Abstract
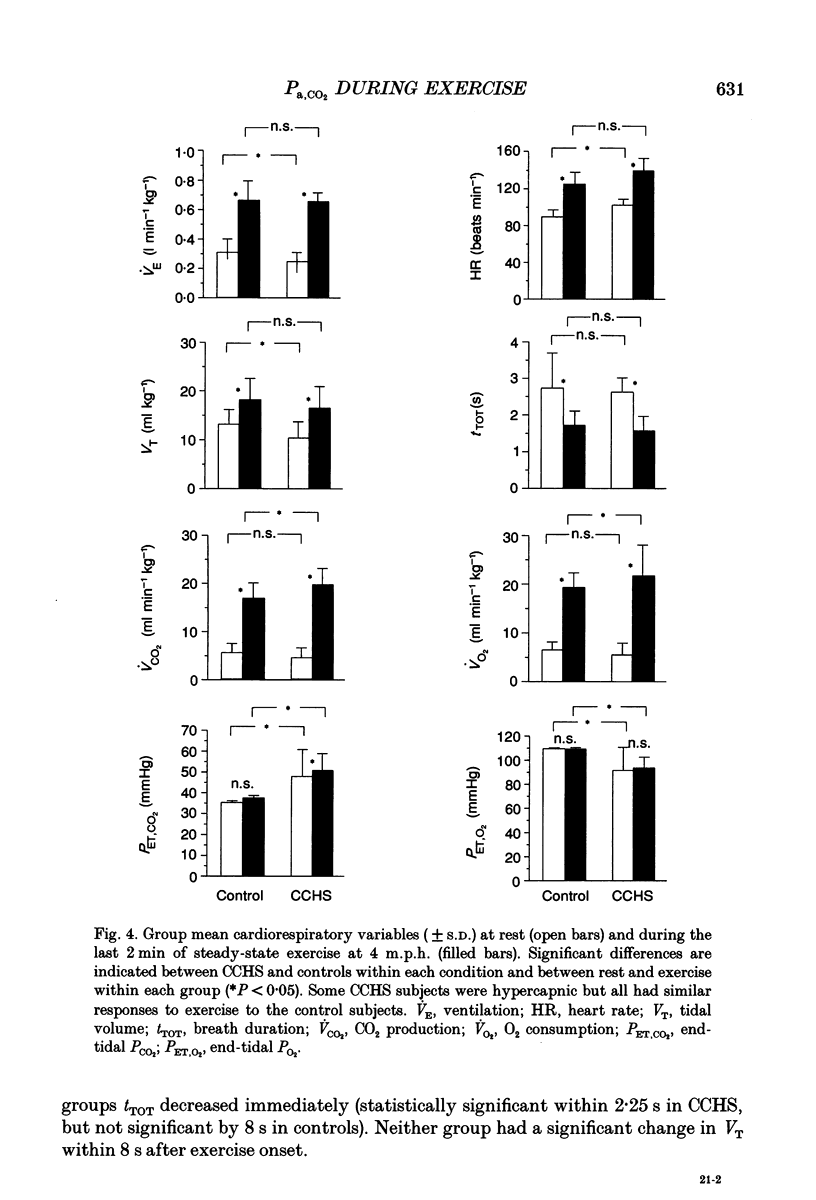
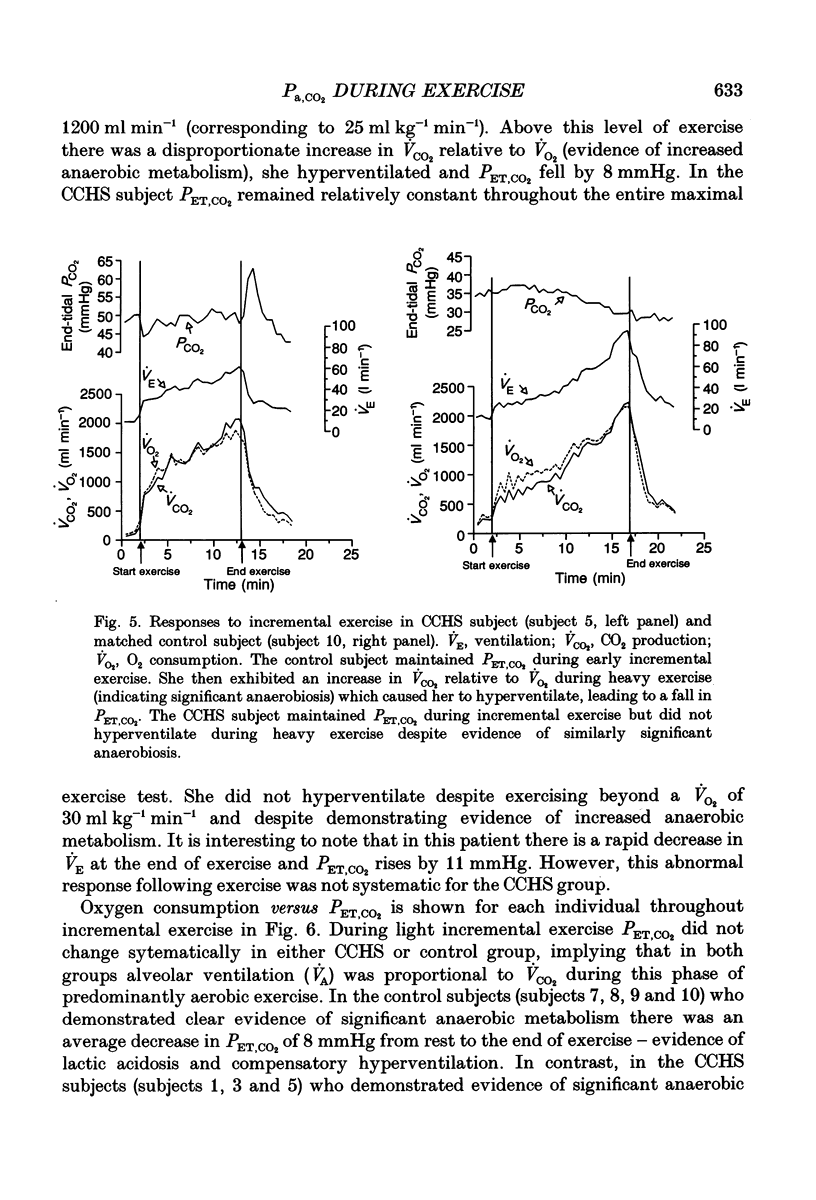
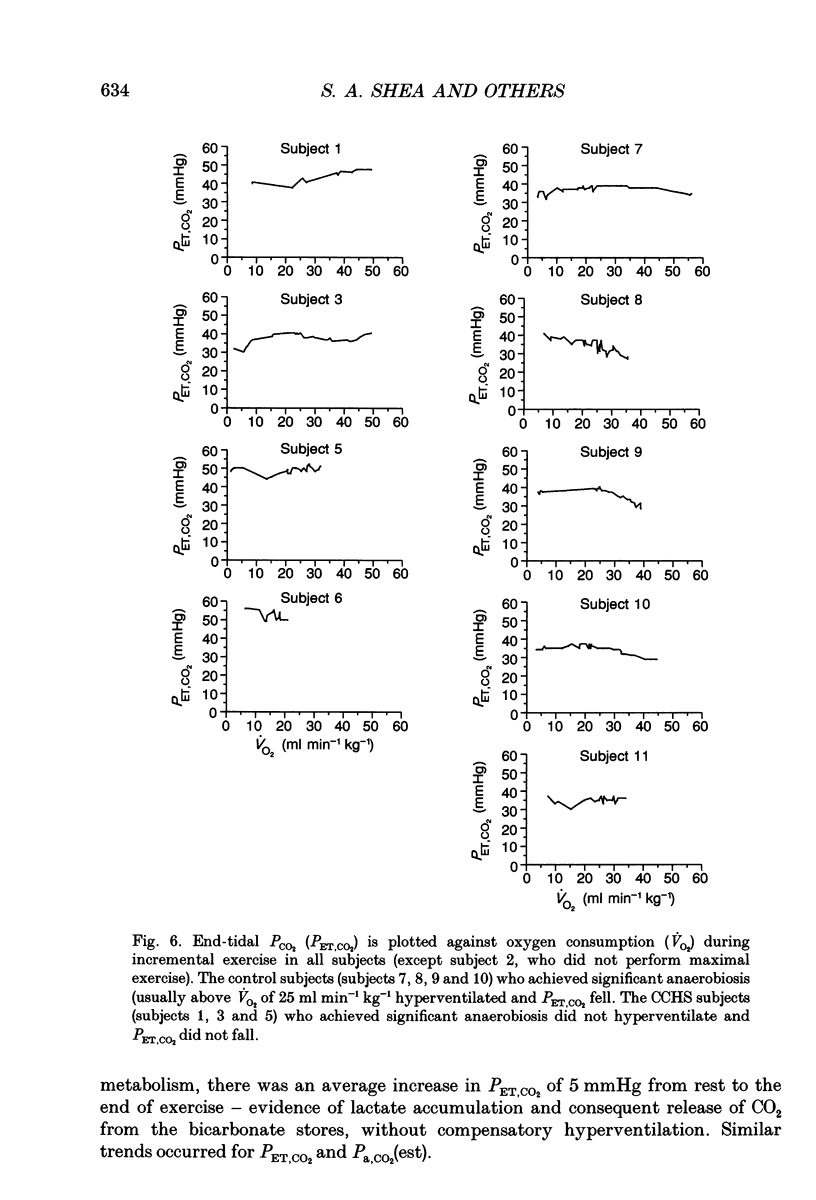
1. In healthy humans during aerobic exercise ventilation increases and mean arterial PCO2 usually remains constant over a wide range of CO2 production. 2. Congenital central hypoventilation syndrome (CCHS) is associated with ineffective chemoreceptor regulation of breathing and severe hypoventilation during sleep (requiring mechanical ventilation) reflecting abnormalities in the brainstem respiratory complex or its chemoreceptor input. Such patients can have adequate spontaneous ventilation during resting wakefulness and participate in normal activities. 3. If children with CCHS have normal ventilatory responses to exercise then chemoreceptors are not necessary for this ventilatory response or the resultant control of Pa,CO2 during exercise. We studied five children with CCHS (aged 8-17 years) with abnormally low ventilatory responses to steady-state increased end-tidal PCO2 (< 9 ml min-1 kg-1 mmHg-1) and five age-matched controls. 4. Depth and rate of breathing, end-tidal PCO2, end-tidal PO2, CO2 production, O2 utilization and heart rate were monitored during the following conditions: whilst subjects stood at rest; following the onset of treadmill exercise (4 m.p.h.); during steady-state exercise (4 m.p.h.); during an incremental maximal exercise test; and during recovery from exercise. 5. There were no significant differences in the ventilatory responses between CCHS subjects and controls during the onset of treadmill exercise, in the dynamic response in achieving the steady-state exercise, during steady-state exercise, in the recovery from steady-state exercise, or during incremental exercise (up to the point of presumed blood lactate accumulation, as indicated by gas exchange criteria). There was a very small mean increase in PCO2 in both groups during steady-state exercise (controls 1.4 mmHg; CCHS 2.2 mmHg). 6. The only differences which emerged between groups were (i) slightly more variability in PCO2 in the CCHS group during steady-state exercise, and (ii) the CCHS subjects did not hyperventilate, as the controls did, at exercise levels above the point of presumed blood lactate accumulation. 7. Breath-by-breath coefficient of variation of ventilation was significantly reduced in both groups during steady-state exercise compared to rest. There were no differences between groups in either state. 8. We conclude that chemoreceptors are not necessary for an appropriate ventilatory response to aerobic exercise. Hence, other stimuli, such as afferent information from the exercising limbs or signals related to activation of the motor cortex, can increase alveolar ventilation in close proportion to CO2 production. 9. The lack of hyperventilatory response to blood lactate accumulation during heavy exercise provides good evidence that these CCHS patients have ineffective peripheral chemoreception.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaver W. L., Wasserman K., Whipp B. J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986 Jun;60(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Cummin A. R., Telford R. J., Saunders K. B. Hypoxia following voluntary hyperventilation during exercise in man. Respir Physiol. 1991 May;84(2):199–207. doi: 10.1016/0034-5687(91)90117-2. [DOI] [PubMed] [Google Scholar]
- Griffiths T. L., Henson L. C., Whipp B. J. Influence of inspired oxygen concentration on the dynamics of the exercise hyperpnoea in man. J Physiol. 1986 Nov;380:387–403. doi: 10.1113/jphysiol.1986.sp016292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. L., Robertson D. G., Kane J. W. Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol Respir Environ Exerc Physiol. 1979 Nov;47(5):954–960. doi: 10.1152/jappl.1979.47.5.954. [DOI] [PubMed] [Google Scholar]
- Kaufman M. P., Rybicki K. J., Waldrop T. G., Ordway G. A. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol Respir Environ Exerc Physiol. 1984 Sep;57(3):644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Maayan C., Carley D. W., Axelrod F. B., Grimes J., Shannon D. C. Respiratory system stability and abnormal carbon dioxide homeostasis. J Appl Physiol (1985) 1992 Mar;72(3):1186–1193. doi: 10.1152/jappl.1992.72.3.1186. [DOI] [PubMed] [Google Scholar]
- Menitove S. M., Rapoport D. M., Epstein H., Sorkin B., Goldring R. M. CO2 rebreathing and exercise ventilatory responses in humans. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):1039–1044. doi: 10.1152/jappl.1984.56.4.1039. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L. Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems. J Appl Physiol (1985) 1986 Oct;61(4):1249–1263. doi: 10.1152/jappl.1986.61.4.1249. [DOI] [PubMed] [Google Scholar]
- Oren J., Kelly D. H., Shannon D. C. Long-term follow-up of children with congenital central hypoventilation syndrome. Pediatrics. 1987 Sep;80(3):375–380. [PubMed] [Google Scholar]
- Oren J., Newth C. J., Hunt C. E., Brouillette R. T., Bachand R. T., Shannon D. C. Ventilatory effects of almitrine bismesylate in congenital central hypoventilation syndrome. Am Rev Respir Dis. 1986 Nov;134(5):917–919. doi: 10.1164/arrd.1986.134.5.917. [DOI] [PubMed] [Google Scholar]
- Paton J. Y., Swaminathan S., Sargent C. W., Keens T. G. Hypoxic and hypercapnic ventilatory responses in awake children with congenital central hypoventilation syndrome. Am Rev Respir Dis. 1989 Aug;140(2):368–372. doi: 10.1164/ajrccm/140.2.368. [DOI] [PubMed] [Google Scholar]
- Rebuck A. S., Jones N. L., Campbell E. J. Ventilatory response to exercise and to CO 2 rebreathing in normal subjects. Clin Sci. 1972 Dec;43(6):861–867. doi: 10.1042/cs0430861. [DOI] [PubMed] [Google Scholar]
- Shannon D. C., Marsland D. W., Gould J. B., Callahan B., Todres I. D., Dennis J. Central hypoventilation during quiet sleep in two infants. Pediatrics. 1976 Mar;57(3):342–346. [PubMed] [Google Scholar]
- Shea S. A., Walter J., Pelley C., Murphy K., Guz A. The effect of visual and auditory stimuli upon resting ventilation in man. Respir Physiol. 1987 Jun;68(3):345–357. doi: 10.1016/s0034-5687(87)80019-1. [DOI] [PubMed] [Google Scholar]
- Solliday N. H., Gaensler E. A., Schwaber J. R., Parker T. F. Impaired central chemoreceptor function and chronic hypoventilation many years following poliomyelitis. Case report. Respiration. 1974;31(2):177–192. doi: 10.1159/000193108. [DOI] [PubMed] [Google Scholar]
- Takano N., Tamura N., Chaki K. Exercise-entrained breathing and non-invasive determination of anaerobic threshold. Respir Physiol. 1991 Dec;86(3):381–392. doi: 10.1016/0034-5687(91)90108-u. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. J., Koyal S. N., Cleary M. G. Effect of carotid body resection on ventilatory and acid-base control during exercise. J Appl Physiol. 1975 Sep;39(3):354–358. doi: 10.1152/jappl.1975.39.3.354. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer D. E., Silvestri J. M., Menzies L. J., Morrow-Kenny A. S., Hunt C. E., Hauptman S. A. Congenital central hypoventilation syndrome: diagnosis, management, and long-term outcome in thirty-two children. J Pediatr. 1992 Mar;120(3):381–387. doi: 10.1016/s0022-3476(05)80901-1. [DOI] [PubMed] [Google Scholar]


