Abstract
For cirrhotic refractory ascites, diuretics combined with albumin and vasoactive drugs are the first-line choice for ascites management. However, their therapeutic effects are limited, and most refractory ascites do not respond to medication treatment, necessitating consideration of drainage or surgical interventions. Consequently, numerous drainage methods for cirrhotic ascites have emerged, including large-volume paracentesis, transjugular intrahepatic portosystemic shunt, peritoneovenous shunt, automated low-flow ascites pump, cell-free and concentrated ascites reinfusion therapy, and peritoneal catheter drainage. This review introduces the advantages and disadvantages of these methods in different aspects, as well as indications and contraindications for this disease.
Keywords: Liver cirrhosis ascites, Large-volume paracentesis, Transjugular intrahepatic portosystemic shunt, Peritoneovenous shunt, Automated low-flow ascites pump, Cell-free and concentrated ascites reinfusion therapy, Peritoneal catheter drainage
Core Tip: For cirrhotic refractory ascites, peritoneovenous shunt is rarely used due to its high complication rate. Initial treatment for most refractory ascites prioritizes large-volume paracentesis combined with albumin infusion and peritoneal catheter drainage. If these treatments are ineffective or result in severe complications, transjugular intrahepatic portosystemic shunt or automated low-flow ascites pump may be considered. Cell-free and concentrated ascites reinfusion therapy requires further validation for suitability.
INTRODUCTION
Ascites denotes the accumulation of excess free fluid in the abdominal cavity, with cirrhosis being the predominant cause, responsible for over 60% of cases. The onset of ascites is a critical marker in the progression of cirrhosis, often signaling a transition from a stable to a more severe clinical phase. This condition frequently accompanies acute decompensation events, such as acute-on-chronic liver failure, bacterial infections, and recurrent hospitalizations, significantly impacting overall treatment outcome[1]. Ascites is associated with multiple interrelated pathogenic mechanisms involving visceral and systemic hemodynamics, as well as dysfunctions in both liver and extrahepatic organs, primarily the kidneys and heart[2]. Portal hypertension is the primary and initiating factor of ascites formation in cirrhosis[3], causing reduced tissue fluid reabsorption and leakage into the abdominal cavity. Additionally, decreased serum albumin levels lead to reduced plasma colloid osmotic pressure, causing fluid to seep into the abdominal cavity or interstitial spaces. Systemic inflammatory response syndrome also significantly contributes to ascites formation. The primary mechanism involves interactions between bacterial products or pathogen-associated molecular patterns and their respective receptors, promoting the formation and release of inflammatory cytokines. This inflammation stimulates the production of endogenous vasodilators, such as endotoxins, vasoactive intestinal peptides, and nitric oxide, leading to vasodilation[4]. This vasodilation results in effective circulating volume (ECV) deficiency, reduced renal blood flow, and activation of the renin-angiotensin system, exacerbating sodium and water retention, thereby promoting ascites formation. Furthermore, the impaired hepatic processing function in cirrhotic patients weakens the inactivation of aldosterone and antidiuretic hormone, further promoting sodium and water retention. Increased pressure within the hepatic sinusoids in cirrhotic patients leads to increased lymph formation. When the volume of returning lymph exceeds the drainage capacity of the thoracic duct, it can seep from the liver surface into the abdominal cavity, forming ascites[5].
METHODS OF DRAINAGE OF ASCITES
Currently, managing and controlling refractory ascites and its related complications remains a significant clinical challenge. Pharmacotherapy, commonly using diuretics (furosemide or spironolactone) combined with albumin and vasoactive drugs, is the first-line choice for ascites management[6]. However, its therapeutic effects are often limited, and most refractory ascites do not respond to drug treatment, necessitating consideration of drainage or surgical interventions. Liver transplantation remains the only curative treatment method. For some patients with refractory ascites, liver transplantation is an option. However, due to various contraindications and the lack of liver donors, it is not feasible for many patients[7]. Consequently, numerous drainage methods for cirrhotic ascites have emerged, including large-volume paracentesis (LVP), transjugular intrahepatic portosystemic shunt (TIPS), peritoneovenous shunt (PVS), automated low-flow ascites pump (alfapump), cell-free and concentrated ascites reinfusion therapy (CART), and peritoneal catheter drainage (Table 1).
Table 1.
Methods and characteristics of ascites drainage in cirrhosis
|
Ascites drainage methods
|
Characteristics
|
| LVP | In the majority of patients suffering from refractory ascites, the preferred treatment approach involves the prioritization of LVP in conjunction with HSA administration. An advisable frequency for this therapeutic regimen is approximately once every two weeks, ensuring that the maximum volume of fluid removed during a single paracentesis does not surpass 5 L. It is imperative to note that repetitive LVP procedures heighten the potential for the development of complications. |
| TIPS | For patients who require frequent paracentesis procedures, frequent hospitalizations, or are awaiting liver transplantation, TIPS can act as a vital bridge therapy, facilitating the transition to definitive liver transplantation. However, due to its associated complications and contraindications, TIPS is typically reserved as a second-line therapeutic option, employed subsequent to the failure of LVP treatment. This approach ensures that the most appropriate and safe treatment pathway is pursued for each individual patient’s unique circumstances. |
| PVS | When addressing refractory ascites, PVS has not demonstrated superiority over repeated LVP in terms of treatment outcomes. Furthermore, the risk of severe complications associated with PVS has rendered this approach virtually obsolete, as it has led to its near-complete abandonment in clinical practice. |
| Alfapump | The alfapump system holds significant potential in drastically reducing the reliance on LVP for patients. Nonetheless, it is important to acknowledge that the complication rate associated with this treatment modality remains relatively high. For those individuals diagnosed with non-malignant refractory ascites who are deemed ineligible for alternative therapies, such as TIPS or liver transplantation, the implantation of an alfapump represents an efficacious and viable treatment option. |
| CART | CART exhibits remarkable efficacy in swiftly alleviating abdominal distension, mitigating the burden of ascites, and enhancing nutritional intake for patients. Nevertheless, this innovative therapy is not without its challenges, including substantial equipment costs, intricate procedural requirements, and potential allergic reactions. Presently, CART is predominantly utilized in Japan, and its universal applicability across global healthcare settings remains an area of ongoing investigation and verification. |
| Peritoneal catheter drainage | The adoption of peritoneal catheter drainage as a management strategy for cirrhotic ascites boasts a high rate of symptom alleviation, coupled with low financial costs and a minimal incidence of associated complications. This approach significantly diminishes the necessity for repeated LVP procedures, positioning it as a potential cornerstone in the evolving landscape of cirrhotic ascites management. Nevertheless, meticulous attention must be paid to minimizing the duration of catheter retention, as this is paramount in preventing the emergence of complications. |
LVP: Large-volume paracentesis; HAS: Human serum albumin; TIPS: Transjugular intrahepatic portosystemic shunt; PVS: Peritoneovenous shunt; Alfapump: Automated low-flow ascites pump; CART: Cell-free and concentrated ascites reinfusion therapy.
LVP
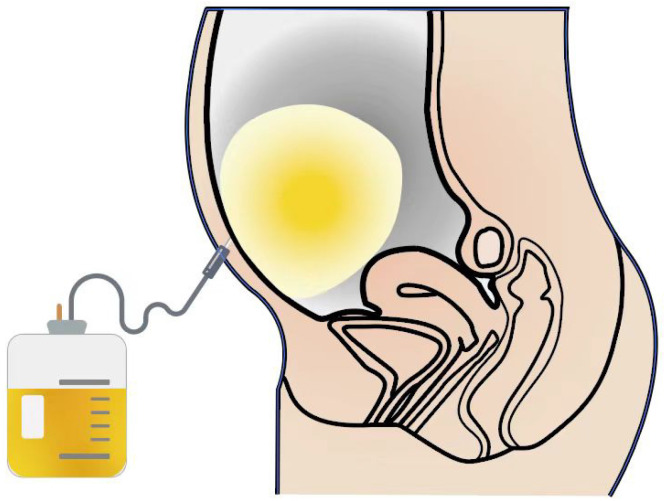
LVP combined with human serum albumin (HSA) supplementation remains the cornerstone of current ascites management (Figure 1)[8]. Defined as the removal of more than 5 L ascites in a single session, LVP can significantly alleviate patient discomfort, such as abdominal distension, by draining 4-6 L per day. Studies indicate that patients undergoing paracentesis exhibit lower in-hospital mortality rates compared to those who do not[9]. Compared with diuretics, LVP rapidly controls large volumes of ascites and shortens hospital stays[10], with fewer complications, such as electrolyte abnormalities, renal dysfunction, and hemodynamic instability[11]. However, LVP requires repeated punctures and has limitations, as it does not address the underlying pathophysiology of ascites formation, leading to rapid recurrence. The reduction in intra-abdominal pressure after LVP often increases the pressure gradient between the liver and abdominal cavity, causing rapid ascites refilling. Thus, repeated LVP is typically necessary, with most patients needing another paracentesis within two weeks. Martin et al[12] found that repeated LVP increases the risk of complications, including hepatorenal syndrome, hepatic encephalopathy, gastrointestinal bleeding, peritoneal infection, electrolyte disorders, and paracentesis-induced circulatory dysfunction (PICD), which can lead to renal failure. The sudden decrease in intra-abdominal pressure and insufficient blood volume after repeated LVP can cause redistribution of circulating volume, reducing effective arterial blood volume and increasing the risk of renal dysfunction, dilutional hyponatremia, and death. This condition is known as PICD[13]. Larger LVP volumes are associated with higher PICD risks. When LVP is performed without volume expanders, up to 80% of cases may develop PICD, whereas intravenous albumin as a volume expander can reduce the PICD incidence to 15-35%[14]. Therefore, many studies recommend infusing 6-8 g of albumin per L of ascites drained after LVP > 5 L to prevent PICD[15-17]. The benefits of HSA infusion stem from its colloid osmotic and non-colloid osmotic properties[18]. The former improves effective hypovolemia by expanding plasma volume, while the latter offers anti-inflammatory, antioxidant, and immunomodulatory effects[19]. Studies show that routine HSA use and limiting LVP to 8 L can prevent renal function impairment[20]. Early HSA therapy reduces ascites incidence[21,22], benefits the prognosis of cirrhotic patients, and significantly lowers mortality in patients with mild cirrhosis[23]. Additionally, HSA treatment decreases the incidence of hepatic encephalopathy, hepatorenal syndrome, spontaneous bacterial peritonitis (SBP), and non-SBP infections[24]. The baseline model for end-stage liver disease score (MELD)-Na score of LVP patients is associated with acute kidney injury (AKI), necessitating caution for those with high MELD-Na scores considering therapeutic paracentesis. In summary, for most patients with refractory ascites, LVP combined with HSA is prioritized, but the frequency of paracentesis should be controlled. A reasonable treatment frequency is about once every two weeks, with a single maximum paracentesis volume not exceeding 5 L.
Figure 1.
Schematic diagram of large-volume paracentesis.
TIPS
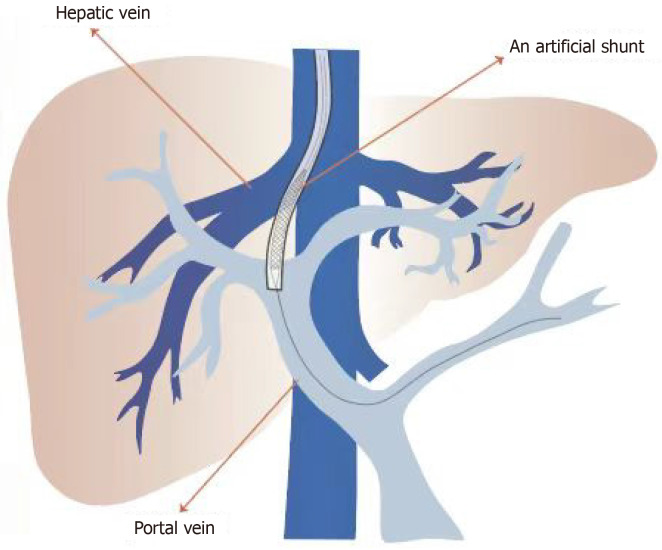
TIPS involves inserting a stent to bridge the portal vein branch and hepatic vein, effectively creating a portosystemic shunt to treat cirrhotic ascites (Figure 2). Unlike paracentesis, this procedure targets the elimination of portal hypertension and its complications rather than merely alleviating symptoms[25]. Successful TIPS insertion lowers portal vein pressure, enhances circulatory function in ascites patients[26], increases visceral blood flow to systemic circulation, mitigates effective arterial blood volume deficiency, and improves heart and kidney functions[27]. Two types of stents are used in TIPS: bare metal stents and covered stents. Approximately 70% of patients with bare metal TIPS stents develop stenosis due to excessive endothelial growth within the stent, narrowing the lumen[28]. TIPS stenosis can be treated by balloon dilatation of the stent, and new stent insertion is required if this fails. Covered stents, coated with polytetrafluoroethylene (PTFE), significantly reduce TIPS stenosis by preventing bile leakage into the shunt lumen and providing a smoother internal surface, allowing uniform endothelial growth and preventing neointimal proliferation and subsequent stent stenosis[29,30]. Bare stents have a higher incidence of shunt dysfunction[31], while covered stents significantly improve long-term shunt patency[32], reduce shunt dysfunction, and enhance clinical outcomes[33]. In patients with cirrhosis and variceal bleeding, covered stents have improved the 1-year survival rate[34]. A randomized controlled trial[35] found that covered stents in TIPS result in lower recurrence rates of ascites and higher long-term patency compared to bare stents, reducing the need for repeat interventions and possibly requiring less frequent monitoring[36]. Covered stents are also more cost-effective[37]. The diameter of covered stents affects clinical outcomes. Using under-expanded, smaller diameter covered stents can significantly reduce hepatic encephalopathy incidence[38]. Patients with 8-mm covered stents showed improved survival rates compared to those with 10-mm stents[39], though some studies suggest that 10- mm stents better control ascites without increasing hepatic encephalopathy[40]. Therefore, initially using small-diameter stents aiming for a modest reduction in portal vein pressure with gradual expansion to a maximum of 10 mm is recommended if an adequate clinical response is not achieved[41]. TIPS can serve as a bridge to liver transplantation for patients needing frequent paracentesis, hospitalizations, or those awaiting liver transplantation[42]. However, TIPS placement can result in complications related to the procedure, stent, or the portosystemic shunt. Procedural complications include bleeding, arrhythmias, hemoperitoneum, liver capsule rupture, and biliary fistulas. Stent-related complications include stent migration, stenosis, hemolytic anemia[43], and stent infection[44]. Portosystemic shunt complications include hepatic encephalopathy[45], progressive liver failure, and heart failure due to exacerbated hyperdynamic circulatory state[46]. TIPS is contraindicated for patients with advanced liver failure, and with serum bilirubin ≥ 45 mg/dL, Child-Pugh score ≥ 11, MELD score ≥ 18, or a history of hepatic encephalopathy. Renal failure, sepsis, and portal vein thrombosis also contraindicate TIPS[47,48]. TIPS placement is not recommended for patients at a high risk of heart failure or severe pulmonary hypertension. High right-sided pressures can eliminate the necessary pressure gradient between the portal and systemic venous systems, rendering TIPS ineffective. In such cases, TIPS placement may exacerbate heart failure[49]. Patients with intrahepatic malignancies should avoid TIPS due to the risk of tumor spread[50]. TIPS has shown significant efficacy in treating ascites[51]. A meta-analysis by Deltenre et al[52] confirmed TIPS’s superiority over LVP in controlling ascites and improving survival rates. Additionally, studies show that TIPS significantly improves survival rates in patients with refractory ascites[53], reduces recurrent ascites and hepatorenal syndrome, but increases hepatic encephalopathy risk[54]. Furthermore, it is necessary to consider the inclusion of bare stents in some studies, as they carry a higher risk of hepatic encephalopathy, which may consequently impact the outcomes. Other studies indicate that compared to LVP, TIPS with covered stents better controls ascites without higher rates of new-onset hepatic encephalopathy in PTFE-TIPS patients[55]. In summary, compared to LVP, TIPS treatment in patients with recurrent ascites can reduce complications and improve survival rates[56], making it a viable alternative. Current clinical guidelines consider TIPS as a second-line treatment, recommending its use only when frequent LVP or other treatments are ineffective[57], due to the high risk of TIPS-related hepatic encephalopathy in decompensated cirrhosis, which has high mortality rates[58]. However, with advancement and experience, especially in self-expanding PTFE-covered stents, TIPS-related complications have been significantly reduced[59], leading to increased TIPS use for refractory ascites. Additionally, TIPS effectively prevents variceal rebleeding, potentially becoming the preferred treatment for cirrhotic ascites in the future.
Figure 2.
Transjugular intrahepatic portosystemic shunt. Establishing an artificial shunt between the hepatic vein and portal vein.
PVS
PVS (LeVeen or Denver), first employed in the 1970s for refractory ascites, has been shown to reduce hospitalization duration, the number of hospitalizations, and diuretic dosage. This method involves implanting a device in the abdominal cavity to collect and filter blood from the peritoneal cavity based on the pressure gradient between the peritoneal cavity and central veins, directing it to the heart for further processing. The primary mechanism aims to reduce ascites volume while expanding plasma volume[60]. Ginès et al[61] have demonstrated that PVS effectively controls ascites compared to LVP combined with albumin infusion. Additionally, PVS has been reported to improve the glomerular filtration rate (GFR) in cirrhotic patients with refractory ascites, particularly those with moderate to severe renal impairment[62]. However, other studies indicate that PVS does not surpass repeated LVP and albumin infusion in treating refractory ascites[63]. Despite its relatively simple operation, PVS can cause serious, even fatal, complications, including infections[64], device blockage, shunt dysfunction, thrombosis, volume overload, disseminated intravascular coagulation, heart failure[65], air embolism, and complications related to surgical insertion[66]. These complications significantly increase patient mortality. Moreover, PVS placement can hinder TIPS procedures and cause peritoneal adhesions, complicating liver transplantation surgery. Although PVS can be used for refractory ascites patients ineligible for TIPS or liver transplantation, its high risk of adverse outcomes has led to its near-total abandonment[67].
Alfapump
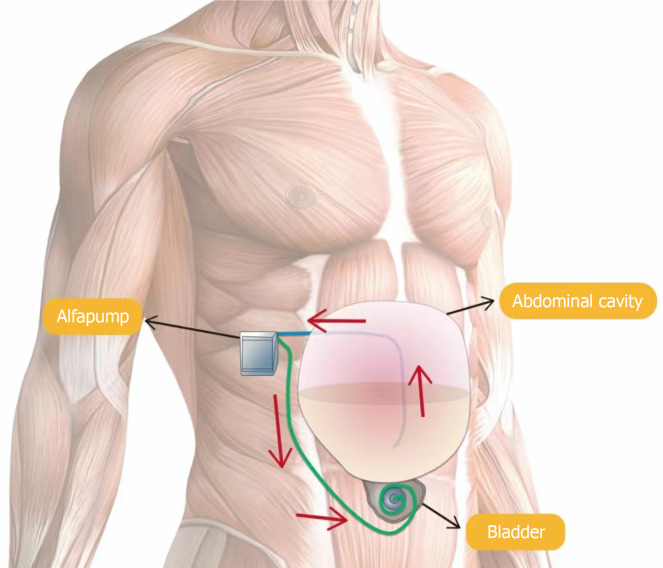
Alfapump is currently an alternative therapy for patients with refractory ascites (Figure 3)[68]. This implanted, battery-powered pump includes two silicone catheters: One in the peritoneum to collect ascites and the other in the bladder to deliver the ascites. The alfapump has four pressure sensors that monitor abdominal and bladder pressure, providing information on flow rates and system behavior. Generally, the pumping cycle starts when bladder pressure is below a certain threshold and stops immediately when peritoneal cavity pressure decreases significantly. This control allows the alfapump to manage the volume of ascites drained as well as the timing and frequency of pump activity. The alfapump’s purpose is to transfer ascites from the abdominal cavity to the bladder, allowing elimination through urination[69], effectively performing continuous small-volume, low-rate paracentesis daily. Despite requiring daily battery charging for less than 20 min, the pump operates for about 16 hours, with an expected battery life of over three years[70]. In managing cirrhotic ascites, the alfapump can significantly reduce the need for LVP[71,72]. A meta-analysis revealed that 62% of patients no longer needed LVP after alfapump implantation, and the number of required LVPs was significantly reduced[73]. Compared to repeated LVP, alfapump is more acceptable to patients with refractory ascites and improves their quality of life[74-76]. Studies show that the alfapump system offers advantages over LVP by reducing or eliminating the need for paracentesis and enhancing quality of life and nutritional status[77]. Although the alfapump system’s implantation cost is high, it stabilizes after intervention, unlike the continually increasing cost of repeated LVP. Therefore, for cirrhotic patients with refractory ascites, the alfapump system can effectively reduce the need for paracentesis and improve health quality. While its impact on survival has not been formally studied, the alfapump controls ascites as effectively as LVP combined with HSA[78]. However, the complication rate is high, including pump system infections, pump failures, catheter displacements, electrolyte abnormalities, and renal complications[79]. Infections are the most common complication, with an incidence exceeding 40%. These include bacterial peritonitis, urinary tract infections, sepsis, and surgical site infections[80], with cellulitis at the pump or catheter site being the most frequent. Hyponatremia is the most common electrolyte abnormality, which is reported to occur due to high pump rate settings, and resolves after lowering the pump rate[81]. Alfapump treatment may significantly activate the endogenous vasoconstrictor system and impair renal function, with up to 30% of patients experiencing AKI[82]. Studies have noted a gradual decline in GFR after alfapump implantation, potentially affecting ECV and leading to vasoconstrictor system activation, similar to circulatory dysfunction after paracentesis[83]. Administering intravenous albumin during alfapump treatment may inhibit vasoconstrictor system activation and prevent renal injury. Bellot et al[84] found that even small continuous removal of ascites without albumin supplementation might further reduce ECV in patients with refractory ascites. Patients with renal insufficiency, serum creatinine > 132 μmol/L, or estimated GFR < 30 mL/min/1.32 m should avoid alfapump implantation. Other contraindications include recent infection, recent abdominal surgery, a history of bladder cancer, a history of solid organ transplantation, and bilirubin levels > 85 μmol/L[85]. Thus, ideal candidates for alfapump treatment are patients with refractory ascites in relatively good nutritional status, with normal liver and kidney function, and no concurrent infection[86]. The total daily volume drained by the alfapump should not exceed 1 L, and dietary measures may be taken to reduce ascites production if necessary. Continuous ascites drainage and increased intra-abdominal pressure in refractory ascites patients can lead to AKI, necessitating albumin infusion during ascites drainage[87]. In cases of suspected infection, immediate antibiotic treatment is recommended to reduce complications. In conclusion, for patients with non-malignant refractory ascites who are unsuitable for alternative treatments such as TIPS or liver transplantation, alfapump implantation is an effective treatment option[88].
Figure 3.
Schematic presentation of the automated low-flow ascites pump system. Ascitic fluid is aspirated through a catheter placed into the abdominal cavity and further transported into the bladder through a subcutaneous catheter. The battery-driven pump is implanted in the right middle quadrant of the abdomen.
CART
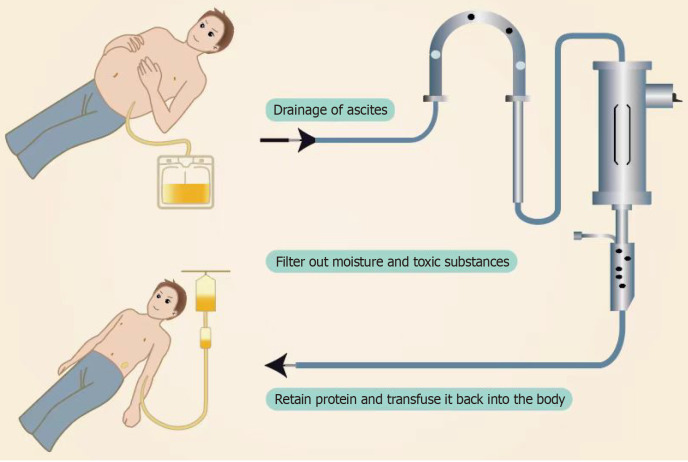
CART was first reported in 1977[89]. It is mainly used for treating patients with ascites due to decompensated cirrhosis (Figure 4). The purpose of CART is to maintain plasma colloid osmotic pressure by reinfusing proteins recovered from ascites[90]. The CART procedure includes several steps: First, paracentesis is performed to remove ascites into a drainage bag. Next, filtration is used to remove pathogens and small molecular harmful substances such as urea nitrogen, creatinine, and bilirubin. Then, excess water is removed through concentration. Finally, the liquid obtained from these steps, including useful proteins like albumin and globulin, is reinfused intravenously[91]. This method avoids the loss of proteins contained in the ascites, reduces the cost of using large amounts of albumin, and avoids the risk of infection from using blood products. The main indication for CART is ascites due to cirrhosis, but it has also been used to treat malignant ascites. Compared to LVP, CART significantly increases the amount of ascites that can be removed in a single session, up to a maximum of 8000 mL. The treatment process lasts about 2-3 hours and can quickly relieve abdominal distension, reduce the burden of ascites, and increase nutritional intake[92]. CART therapy has been reported to reduce abdominal circumference, improve diuretic resistance, and enhance the patients’ quality of life[93]. Compared to continued use of diuretics, CART reduces the risk of complications and increases serum albumin levels[94]. Studies have found that CART prevents the loss of albumin and globulin, thereby improving nutritional and immune status[95]. Yamada et al[96] reported that CART raised albumin levels more effectively than simple paracentesis or albumin infusion alone. The reinfusion of albumin increases plasma colloid osmotic pressure, corrects insufficient renal blood perfusion, improves renal function, and promotes diuresis[97]. Jatoi et al[98] showed significant increases in albumin, total protein, and eGFR after concentrated ascites reinfusion, suggesting a better effect than LVP alone. CART has better safety compared to previous ascites reinfusion or PVS surgeries, with efficacy comparable to LVP combined with albumin[99]. Moreover, Matsusaki et al[100] developed a new CART system with a membrane cleaning function (KM-CART) that can handle larger volumes of refractory ascites. Since most patients with decompensated cirrhosis also have chronic kidney disease, large-volume ascites drainage can quickly reduce intra-abdominal pressure, worsening effective blood volume circulation. Thus, it is crucial to shorten the dehydration time as much as possible[101]. KM-CART operates at high speed, shortening the dehydration time caused by large-volume ascites drainage, reducing the impact on renal function, and increasing urine output. However, CART also has disadvantages such as high equipment costs, complex procedures, and allergic reactions[102]. Complications may include hypotension, decreased fibrinogen, and fever[103]. Fever is a relatively common adverse event after concentrated ascites reinfusion, but pre-treatment with steroids and/or nonsteroidal anti-inflammatory drugs can prevent it[104]. CART should be used with caution in patients with severe cardiac disease, electrolyte imbalances, renal failure, severe abdominal or peritoneal skin infections, bleeding tendencies, and hepatic encephalopathy. Overall, CART maintains plasma colloid osmotic pressure, improves nutrition, and enhances the quality of life for patients with refractory ascites due to decompensated cirrhosis without significantly impacting renal function, making it an effective palliative treatment[105]. However, CART is currently mainly used in Japan, and its global applicability remains to be verified[106].
Figure 4.
Schematic illustration of the concentrated ascites reinfusion therapy process.
Peritoneal catheter drainage
Peritoneal catheter drainage serves as an effective method for managing cirrhotic ascites. Studies suggest that long-term abdominal drainage provides a safe and effective palliative intervention for end-stage liver disease (Figure 5)[107]. Various catheter placements have been recommended as an alternative to traditional treatments for refractory ascites[108]. This approach involves inserting a peritoneal puncture catheter kit into the abdominal cavity under local anesthesia guided by ultrasound. The drainage amount is adjusted based on the patient’s condition, along with basic treatments such as liver protection, diuretics, and albumin supplementation. The catheter is removed once ascites is no longer present or ultrasound indicates near-total absorption. Typically, daily drainage is limited to 5 hours, with volumes controlled between 800 and 1500 mL over 3 to 7 days. Research indicates that peritoneal indwelling central venous catheters can provide continuous drainage, reducing the need for multiple punctures[109,110]. Adjusting the drainage volume according to the patient’s blood volume and renal function helps avoid circulatory dysfunction and the complications of repeated punctures, thereby minimizing patient discomfort. This method significantly alleviates symptoms like abdominal distension, poor appetite, and dyspnea, improving quality of life. Compared to LVP, continuous drainage via peritoneal catheter reduces the number of punctures, prevents ascites accumulation, and avoids circulatory dysfunction, thus decreasing the incidence of complications. Studies confirm that peritoneal catheter drainage does not increase the risk of renal function impairment, and plasma blood urea nitrogen levels decrease significantly after two weeks of catheterization[111]. The soft texture and high compatibility of the indwelling catheter with human tissue minimize damage to abdominal organs. Connecting the catheter to a drainage bag and monitoring blood pressure and intra-abdominal pressure enables slow, continuous ascites drainage, leading to gradual reduction in abdominal pressure. This approach prevents sudden fluctuations in blood volume and internal environment, making the procedure safer and more reliable, reducing patient discomfort, and improving tolerance. Faster resolution of ascites and better control of abdominal infections can shorten hospital stays. Active volume expansion therapy, such as daily supplementation with 10 g human albumin after ascites drainage, effectively prevents puncture-induced circulatory dysfunction. Studies show no significant changes in renal function, indicated by stable creatinine levels, before and after puncture with regular albumin supplementation[112]. Given these advantages, peritoneal catheter drainage has significant clinical application value. However, complications such as abdominal infection, hyponatremia, hepatic encephalopathy, and subcutaneous or intramuscular hematoma hematomas can occur[113]. The most critical issue is the risk of SBP due to prolonged drainage[114]. Ascitic fluid serves as an excellent culture medium for bacteria, and cirrhotic patients have weakened immunity, leading to a higher incidence of SBP. Studies indicate that draining ascites for more than 24 hours increases the risk of ascites-related bacterial peritonitis and AKI. Therefore, completing ascites drainage within 24 hours is recommended, especially for cirrhotic patients with a Child-Pugh C grade or higher MELD score[115]. Stratmann et al[116] suggest a maximum catheter retention time of 72 hours, although they found no direct link between retention time and infection complications. Other studies confirm the theoretical adverse effects of prolonged catheter retention on infection and survival in cirrhotic patients[117]. Repeated monitoring of white blood cell and neutrophil counts in drained ascites is necessary during catheter retention, and prophylactic antibiotics may be required to prevent infection. In conclusion, peritoneal catheter drainage for cirrhotic ascites offers a high symptom relief rate, low cost, and low incidence of related complications, reducing the need for repeated paracentesis procedures[118]. It can effectively remove ascites before procedures like TIPS or liver transplantation, potentially becoming a cornerstone in the future management of cirrhotic ascites. However, minimizing catheter retention time is crucial to prevent complications.
Figure 5.
Peritoneal catheterization for the drainage of ascites.
CONCLUSION
Different drainage methods for cirrhotic ascites have their advantages and disadvantages. PVS is rarely used currently due to its high complication rate. Initial treatment for most refractory ascites should prioritize LVP combined with albumin infusion and peritoneal catheter drainage. If these treatments are ineffective or cause severe complications, TIPS or alfapump may be considered. CART, primarily used in Japan, requires further validation for suitability in other populations. Clinicians must evaluate the patient’s specific condition and circumstances to determine the most appropriate treatment.
ACKNOWLEDGEMENTS
We would like to thank our colleagues for their valuable contributions to this review. We gratefully acknowledge Jinan University and Shenzhen People’s Hospital for providing the necessary support for this study.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Yoshioka K S-Editor: Fan M L-Editor: Ma JY P-Editor: Zhang L
Contributor Information
Jia-Xing Yang, Department of Gastroenterology, The Second Clinical Medical College, Jinan University, Shenzhen 518000, Guangdong Province, China.
Yue-Ming Peng, Department of Nursing, Shenzhen People’s Hospital, The Second Clinical Medical College, Jinan University, Shenzhen 518000, Guangdong Province, China.
Hao-Tian Zeng, Department of Gastroenterology, The Second Clinical Medical College, Jinan University, Shenzhen 518000, Guangdong Province, China.
Xi-Min Lin, Department of Gastroenterology, The Second Clinical Medical College, Jinan University, Shenzhen 518000, Guangdong Province, China.
Zheng-Lei Xu, Department of Gastroenterology, Shenzhen People’s Hospital, The Second Clinical Medical College, Jinan University, Shenzhen 518000, Guangdong Province, China. 78249073@qq.com.
References
- 1.Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, Wilkes EA, Moore K, Leithead JA, Hayes PC, O'Brien AJ, Verma S. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9–29. doi: 10.1136/gutjnl-2020-321790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen JS, Bendtsen F, Møller S. Management of cirrhotic ascites. Ther Adv Chronic Dis. 2015;6:124–137. doi: 10.1177/2040622315580069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larrue H, Vinel JP, Bureau C. Management of Severe and Refractory Ascites. Clin Liver Dis. 2021;25:431–440. doi: 10.1016/j.cld.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Neong SF, Adebayo D, Wong F. An update on the pathogenesis and clinical management of cirrhosis with refractory ascites. Expert Rev Gastroenterol Hepatol. 2019;13:293–305. doi: 10.1080/17474124.2018.1555469. [DOI] [PubMed] [Google Scholar]
- 6.Chinese Society of Hepatology Chinese Medical Association. [Guidelines on the management of ascites in cirrhosis (2023 version)] Zhonghua Gan Zang Bing Za Zhi. 2023;31:813–826. doi: 10.3760/cma.j.cn501113-20230719-00011. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Zhao R, Lu J, Shi Y, Zhao H, Xu K, Sheng J. Current management of refractory ascites in patients with cirrhosis. J Int Med Res. 2018;46:1138–1145. doi: 10.1177/0300060517735231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orman ES, Hayashi PH, Bataller R, Barritt AS 4th. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12:496–503.e1. doi: 10.1016/j.cgh.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaccherini G, Tufoni M, Iannone G, Caraceni P. Management of Ascites in Patients with Cirrhosis: An Update. J Clin Med. 2021;10 doi: 10.3390/jcm10225226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginés P, Arroyo V, Quintero E, Planas R, Bory F, Cabrera J, Rimola A, Viver J, Camps J, Jiménez W. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234–241. doi: 10.1016/0016-5085(87)91007-9. [DOI] [PubMed] [Google Scholar]
- 12.Martin DK, Walayat S, Jinma R, Ahmed Z, Ragunathan K, Dhillon S. Large-volume paracentesis with indwelling peritoneal catheter and albumin infusion: a community hospital study. J Community Hosp Intern Med Perspect. 2016;6:32421. doi: 10.3402/jchimp.v6.32421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong F. Management of refractory ascites. Clin Mol Hepatol. 2023;29:16–32. doi: 10.3350/cmh.2022.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay AJ, Burton J, Ray CE Jr. Paracentesis-induced circulatory dysfunction: a primer for the interventional radiologist. Semin Intervent Radiol. 2014;31:276–278. doi: 10.1055/s-0034-1382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandna S, Zarate ER, Gallegos-Orozco JF. Management of Decompensated Cirrhosis and Associated Syndromes. Surg Clin North Am. 2022;102:117–137. doi: 10.1016/j.suc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Harvey JJ, Prentice R, George J. Diagnostic and therapeutic abdominal paracentesis. Med J Aust. 2023;218:18–21. doi: 10.5694/mja2.51795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsochatzis EA, Gerbes AL. Diagnosis and treatment of ascites. J Hepatol. 2017;67:184–185. doi: 10.1016/j.jhep.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X, Bai Z, Wang T, Romeiro FG, Mancuso A, Philips CA, Wong YJ, Nery FG, Qi X. Human Albumin Infusion for the Management of Liver Cirrhosis and Its Complications: An Overview of Major Findings from Meta-analyses. Adv Ther. 2023;40:1494–1529. doi: 10.1007/s12325-023-02430-3. [DOI] [PubMed] [Google Scholar]
- 19.Caraceni P, Abraldes JG, Ginès P, Newsome PN, Sarin SK. The search for disease-modifying agents in decompensated cirrhosis: From drug repurposing to drug discovery. J Hepatol. 2021;75 Suppl 1:S118–S134. doi: 10.1016/j.jhep.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Tan HK, James PD, Wong F. Albumin May Prevent the Morbidity of Paracentesis-Induced Circulatory Dysfunction in Cirrhosis and Refractory Ascites: A Pilot Study. Dig Dis Sci. 2016;61:3084–3092. doi: 10.1007/s10620-016-4140-3. [DOI] [PubMed] [Google Scholar]
- 21.Piano S, Tonon M, Angeli P. Management of ascites and hepatorenal syndrome. Hepatol Int. 2018;12:122–134. doi: 10.1007/s12072-017-9815-0. [DOI] [PubMed] [Google Scholar]
- 22.Romanelli RG, La Villa G, Barletta G, Vizzutti F, Lanini F, Arena U, Boddi V, Tarquini R, Pantaleo P, Gentilini P, Laffi G. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol. 2006;12:1403–1407. doi: 10.3748/wjg.v12.i9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai Z, Wang L, Wang R, Zou M, Méndez-Sánchez N, Romeiro FG, Cheng G, Qi X. Use of human albumin infusion in cirrhotic patients: a systematic review and meta-analysis of randomized controlled trials. Hepatol Int. 2022;16:1468–1483. doi: 10.1007/s12072-022-10374-z. [DOI] [PubMed] [Google Scholar]
- 24.Di Pascoli M, Fasolato S, Piano S, Bolognesi M, Angeli P. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019;39:98–105. doi: 10.1111/liv.13968. [DOI] [PubMed] [Google Scholar]
- 25.Møller S, Henriksen JH, Bendtsen F. Ascites: pathogenesis and therapeutic principles. Scand J Gastroenterol. 2009;44:902–911. doi: 10.1080/00365520902912555. [DOI] [PubMed] [Google Scholar]
- 26.Adebayo D, Neong SF, Wong F. Refractory Ascites in Liver Cirrhosis. Am J Gastroenterol. 2019;114:40–47. doi: 10.1038/s41395-018-0185-6. [DOI] [PubMed] [Google Scholar]
- 27.Wong F. Management of ascites in cirrhosis. J Gastroenterol Hepatol. 2012;27:11–20. doi: 10.1111/j.1440-1746.2011.06925.x. [DOI] [PubMed] [Google Scholar]
- 28.Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, DeMeo J, Cole PE, Tisnado J. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997;112:889–898. doi: 10.1053/gast.1997.v112.pm9041251. [DOI] [PubMed] [Google Scholar]
- 29.Barrio J, Ripoll C, Bañares R, Echenagusia A, Catalina MV, Camúñez F, Simó G, Santos L. Comparison of transjugular intrahepatic portosystemic shunt dysfunction in PTFE-covered stent-grafts versus bare stents. Eur J Radiol. 2005;55:120–124. doi: 10.1016/j.ejrad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhu P, Dong S, Sun P, Belgaumkar AP, Sun Y, Cheng X, Zheng Q, Li T. Expanded polytetrafluoroethylene (ePTFE)-covered stents versus bare stents for transjugular intrahepatic portosystemic shunt in people with liver cirrhosis. Cochrane Database Syst Rev. 2023;8:CD012358. doi: 10.1002/14651858.CD012358.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommer CM, Gockner TL, Stampfl U, Bellemann N, Sauer P, Ganten T, Weitz J, Kauczor HU, Radeleff BA. Technical and clinical outcome of transjugular intrahepatic portosystemic stent shunt: bare metal stents (BMS) versus viatorr stent-grafts (VSG) Eur J Radiol. 2012;81:2273–2280. doi: 10.1016/j.ejrad.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Angermayr B, Cejna M, Koenig F, Karnel F, Hackl F, Gangl A, Peck-Radosavljevic M Vienna TIPS Study Group. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stentgrafts versus bare stents. Hepatology. 2003;38:1043–1050. doi: 10.1053/jhep.2003.50423. [DOI] [PubMed] [Google Scholar]
- 33.Bureau C, Garcia Pagan JC, Layrargues GP, Metivier S, Bellot P, Perreault P, Otal P, Abraldes JG, Peron JM, Rousseau H, Bosch J, Vinel JP. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27:742–747. doi: 10.1111/j.1478-3231.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 34.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 35.Perarnau JM, Le Gouge A, Nicolas C, d'Alteroche L, Borentain P, Saliba F, Minello A, Anty R, Chagneau-Derrode C, Bernard PH, Abergel A, Ollivier-Hourmand I, Gournay J, Ayoub J, Gaborit C, Rusch E, Giraudeau B STIC-TIPS group. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014;60:962–968. doi: 10.1016/j.jhep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Kwan SW, Allison SK, Gold LS, Shin DS. Cost-Effectiveness of Transjugular Intrahepatic Portosystemic Shunt versus Large-Volume Paracentesis in Refractory Ascites: Results of a Markov Model Incorporating Individual Patient-Level Meta-Analysis and Nationally Representative Cost Data. J Vasc Interv Radiol. 2018;29:1705–1712. doi: 10.1016/j.jvir.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triantafyllou T, Aggarwal P, Gupta E, Svetanoff WJ, Bhirud DP, Singhal S. Polytetrafluoroethylene-Covered Stent Graft Versus Bare Stent in Transjugular Intrahepatic Portosystemic Shunt: Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2018;28:867–879. doi: 10.1089/lap.2017.0560. [DOI] [PubMed] [Google Scholar]
- 38.Schepis F, Vizzutti F, Garcia-Tsao G, Marzocchi G, Rega L, De Maria N, Di Maira T, Gitto S, Caporali C, Colopi S, De Santis M, Arena U, Rampoldi A, Airoldi A, Cannavale A, Fanelli F, Mosconi C, Renzulli M, Agazzi R, Nani R, Quaretti P, Fiorina I, Moramarco L, Miraglia R, Luca A, Bruno R, Fagiuoli S, Golfieri R, Torricelli P, Di Benedetto F, Belli LS, Banchelli F, Laffi G, Marra F, Villa E. Under-dilated TIPS Associate With Efficacy and Reduced Encephalopathy in a Prospective, Non-randomized Study of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2018;16:1153–1162.e7. doi: 10.1016/j.cgh.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Trebicka J, Bastgen D, Byrtus J, Praktiknjo M, Terstiegen S, Meyer C, Thomas D, Fimmers R, Treitl M, Euringer W, Sauerbruch T, Rössle M. Smaller-Diameter Covered Transjugular Intrahepatic Portosystemic Shunt Stents Are Associated With Increased Survival. Clin Gastroenterol Hepatol. 2019;17:2793–2799.e1. doi: 10.1016/j.cgh.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 40.Miraglia R, Maruzzelli L, Tuzzolino F, Petridis I, D'Amico M, Luca A. Transjugular Intrahepatic Portosystemic Shunts in Patients with Cirrhosis with Refractory Ascites: Comparison of Clinical Outcomes by Using 8- and 10-mm PTFE-covered Stents. Radiology. 2017;284:281–288. doi: 10.1148/radiol.2017161644. [DOI] [PubMed] [Google Scholar]
- 41.Bucsics T, Hoffman S, Grünberger J, Schoder M, Matzek W, Stadlmann A, Mandorfer M, Schwabl P, Ferlitsch A, Peck-Radosavljevic M, Trauner M, Karner J, Karnel F, Reiberger T. ePTFE-TIPS vs repetitive LVP plus albumin for the treatment of refractory ascites in patients with cirrhosis. Liver Int. 2018;38:1036–1044. doi: 10.1111/liv.13615. [DOI] [PubMed] [Google Scholar]
- 42.Fagiuoli S, Bruno R, Debernardi Venon W, Schepis F, Vizzutti F, Toniutto P, Senzolo M, Caraceni P, Salerno F, Angeli P, Cioni R, Vitale A, Grosso M, De Gasperi A, D'Amico G, Marzano A AISF TIPS Special Conference. Consensus conference on TIPS management: Techniques, indications, contraindications. Dig Liver Dis. 2017;49:121–137. doi: 10.1016/j.dld.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Sanyal AJ, Freedman AM, Purdum PP, Shiffman ML, Luketic VA. The hematologic consequences of transjugular intrahepatic portosystemic shunts. Hepatology. 1996;23:32–39. doi: 10.1002/hep.510230105. [DOI] [PubMed] [Google Scholar]
- 44.Mizrahi M, Adar T, Shouval D, Bloom AI, Shibolet O. Endotipsitis-persistent infection of transjugular intrahepatic portosystemic shunt: pathogenesis, clinical features and management. Liver Int. 2010;30:175–183. doi: 10.1111/j.1478-3231.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 45.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 46.Siramolpiwat S. Transjugular intrahepatic portosystemic shunts and portal hypertension-related complications. World J Gastroenterol. 2014;20:16996–17010. doi: 10.3748/wjg.v20.i45.16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerbes AL, Gülberg V. Benefit of TIPS for patients with refractory or recidivant ascites: serum bilirubin may make the difference. Hepatology. 2005;41:217. doi: 10.1002/hep.20509. [DOI] [PubMed] [Google Scholar]
- 48.Rudler M, Mallet M, Sultanik P, Bouzbib C, Thabut D. Optimal management of ascites. Liver Int. 2020;40 Suppl 1:128–135. doi: 10.1111/liv.14361. [DOI] [PubMed] [Google Scholar]
- 49.Boyer TD, Haskal ZJ American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 50.Khungar V, Saab S. Cirrhosis with refractory ascites: serial large volume paracentesis, TIPS, or transplantation? Clin Gastroenterol Hepatol. 2011;9:931–5; quiz e121. doi: 10.1016/j.cgh.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 51.Lan T, Chen M, Tang C, Deltenre P. Recent developments in the management of ascites in cirrhosis. United European Gastroenterol J. 2024;12:261–272. doi: 10.1002/ueg2.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deltenre P, Mathurin P, Dharancy S, Moreau R, Bulois P, Henrion J, Pruvot FR, Ernst O, Paris JC, Lebrec D. Transjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysis. Liver Int. 2005;25:349–356. doi: 10.1111/j.1478-3231.2005.01095.x. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Tsao G. The transjugular intrahepatic portosystemic shunt for the management of cirrhotic refractory ascites. Nat Clin Pract Gastroenterol Hepatol. 2006;3:380–389. doi: 10.1038/ncpgasthep0523. [DOI] [PubMed] [Google Scholar]
- 54.Bai M, Qi XS, Yang ZP, Yang M, Fan DM, Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704–2714. doi: 10.3748/wjg.v20.i10.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iannone G, Pompili E, De Venuto C, Pratelli D, Tedesco G, Baldassarre M, Caraceni P, Zaccherini G. The Role of Transjugular Intrahepatic Portosystemic Shunt for the Management of Ascites in Patients with Decompensated Cirrhosis. J Clin Med. 2024;13 doi: 10.3390/jcm13051349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, Mathurin P, Otal P, Cabarrou P, Péron JM, Vinel JP. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology. 2017;152:157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 57.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Fonio P, Discalzi A, Calandri M, Doriguzzi Breatta A, Bergamasco L, Martini S, Ottobrelli A, Righi D, Gandini G. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol Med. 2017;122:713–721. doi: 10.1007/s11547-017-0770-6. [DOI] [PubMed] [Google Scholar]
- 59.Bercu ZL, Fischman AM, Kim E, Nowakowski FS, Patel RS, Schiano TD, Chang CY, Lookstein RA. TIPS for refractory ascites: a 6-year single-center experience with expanded polytetrafluoroethylene-covered stent-grafts. AJR Am J Roentgenol. 2015;204:654–661. doi: 10.2214/AJR.14.12885. [DOI] [PubMed] [Google Scholar]
- 60.Vadeyar HJ, Doran JD, Charnley R, Ryder SD. Saphenoperitoneal shunts for patients with intractable ascites associated with chronic liver disease. Br J Surg. 1999;86:882–885. doi: 10.1046/j.1365-2168.1999.01156.x. [DOI] [PubMed] [Google Scholar]
- 61.Ginès P, Arroyo V, Vargas V, Planas R, Casafont F, Panés J, Hoyos M, Viladomiu L, Rimola A, Morillas R. Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. N Engl J Med. 1991;325:829–835. doi: 10.1056/NEJM199109193251201. [DOI] [PubMed] [Google Scholar]
- 62.Segawa T, Kato K, Kawashima K, Suzuki T, Ehara S. The influence of a peritoneovenous shunt for cirrhotic and malignant intractable ascites on renal function. Acta Radiol Open. 2018;7:2058460118764208. doi: 10.1177/2058460118764208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ginès A, Planas R, Angeli P, Guarner C, Salerno F, Ginès P, Saló J, Rodriguez N, Domènech E, Soriano G. Treatment of patients with cirrhosis and refractory ascites using LeVeen shunt with titanium tip: comparison with therapeutic paracentesis. Hepatology. 1995;22:124–131. doi: 10.1002/hep.1840220120. [DOI] [PubMed] [Google Scholar]
- 64.Koyama S, Nogami A, Yoneda M, Cheng S, Koike Y, Takeuchi Y, Iwaki M, Kobayashi T, Saito S, Utsunomiya D, Nakajima A. Chronological Course and Clinical Features after Denver Peritoneovenous Shunt Placement in Decompensated Liver Cirrhosis. Tomography. 2024;10:471–479. doi: 10.3390/tomography10040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukui H, Kawaratani H, Kaji K, Takaya H, Yoshiji H. Management of refractory cirrhotic ascites: challenges and solutions. Hepat Med. 2018;10:55–71. doi: 10.2147/HMER.S136578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White MA, Agle SC, Padia RK, Zervos EE. Denver peritoneovenous shunts for the management of malignant ascites: a review of the literature in the post LeVeen Era. Am Surg. 2011;77:1070–1075. doi: 10.1177/000313481107700830. [DOI] [PubMed] [Google Scholar]
- 67.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Wong F. Innovative approaches to the management of ascites in cirrhosis. JHEP Rep. 2023;5:100749. doi: 10.1016/j.jhepr.2023.100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dembinski J, Aranovich D, Banz V, Ehmann T, Klein I, Malago M, Richter N, Schnitzbauer AA, Staszewicz W, Tautenhahn HM, Capel J, Regimbeau JM. Surgical technique for placement of the automated low flow ascites pump (Alfapump) Langenbecks Arch Surg. 2020;405:117–123. doi: 10.1007/s00423-019-01822-w. [DOI] [PubMed] [Google Scholar]
- 70.Stirnimann G, Banz V, Storni F, De Gottardi A. Automated low-flow ascites pump for the treatment of cirrhotic patients with refractory ascites. Therap Adv Gastroenterol. 2017;10:283–292. doi: 10.1177/1756283X16684688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fotopoulou C, Berg T, Hausen A, Hennig R, Jalan R, Malagó M, Capel J, De Gottardi A, Stirnimann G. Continuous low flow ascites drainage through the urinary bladder via the Alfapump system in palliative patients with malignant ascites. BMC Palliat Care. 2019;18:109. doi: 10.1186/s12904-019-0497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lepida A, Marot A, Trépo E, Degré D, Moreno C, Deltenre P. Systematic review with meta-analysis: automated low-flow ascites pump therapy for refractory ascites. Aliment Pharmacol Ther. 2019;50:978–987. doi: 10.1111/apt.15502. [DOI] [PubMed] [Google Scholar]
- 73.Thomas MN, Sauter GH, Gerbes AL, Stangl M, Schiergens TS, Angele M, Werner J, Guba M. Automated low flow pump system for the treatment of refractory ascites: a single-center experience. Langenbecks Arch Surg. 2015;400:979–983. doi: 10.1007/s00423-015-1356-1. [DOI] [PubMed] [Google Scholar]
- 74.Wong F, Bendel E, Sniderman K, Frederick T, Haskal ZJ, Sanyal A, Asrani SK, Capel J, Kamath PS. Improvement in Quality of Life and Decrease in Large-Volume Paracentesis Requirements With the Automated Low-Flow Ascites Pump. Liver Transpl. 2020;26:651–661. doi: 10.1002/lt.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stepanova M, Nader F, Bureau C, Adebayo D, Elkrief L, Valla D, Peck-Radosavljevic M, McCune A, Vargas V, Simon-Talero M, Cordoba J, Angeli P, Rossi S, MacDonald S, Capel J, Jalan R, Younossi ZM. Patients with refractory ascites treated with alfapump® system have better health-related quality of life as compared to those treated with large volume paracentesis: the results of a multicenter randomized controlled study. Qual Life Res. 2018;27:1513–1520. doi: 10.1007/s11136-018-1813-8. [DOI] [PubMed] [Google Scholar]
- 76.Weil-Verhoeven D, Di Martino V, Stirnimann G, Cervoni JP, Nguyen-Khac E, Thévenot T. Alfapump(®) implantable device in management of refractory ascites: An update. World J Hepatol. 2022;14:1344–1356. doi: 10.4254/wjh.v14.i7.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garbuzenko DV, Arefyev NO. Current approaches to the management of patients with cirrhotic ascites. World J Gastroenterol. 2019;25:3738–3752. doi: 10.3748/wjg.v25.i28.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Will V, Rodrigues SG, Berzigotti A. Current treatment options of refractory ascites in liver cirrhosis - A systematic review and meta-analysis. Dig Liver Dis. 2022;54:1007–1014. doi: 10.1016/j.dld.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 79.Bendel EC, Sniderman K, Shaw C, Frederick RT, Wong F, Sanyal A, Asrani SK, Kamath PS, Capel J, Haskal ZJ. Feasibility and Procedural Safety of alfapump System Implantation by IR: Experience from the MOSAIC Study, a Multicenter, Open-Label Prospective Study in Cirrhotic Patients with Refractory Ascites. J Vasc Interv Radiol. 2020;31:1256–1262.e3. doi: 10.1016/j.jvir.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Solbach P, Höner Zu Siederdissen C, Wellhöner F, Richter N, Heidrich B, Lenzen H, Kerstin P, Hueper K, Manns MP, Wedemeyer H, Jaeckel E. Automated low-flow ascites pump in a real-world setting: complications and outcomes. Eur J Gastroenterol Hepatol. 2018;30:1082–1089. doi: 10.1097/MEG.0000000000001149. [DOI] [PubMed] [Google Scholar]
- 81.Bureau C, Adebayo D, Chalret de Rieu M, Elkrief L, Valla D, Peck-Radosavljevic M, McCune A, Vargas V, Simon-Talero M, Cordoba J, Angeli P, Rosi S, MacDonald S, Malago M, Stepanova M, Younossi ZM, Trepte C, Watson R, Borisenko O, Sun S, Inhaber N, Jalan R. Alfapump® system vs. large volume paracentesis for refractory ascites: A multicenter randomized controlled study. J Hepatol. 2017;67:940–949. doi: 10.1016/j.jhep.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Will V, Rodrigues SG, Stirnimann G, Gottardi A, Bosch J, Berzigotti A. Transjugular intrahepatic portosystemic shunt and alfapump® system for refractory ascites in liver cirrhosis: Outcomes and complications. United European Gastroenterol J. 2020;8:961–969. doi: 10.1177/2050640620938525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Solà E, Sanchez-Cabús S, Rodriguez E, Elia C, Cela R, Moreira R, Pose E, Sánchez-Delgado J, Cañete N, Morales-Ruiz M, Campos F, Balust J, Guevara M, García-Valdecasas JC, Ginès P. Effects of alfapump™ system on kidney and circulatory function in patients with cirrhosis and refractory ascites. Liver Transpl. 2017;23:583–593. doi: 10.1002/lt.24763. [DOI] [PubMed] [Google Scholar]
- 84.Bellot P, Welker MW, Soriano G, von Schaewen M, Appenrodt B, Wiest R, Whittaker S, Tzonev R, Handshiev S, Verslype C, Moench C, Zeuzem S, Sauerbruch T, Guarner C, Schott E, Johnson N, Petrov A, Katzarov K, Nevens F, Zapater P, Such J. Automated low flow pump system for the treatment of refractory ascites: a multi-center safety and efficacy study. J Hepatol. 2013;58:922–927. doi: 10.1016/j.jhep.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 85.Aagaard NK, Malago M, De Gottardi A, Thomas M, Sauter G, Engelmann C, Aranovich D, Cohen M, Thévenot T, Ehmann T, Capel J, Angeli P, Jalan R, Stirnimann G. Consensus care recommendations for alfapump(®) in cirrhotic patients with refractory or recurrent ascites. BMC Gastroenterol. 2022;22:111. doi: 10.1186/s12876-022-02173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stirnimann G, Berg T, Spahr L, Zeuzem S, McPherson S, Lammert F, Storni F, Banz V, Babatz J, Vargas V, Geier A, Stallmach A, Engelmann C, Trepte C, Capel J, De Gottardi A. Treatment of refractory ascites with an automated low-flow ascites pump in patients with cirrhosis. Aliment Pharmacol Ther. 2017;46:981–991. doi: 10.1111/apt.14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shrestha DB, Budhathoki P, Sedhai YR, Baniya R, Awal S, Yadav J, Awal L, Davis B, Kashiouris MG, Cable CA. Safety and efficacy of human serum albumin treatment in patients with cirrhotic ascites undergoing paracentesis: A systematic review and meta-analysis. Ann Hepatol. 2021;26:100547. doi: 10.1016/j.aohep.2021.100547. [DOI] [PubMed] [Google Scholar]
- 88.Stirnimann G, Berg T, Spahr L, Zeuzem S, McPherson S, Lammert F, Storni F, Banz V, Babatz J, Vargas V, Geier A, Engelmann C, Herber A, Trepte C, Capel J, De Gottardi A. Final safety and efficacy results from a 106 real-world patients registry with an ascites-mobilizing pump. Liver Int. 2022;42:2247–2259. doi: 10.1111/liv.15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inoue N, Yamazaki Z, Oda T, Sugiura M, Wada T. Treatment of intractable ascites by continuous reinfusion of the sterilized, cell-free and concentrated ascitic fluid. Trans Am Soc Artif Intern Organs. 1977;23:699–702. [PubMed] [Google Scholar]
- 90.Iwasa M, Ishihara T, Kato M, Isoai A, Kobayashi R, Torii N, Soneda N, Takei Y. Cell-free and Concentrated Ascites Reinfusion Therapy for Refractory Ascites in Cirrhosis in Post-marketing Surveillance and the Role of Tolvaptan. Intern Med. 2019;58:3069–3075. doi: 10.2169/internalmedicine.3091-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ito T, Hanafusa N. CART: Cell-free and Concentrated Ascites Reinfusion Therapy against malignancy-related ascites. Transfus Apher Sci. 2017;56:703–707. doi: 10.1016/j.transci.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 92.Ito T, Hanafusa N, Fukui M, Yamamoto H, Watanabe Y, Noiri E, Iwase S, Miyagawa K, Fujita T, Nangaku M. Single center experience of cell-free and concentrated ascites reinfusion therapy in malignancy related ascites. Ther Apher Dial. 2014;18:87–92. doi: 10.1111/1744-9987.12049. [DOI] [PubMed] [Google Scholar]
- 93.Ito T, Hanafusa N, Iwase S, Noiri E, Nangaku M, Nakagawa K, Miyagawa K. Effects of cell-free and concentrated ascites reinfusion therapy (CART) on symptom relief of malignancy-related ascites. Int J Clin Oncol. 2015;20:623–628. doi: 10.1007/s10147-014-0750-y. [DOI] [PubMed] [Google Scholar]
- 94.Chen H, Ishihara M, Horita N, Tanzawa S, Kazahari H, Ochiai R, Sakamoto T, Honda T, Ichikawa Y, Watanabe K, Seki N. Effectiveness of Cell-Free and Concentrated Ascites Reinfusion Therapy in the Treatment of Malignancy-Related Ascites: A Systematic Review and Meta-Analysis. Cancers (Basel) 2021;13 doi: 10.3390/cancers13194873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kozaki K, IInuma M, Takagi T, Fukuda T, Sanpei T, Terunuma Y, Yatabe Y, Akano K. Cell-Free and Concentrated Ascites Reinfusion Therapy for Decompensated Liver Cirrhosis. Ther Apher Dial. 2016;20:376–382. doi: 10.1111/1744-9987.12469. [DOI] [PubMed] [Google Scholar]
- 96.Yamada Y, Inui K, Hara Y, Fuji K, Sonoda K, Hashimoto K, Kamijo Y. Verification of serum albumin elevating effect of cell-free and concentrated ascites reinfusion therapy for ascites patients: a retrospective controlled cohort study. Sci Rep. 2019;9:10195. doi: 10.1038/s41598-019-46774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanada R, Yokomichi N, Kato C, Miki K, Oyama S, Morita T, Kawahara R. Efficacy and safety of reinfusion of concentrated ascitic fluid for malignant ascites: a concept-proof study. Support Care Cancer. 2018;26:1489–1497. doi: 10.1007/s00520-017-3980-5. [DOI] [PubMed] [Google Scholar]
- 98.Jatoi A, Nieva JJ, Qin R, Loprinzi CL, Wos EJ, Novotny PJ, Moore DF Jr, Mowat RB, Bechar N, Pajon ER Jr, Hartmann LC. A pilot study of long-acting octreotide for symptomatic malignant ascites. Oncology. 2012;82:315–320. doi: 10.1159/000337246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graziotto A, Rossaro L, Inturri P, Salvagnini M. Reinfusion of concentrated ascitic fluid versus total paracentesis. A randomized prospective trial. Dig Dis Sci. 1997;42:1708–1714. doi: 10.1023/a:1018865516168. [DOI] [PubMed] [Google Scholar]
- 100.Japanese CART Study Group. Matsusaki K, Ohta K, Yoshizawa A, Gyoda Y. Novel cell-free and concentrated ascites reinfusion therapy (KM-CART) for refractory ascites associated with cancerous peritonitis: its effect and future perspectives. Int J Clin Oncol. 2011;16:395–400. doi: 10.1007/s10147-011-0199-1. [DOI] [PubMed] [Google Scholar]
- 101.Shimizu S, Ohira M, Nakano R, Imaoka Y, Sato K, Tahara H, Ide K, Kobayashi T, Kuroda S, Ono H, Tanaka Y, Ohdan H. Management of Refractory Ascites for Liver Transplant Candidates: A Novel Cell-free and Concentrated Ascites Reinfusion Therapy. Transplant Proc. 2019;51:2740–2744. doi: 10.1016/j.transproceed.2019.02.060. [DOI] [PubMed] [Google Scholar]
- 102.Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, Kawaguchi T, Kurosaki M, Sakaida I, Shimizu M, Taniai M, Terai S, Nishikawa H, Hiasa Y, Hidaka H, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for liver cirrhosis 2020. Hepatol Res. 2021;51:725–749. doi: 10.1111/hepr.13678. [DOI] [PubMed] [Google Scholar]
- 103.Zaak D, Paquet KJ, Kuhn R. Prospective study comparing human albumin vs. reinfusion of ultrafiltrate-ascitic fluid after total paracentesis in cirrhotic patients with tense ascites. Z Gastroenterol. 2001;39:5–10. doi: 10.1055/s-2001-10707. [DOI] [PubMed] [Google Scholar]
- 104.Hanafusa N, Isoai A, Ishihara T, Inoue T, Ishitani K, Utsugisawa T, Yamaka T, Ito T, Sugiyama H, Arakawa A, Yamada Y, Itano Y, Onodera H, Kobayashi R, Torii N, Numata T, Kashiwabara T, Matsuno Y, Kato M. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in refractory ascites: Post-marketing surveillance results. PLoS One. 2017;12:e0177303. doi: 10.1371/journal.pone.0177303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ito T, Hanafusa N, Soneda N, Isoai A, Kobayashi R, Torii N, Kato M. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy against cirrhotic ascites in comparison with malignancy-related ascites. J Gastroenterol Hepatol. 2021;36:3224–3232. doi: 10.1111/jgh.15620. [DOI] [PubMed] [Google Scholar]
- 106.Yorioka N, Namisaki T, Shibamoto A, Suzuki J, Kubo T, Iwai S, Tomooka F, Tanaka M, Takeda S, Fujimoto Y, Enomoto M, Muarata K, Inoue T, Tsuji Y, Fujinaga Y, Nishimura N, Kitagawa K, Takaya H, Kaji K, Kawaratani H, Akahane T, Mitoro A, Yamazaki M, Yoshiji H. Changes in Coagulation and Fibrinolytic Factors in Patients With Cirrhotic Refractory Ascites Undergoing Cell-free and Concentrated Ascites Reinfusion Therapy: A Retrospective Observational Study in Japan. In Vivo. 2023;37:1226–1235. doi: 10.21873/invivo.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Macken L, Joshi D, Messenger J, Austin M, Tibble J, Mason L, Verma S. Palliative long-term abdominal drains in refractory ascites due to end-stage liver disease: A case series. Palliat Med. 2017;31:671–675. doi: 10.1177/0269216316671281. [DOI] [PubMed] [Google Scholar]
- 108.Van Thiel DH, Moore CM, Garcia M, George M, Nadir A. Continuous peritoneal drainage of large-volume ascites. Dig Dis Sci. 2011;56:2723–2727. doi: 10.1007/s10620-011-1792-x. [DOI] [PubMed] [Google Scholar]
- 109.Caldwell J, Edriss H, Nugent K. Chronic peritoneal indwelling catheters for the management of malignant and nonmalignant ascites. Proc (Bayl Univ Med Cent) 2018;31:297–302. doi: 10.1080/08998280.2018.1461525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ratre BK, Suvvari P, Hoda W, Roychoudhury P, Bharti SJ, Bhatnagar S. Central Venous Catheter as Peritoneal Indwelling Catheter for the Management of Recurrent Malignant Ascites: A Case Series. Indian J Palliat Care. 2019;25:57–60. doi: 10.4103/IJPC.IJPC_145_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Solbach P, Höner Zu Siederdissen C, Taubert R, Ziegert S, Port K, Schneider A, Hueper K, Manns MP, Wedemeyer H, Jaeckel E. Home-based drainage of refractory ascites by a permanent-tunneled peritoneal catheter can safely replace large-volume paracentesis. Eur J Gastroenterol Hepatol. 2017;29:539–546. doi: 10.1097/MEG.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 112.Leache L, Gutiérrez-Valencia M, Saiz LC, Uriz J, Bolado F, García-Erce JA, Cantarelli L, Erviti J. Meta-analysis: Efficacy and safety of albumin in the prevention and treatment of complications in patients with cirrhosis. Aliment Pharmacol Ther. 2023;57:620–634. doi: 10.1111/apt.17344. [DOI] [PubMed] [Google Scholar]
- 113.Macken L, Bremner S, Gage H, Touray M, Williams P, Crook D, Mason L, Lambert D, Evans CJ, Cooper M, Timeyin J, Steer S, Austin M, Parnell N, Thomson SJ, Sheridan D, Wright M, Isaacs P, Hashim A, Verma S. Randomised clinical trial: palliative long-term abdominal drains vs large-volume paracentesis in refractory ascites due to cirrhosis. Aliment Pharmacol Ther. 2020;52:107–122. doi: 10.1111/apt.15802. [DOI] [PubMed] [Google Scholar]
- 114.Nadir A, Van Thiel DH. Frequency of peritoneal infections among patients undergoing continuous paracentesis with an indwelling catheter. J Ayub Med Coll Abbottabad. 2010;22:37–41. [PubMed] [Google Scholar]
- 115.Wong YJ, Lum HM, Tan PT, Teo EK, Tan J, Kumar R, Thurairajah PH. Clinical implications of prompt ascitic drain removal in cirrhosis with refractory ascites. Singapore Med J. 2021;62:659–664. doi: 10.11622/smedj.2021049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stratmann K, Fitting D, Zeuzem S, Bojunga J, Trebicka J, Friedrich-Rust M, Dultz G. Establishing an indwelling peritoneal catheter as a standard procedure for hospitalized patients with ascites: Retrospective data on feasibility, effectiveness and safety. United European Gastroenterol J. 2019;7:673–681. doi: 10.1177/2050640619842442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kathpalia P, Bhatia A, Robertazzi S, Ahn J, Cohen SM, Sontag S, Luke A, Durazo-Arvizu R, Pillai AA. Indwelling peritoneal catheters in patients with cirrhosis and refractory ascites. Intern Med J. 2015;45:1026–1031. doi: 10.1111/imj.12843. [DOI] [PubMed] [Google Scholar]
- 118.Kaur S, Motta RV, Chapman B, Wharton V, Collier JD, Saffioti F. Palliative long-term abdominal drains vs large volume paracenteses for the management of refractory ascites in end-stage liver disease. World J Hepatol. 2024;16:428–438. doi: 10.4254/wjh.v16.i3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]