Abstract
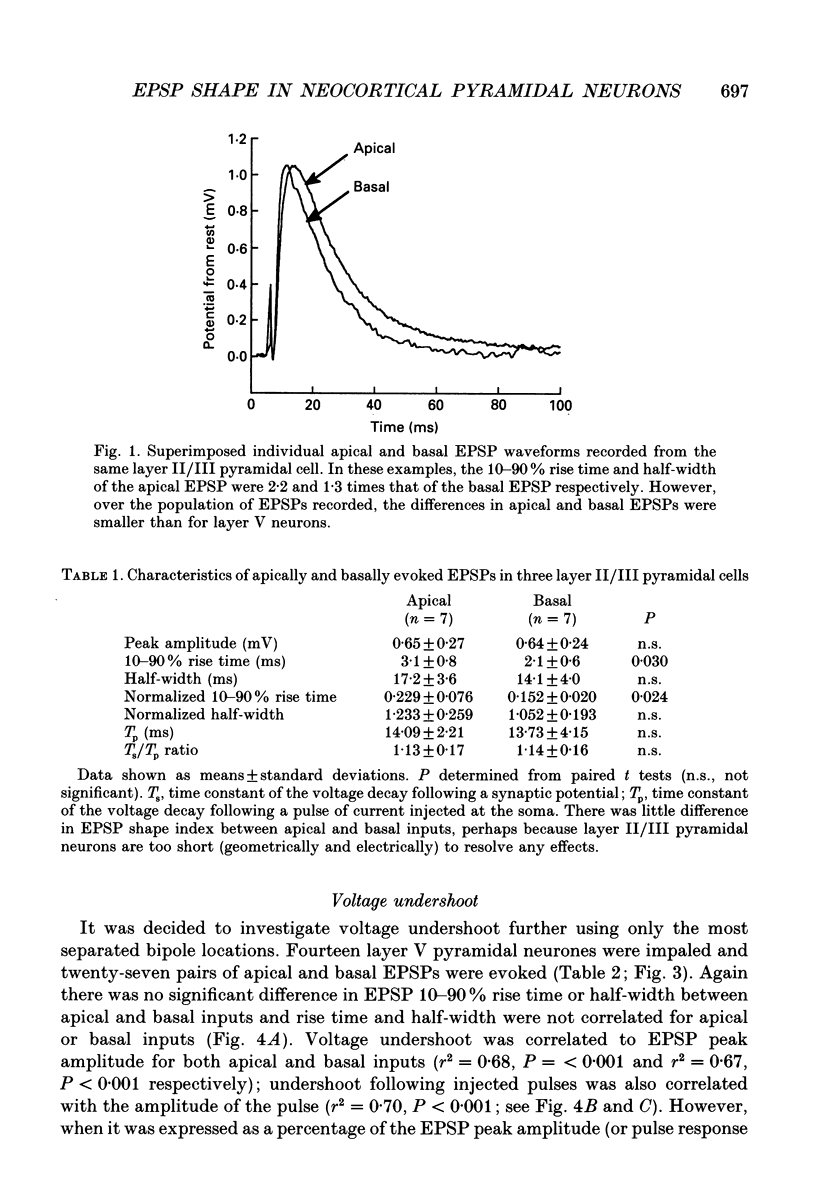
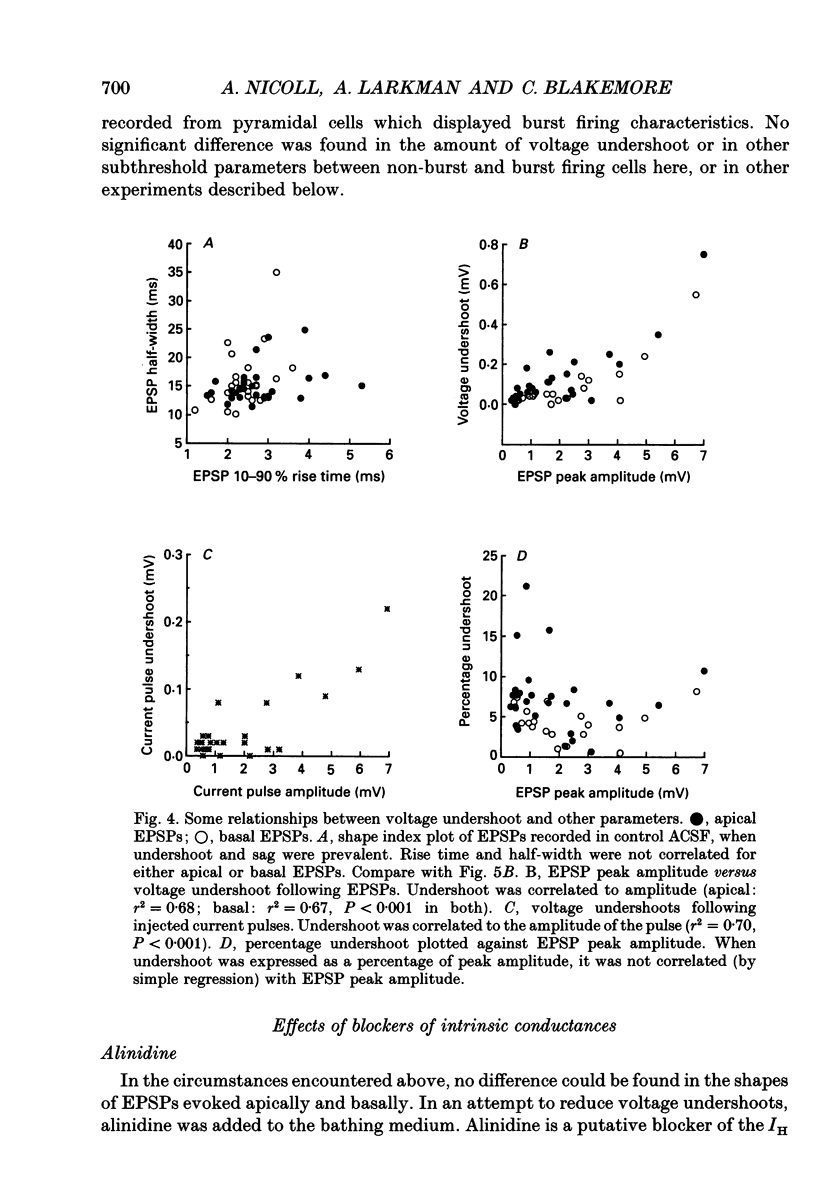
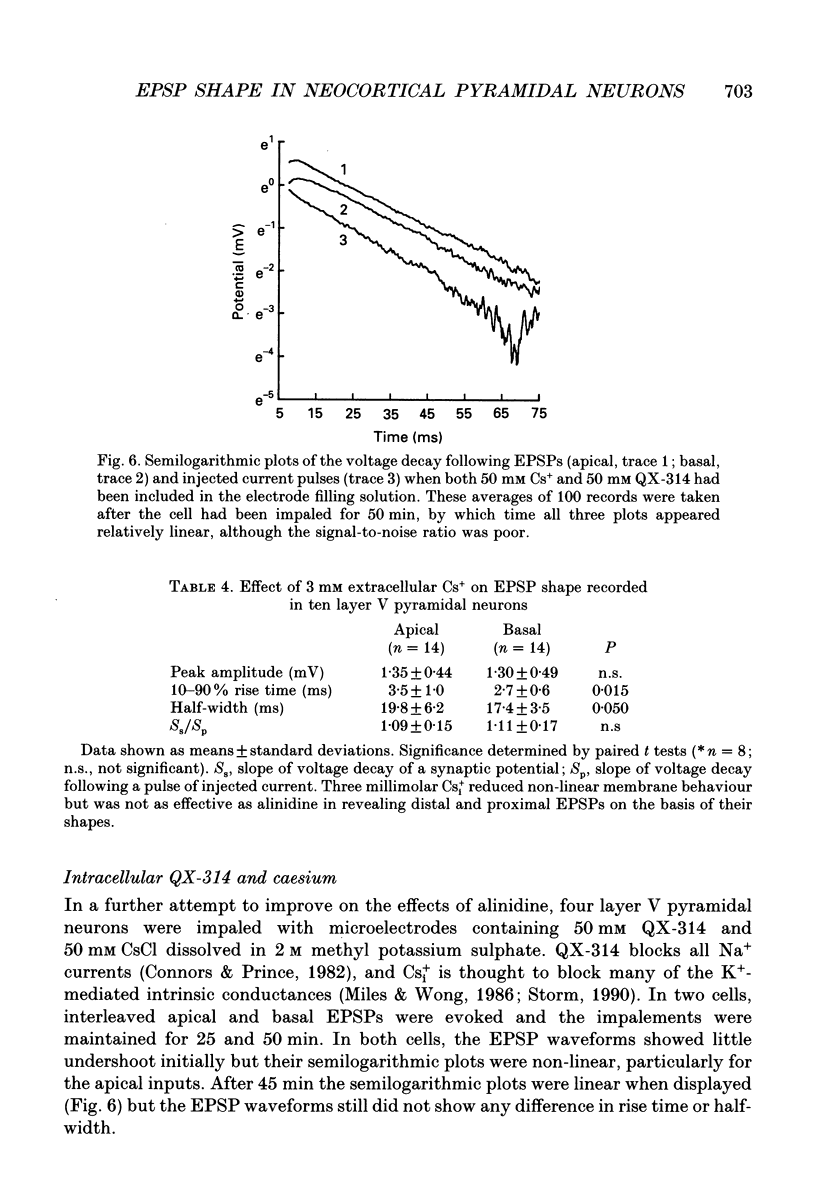
1. Intracellular recordings were made from pyramidal neurons in layers II/III and V of rat visual cortical slices. Distal and proximal excitatory postsynaptic potentials (EPSPs) were evoked using extracellular bipolar electrodes placed on the slice horizontal to each cell, near the apical and basal dendrites respectively. Experiments were conducted in the presence of 2-amino-5-phosphonopentanoate, picrotoxin and, in most cases, 2-hydroxy-saclofen. 2. For layer II/III pyramidal neurons, voltage undershoots following distal and proximal EPSPs (n = 7 pairs) and injected somatic pulses were rarely apparent. In layer V pyramidal neurons substantial voltage undershoots were recorded following distal and proximal EPSPs (n = 27 pairs) and injected somatic pulses, with undershoot being greatest for apical inputs (P = 0.001). The greater undershoots following apical EPSPs were also apparent in semilogarithmic plots of voltage decay where the slope of decay for apical EPSPs was quicker than the voltage decay following pulses of current injected at the soma. There was no significant difference in the shapes of distal and proximal EPSPs in layer II/III or layer V pyramidal cells under control conditions. 3. Pharmacological agents were used to reduce voltage undershoots. The most successful of these was alinidine, a putative blocker of the slow inward rectifier (IH) conductance. In the presence of bath-applied 100 microM alinidine, undershoots were significantly reduced and it became possible to distinguish the relative origins of EPSPs on the basis of their shape. Distally generated EPSPs (n = 14) had rise times and half-widths that were 2.8 and 1.5 times longer respectively than those evoked proximally (n = 10; P = 0.001 for both parameters). 4. These results confirm previous theoretical simulations of somatic recordings in passive model neurons where distal EPSPs display slower rise times and longer half-widths than proximal EPSPs. The present results suggest that, at least in pyramidal neurons of layer V, distal synaptic inputs can be specifically modulated by intrinsic membrane conductances.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Silfvenius H., Sundberg S. H., Sveen O. A comparison of distal and proximal dendritic synapses on CAi pyramids in guinea-pig hippocampal slices in vitro. J Physiol. 1980 Oct;307:273–299. doi: 10.1113/jphysiol.1980.sp013435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander O., Douglas R. J., Martin K. A., Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Redman S. J. Cable properties of cat spinal motoneurones measured by combining voltage clamp, current clamp and intracellular staining. J Physiol. 1989 Feb;409:63–87. doi: 10.1113/jphysiol.1989.sp017485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Prince D. A. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982 Mar;220(3):476–481. [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Holmes W. R., Woody C. D. Effects of uniform and non-uniform synaptic 'activation-distributions' on the cable properties of modeled cortical pyramidal neurons. Brain Res. 1989 Dec 25;505(1):12–22. doi: 10.1016/0006-8993(89)90110-8. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Midtgaard J. Dendrite processing in more ways than one . Trends Neurosci. 1989 Sep;12(9):313–315. doi: 10.1016/0166-2236(89)90036-2. [DOI] [PubMed] [Google Scholar]
- Hwa G. G., Avoli M. Hyperpolarizing inward rectification in rat neocortical neurons located in the superficial layers. Neurosci Lett. 1991 Mar 11;124(1):65–68. doi: 10.1016/0304-3940(91)90823-c. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Hablitz J. J., Wilson W. A. Voltage clamp discloses slow inward current in hippocampal burst-firing neurones. Nature. 1980 Jul 24;286(5771):391–393. doi: 10.1038/286391a0. [DOI] [PubMed] [Google Scholar]
- Larkman A. U., Major G., Stratford K. J., Jack J. J. Dendritic morphology of pyramidal neurones of the visual cortex of the rat. IV: Electrical geometry. J Comp Neurol. 1992 Sep 8;323(2):137–152. doi: 10.1002/cne.903230202. [DOI] [PubMed] [Google Scholar]
- Larkman A. U., Mason A., Blakemore C. The in vitro slice preparation for combined morphological and electrophysiological studies of rat visual cortex. Neurosci Res. 1988 Oct;6(1):1–19. doi: 10.1016/0168-0102(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Larkman A., Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci. 1990 May;10(5):1407–1414. doi: 10.1523/JNEUROSCI.10-05-01407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Lillie C., Kobinger W. Decrease in bradycardic effect of AQ-A 39 and alinidine in guinea-pig sinoatrial node depolarized by high external K+-concentration. Naunyn Schmiedebergs Arch Pharmacol. 1984 Dec;328(2):210–213. doi: 10.1007/BF00512075. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A., Larkman A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J Neurosci. 1990 May;10(5):1415–1428. doi: 10.1523/JNEUROSCI.10-05-01415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll A., Larkman A., Blakemore C. EPSPs in rat neocortical pyramidal neurones in vitro are prolonged by NMDA receptor-mediated currents. Neurosci Lett. 1992 Aug 31;143(1-2):5–9. doi: 10.1016/0304-3940(92)90220-2. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Redman S., Walmsley B. The time course of synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:117–133. doi: 10.1113/jphysiol.1983.sp014884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Crill W. E. Calcium-dependent potassium currents in neurons from cat sensorimotor cortex. J Neurophysiol. 1992 Jan;67(1):216–226. doi: 10.1152/jn.1992.67.1.216. [DOI] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Post-inhibitory excitation and inhibition in layer V pyramidal neurones from cat sensorimotor cortex. J Physiol. 1991 Mar;434:609–626. doi: 10.1113/jphysiol.1991.sp018489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Storm J. F. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989 Feb;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Girdlestone D., West D. C. Voltage-dependent currents prolong single-axon postsynaptic potentials in layer III pyramidal neurons in rat neocortical slices. J Neurophysiol. 1988 Dec;60(6):1896–1907. doi: 10.1152/jn.1988.60.6.1896. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Buzsáki G. Computer simulation of carbachol-driven rhythmic population oscillations in the CA3 region of the in vitro rat hippocampus. J Physiol. 1992;451:653–672. doi: 10.1113/jphysiol.1992.sp019184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. A. Waveform and amplitude characteristics of evoked responses to dendritic stimulation of CA1 guinea-pig pyramidal cells. J Physiol. 1988 Jan;395:419–439. doi: 10.1113/jphysiol.1988.sp016927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B., Stuklis R. Effects of spatial and temporal dispersion of synaptic input on the time course of synaptic potentials. J Neurophysiol. 1989 Apr;61(4):681–687. doi: 10.1152/jn.1989.61.4.681. [DOI] [PubMed] [Google Scholar]


