Abstract
1. Single Purkinje cells, enzymatically isolated from rabbit ventricle, were studied under whole-cell voltage clamp and internally perfused with the fluorescent Ca2+ indicator, indo-1 (100 microM). 2. Fast [Ca2+]i transients were elicited by brief depolarizations from a holding voltage of -45 mV and by repolarization from very positive potentials. The peak [Ca2+]i-voltage relation was bell-shaped with a peak around +10 mV. 3. [Ca2+]i transients were completely blocked by the Ca2+ channel antagonist, nisoldipine (10 microM) and were very small when Ca2+ release from the sarcoplasmic reticulum (SR) was prevented by superfusion of cells by caffeine (1 mM) or ryanodine (10 microM). A fast application of caffeine induced a transient increase in [Ca2+]i. These results suggest [Ca2+]i transients are due to Ca(2+)-induced Ca2+ release from the SR. 4. Rate of decline of the [Ca2+]i transient was voltage dependent, suggesting contribution of the Na(+)-Ca2+ exchanger to Ca2+ efflux. At very positive potentials (> +60 mV), Ca2+ influx through the Na(+)-Ca2+ exchanger could be observed. 5. A transient outward current was observed at potentials positive to +10 mV, but only if depolarizing pulses were accompanied by a [Ca2+]i transient. 6. When the amplitude of the [Ca2+]i transient was changed by (1) changes in [Ca2+]o, (2) changes in frequency of depolarization or (3) conditioning prepulses, the amplitude of the outward current changed in the same direction. This suggests activation of the current is dependent on and graded by [Ca2+]i. 7. The outward current was observed in K(+)-free solutions, in the presence of Cs+ and TEA+, and was not blocked by 4-aminopyridine (10 mM). In contrast, DIDS (100 microM) decreased the outward current by 70 +/- 20% (mean +/- S.D., n = 9), without affecting [Ca2+]i. 8. When external Cl- was lowered, the amplitude of the outward current decreased; when internal Cl- was replaced by aspartate, it became apparent at more negative potentials. These interventions strongly suggest the current was carried by Cl-; it can therefore be referred to as a [Ca2+]i-activated Cl- current or ICl(Ca). 9. When ICl(Ca) was maximally activated during a conditioning step, steps to negative potentials revealed inward currents through ICl(Ca) (in symmetrical Cl- solutions). The fully activated I-V relation was linear. 10. ICl(Ca) could be activated at membrane potentials between -80 and +80 mV by a fast application of caffeine (10 mM), inducing Ca2+ release from the SR, demonstrating that ICl(Ca) does not require membrane depolarization or Ca2+ influx through the Ca2+ channel for its activation.(ABSTRACT TRUNCATED AT 400 WORDS)
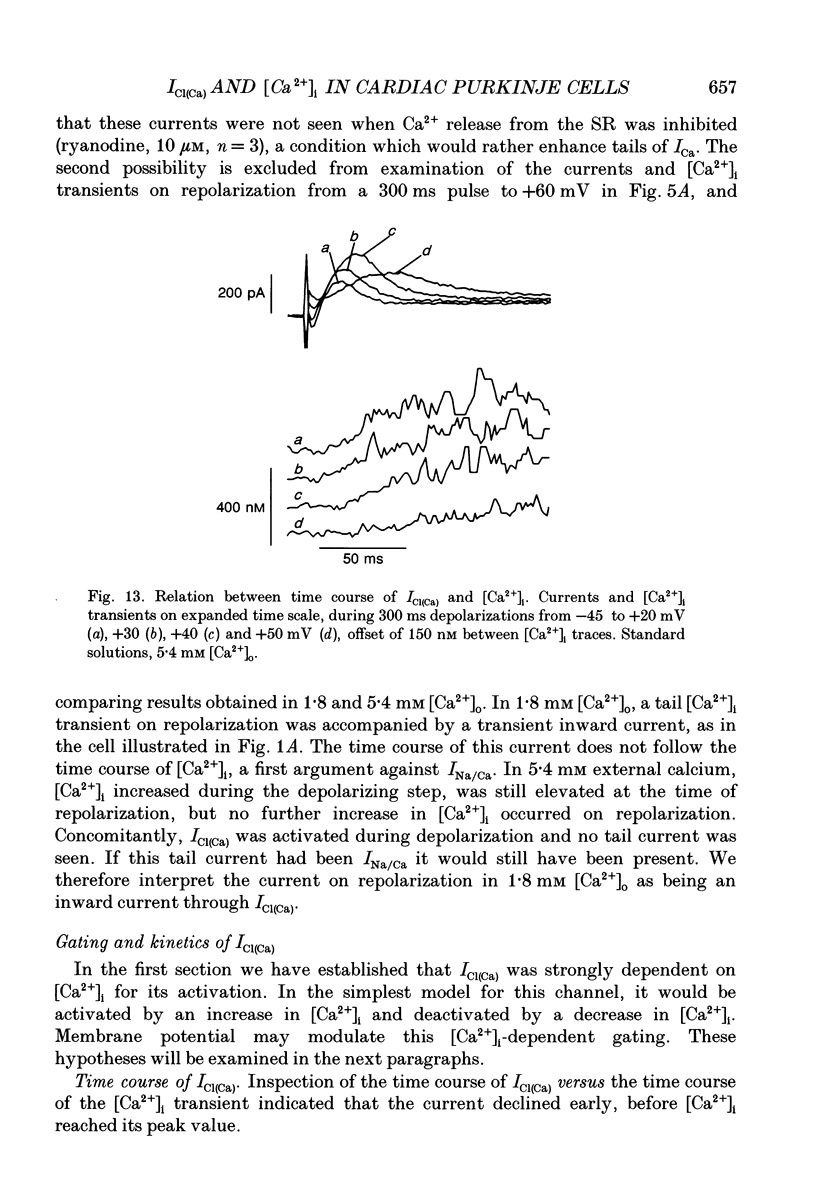
Full text
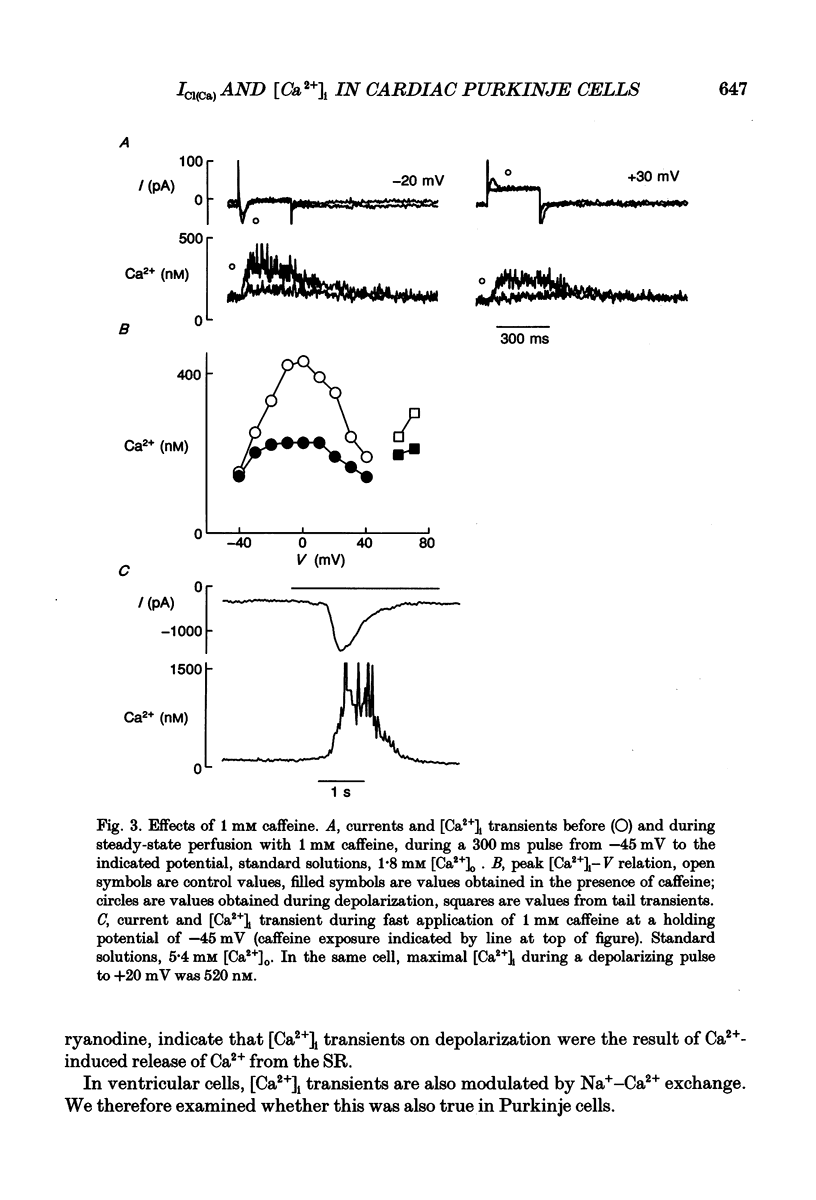
PDF





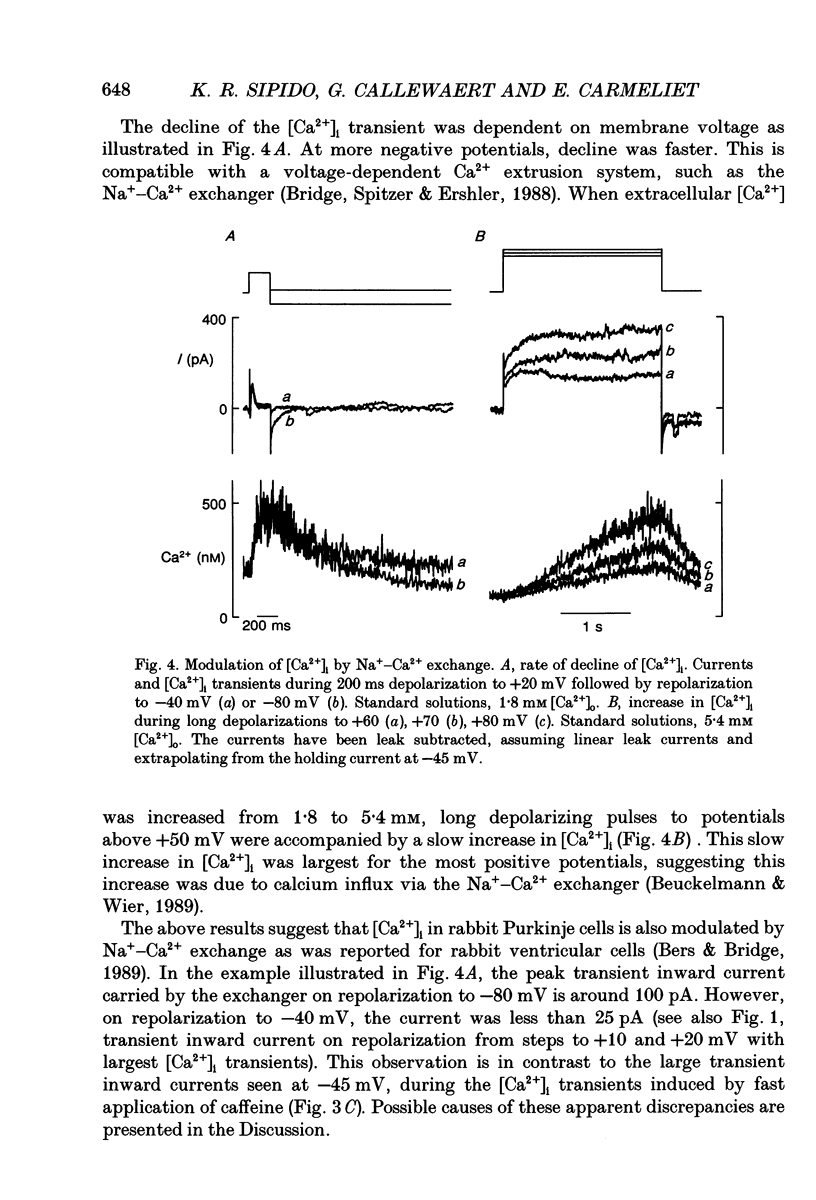












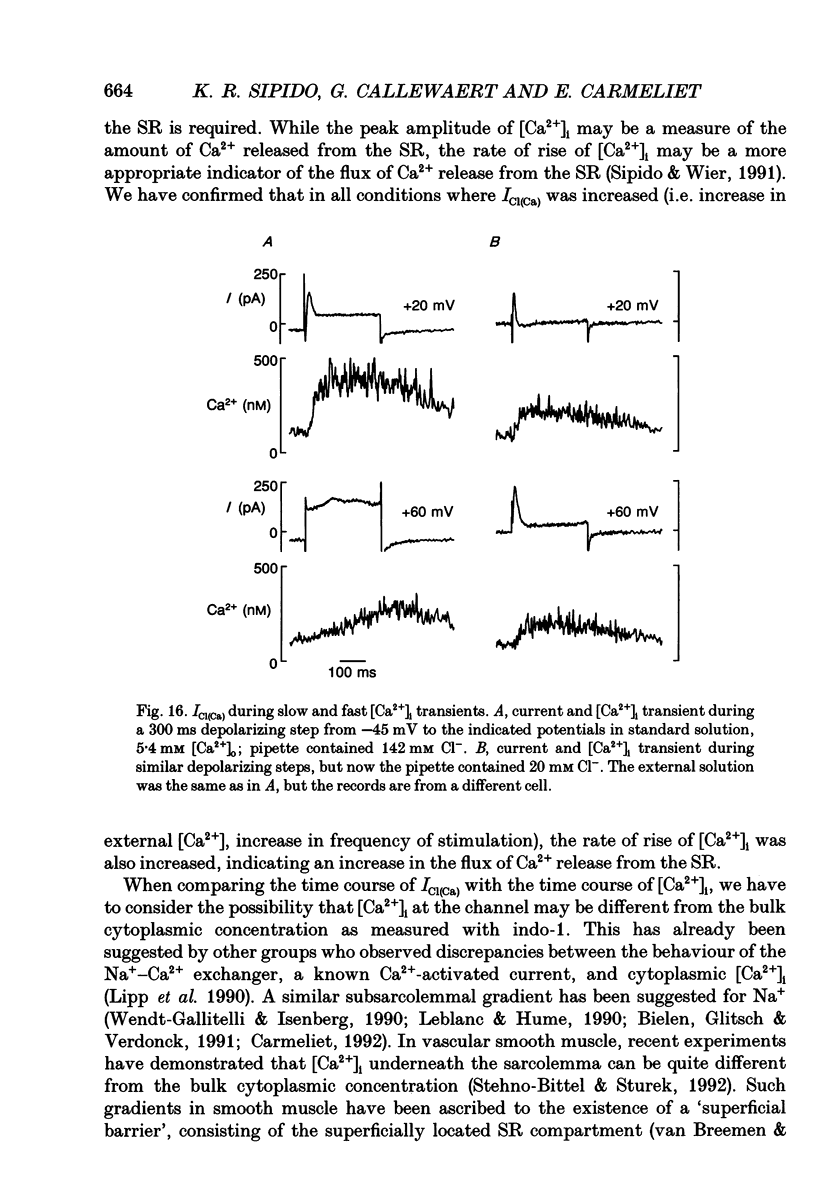



Selected References
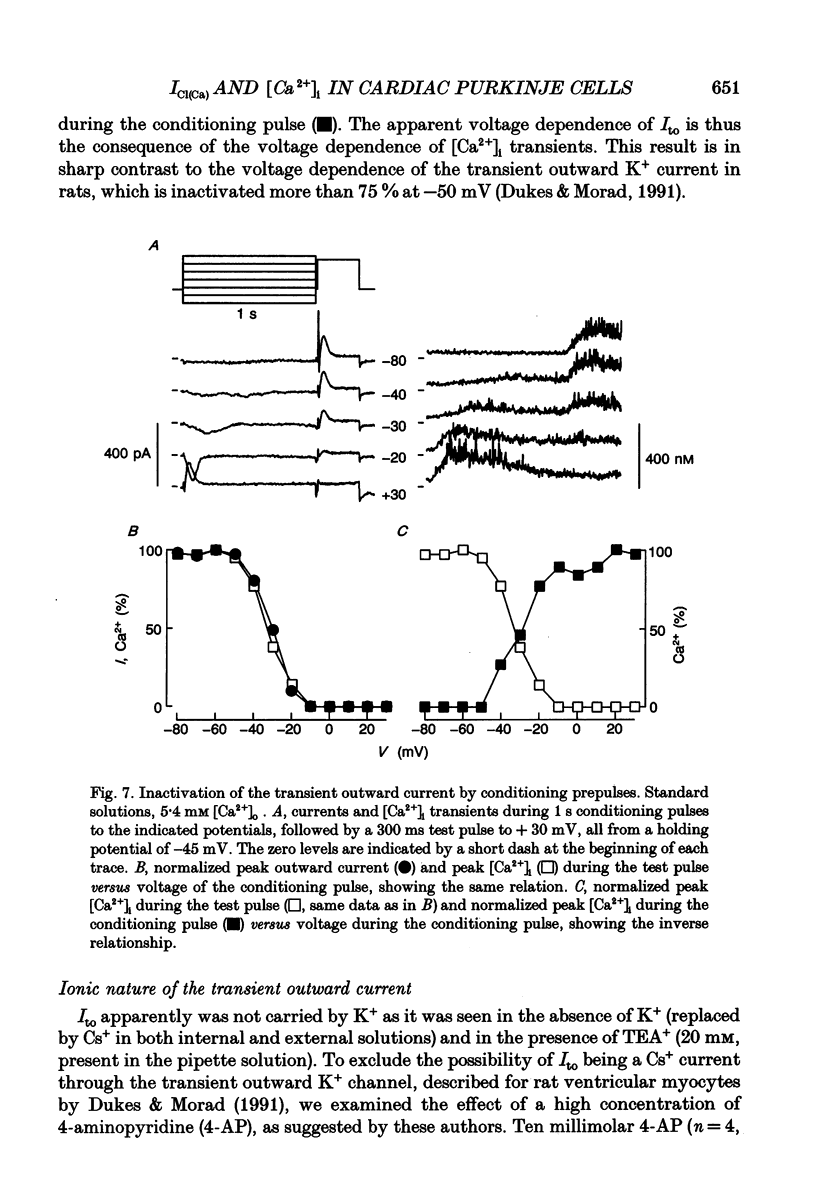
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ Res. 1989 Aug;65(2):334–342. doi: 10.1161/01.res.65.2.334. [DOI] [PubMed] [Google Scholar]
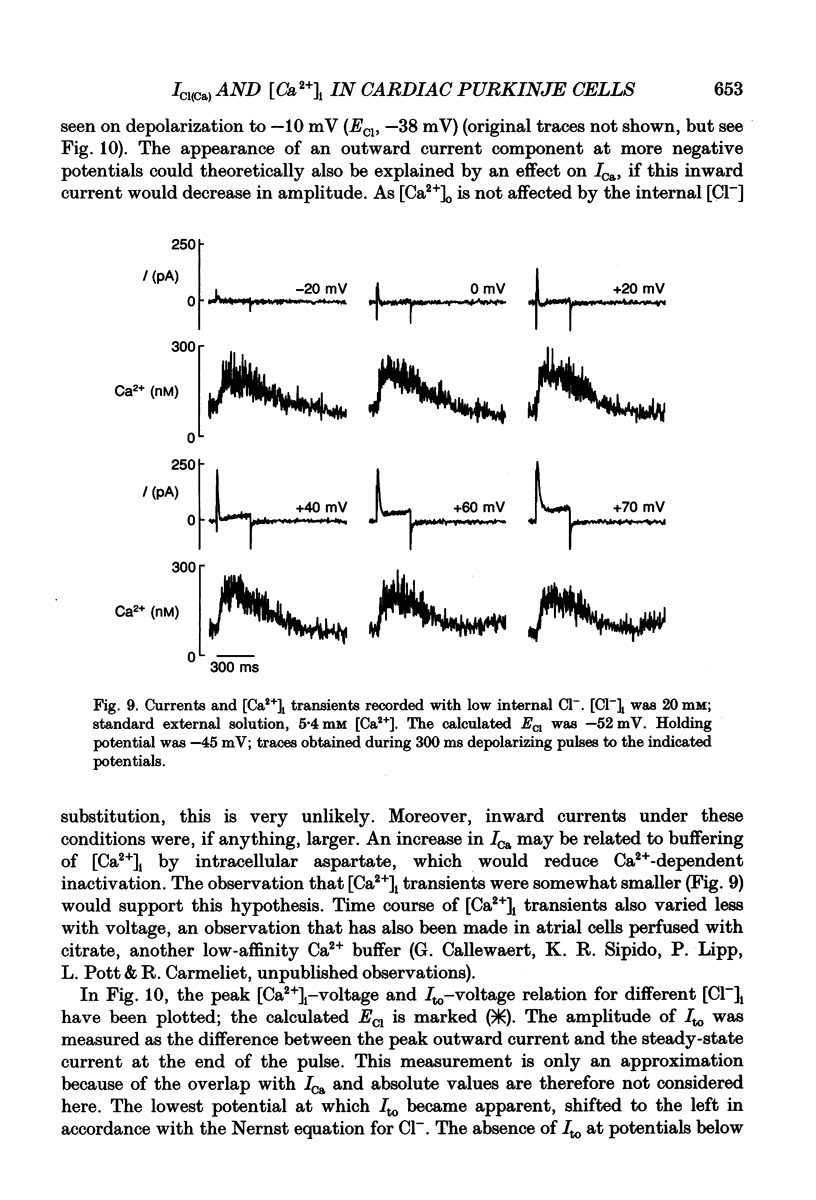
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in guinea-pig cardiac cells: exchange current and changes in intracellular Ca2+. J Physiol. 1989 Jul;414:499–520. doi: 10.1113/jphysiol.1989.sp017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielen F. V., Glitsch H. G., Verdonck F. Changes of the subsarcolemmal Na+ concentration in internally perfused cardiac cells. Biochim Biophys Acta. 1991 Jun 18;1065(2):269–271. doi: 10.1016/0005-2736(91)90239-5. [DOI] [PubMed] [Google Scholar]
- Bridge J. H., Spitzer K. W., Ershler P. R. Relaxation of isolated ventricular cardiomyocytes by a voltage-dependent process. Science. 1988 Aug 12;241(4867):823–825. doi: 10.1126/science.3406740. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol. 1989 Jul;257(1 Pt 1):C147–C152. doi: 10.1152/ajpcell.1989.257.1.C147. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Epinephrine enhances Ca2+ current-regulated Ca2+ release and Ca2+ reuptake in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Mar;85(6):2009–2013. doi: 10.1073/pnas.85.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Lipp P., Pott L., Carmeliet E. High-resolution measurement and calibration of Ca(2+)-transients using Indo-1 in guinea-pig atrial myocytes under voltage clamp. Cell Calcium. 1991 Apr;12(4):269–277. doi: 10.1016/0143-4160(91)90001-u. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Vereecke J., Carmeliet E. Existence of a calcium-dependent potassium channel in the membrane of cow cardiac Purkinje cells. Pflugers Arch. 1986 Apr;406(4):424–426. doi: 10.1007/BF00590947. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Lederer W. J. The arrhythmogenic current ITI in the absence of electrogenic sodium-calcium exchange in sheep cardiac Purkinje fibres. J Physiol. 1986 May;374:201–219. doi: 10.1113/jphysiol.1986.sp016075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells? Cardiovasc Res. 1992 May;26(5):433–442. doi: 10.1093/cvr/26.5.433. [DOI] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. J Physiol. 1991 Jan;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Dukes I. D., Morad M. The transient K+ current in rat ventricular myocytes: evaluation of its Ca2+ and Na+ dependence. J Physiol. 1991 Apr;435:395–420. doi: 10.1113/jphysiol.1991.sp018516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T., Noma A., Ono K. Calcium-activated non-selective cation channel in ventricular cells isolated from adult guinea-pig hearts. J Physiol. 1988 Sep;403:117–133. doi: 10.1113/jphysiol.1988.sp017242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Cannell M. B., Cohen N. M., Berlin J. R. Excitation-contraction coupling in heart muscle. Mol Cell Biochem. 1989 Sep 7;89(2):115–119. doi: 10.1007/BF00220762. [DOI] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Simultaneous recording of Indo-1 fluorescence and Na+/Ca2+ exchange current reveals two components of Ca2(+)-release from sarcoplasmic reticulum of cardiac atrial myocytes. FEBS Lett. 1990 Nov 26;275(1-2):181–184. doi: 10.1016/0014-5793(90)81467-3. [DOI] [PubMed] [Google Scholar]
- Marban E., Wier W. G. Ryanodine as a tool to determine the contributions of calcium entry and calcium release to the calcium transient and contraction of cardiac Purkinje fibers. Circ Res. 1985 Jan;56(1):133–138. doi: 10.1161/01.res.56.1.133. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S. Calcium-activated potassium channels in rat muscle inactivate from a short-duration open state. J Physiol. 1985 Jun;363:501–516. doi: 10.1113/jphysiol.1985.sp015724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido K. R., Wier W. G. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. J Physiol. 1991 Apr;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro C. R., Lingle C. J. Trypsin-sensitive, rapid inactivation of a calcium-activated potassium channel. Science. 1992 Sep 18;257(5077):1694–1698. doi: 10.1126/science.1529355. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C., Latorre R. Kinetics of Ca2+-activated K+ channels from rabbit muscle incorporated into planar bilayers. Evidence for a Ca2+ and Ba2+ blockade. J Gen Physiol. 1983 Oct;82(4):543–568. doi: 10.1085/jgp.82.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G. Calcium transients during excitation-contraction coupling in mammalian heart: aequorin signals of canine Purkinje fibers. Science. 1980 Mar 7;207(4435):1085–1087. doi: 10.1126/science.7355274. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Isenberg G. Intracellular [Ca2+] transients in voltage clamped cardiac Purkinje fibers. Pflugers Arch. 1982 Jan;392(3):284–290. doi: 10.1007/BF00584312. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Properties of the calcium-activated chloride current in heart. J Gen Physiol. 1992 Mar;99(3):391–414. doi: 10.1085/jgp.99.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


