Abstract
1. Single skeletal muscle fibres were enzymatically isolated from the flexor digitorum brevis muscles (FDB) of dystrophic mdx and control C57BL/10 mice aged 3-9 weeks. In this age range the majority (> 95%) of the mdx fibres were morphologically normal. 2. There was no significant difference between the resting membrane potential (RMP) of mdx and control mice, -71.2 +/- 1.21 (n = 26) and -70.6 +/- 1.15 mV (n = 42), respectively. 3. At RMP more negative than -60 mV the resting calcium (recorded with fura-2, free acid ionophoresed into cell) in the dystrophic mdx cells was not significantly different from the normal animals, 45.7 +/- 4.1 (n = 10) and 46.2 +/- 3.9 nM (n = 9), respectively. 4. The resting cytosolic calcium concentration was measured simultaneously with the RMP. At RMP between -60 to -17 mV there was an increase in the resting calcium concentration in both mdx and control ranging from 79.3 to 252 nM. This increase was most probably due to the activation of the slow calcium current. 5. Fura-2 calcium transients were produced via single action potential stimulation using an intracellular microelectrode both to stimulate the cell and record potential changes. There was no significant difference between the rise time (Tp) or half-decay time (T1/2) at 22 degrees C of the calcium transient in response to a single action potential in mdx compared to normal animals, 5.9 +/- 0.34 (n = 8) and 5.4 +/- 0.36 ms (n = 7); 39.5 +/- 2.9 (n = 8) and 40.75 +/- 3.7 ms (n = 7), respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


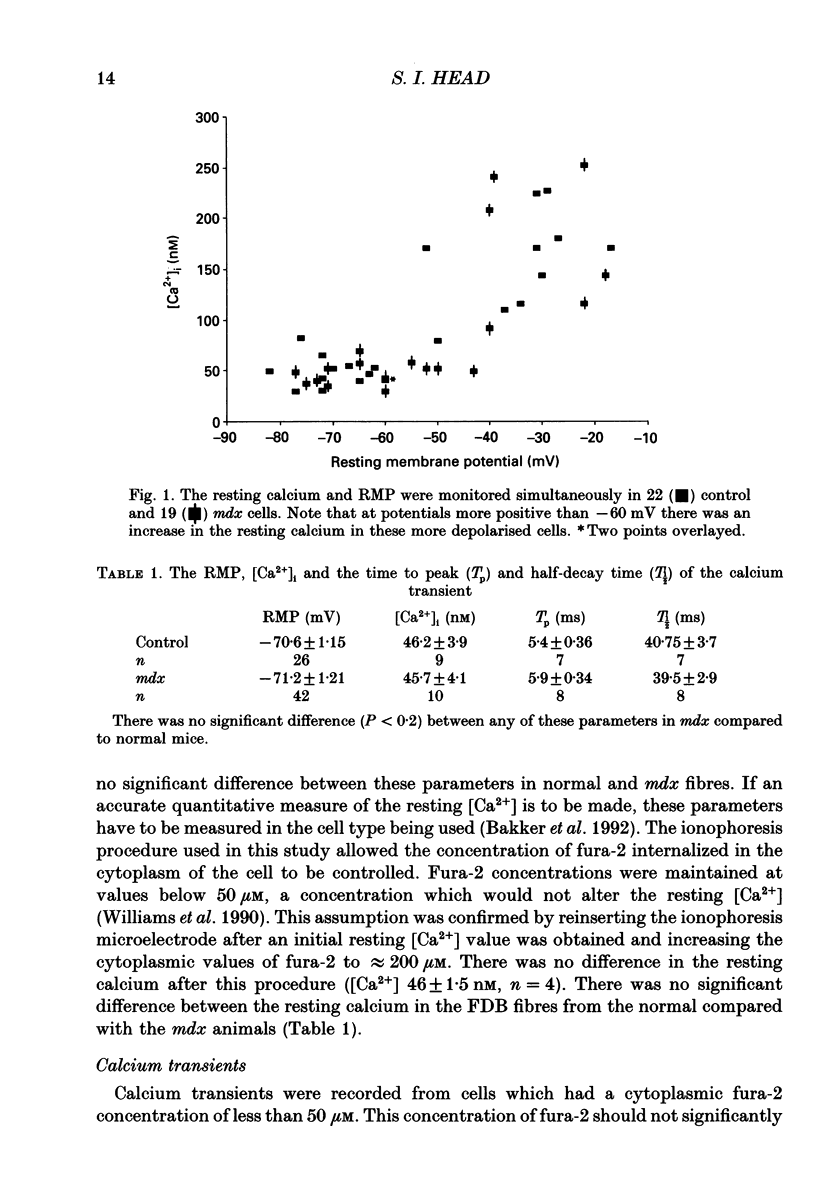
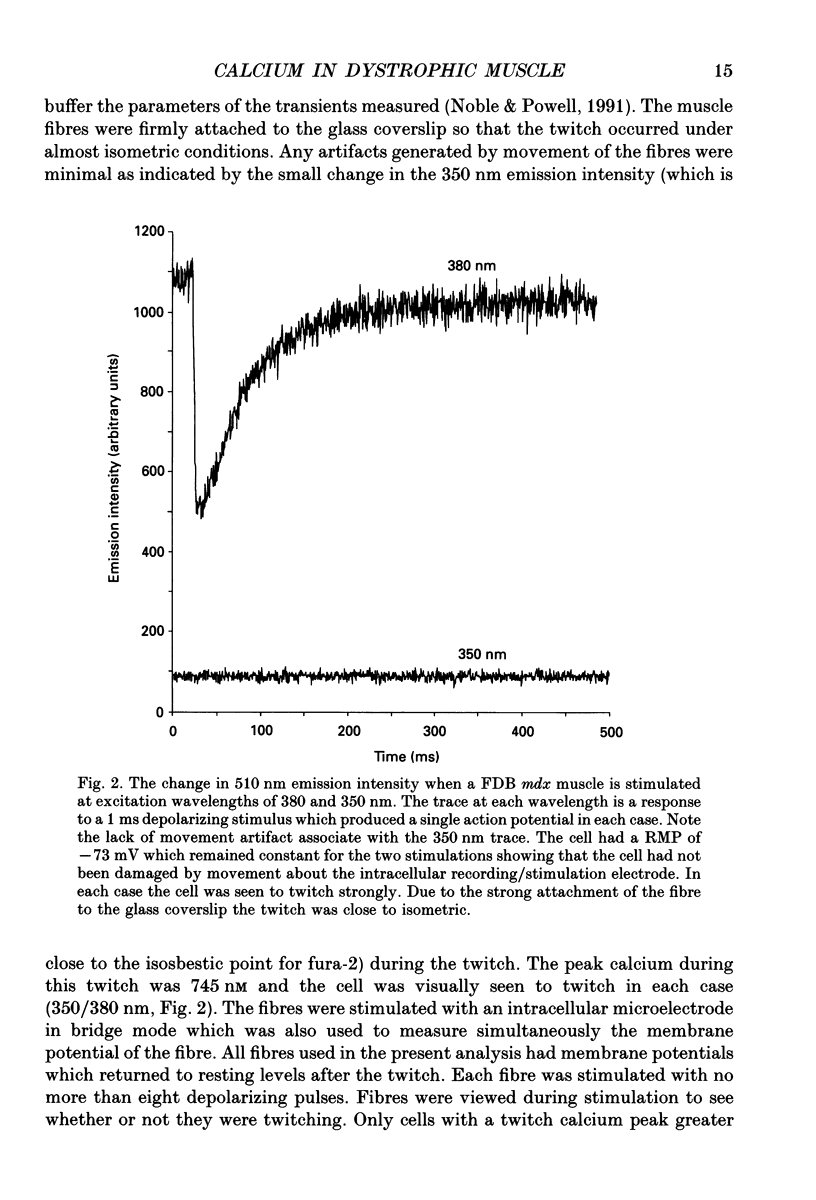
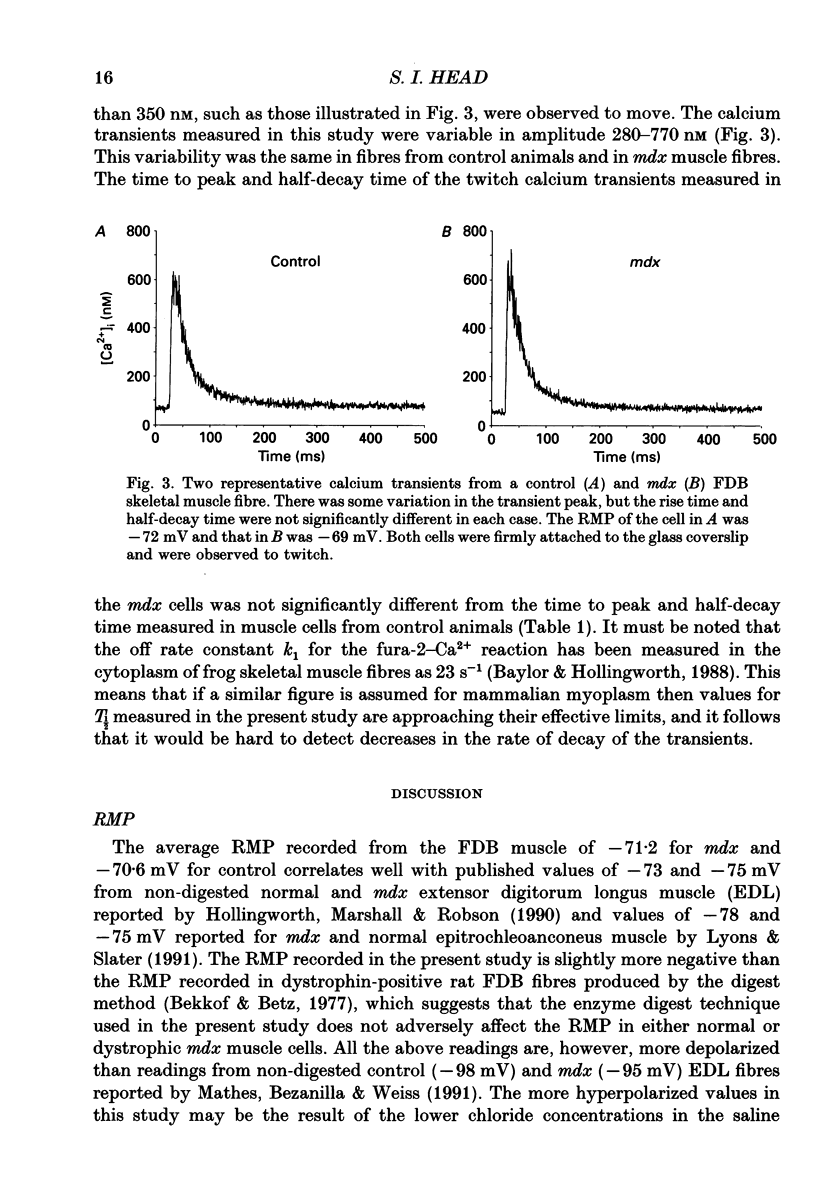



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker A. J., Head S. I., Williams D. A., Stephenson D. G. Ca2+ levels in myotubes grown from the skeletal muscle of dystrophic (mdx) and normal mice. J Physiol. 1993 Jan;460:1–13. doi: 10.1113/jphysiol.1993.sp019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A., Betz W. J. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 1977 Sep;271(1):25–40. doi: 10.1113/jphysiol.1977.sp011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnwath J. W., Shotton D. M. Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J Neurol Sci. 1987 Aug;80(1):39–54. doi: 10.1016/0022-510x(87)90219-x. [DOI] [PubMed] [Google Scholar]
- DiMario J. X., Uzman A., Strohman R. C. Fiber regeneration is not persistent in dystrophic (MDX) mouse skeletal muscle. Dev Biol. 1991 Nov;148(1):314–321. doi: 10.1016/0012-1606(91)90340-9. [DOI] [PubMed] [Google Scholar]
- Fong P. Y., Turner P. R., Denetclaw W. F., Steinhardt R. A. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990 Nov 2;250(4981):673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- Franco A., Jr, Lansman J. B. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature. 1990 Apr 12;344(6267):670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haws C. M., Lansman J. B. Developmental regulation of mechanosensitive calcium channels in skeletal muscle from normal and mdx mice. Proc Biol Sci. 1991 Sep 23;245(1314):173–177. doi: 10.1098/rspb.1991.0105. [DOI] [PubMed] [Google Scholar]
- Head S. I., Stephenson D. G., Williams D. A. Properties of enzymatically isolated skeletal fibres from mice with muscular dystrophy. J Physiol. 1990 Mar;422:351–367. doi: 10.1113/jphysiol.1990.sp017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head S. I., Williams D. A., Stephenson D. G. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci. 1992 May 22;248(1322):163–169. doi: 10.1098/rspb.1992.0058. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hollingworth S., Marshall M. W., Robson E. Excitation contraction coupling in normal and mdx mice. Muscle Nerve. 1990 Jan;13(1):16–20. doi: 10.1002/mus.880130105. [DOI] [PubMed] [Google Scholar]
- Knudson C. M., Hoffman E. P., Kahl S. D., Kunkel L. M., Campbell K. P. Evidence for the association of dystrophin with the transverse tubular system in skeletal muscle. J Biol Chem. 1988 Jun 15;263(17):8480–8484. [PubMed] [Google Scholar]
- Lamb G. D., Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987 Dec;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons P. R., Slater C. R. Structure and function of the neuromuscular junction in young adult mdx mice. J Neurocytol. 1991 Dec;20(12):969–981. doi: 10.1007/BF01187915. [DOI] [PubMed] [Google Scholar]
- Mathes C., Bezanilla F., Weiss R. E. Sodium current and membrane potential in EDL muscle fibers from normal and dystrophic (mdx) mice. Am J Physiol. 1991 Oct;261(4 Pt 1):C718–C725. doi: 10.1152/ajpcell.1991.261.4.C718. [DOI] [PubMed] [Google Scholar]
- McArdle A., Edwards R. H., Jackson M. J. Accumulation of calcium by normal and dystrophin-deficient mouse muscle during contractile activity in vitro. Clin Sci (Lond) 1992 Apr;82(4):455–459. doi: 10.1042/cs0820455. [DOI] [PubMed] [Google Scholar]
- Menke A., Jockusch H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature. 1991 Jan 3;349(6304):69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- Mongini T., Ghigo D., Doriguzzi C., Bussolino F., Pescarmona G., Pollo B., Schiffer D., Bosia A. Free cytoplasmic Ca++ at rest and after cholinergic stimulus is increased in cultured muscle cells from Duchenne muscular dystrophy patients. Neurology. 1988 Mar;38(3):476–480. doi: 10.1212/wnl.38.3.476. [DOI] [PubMed] [Google Scholar]
- Noble D., Powell T. The slowing of Ca2+ signals by Ca2+ indicators in cardiac muscle. Proc Biol Sci. 1991 Nov 22;246(1316):167–172. doi: 10.1098/rspb.1991.0140. [DOI] [PubMed] [Google Scholar]
- Suda N., Kurihara S. Intracellular calcium signals measured with fura-2 and aequorin in frog skeletal muscle fibers. Jpn J Physiol. 1991;41(2):277–295. doi: 10.2170/jjphysiol.41.277. [DOI] [PubMed] [Google Scholar]
- Turner P. R., Fong P. Y., Denetclaw W. F., Steinhardt R. A. Increased calcium influx in dystrophic muscle. J Cell Biol. 1991 Dec;115(6):1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Westwood T., Regen C. M., Steinhardt R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988 Oct 20;335(6192):735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991 Sep;98(3):615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Head S. I., Bakker A. J., Stephenson D. G. Resting calcium concentrations in isolated skeletal muscle fibres of dystrophic mice. J Physiol. 1990 Sep;428:243–256. doi: 10.1113/jphysiol.1990.sp018210. [DOI] [PMC free article] [PubMed] [Google Scholar]


