Abstract
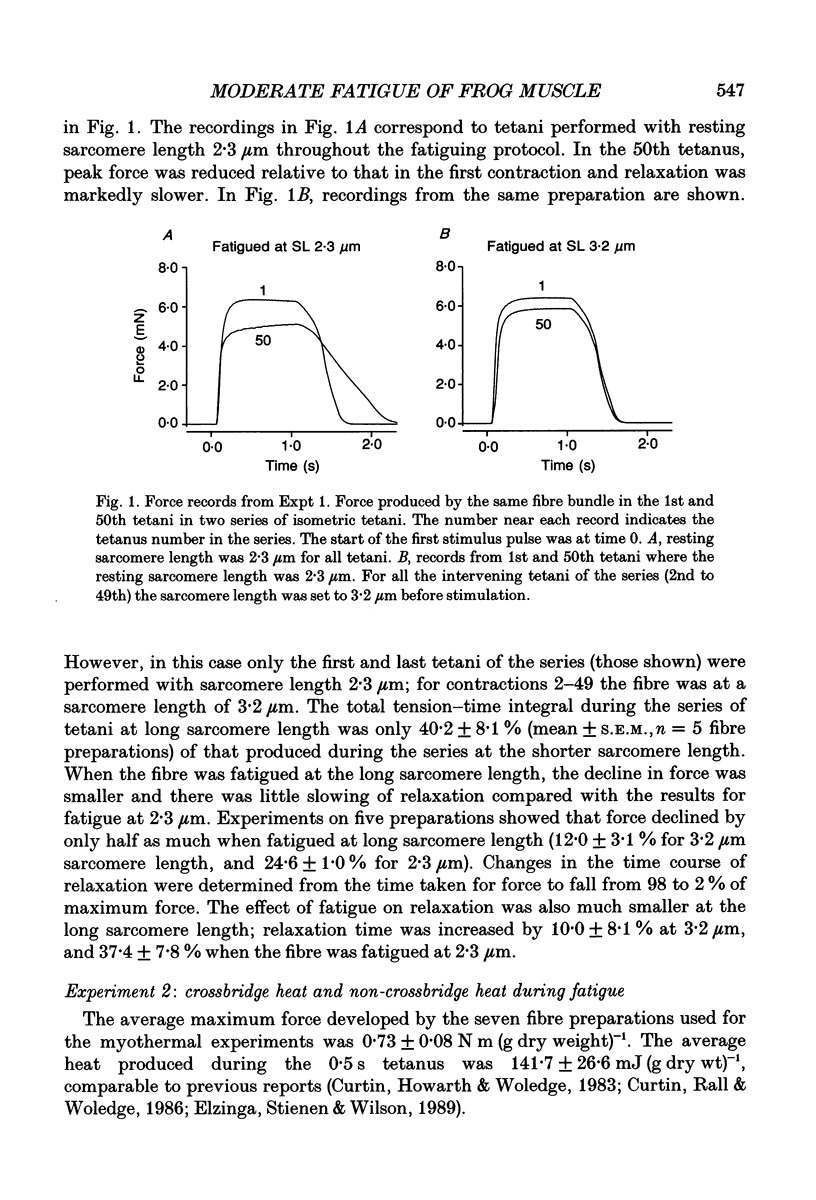
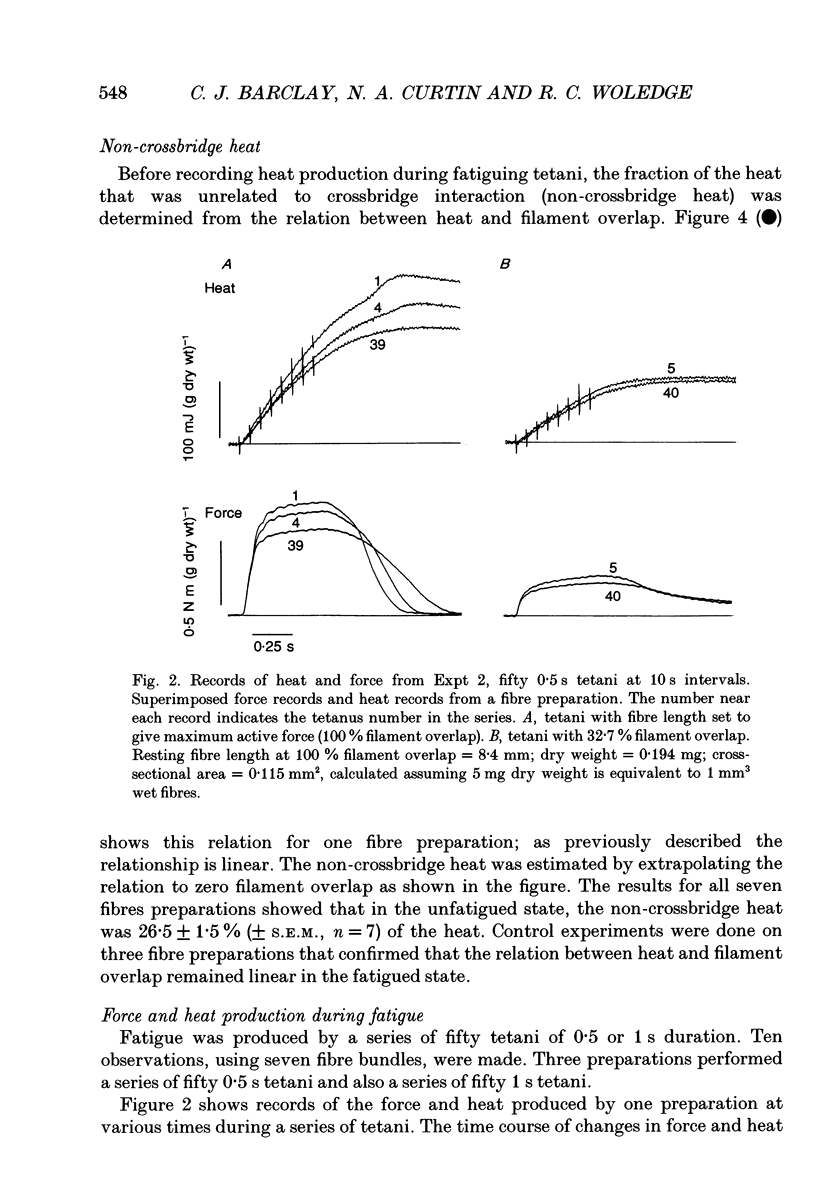
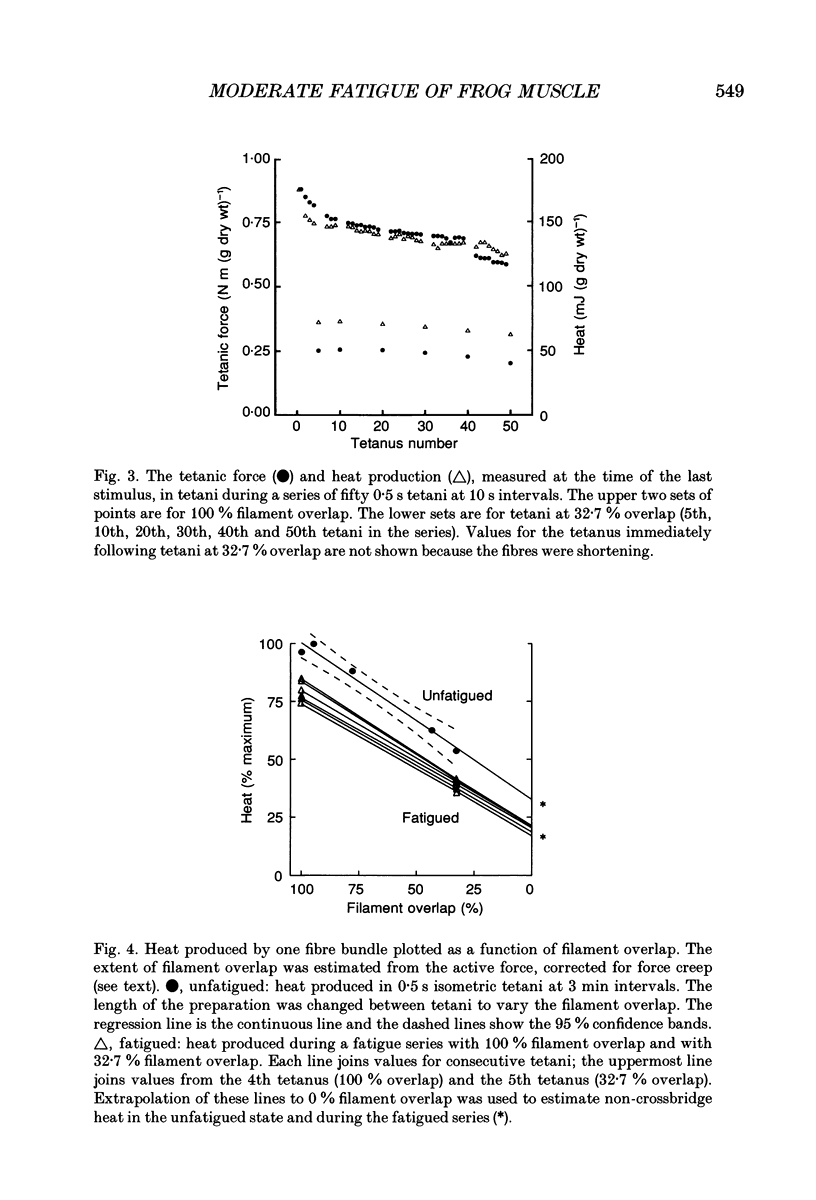
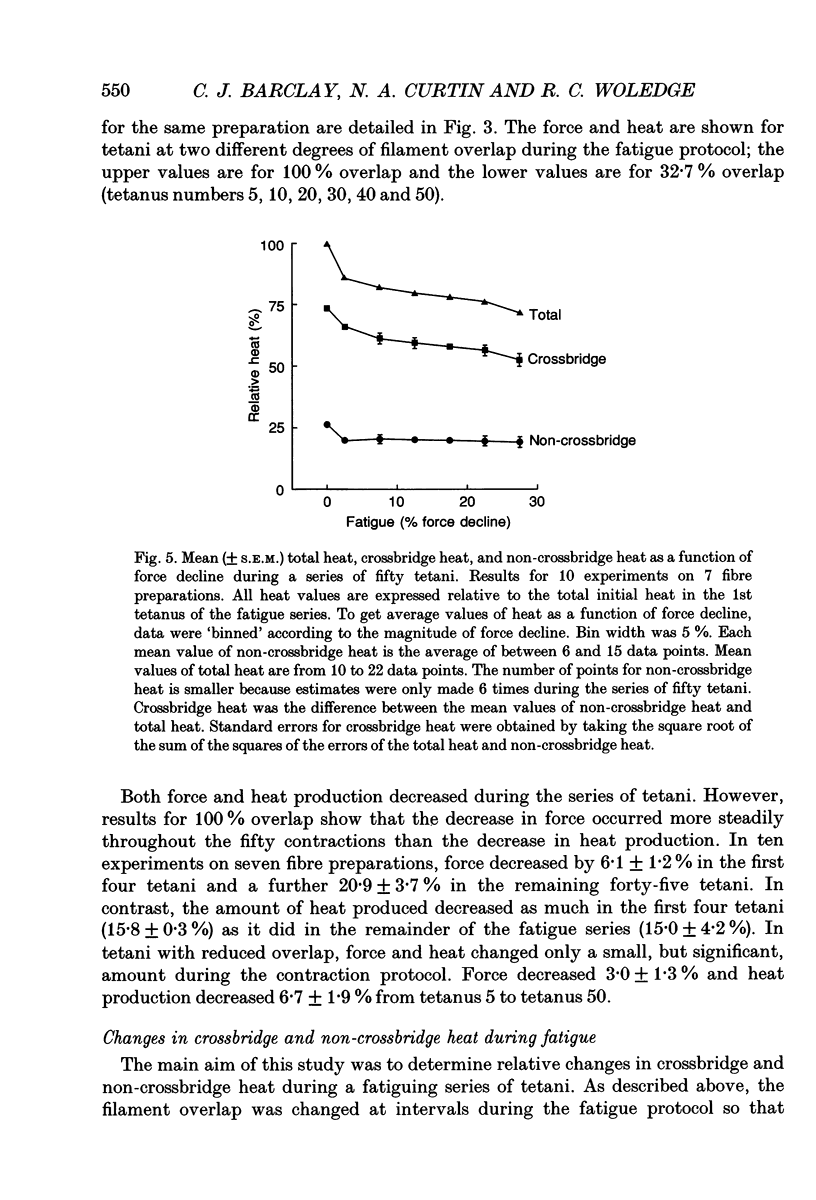
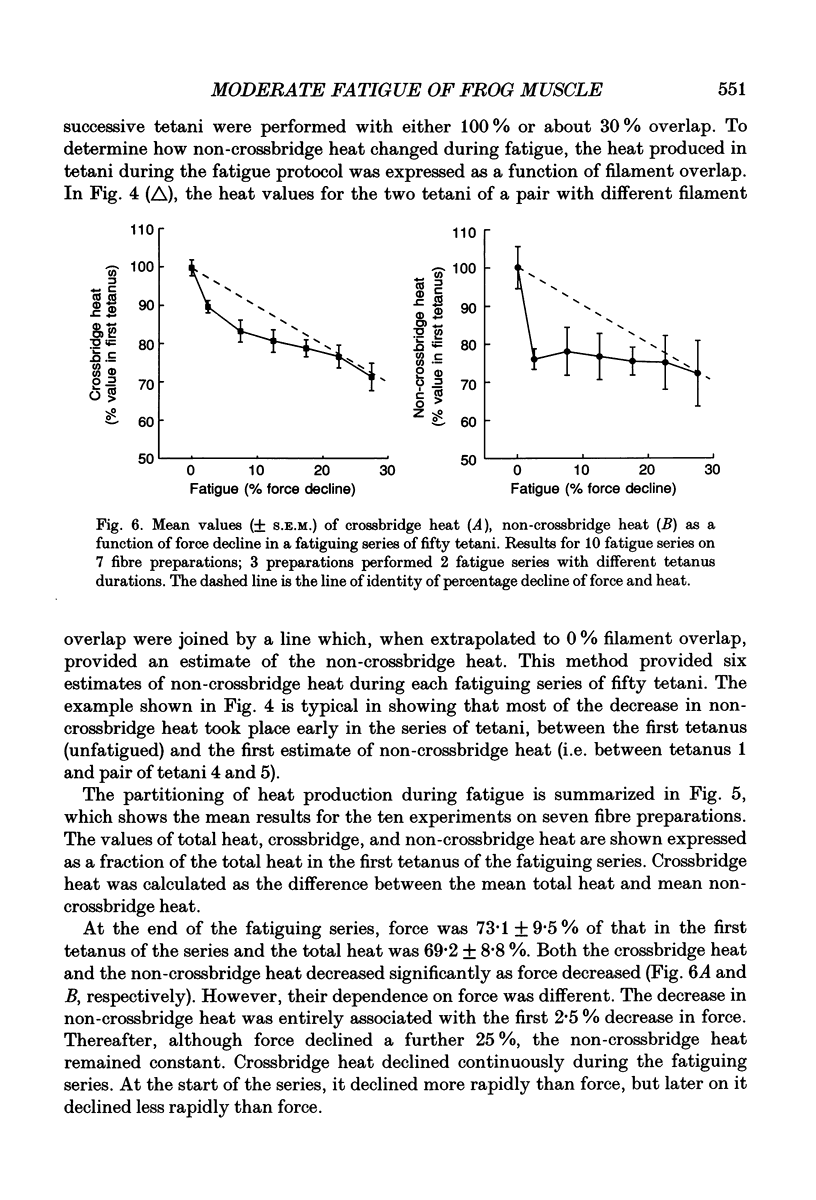
1. The effect of sarcomere length (SL) during a fatiguing series of isometric tetani of frog muscle fibres was investigated. Tetani at 2.3 microns SL were more fatiguing than tetani at 3.2 microns SL, in that force declined twice as much as relaxation became much slower. 2. In a second set of experiments the force and heat production were measured during a series of fatiguing tetani. Heat was separated into two components: (a) crossbridge heat which is dependent on filament overlap and interaction, and (b) non-crossbridge heat which is independent of filament overlap and due to Ca2+ turnover. 3. In a series of fifty tetani, force, crossbridge heat and non-crossbridge heat each declined by 25-30% of its initial value. 4. The 25% reduction in non-crossbridge heat occurred completely during the first few tetani of the fatiguing series while force declined by less than 3%. This may be due to a reduction in Ca2+ binding to parvalbumin and to Ca2+ remaining bound during the remainder of the fatigue series. 5. After the first few tetani of the fatigue series the non-crossbridge heat hardly changed as force declined by a further 25% of its initial value. Continuing reduction of force with constant Ca2+ turnover indicates a reduction in the Ca2+ sensitivity of the filaments, and/or a reduction in the average force per attached crossbridge. 6. At the start of the fatiguing series, as force declines by about 7.5% there is a much larger decline of crossbridge heat (17%). The reason for this is unknown. Later in the series, force declined more rapidly than heat. This is probably due to a progressive accumulation of inorganic phosphate which acts by depressing force more than it depresses ATP breakdown.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989 Aug;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowater R., Sleep J. Demembranated muscle fibers catalyze a more rapid exchange between phosphate and adenosine triphosphate than actomyosin subfragment 1. Biochemistry. 1988 Jul 12;27(14):5314–5323. doi: 10.1021/bi00414a055. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Rall J. A., Wilson M. G., Woledge R. C. Absolute values of myothermic measurements on single muscle fibres from frog. J Muscle Res Cell Motil. 1986 Aug;7(4):327–332. doi: 10.1007/BF01753653. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Woledge R. C. Heat production by single fibres of frog muscle. J Muscle Res Cell Motil. 1983 Apr;4(2):207–222. doi: 10.1007/BF00712031. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Kometani K., Woledge R. C. Effect of intracellular pH on force and heat production in isometric contraction of frog muscle fibres. J Physiol. 1988 Feb;396:93–104. doi: 10.1113/jphysiol.1988.sp016952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Lou F. Changes in force and stiffness induced by fatigue and intracellular acidification in frog muscle fibres. J Physiol. 1990 May;424:133–149. doi: 10.1113/jphysiol.1990.sp018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Elzinga G., Stienen G. J., Wilson M. G. Isometric force production before and after chemical skinning in isolated muscle fibres of the frog Rana temporaria. J Physiol. 1989 Mar;410:171–185. doi: 10.1113/jphysiol.1989.sp017527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Kean C. J. Skeletal muscle energetics and metabolism. Annu Rev Physiol. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- Homsher E., Mommaerts W. F., Ricchiuti N. V., Wallner A. Activation heat, activation metabolism and tension-related heat in frog semitendinosus muscles. J Physiol. 1972 Feb;220(3):601–625. doi: 10.1113/jphysiol.1972.sp009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol. 1991 Sep;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R., Moss R. L. Sarcomere length non-uniformity in relation to tetanic responses of stretched skeletal muscle fibres. Proc R Soc Lond B Biol Sci. 1978 Jan 24;200(1138):109–116. doi: 10.1098/rspb.1978.0009. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Tanokura M., Yamada K. Phosphorus nuclear magnetic resonance studies on the effect of duration of contraction in bull-frog skeletal muscles. J Physiol. 1988 Dec;407:243–261. doi: 10.1113/jphysiol.1988.sp017413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. A., Westerblad H., Allen D. G. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol. 1991 Feb;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulieri L. A., Luhr G., Trefry J., Alpert N. R. Metal-film thermopiles for use with rabbit right ventricular papillary muscles. Am J Physiol. 1977 Nov;233(5):C146–C156. doi: 10.1152/ajpcell.1977.233.5.C146. [DOI] [PubMed] [Google Scholar]
- Rall J. A. Effects of deuterium oxide on mechanics and energetics of skeletal muscle contraction. Am J Physiol. 1980 Sep;239(3):C105–C111. doi: 10.1152/ajpcell.1980.239.3.C105. [DOI] [PubMed] [Google Scholar]
- Rall J. A. Effects of temperature on tension, tension-dependent heat, and activation heat in twitches of frog skeletal muscle. J Physiol. 1979 Jun;291:265–275. doi: 10.1113/jphysiol.1979.sp012811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall J. A. Energetics of Ca2+ cycling during skeletal muscle contraction. Fed Proc. 1982 Feb;41(2):155–160. [PubMed] [Google Scholar]
- Smith I. C. Energetics of activation in frog and toad muscle. J Physiol. 1972 Feb;220(3):583–599. doi: 10.1113/jphysiol.1972.sp009724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lee J. A., Lännergren J., Allen D. G. Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol. 1991 Aug;261(2 Pt 1):C195–C209. doi: 10.1152/ajpcell.1991.261.2.C195. [DOI] [PubMed] [Google Scholar]


