes | 0 | 1 |
| Exagamglogene autotemcel (exa-cel) [171] | No | No | 3 | 0 |
| Lovotibeglogene autotemcel (lovo-cel) [172] | No | Yes | 2 | 0 |
| Donislecel-jujn [146] | No | No | 0 | 0 |
| Valoctocogene roxaparvovec-rvox [147] | No | No | 0 | 0 |
| Delandistrogene moxeparvovec-rokl [148] | No | No | 1 | 1 |
| Beremagene geperpavec [149] | No | No | 0 | 0 |
| Nadofaragene firadenovec-vncg [150] | No | No | 0 | 1 |
| Etranacogene dezaparvovec-drlb [151] | No | No | 2 | 0 |
| Elivaldogene autotemcel [152] | No | Yes | 5 | 0 |
| Betibeglogene autotemcel [153] | No | No | 2 | 0 |
| Ciltacabtagene autoleucel [154] | Yes | Yes | 1 | 0 |
| Allogeneic processed thymus tissue–agdc [155] | No | No | 0 | 0 |
| Allogeneic cultured keratinocytes and dermal fibroblasts in murine [156] | No | No | 1 | 0 |
| Idecabtagene vicleucel [157] | Yes | Yes | 1 | 1 |
| Lisocabtagene maraleucel [158] | Yes | Yes | 1 | 0 |
| Brexucabtagene autoleucel [159] | Yes | Yes | 3 | 0 |
| Onasemnogene abeparvovec [160] | No | Yes | 0 | 0 |
| Tisagenlecleucel [161] | Yes | Yes | 1 | 0 |
| Voretigene neparvovec-rzyl [162] | No | No | 0 | 0 |
| Axicabtagene ciloleucel [163] | Yes | Yes | 1 | 0 |
| Autologous cultured chondrocytes on a porcine collagen membrane [164] | No | No | 1 | 0 |
| Talimogene laherparepvec [165] | No | No | 1 | 0 |
| Allogeneic cultured keratinocytes and fibroblasts in bovine collagen [166] | No | No | 1 | 0 |
| Sipuleucel-T [167] | No | No | 0 | 0 |
| Total | 6 | 10 | 29 | 4 |
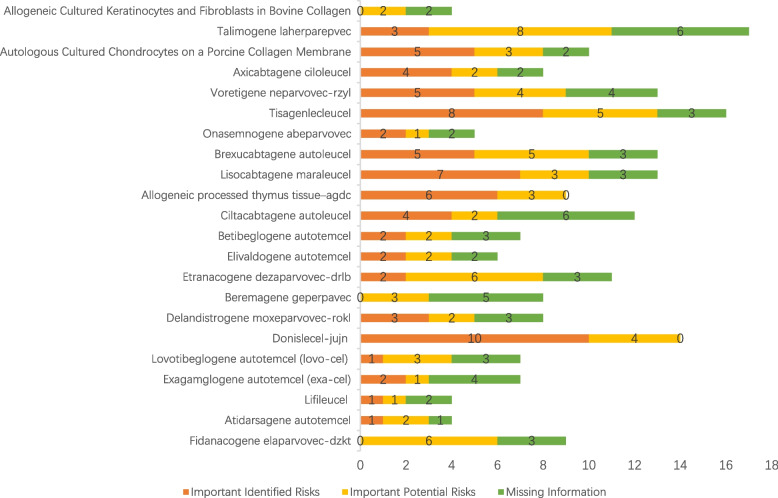
No detailed PVP review documents of 5 products (sipuleucel-T [167], idecabtagene vicleucel [157], allogeneic cultured keratinocytes and dermal fibroblasts in murine [156], nadofaragene firadenovec-vncg [150], and valoctocogene roxaparvovec-rvox [147]) were found on the FDA website. Therefore, they are not included in the statistics of various safety issues. From the PVP review of the remaining 22 products, important identified risks, important potential risks, and missing information are listed in Fig. 3. As shown in Fig. 3, 10 safety concerns need to be considered for each product on average, except for nadofaragene firadenovec-vncg and allogeneic processed thymus tissue–agdc (12%), with no missing information, and for talimogene laherparepvec (6%), with no proposed measures for one piece of missing information. For the remaining 14 products (82%), relevant PVP or RMM are listed for each risk or missing information.
Fig. 3.
The identified risks of the CGTPs approved in the US
The implementation of PMS for the CGTPs approved in Japan
Japan promotes and accelerates the development, approval of regenerative medical products through orphan drugs designation, Sakigake, priority review, conditional and time-limited approvals, etc. To date, 10 CTPs and 9 GTPs have been approved in Japan, among which 4 products (21%) received priority review and approved through conditional and time-limited approval, and 13 products received orphan drug designation, with 3 products (16%) included in Sakigake program (Additional file 1: Table S9) [173–191].
For PMS measures, 15 products (79%) are required to conduct all-case monitoring, and a re-examination period is stipulated for 10 products (53%). Sixteen products (84%) should be used under the supervision of physicians with adequate knowledge and experience or at qualified medical institutions. Thirteen products (68%) are required to disseminate guidelines for proper use of drugs (Table 9). Since only a limited number of patients participated in the clinical studies, the applicant needs to conduct a use-results survey after the market launch, take necessary measures to ensure 30-year retention of a sample of the finished product and a record of use, and conduct RMP.
Table 9.
The implementation of PMS for the CGTPs approved in Japan
| Name | All-case surveillance | Re-examination period | Requirements for doctors and medical institution | Dissemination of guideline for proper use |
|---|---|---|---|---|
| Neltependocel [173] | Yes | 10 | Yes | Yes |
| Melanocyte-containing human (autologous) epidermis-derived cell sheet [174] | Yes | 8 | No | No |
| Ciltacabtagene autoleucel [175] | Yes | 10 | Yes | Yes |
| Idecabtagene vicleucel [176] | Yes | 10 | Yes | Yes |
| Human (autologous) oral mucosa-derived epithelial cell sheet using human amniotic membrane substrate [177] | Yes | No | Yes | Yes |
| Darvadstrocel [178] | Yes | No | Yes | Yes |
| Teserpaturev [179] | Yes | No | Yes | Yes |
| Human (autologous) oral mucosa-derived epithelial cell sheet [180] | Yes | No | Yes | Yes |
| Lisocabtagene maraleucel [181] | Yes | No | Yes | Yes |
| Axicabtagene ciloleucel [182] | Yes | 10 | Yes | Yes |
| Onasemnogene abeparvovec [183] | Yes | 10 | Yes | Yes |
| Human (autologous) corneal limbus-derived corneal epithelial cell sheet [184] | Yes | 10 | Yes | Yes |
| Beperminogene perplasmid [185] | Yes | No | Yes | Yes |
| Tisagenlecleucel [186] | No | 10 | Yes | Yes |
| Human (autologous) bone marrow-derived mesenchymal stem cells [187] | Yes | No | Yes | No |
| Human (autologous) epidermal cell sheet [188] | Yes | 10 | Yes | No |
| Human (autologous) skeletal myoblast-derived cell sheet [189] | No | No | Yes | No |
| Human (allogeneic) bone marrow-derived mesenchymal stem cells [190] | No | 10 | No | No |
| Human autologous tissue for transplantation [191] | No | No | No | No |
| Total | 15 | 10 | 16 | 13 |
A use-results survey is required for the 19 products, which should cover all Japanese patients treated with the product after the market launch, to promptly collect safety and efficacy data. The number of patients in the control and treatment groups, the duration, risks requiring special attention, and endpoints are discussed in detail in the survey. In addition, the applicant needs to provide other post-marketing monitoring plans, such as the proposed marketing time, period of registration, observation period of a single patient, proposed reapplication time, production process, and quality control.
The implementation of PMS for the CGTPs approved in South Korea and China
The detailed implementation information of PMS for the CGTPs approved in South Korea and China was not found on the official website.
Comparison for the PMS implementation of CGTPs in the EU, the US, and Japan
Each region has established PMS measures for CGTPs. In the EU, it mainly involves post-marketing risk management, including safety concerns, routine/additional RMM, routine/additional PV activities, and additional monitoring. In the US, it primarily refers to REMS, black box warnings, PMR, and PMC. In Japan, it principally encompasses all-case surveillance, re-examination periods, requirements for doctors and medical institutions, and dissemination of guidelines for proper use.
The important identified risks for CGTPs authorized in the EU and the US are summarized in Additional file 1: Table S10 and S11, respectively. Among the 16 CGTPs marketed in both the EU and the US, 13 products have similar important identified risks in these two regions. The other 3 CGTPs (valoctocogene roxaparvovec-rvox, idecabtagene vicleucel, and sipuleucel-T) were not included in the comparison due to the unavailability of PVP for these 3 products on the official website of the FDA. The most common important identified risks are infections, cytopenia, neurologic toxicities, cytokine release syndrome, disorders, and hypogammaglobulinemia. In addition, the important identified risks of CGTPs granted in Japan were not clarified in the review reports of each product and therefore were not been not included in the analysis.
Discussion
The guidelines of PMS for CGTPs in the EU are the most comprehensive, which has not only a regulatory framework specific to CGTPs but also relevant supporting guidelines of PMS for CGTPs. Although Japan and South Korea have not specifically formulated regulations of PMS for CGTPs, their existing PMS and data collection systems are well developed. In Japan, for further risk control, early post-marketing phase vigilance is used to identify and evaluate safety signals in the early stage after marketing, and appropriate safety measures can be taken for adverse events when necessary.
The PMS for the marketed CGTPs in the EU is more systematic than that in other regions, including approval conditions, quality control, and RMP. Although the regulatory measures of the US are not as comprehensive as those of the EU, it has its own developed system, providing a relative supervision strategy for each listed risk. PMS in Japan is also comparatively comprehensive; related supervision measures such as quality control and post-marketing use-results surveys are carried out for each product, and product risks are also identified. Post-marketing regulatory policies have instructive significance for actual regulatory implementation, and more comprehensive regulatory policies will bring about more specific and systematic supervision in practice.
There is still significant room for improvement in regard of the development, study protocols, and effectiveness evaluation of RMP in China. For example, the current RMP writing guidance in China provides only general considerations and writing principles (including safety overview, PVP, and risk control measures) as well as a RMP template, without detailed guidance for risk management based on the different stages of the drug lifecycle. Moreover, there is no detailed specification for the update of post-marketing RMPs and the evaluation of RMM effectiveness for CGTPs. Therefore, certain guidelines could be issued to address these problems. Technical guidelines for the dynamic update of RMP throughout the product lifecycle and the evaluation of RMM effectiveness could be published. Making all the detailed documents of RMP, post-marketing study plans and reports publicly available would allow healthcare professionals, patients, pharmaceutical companies, and other stakeholders to access pertinent risk information.
To enhance the efficiency of life-cycle dynamic risk management, including post-marketing risk, there is a significant need for detailed guidance on how the MAH can effectively perform post-marketing studies, transitioning from the RMP at a marketing authorization application stage to the post-marketing stage based on the benefit/risk knowledge of the CGTPs after approval. In China, current regulations and guidelines require the MAH to conduct post-marketing research when necessary. China could issue detailed guidance post-marketing research plans, including submission requirements, evaluation procedures, and standards, referring to that of the EMA.
The MAH/applicants should cooperate with medical institutions, medicine manufacturers, and regulatory authorities to establish PV for CGTPs so as to obtain high-quality data from multiple sources to assess product risks more comprehensively and scientifically, strengthening the application of new technologies in PV practices. The MAH is also encouraged to cooperate with scientific research institutes and industry associations to promote PV activities, i.e., entrusting institutions and associations to conduct long-term follow-up of patients from the clinical trial population or a cohort study to provide additional characterization of the long-term safety of the medicinal product. Furthermore, led by the NMPA, MAH can also collaborate with healthcare institutions and industry associations to establish real-world medical databases, by integrating real-world data from the various networks for the real-world evidence generation. Above all, a proper PMS framework for CGTPs should be established to meet the fast-growing CGTPs development in China.
This study has limitations as follows: (1) some documents related to RMP for CGTPs in China and South Korea are not publicly accessible, and (2) systematic analysis of issues such as changes in product labeling and safety concerns resulting from the real-world application of marketed CGTPs are not included, which could be carried out in the future.
Conclusions
At present, the practice of PMS in the EU, the US, and Japan are detailed, specific, and focused. Although Chemistry, Manufacturing and Controls (CMC) and clinical guidelines for CTPs have already been issued in China, more detailed guidelines of PMS for CGTPs need to be published. It is recommended that China develops guidance for RMP writing that considers various stages of the drug lifecycle. To enhance regulatory actions, China should establish technical guidelines for the dynamic update of RMPs throughout the product lifecycle and provide clear instructions for evaluating the effectiveness of RMM. Additionally, increasing the frequency of PSUR submissions would enhance the surveillance for safety issues. Making the relevant documents of RMP, post-marketing study plans and reports publicly available would allow healthcare professionals, patients, pharmaceutical companies, and other stakeholders to access pertinent risk information. This transparency fosters a more informed and collaborative approach to managing risks associated with CGTPs.
Supplementary Information
Additional File 1: Tables S1-S12. Table S1 – Methods for obtaining the lists of marketed CGTPs in the EU, the US, Japan, South Korea and China. Table S2 –The guidelines of RMP in the EU. Table S3 – Definitions of the key elements related to RMP. Table S4 – The guidelines of REMS in the US. Table S5 – The regulatory frameworks of CGTPs in the EU, the US, Japan, China, and South Korea. Table S6 – The marketed CGTPs in the EU, the US, Japan, South Korea and China. Table S7 – The support mechanisms for the accelerated marketing of CGTPs in the EU. Table S8 – The support mechanisms for the accelerated marketing of CGTPs in the US. Table S9 – The support mechanisms for the accelerated marketing of CGTPs in Japan. Table S10 – Important identified risks of CGTPs approved in the EU. Table S11 – Important identified risks of CGTPs approved in the US. Table S12 – Other related regulations and guidelines of PMS for CGTPs in different regions.
Acknowledgements
We are grateful to the invaluable suggestions provided by Dr. Jin Xu from Shanghai Roche Pharmaceutical Ltd.
Abbreviations
- CGTPs
Cell and gene products
- EU
European Union
- US
United States
- PMS
Post-marketing surveillance
- EMA
European Medicines Agency
- FDA
Food and Drug Administration (FDA)
- PMDA
Pharmaceuticals and Medical Device Agency
- MFDS
Ministry of Food and Drug Safety
- NMPA
National Medical Products Administration
- ATMPs
Advanced therapy medicinal products
- GMP
Good Manufacturing Practice
- RMP
Risk management plan
- MAH
Marketing authorization holders
- PASS
Post-marketing safety studies
- PEAS
Post-marketing effectiveness studies
- GVP
Good pharmacovigilance practices
- REMS
Risk assessment and mitigation strategies
- MHLW
The Ministry of Health, Labor and Welfare
- PMD
Pharmaceuticals and Medical
- ASRM
The Act on the Safety of Regenerative Medicine
- CDE
The Center for Drug Evaluation
- PVP
Pharmacovigilance plan/activities
- CAR-T
Chimeric antigen Receptor T cell
- CTPs
Cell therapy products
- GTPs
Gene therapy products
- PSUR
Periodic safety update reports
- PMR
Post-marketing requirements
- LTFU
Long-term follow-up
Authors’ contributions
Y.C., L.S., and Y.G. conceived and designed the conception of the manuscript. Y.C., L.S., and J.W. drafted the manuscript. W.Q., Y.P., L.G., and W.W. gave the comments and revised the manuscript. All authors contributed to the general discussion. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82272769, 81972392).
Availability of data and materials
The data sources are from the official website of FDA, EMA, PMDA, NMPA and MFDS. The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuxin Cai, Lijuan Sui and Jingjing Wang contributed equally to this work.
References
- 1.Pizevska M, Kaeda J, Fritsche E, Elazaly H, Reinke P, Amini L. Advanced therapy medicinal products’ translation in europe: a developers’ perspective. Front Med (Lausanne). 2022;9:757647.10.3389/fmed.2022.757647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health Surveillance Agency (Anvisa). It provides on the rules for conducting clinical trials with investigational Advanced Therapy Medicinal Products in Brazil and other provisions. 2021. http://antigo.anvisa.gov.br/documents/10181/6278627/RDC_506_2021_.pdf/e932e631-4054-4014-9ac99813474e44a4. Accessed 21 Dec 2023.
- 3.Gomes K, da Silva R, da Silva J, Bosio C, Novaes M. Post-marketing authorisation safety and efficacy surveillance of advanced therapy medicinal products in Brazil, the European Union, the United States and Japan. Cytotherapy. 2023;25(10):1113–23. 10.1016/j.jcyt.2023.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Trisha G. Hemophilia: after a setback, gene therapy progresses gingerly. Science. 2001;291:1692–7. 10.1126/science.291.5509.1692. [DOI] [PubMed] [Google Scholar]
- 5.Wu W, Wang Y, Tang Z, Gao Y, Huo Y. Regulatory oversight of cell-based therapy in China: government’s efforts in patient access and therapeutic innovation. Pharmacol Res. 2020;158:104889. [DOI] [PubMed]
- 6.Gang W. Comparison of regulatory policies for cell and gene therapy products between China and America. Chin Food Drug Admin Mag. 2019;187(08):20–5. 10.3969/j.issn.1673-5390.2019.08.02. [Google Scholar]
- 7.Center for Biologics Evaluation and Research (CBER). Guidance for human somatic cell therapy and gene therapy. 1998. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-human-somatic-cell-therapy-and-gene-therapy. Accessed 18 Sept 2023.
- 8.Lyu M, Chen J, Peng Y, Han F, Gong L, Guo J, et al. The global patent landscape of mRNA for diagnosis and therapy. Nat Biotechnol. 2023;41(9):1193–9. 10.1038/s41587-023-01925-2. [DOI] [PubMed] [Google Scholar]
- 9.Wu W, Huo Y, Ding X, Zhou Y, Gu S, Gao Y. Identification of the risks in CAR T-celtherapy clinical trials in China: a Delphi study. Ther Adv Med Oncol. 2020;12:1–12. 10.1177/1758835920966574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goula A, Gkioka V, Michalopoulos E, Katsimpoulas M, Noutsias M, Sarri EF, et al. Advanced therapy medicinal products challenges and perspectives in regenerative medicine. J Clin Med Res. 2020;12(12):780–6. 10.14740/jocmr3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicines Agency (EMA). CHMP: agendas, minutes and highlights. https://www.ema.europa.eu/en/committees/chmp/chmp-agendas-minutes-highlights. Accessed 7 Sept 2022.
- 12.U.S. Food & Drug Administration (FDA). Approved cellular and gene therapy products. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products. Accessed 10 Sept 2022.
- 13.Pharmaceuticals and Medical Device Agency (PMDA). Regenerative medical products. https://www.pmda.go.jp/english/review-services/reviews/approved-information/0003.html. Accessed 7 Sept 2022.
- 14.Ministry of Food and Drug Safety (MFDS). 2022 Drug Approval Report. 2023. https://www.mfds.go.kr/eng/brd/m_19/view.do?seq=70438. Accessed 28 Dec 2023.
- 15.The American Society of Gene&Cell Therapy (ASGCT). Gene, cell & RNA therapy landscape report. 2023. https://asgct.org/global/documents/asgct-citeline-q3-2023-report.aspx. Accessed 28 Dec 2023.
- 16.U.S. Food & Drug Administration (FDA). Postmarket requirements and commitments. https://www.accessdata.fda.gov/scripts/cder/pmc/index.cfm. Accessed 2 Mar 2023.
- 17.European Medicines Agency (EMA). How EMA evaluates medicines for human use. 2022. https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines/how-ema-evaluates-medicines-human-use. Accessed 9 Mar 2022.
- 18.European Medicines Agency (EMA). Support for early access. 2016. https://www.ema.europa.eu/en/human-regulatory-overview/support-early-access. Accessed 12 Dec 2022.
- 19.European Medicines Agency (EMA). PRIME: priority medicines. 2016. https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/prime-priority-medicines. Accessed 12 Dec 2022.
- 20.European Medicines Agency (EMA). Pharmacovigilance Risk Assessment Committee (PRAC). 2022. https://www.ema.europa.eu/en/committees/pharmacovigilance-risk-assessment-committee-prac. Accessed 23 Jan 2022.
- 21.European Medicines Agency (EMA). Guideline on safety and efficacy follow-up-risk management of advanced therapy medicinal products. 2007. https://www.ema.europa.eu/en/guideline-safety-efficacy-follow-risk-management-advanced-therapy-medicinal-products-scientific. Accessed 10 Dec 2020.
- 22.European Medicines Agency (EMA). Guideline on safety and efficacy follow-up and risk management of advanced therapy medicinal products draft. 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-safety-efficacy-follow-risk-management-advanced-therapy-medicinal-products-revision_en.pdf. Accessed 9 Aug 2022.
- 23.European Medicines Agency (EMA). Clinical trials in small populations. 2006. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-trials-small-populations_en.pdf. Accessed 7 Sept 2022.
- 24.Ma Y, Wu H, Zhang L, Song H, Huang J, Yang T. Analysis and reflection on the regulatory system for post-marketing safety research of EU drugs. Drug Eval Res. 2021;44(06):1141–8. 10.7501/j.issn.1674-6376.2021.06.002. [Google Scholar]
- 25.European Medicines Agency (EMA). Guideline on scientific requirements for the environmental risk assessment of gene therapy medicinal products. 2008. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-scientific-requirements-environmental-risk-assessment-gene-therapy-medicinal-products_en.pdf. Accessed 15 Dec 2022.
- 26.European Medicines Agency (EMA). Reflection paper on management of clinical risks deriving from insertional mutagenesis. 2013. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-management-clinical-risks-deriving-insertional-mutagenesis_en.pdf. Accessed 5 Dec 2022. [DOI] [PubMed]
- 27.European Medicines Agency (EMA). Guideline on follow-up of patients administered with gene therapy medicinal products. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-follow-patients-administered-gene-therapy-medicinal-products_en.pdf. Accessed 6 Dec 2022.
- 28.European Medicines Agency (EMA). Guideline on potency testing of cell-based immunotherapy medicinal products for the treatment of cancer. https://www.ema.europa.eu/en/potency-testing-cell-based-immunotherapy-medicinal-products-treatment-cancer-scientific-guideline. Accessed 1 Nov 2022.
- 29.European Medicines Agency (EMA). Guideline on Good Pharmacovigilance Practices (GVP) Module VIII – Post-authorisation Safety Studies (Rev 3). 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-viii-post-authorisation-safety-studies-rev-3_en.pdf. Accessed 27 July 2022.
- 30.European Medicines Agency (EMA). Guideline on Good Pharmacovigilance Practices (GVP) Module V – Risk Management Systems (Rev 2) 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-v-risk-management-systems-rev-2_en.pdf. Accessed 12 Dec 2021.
- 31.European Medicines Agency (EMA). Guideline on Good Pharmacovigilance Practices (GVP) Module XVI Risk Minimisation Measures: Selection of Tools and Effectiveness Indicators (Rev 2). 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-goodpharmacovigilance-practices-module-xvi-risk-minimisation-measures-selection-tools_en-3.pdf. Accessed 28 Mar 2017.
- 32.European Medicines Agency (EMA). Draft guideline on Core SmPC, labelling and package leaflet for advanced therapy medicinal products (ATMPs) containing genetically modified cells. 2021. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-core-smpc-labelling-package-leaflet-advanced-therapy-medicinal-products-atmps_en.pdf. Accessed 8 Aug 2022.
- 33.European Medicines Agency (EMA). Risk management plans (RMP) in post-authorisation phase: questions and answers. 2020. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/risk-management-plans-rmp-post-authorisation-phase-questions-answers. Accessed 1 March 2022.
- 34.European Medicines Agency (EMA). European Medicines Agency post-authorisation procedural advice for users of the centralised procedure. 2022. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/european-medicines-agency-post-authorisation-procedural-advice-users-centralised-procedure_en.pdf. Accessed 21 Dec 2022.
- 35.European Medicines Agency (EMA). Guidance on the format of the risk management plan (RMP) in the EU–in integrated format. 2018. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidance-format-risk-management-plan-rmp-eu-integrated-format-rev-201_en.pdf. Accessed 13 Dec 2021.
- 36.European Medicines Agency (EMA). Questions and answers on the risk management plan (RMP) summary. 2017. https://www.ema.europa.eu/en/documents/other/questions-answers-risk-management-plan-rmp-summary_en.pdf. Accessed 30 Mar 2017.
- 37.European Medicines Agency (EMA). Anonymisation of protected personal data and assessment of commercially confidential information during the preparation of risk management plans (main body and annexes 4 and 6) general guidance. 2022. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidance-anonymisation-protected-personal-data-and-assessment-commercially-confidential-information-during-preparation-rmps-main-body-and-annexes-4-and-6_en.pdf. Accessed 15 Oct 2022.
- 38.European Medicines Agency (EMA). Guideline on Good Pharmacovigilance Practices (GVP) - Module VI – Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev. 2) 2017. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-and-submission-reports-suspected-adverse-reactions-medicinal-products-rev-2_en.pdf. Accessed 25 Dec 2022.
- 39.european Medicines Agency (EMA). Guideline on Good Pharmacovigilance Practices (GVP) Module IX – Signal Management (Rev 1). 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-ix-signal-management-rev-1_en.pdf. Accessed 25 Dec 2022.
- 40.European Medicines Agency (EMA). Guideline on Good Pharmacovigilance Practices (GVP) Module X – Additional Monitoring. 2013. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-x-additional-monitoring_en.pdf. Accessed 8 Aug 2022.
- 41.European Medicines Agency (EMA). Guideline on Good Pharmacovigilance Practices (GVP) Module XV – Safety Communication (Rev 1). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-xv-safety-communication-rev-1_en.pdf. Accessed 25 Dec 2022.
- 42.European Medicines Agency (EMA). Guidance for the format and content of the final study report of non-interventional post-authorisation safety studies. 2013. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidance-format-content-final-study-report-non-interventional-post-authorisation-safety-studies_en.pdf. Accessed 28 July 2022.
- 43.European Medicines Agency (EMA). Hemgenix. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/hemgenix. Accessed 9 Sept 2023.
- 44.Holm J, Ruppert J, Ramsden S. Impact of changing regulations and the dynamic nature of european risk management plans for human medicines on the lifecycle of safety concerns. Pharmaceut Med. 2022;36(1):33–46. 10.1007/s40290-021-00414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro A, Genov G, Spooner A, Raine J, Arlett P. Promoting and protecting public health: how the European Union pharmacovigilance system works. Drug Saf. 2017;40(10):855–69. 10.1007/s40264-017-0572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.European Medicines Agency (EMA). Periodic safety update reports (PSURs). 2022. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/periodicsafety-update-reports-psurs. Accessed 12 Sept 2022.
- 47.Francisca RDC, Baba E, Hoeve CE, Zomerdijk IM, Sturkenboom M, Straus S. Introduction or discontinuation of additional risk minimisation measures during the life cycle of medicines in Europe. Drug Saf. 2021;44(1):63–72. 10.1007/s40264-020-00993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.European Medicines Agency (EMA). Scientific guidance on post-authorisation efficacy studies. 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/scientific-guidance-post-authorisation-efficacy-studies-first-version_en.pdf. Accessed 27 Aug 2022.
- 49.U.S. Food & Drug Administration (FDA). Risk evaluation and mitigation strategies | REMS. 2021. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems. Accessed 17 Dec 2021.
- 50.U.S. Food & Drug Administration (FDA). Postmarketing requirements and commitments: introduction. 2016. https://www.fda.gov/drugs/guidance-compliance-regulatory-information/postmarket-requirements-and-commitments. Accessed 22 Feb 2022.
- 51.Ministry of Food and Drug Safety (MFDS). Guideline on re-examination affairs of new drugs etc. 2021. https://www.mfds.go.kr/docviewer/skin/doc.html?fn=20230327042331364.pdf&rs=/docviewer/result/eng0003/71532/1/202312. Accessed 4 Dec 2022.
- 52.Kondo H, Masamune K. Effectiveness of drug postmarketing all-case surveillance as a safety measure in Japan. Ther Adv Drug Saf. 2021;12:20420986211065216. 10.1177/20420986211065215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ministry of Food and Drug Safety (MFDS). Regulation on the execution of re-evaluation of pharmaceuticals. 2022. https://www.mfds.go.kr/eng/brd/m_18/view.do?seq=71508&srchFr=&srchTo=&srchWord=&srchTp=7&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=3. Accessed 25 Jan 2022.
- 54.European Medicines Agency (EMA). Post-authorisation Safety Studies (PASS). https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/post-authorisation-safety-studies-pass. Accessed 4 Dec 2023.
- 55.European Medicines Agency (EMA). Guidance for the format and content of the protocol of non-interventional post-authorisation safety studies. 2012. https://www.ema.europa.eu/en/documents/other/guidance-format-content-protocol-non-interventional-post-authorisation-safety-studies_en.pdf. Accessed 12 Aug 2022.
- 56.European Medicines Agency (EMA). Guidance for the format and content of the final study report of non-interventional postauthorisation safety studies. 2013. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidance-formatcontent-final-study-report-non-interventional-post-authorisation-safety-studies_en.pdf. Accessed 12 Oct 2022.
- 57.European Medicines Agency (EMA). <PRAC><Rapporteur><preliminary><updated> non-interventional imposed PASS final study report <and RMP> assessment report. 2015. https://www.ema.europa.eu/en/documents/template-form/template-prac-assessment-report-non-interventional-imposed-pass-final-study-report_en.pdf. Accessed 9 Sept 2022.
- 58.U.S. Food & Drug Administration (FDA). Human cells, tissues, and cellular and tissue-based products, 21 CFR 1271. 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm? CFRPart=1271&showFR=1. Accessed 30 Dec 2020.
- 59.U.S. Food & Drug Administration (FDA). What is gene therapy? 2018. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy. Accessed 3 Dec 2020.
- 60.U.S. Food & Drug Administration (FDA). Guidance for industry: expedited programs for serious conditions--Drugs and Biologics. 2014. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-serious-conditions-drugs-and-biologics. Accessed 7 Aug 2022.
- 61.U.S. Food & Drug Administration (FDA). Expedited programs for regenerative medicine therapies for serious conditions guidance for industry. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-regenerative-medicine-therapies-serious-conditions. Accessed 16 Sept 2022.
- 62.Hurley EA, Hull D, Shriver SP. The next phase of human gene-therapy oversight. N Engl J Med. 2019;380(4):401–2. 10.1056/NEJMc1815048. [DOI] [PubMed] [Google Scholar]
- 63.Gene Therapy Net. Gene therapy legislation in the US. 2021. http://www.genetherapynet.com/united-states-of-america.html. Accessed 12 Jan 2021.
- 64.U.S. Health & Human Services (HHS). Register IRBs & Obtain FWAs. 2021. https://www.hhs.gov/ohrp/register-irbs-and-obtain-fwas/index.html. Accessed 10 Jan 2021.
- 65.U.S. Food & Drug Administration (FDA). Cellular & gene therapy guidances. 2021. https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances Accessed 10 Sept 2023.
- 66.U.S. Food & Drug Administration (FDA). Guidance for industry: considerations for the design of early-phase clinical trials of cellular and gene therapy products. 2015. https://www.fda.gov/media/106369/download. Accessed 4 Dec 2020.
- 67.U.S. Food & Drug Administration (FDA). FDA’s role in managing medication risks. https://www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/fdas-role-managingmedication-risks. Accessed 17 Sept 2022.
- 68.U.S. Food & Drug Administration (FDA). Guidance for industry: long term follow-up after administration of human gene therapy products. 2020. https://www.fda.gov/media/113768/download. Accessed 26 Dec 2020.
- 69.U.S. Food & Drug Administration (FDA). Guidance for industry and health: testing of retroviral vector-based human gene therapy products for replication competent retrovirus during product manufacture and patient follow-up. 2020. https://www.fda.gov/media/113790/download. Accessed 13 May 2022.
- 70.U.S. Food & Drug Administration (FDA). Guidance for industry: post-marketing studies and clinical trials —implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act. 2011. https://www.fda.gov/media/131980/download. Accessed 1 Sept. 2022.
- 71.U.S. Food & Drug Administration (FDA). Draft guidance: post-marketing studies and clinical trials—implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act Guidance for Industry. 2019. https://www.fda.gov/media/148646/download. Accessed 1 Sept. 2022.
- 72.U.S. Food & Drug Administration (FDA). What’s in a REMS? 2018. https://www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/whats-rems. Accessed 1 Sept. 2022.
- 73.U.S. Food & Drug Administration (FDA). REMS: FDA’s application of statutory factors in determining when a rems is necessary guidance for industry. 2019. https://www.fda.gov/media/100307/download. Accessed 8 Oct. 2022.
- 74.U.S. Food & Drug Administration (FDA). Use of a Drug Master File for shared system REMS submissions guidance for industry draft guidance. 2017. https://www.fda.gov/media/109124/download. Accessed 20 Oct. 2022.
- 75.U.S. Food & Drug Administration (FDA). Medication guides — distribution requirements and inclusion in risk evaluation and mitigation strategies (REMS). 2011. https://www.fda.gov/media/79776/download. Accessed 11 Oct. 2022.
- 76.U.S. Food & Drug Administration (FDA). Format and content of a REMS document guidance for industry. 2023. https://www.fda.gov/media/79776/download. Accessed 1 Feb. 2023.
- 77.U.S. Food & Drug Administration (FDA). Providing regulatory submissions in electronic format — content of the risk evaluation and mitigation strategies document using structured product labeling guidance for industry. 2020. https://www.fda.gov/media/107302/download. Accessed 5 Mar. 2022.
- 78.U.S. Food & Drug Administration (FDA). Risk evaluation and mitigation strategies: modifications and revisions guidance for industry. 2020. https://www.fda.gov/media/128651/download. Accessed 5 Mar 2022.
- 79.U.S. Food & Drug Administration (FDA). Policy for certain REMS requirements during the COVID-19 public health emergency guidance for industry and health care professionals. 2020. https://www.fda.gov/media/136317/download. Accessed 5 Mar 2022.
- 80.U.S. Food & Drug Administration (FDA). REMS document technical conformance guide. 2023. https://www.fda.gov/media/164344/download. Accessed 1 Feb 2023.
- 81.U.S. Food & Drug Administration (FDA). Survey methodologies to assess REMS goals that relate to knowledge guidance for industry DRAFT GUIDANCE. 2019. https://www.fda.gov/media/119789/download. Accessed 15 Nov 2022.
- 82.U.S. Food & Drug Administration (FDA). Development of a shared system REMS guidance for industry DRAFT GUIDANCE. 2018. https://www.fda.gov/media/113869/download. Accessed 23 Dec 2022.
- 83.U.S. Food & Drug Administration (FDA). REMS assessment: planning and reporting guidance for industry DRAFT GUIDANCE. 2019. https://www.fda.gov/media/119790/download. Accessed 27 Dec 2022.
- 84.The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Periodic benefit-risk evaluation report. 2012. https://database.ich.org/sites/default/files/E2C_R2_Guideline.pdf. Accessed 17 Sept 2022.
- 85.Chen J, Yu Z. Research on the safety summary report system after foreign listing and its enlightenment for China. Modern Commercial Industry. 2021;42(11):51–3. 10.19311/j.cnki.1672-3198.2021.11.026. [Google Scholar]
- 86.U.S. Food & Drug Administration (FDA). Providing postmarket periodic safety reports in the ICH E2C(R2) format (Periodic Benefit-Risk Evaluation Report). 2016. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/providing-postmarket-periodic-safety-reports-ich-e2cr2-format-periodic-benefit-risk-evaluation. Accessed 5 Jan 2024.
- 87.U.S. Food & Drug Administration (FDA). FDA investigating serious risk of T-cell malignancy following BCMA-directed or CD19-directed autologous chimeric antigen receptor (CAR) T cell immunotherapies. 2023. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-t-cell-malignancy-following-bcma-directed-or-cd19-directed-autologous. Accessed 3 Dec 2023.
- 88.Konishi A, Sakushima K, Isobe S, Sato D. First approval of regenerative medical products under the PMD act in Japan. Cell Stem Cell. 2016;18(4):434–5. 10.1016/j.stem.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Pharmaceuticals and Medical Device Agency (PMDA). Services of PMDA. 2022. https://www.pmda.go.jp/english/about-pmda/outline/0006.html. Accessed 8 Oct 2022.
- 90.Pharmaceuticals and Medical Device Agency (PMDA). SATO D. ACTO Satellite symposium - Japanese regulation of regenerative medicine. 2014. https://www.pmda.go.jp/files/000199507.pdf. Accessed 9 Dec 2022.
- 91.Diao Y, Liang Y. Management of cell therapy products in Japan and its implications for China. Prog Pharm Sci. 2019;43(12):908–13. [Google Scholar]
- 92.Ministry of Health LaWoJM. Strategy of SAKIGAKE. https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/140729-01.html. Accessed 20 Oct 2022.
- 93.Pharmaceuticals and Medical Device Agency (PMDA). Regulatory update from MHLW/PMDA. 2017. https://www.pmda.go.jp/files/000221880.pdf. Accessed 22 Oct 2022.
- 94.Azuma K. Regulatory landscape of regenerative medicine in Japan. Current Stem Cell Reports. 2015;1(2):118–28. 10.1007/s40778-015-0012-6. [Google Scholar]
- 95.Pharmaceuticals and Medical Device Agency (PMDA). Procedures for developing post-marketing study plan. 2018. https://www.pmda.go.jp/files/000226080.pdf. Accessed 9 Feb 2024.
- 96.Pharmaceuticals and Medical Device Agency (PMDA). Risk management plan guidance. 2012. https://www.pmda.go.jp/files/000153333.pdf. Accessed 9 Dec 2023.
- 97.Pharmaceuticals and Medical Device Agency (PMDA). Implementation methods, etc. of early post-marketing phase vigilance for prescription drugs. 2006. https://www.pmda.go.jp/files/000209204.pdf. Accessed 9 Feb 2024.
- 98.Pharmaceuticals and Medical Device Agency (PMDA). Risk management plan (RMP). https://www.pmda.go.jp/english/safety/info-services/drugs/rmp/0001.html. Accessed 29 Dec 2022.
- 99.TAWARAGI T. New streams of risk management. 2015. http://www.pmda.go.jp/files/000205832.pdf. Accessed 12 Oct 2022.
- 100.Ministry of Food and Drug Safety (MFDS). Biological products. 2023. https://www.mfds.go.kr/eng/wpge/m_22/de011012l001.do. Accessed 12 Sept 2023.
- 101.Ministry of Food and Drug Safety (MFDS). Pharmaceutical Affairs Act. 2019. https://www.mfds.go.kr/docviewer/skin/doc.html?fn=20211013112528933.pdf&rs=/docviewer/result/eng0003/71500/1/202312. Accessed 24 Oct 2022.
- 102.Choi M, Han E, Lee S, Kim T, Shin W. Regulatory oversight of gene therapy and cell therapy products in Korea. Regulatory Aspects Gene Therapy Cell Therapy Products. Advances in Experimental Medicine and Biology2015. p. 163–179. [DOI] [PubMed]
- 103.E H, Won S. Regulation of cell therapy products in Korea. ISBT Science Series. 2015; 10(Suppl. 1):129–133. 10.1111/voxs.12158.
- 104.Zhang Y, Jiang Y. Introduction and Insights into the Korean Pharmacovigilance System. Chinese J Pharmacovigilance. 2022;19(10):1082–6. 10.19803/j.1672-8629.20210331. [Google Scholar]
- 105.Ministry of Food and Drug Safety (MFDS). Standard for re-examination of new drugs. 2022. https://www.mfds.go.kr/eng/brd/m_18/view.do?seq=71517&srchFr=&srchTo=&srchWord=&srchTp=7&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=2. Accessed 9 Feb 2022.
- 106.Ministry of Food and Drug Safety (MFDS). Regulation for pharmaceutical approvals, notifications and reviews. 2021. https://www.mfds.go.kr/docviewer/skin/doc.html?fn=20230327035644903.pdf&rs=/docviewer/result/eng0003/71524/1/202312. Accessed 4 Oct 2022.
- 107.Ministry of Food and Drug Safety (MFDS). Regulation on safety information control of medicinal products. 2016. https://www.mfds.go.kr/eng/brd/m_27/view.do?seq=70881&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=2. Accessed 26 Sept 2021.
- 108.Ministry of Food and Drug Safety (MFDS). Enforcement Decree of the Pharmaceutical Affairs Act. 2021. https://www.mfds.go.kr/eng/brd/m_18/view.do?seq=71501&srchFr=&srchTo=&srchWord=&srchTp=7&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=3. Accessed 13 Oct 2021.
- 109.National Medical Products Administration (NMPA). Main responsibilities of the National Drug Administration. 2022. https://www.nmpa.gov.cn/jggk/jgzhn/zhyzhz/index.html. Accessed 15 Nov 2022.
- 110.CENTER FOR DRUG EVALUATION (CDE). Role of the center. https://www.cde.org.cn/main/jgzn/contentpage/fc528384dbd9f6691ff56f7e1167b4ad. Accessed 25 Sept 2022.
- 111.State Adminitration for Market Regulation. Measures for the administration of drug registration. 2020. https://www.samr.gov.cn/zw/zfxxgk/fdzdgknr/fgs/art/2023/art_3275cb2a929d4c34ac8c0421b2a9c257.html. Accessed 9 Feb 2023.
- 112.Lu J, Xu L, Wei W, He W. Advanced therapy medicinal products in China: regulation and development. MedComm (2020). 2023; 4(3):e251. 10.1002/mco2.251. [DOI] [PMC free article] [PubMed]
- 113.National medical Products Administration (NMPA). Procedures for review and approval of applications for conditional marketing authorization of medicinal products (Trial Implementation) (draft for soliciting comments). 2023. https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjyp/20230825104212129.html. Accessed 15 Feb 2024.
- 114.National Medical Products Administration (NMPA). Drug Administration Law. 2019. https://www.nmpa.gov.cn/xxgk/fgwj/flxzhfg/20190827083801685.html. Accessed 27 Aug 2022.
- 115.National Medical Products Administration (NMPA). Announcement of the State Food and Drug Administration on the issuance of the “Quality Management Standards for Pharmacovigilance"”(No. 65 of 2021). 2021. https://www.nmpa.gov.cn/yaopin/ypfgwj/ypfggzwj/20210513151827179.html. Accessed 13 May 2021.
- 116.Center for Drug Evaluation (CDE). Technical guidelines for long-term follow-up clinical studies of gene therapy products. 2021. https://www.cde.org.cn/main/news/viewInfoCommon/c9de887410ddcc291ce5a1c039a241c6. Accessed 3 Dec 2021.
- 117.Center for Drug Evaluation (CDE). Technical guidelines for clinical risk management plan of chimeric antigen receptor T cell (CAR-T) Therapeutic Products. 2022. https://www.cde.org.cn/main/news/viewInfoCommon/574e71202540d2b38cf34dfeb5673a86. Accessed 29 Jan 2022.
- 118.Center for Drug Evaluation (CDE). Guidance for writing a clinical risk management plan (for Trial Implementation). 2022. https://www.cde.org.cn/main/news/viewInfoCommon/77e34e30c7141b2770ddd6f80e80f9ff. Accessed 6 Jan 2022.
- 119.National Medical Products Administration (NMPA). Guidelines for pharmacovigilance examination. 2022. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20220415102743184.html. Accessed 12 Sept 2023.
- 120.QVigilance. a guide to the US FDA safety requirements for pharmacovigilance. 2019. https://www.qvigilance.com/blog/usa-fda-safety-requirements-pharmacovigilance. Accessed 17 Feb 2024.
- 121.European Medicines Agency (EMA). Ebvallo. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/ebvallo. Accessed 9 Sept 2023.
- 122.European Medicines Agency (EMA). Breyanzi. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/breyanzi. Accessed 9 Sept 2023.
- 123.European Medicines Agency (EMA). Roctavian. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/roctavian-0. Accessed 1 Feb 2023.
- 124.European Medicines Agency (EMA). Upstaza. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/upstaza. Accessed 1 Feb 2023.
- 125.European Medicines Agency (EMA). Carvykti. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/carvykti. Accessed 1 Feb 2023.
- 126.European Medicines Agency (EMA). Abecma. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/abecma. Accessed 21 Dec 2022.
- 127.European Medicines Agency (EMA). Skysona. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/skysona. Accessed 1 Mar 2023.
- 128.European Medicines Agency (EMA). Libmeldy. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/libmeldy. Accessed 1 Feb 2023.
- 129.European Medicines Agency (EMA). Tecartus. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/tecartus. Accessed 30 Jan 2023.
- 130.European Medicines Agency (EMA). Zolgensma. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma. Accessed 17 Feb 2023.
- 131.European Medicines Agency (EMA). Zynteglo. 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/zynteglo. Accessed 30 Nov 2022.
- 132.European Medicines Agency (EMA). Luxturna. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/luxturna. Accessed 19 Aug 2022.
- 133.European Medicines Agency (EMA). Yescarta. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta. Accessed 30 Jan 2023.
- 134.European Medicines Agency (EMA). Kymriah. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah. Accessed 7 Nov 2022.
- 135.European Medicines Agency (EMA). Alofisel. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel. Accessed 12 Jan 2023.
- 136.European Medicines Agency (EMA). Spherox. 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/spherox. Accessed 16 Jan 2023.
- 137.European Medicines Agency (EMA). Strimvelis. 2016. https://www.ema.europa.eu/en/medicines/human/EPAR/strimvelis. Accessed 1 Aug 2022.
- 138.European Medicines Agency (EMA). Zalmoxis. 2016. https://www.ema.europa.eu/en/medicines/human/EPAR/zalmoxis. Accessed 14 Feb 2020.
- 139.European Medicines Agency (EMA). Imlygic. 2015. https://www.ema.europa.eu/en/medicines/human/EPAR/imlygic. Accessed 15 Dec 2022.
- 140.European Medicines Agency (EMA). Holoclar. 2015. https://www.ema.europa.eu/en/medicines/human/EPAR/holoclar. Accessed 6 Jan 2022.
- 141.European Medicines Agency (EMA). Provenge. 2013. https://www.ema.europa.eu/en/medicines/human/EPAR/provenge. Accessed 19 May 2015.
- 142.European Medicines Agency (EMA). Maci. 2013. https://www.ema.europa.eu/en/medicines/human/EPAR/maci. Accessed 5 July 2018.
- 143.European Medicines Agency (EMA). Glybera. 2012. https://www.ema.europa.eu/en/medicines/human/EPAR/glybera. Accessed 30 Oct 2017.
- 144.European Medicines Agency (EMA). Chondrocelect. 2009. https://www.ema.europa.eu/en/medicines/human/EPAR/chondrocelect. Accessed 28 May 2019.
- 145.European Medicines Agency (EMA). Casgevy. 2023. https://www.ema.europa.eu/en/documents/assessment-report/casgevy-epar-public-assessment-report_en.pdf. Accessed 21 June 2024.
- 146.US Food and Drug Administration (FDA). LANTIDRA. 2023. https://www.fda.gov/vaccines-blood-biologics/lantidra. Accessed 10 Sept 2023.
- 147.US Food and Drug Administration (FDA). ROCTAVIAN. 2023. US Food and Drug Administration (FDA). Accessed 10 Sept 2023.
- 148.US Food and Drug Administration (FDA). ELEVIDYS. 2023. https://www.fda.gov/vaccines-blood-biologics/tissue-tissue-products/elevidys. Accessed 10 Sept 2023.
- 149.US Food and Drug Administration (FDA). VYJUVEK. 2023. https://www.fda.gov/vaccines-blood-biologics/vyjuvek. Accessed 10 Sept 2023.
- 150.US Food and Drug Administration (FDA). ADSTILADRIN. 2022. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/adstiladrin. Accessed 7 Jan 2023.
- 151.US Food and Drug Administration (FDA). HEMGENIX. 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/hemgenix. Accessed 8 Sept 2023.
- 152.US Food and Drug Administration (FDA). SKYSONA. 2022. https://www.fda.gov/vaccines-blood-biologics/skysona. Accessed 25 Oct 2022.
- 153.US Food and Drug Administration (FDA). ZYNTEGLO. 2022. https://www.fda.gov/vaccines-blood-biologics/zynteglo. Accessed 7 Jan 2023.
- 154.US Food and Drug Administration (FDA). CARVYKTI. 2022. https://www.fda.gov/vaccines-blood-biologics/carvykti. Accessed 7 Jan 2023.
- 155.US Food and Drug Administration (FDA). RETHYMIC. 2021. https://www.fda.gov/vaccines-blood-biologics/rethymic. Accessed 4 Nov 2021.
- 156.US Food and Drug Administration (FDA). STRATAGRAFT. 2021. https://www.fda.gov/vaccines-blood-biologics/stratagraft. Accessed 13 July 2021.
- 157.US Food and Drug Administration (FDA). ABECMA (idecabtagene vicleucel). 2021. https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel. Accessed January 21, 2021.
- 158.US Food and Drug Administration (FDA). BREYANZI (lisocabtagene maraleucel). 2021. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/breyanzi-lisocabtagene-maraleucel. Accessed July 1, 2022.
- 159.US Food and Drug Administration (FDA). TECARTUS (brexucabtagene autoleucel). 2020. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus-brexucabtagene-autoleucel. Accessed April 13, 2022.
- 160.US Food and Drug Administration (FDA). ZOLGENSMA. 2019. https://www.fda.gov/vaccines-blood-biologics/zolgensma. Accessed February 16, 2023.
- 161.US Food and Drug Administration (FDA). KYMRIAH (tisagenlecleucel). 2018. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel. Accessed July 7, 2022.
- 162.US Food and Drug Administration (FDA). LUXTURNA. 2017. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/luxturna. Accessed June 9, 2022.
- 163.US Food and Drug Administration (FDA). YESCARTA (axicabtagene ciloleucel). 2017. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel. Accessed November 4, 2022.
- 164.US Food and Drug Administration (FDA). MACI (Autologous Cultured Chondrocytes on a Porcine Collagen Membrane). 2016. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/maci-autologous-cultured-chondrocytes-porcine-collagen-membrane. Accessed June 30, 2021.
- 165.US Food and Drug Administration (FDA). IMLYGIC. 2015. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/imlygic. Accessed February 15, 2023.
- 166.US Food and Drug Administration (FDA). GINTUIT (allogeneic cultured keratinocytes and fibroblasts in bovine collagen). 2012. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/gintuit-allogeneic-cultured-keratinocytes-and-fibroblasts-bovine-collagen. Accessed 20 Feb 2018.
- 167.US Food and Drug Administration (FDA). PROVENGE (sipuleucel-T). 2010. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/provenge-sipuleucel-t. Accessed 28 May 2019.
- 168.US Food and Drug Administration (FDA). BEQVEZ. 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/beqvez. Accessed 21 June 2024.
- 169.U.S. Food & Drug Administration (FDA). LENMELDY. 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/lenmeldy. Accessed 21 June 2024.
- 170.U.S. Food & Drug Administration (FDA). AMTAGVI. 2024. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/amtagvi. Accessed 21 June 2024.
- 171.U.S. Food & Drug Administration (FDA). CASGEVY. 2023. https://www.fda.gov/vaccines-blood-biologics/casgevy. Accessed 21 June 2024.
- 172.U.S. Food & Drug Administration (FDA). LYFGENIA. 2023. https://www.fda.gov/vaccines-blood-biologics/lyfgenia. Accessed 21 June 2024.
- 173.Pharmaceuticals and Medical Device Agency (PMDA). Vyznova. 2023. https://www.pmda.go.jp/files/000263641.pdf. Accessed 10 Sept 2023.
- 174.Pharmaceuticals and Medical Device Agency (PMDA). JACEMIN. 2023. https://www.pmda.go.jp/files/000264109.pdf. Accessed 10 Sept 2023.
- 175.Pharmaceuticals and Medical Device Agency (PMDA). Carvykti. 2022. https://www.pmda.go.jp/files/000252610.pdf. Accessed 3 Aug 2022.
- 176.Pharmaceuticals and Medical Device Agency (PMDA). Abecma. 2021. https://www.pmda.go.jp/files/000250563.pdf. Accessed 6 Dec 2021.
- 177.Pharmaceuticals and Medical Device Agency (PMDA). Sakracy. 2021. https://www.pmda.go.jp/files/000247920.pdf. Accessed 6 Dec 2021.
- 178.Pharmaceuticals and Medical Device Agency (PMDA). Alofisel. 2021. https://www.pmda.go.jp/files/000246420.pdf. Accessed 6 Sept 2021.
- 179.Pharmaceuticals and Medical Device Agency (PMDA). Delytact. 2021. https://www.pmda.go.jp/files/000242808.pdf. Accessed 24 May 2021.
- 180.Pharmaceuticals and Medical Device Agency (PMDA). Ocural. 2021. https://www.pmda.go.jp/files/000246286.pdf. Accessed 24 May 2021.
- 181.Pharmaceuticals and Medical Device Agency (PMDA). Breyanzi. 2021. https://www.pmda.go.jp/files/000243465.pdf. Accessed 5 Mar 2021.
- 182.Pharmaceuticals and Medical Device Agency (PMDA). YESCARTA. 2021. https://www.pmda.go.jp/files/000243495.pdf. Accessed 3 Dec 2021.
- 183.Pharmaceuticals and Medical Device Agency (PMDA). ZOLGENSMA. 2020. https://www.pmda.go.jp/files/000237139.pdf. Accessed 26 Feb 2020.
- 184.Pharmaceuticals and Medical Device Agency (PMDA). Nepic. 2020. https://www.pmda.go.jp/files/000237511.pdf. Accessed 7 Feb 2022.
- 185.Pharmaceuticals and Medical Device Agency (PMDA). Collategene. 2019. https://www.pmda.go.jp/files/000231386.pdf. Accessed 20 Feb 2019.
- 186.Pharmaceuticals and Medical Device Agency (PMDA). Kymriah. 2018. https://www.pmda.go.jp/files/000231651.pdf. Accessed Feb 2019.
- 187.Pharmaceuticals and Medical Device Agency (PMDA). Stemirac. 2018. https://www.pmda.go.jp/files/000231946.pdf. Accessed 21 Nov 2019.
- 188.Pharmaceuticals and Medical Device Agency (PMDA). JACE. 2016. https://www.pmda.go.jp/files/000223079.pdf. Accessed 6 Aug 2022.
- 189.Pharmaceuticals and Medical Device Agency (PMDA). HeartSheet. 2015. https://www.pmda.go.jp/files/000215222.pdf. Accessed 8 Sept 2022.
- 190.Pharmaceuticals and Medical Device Agency (PMDA). Temcell. 2015. https://www.pmda.go.jp/files/000215658.pdf. Accessed 2 Sept 2022.
- 191.Pharmaceuticals and Medical Device Agency (PMDA). JACC. 2012. https://www.pmda.go.jp/files/000229937.pdf. Accessed 6 Sept 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1: Tables S1-S12. Table S1 – Methods for obtaining the lists of marketed CGTPs in the EU, the US, Japan, South Korea and China. Table S2 –The guidelines of RMP in the EU. Table S3 – Definitions of the key elements related to RMP. Table S4 – The guidelines of REMS in the US. Table S5 – The regulatory frameworks of CGTPs in the EU, the US, Japan, China, and South Korea. Table S6 – The marketed CGTPs in the EU, the US, Japan, South Korea and China. Table S7 – The support mechanisms for the accelerated marketing of CGTPs in the EU. Table S8 – The support mechanisms for the accelerated marketing of CGTPs in the US. Table S9 – The support mechanisms for the accelerated marketing of CGTPs in Japan. Table S10 – Important identified risks of CGTPs approved in the EU. Table S11 – Important identified risks of CGTPs approved in the US. Table S12 – Other related regulations and guidelines of PMS for CGTPs in different regions.
Data Availability Statement
The data sources are from the official website of FDA, EMA, PMDA, NMPA and MFDS. The datasets used and/or analyzed during the current study are available from the corresponding author on request.