Abstract
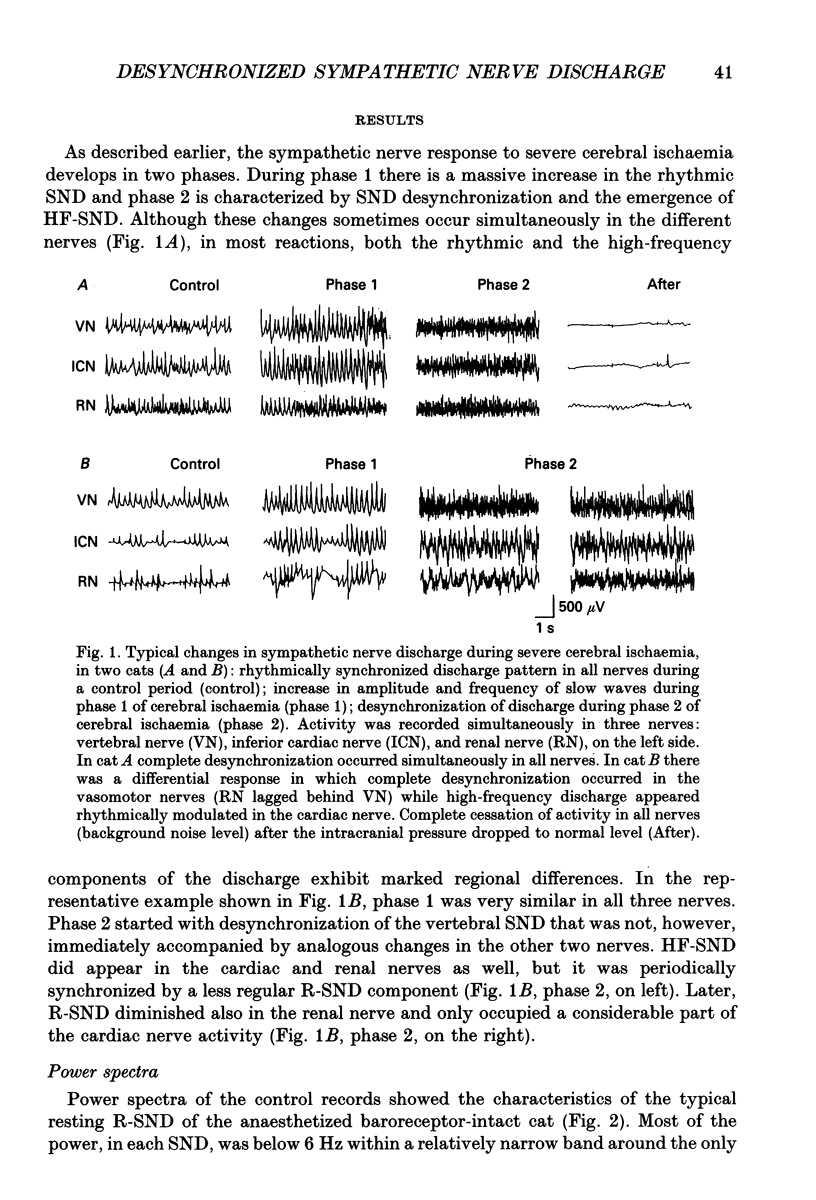
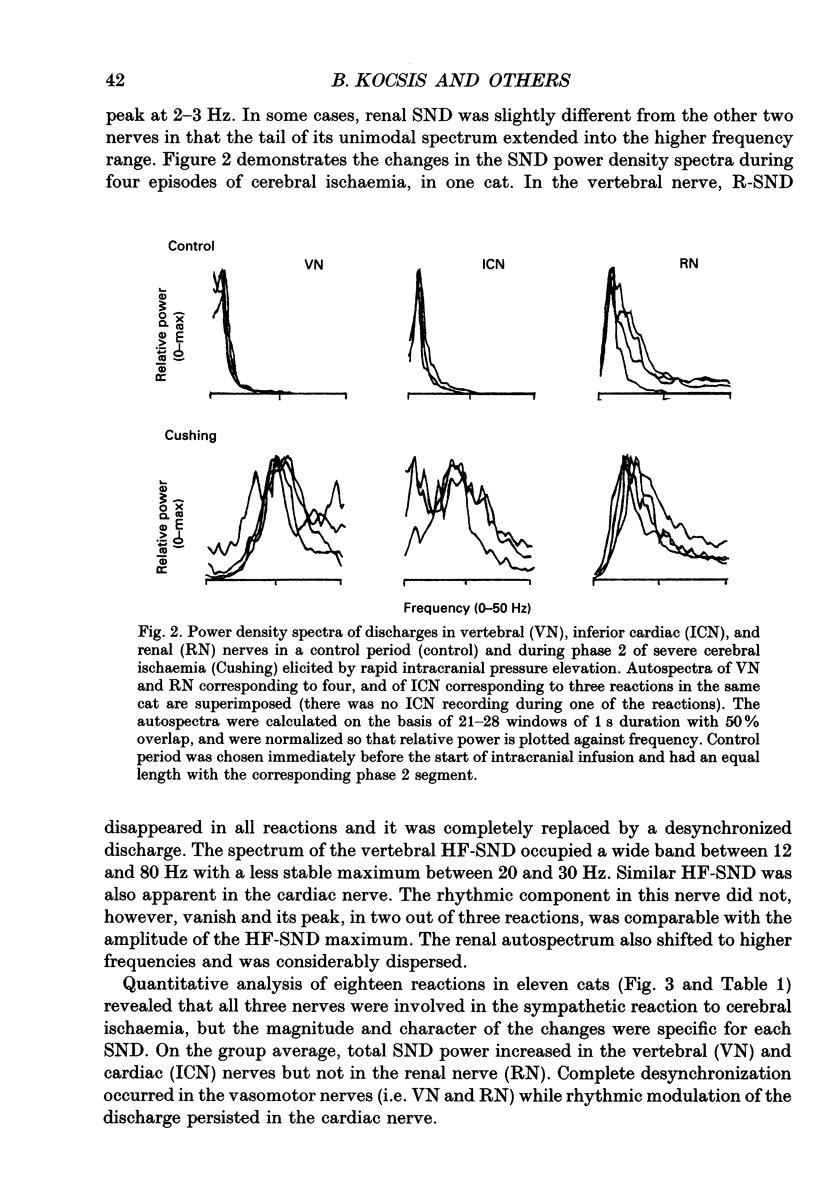
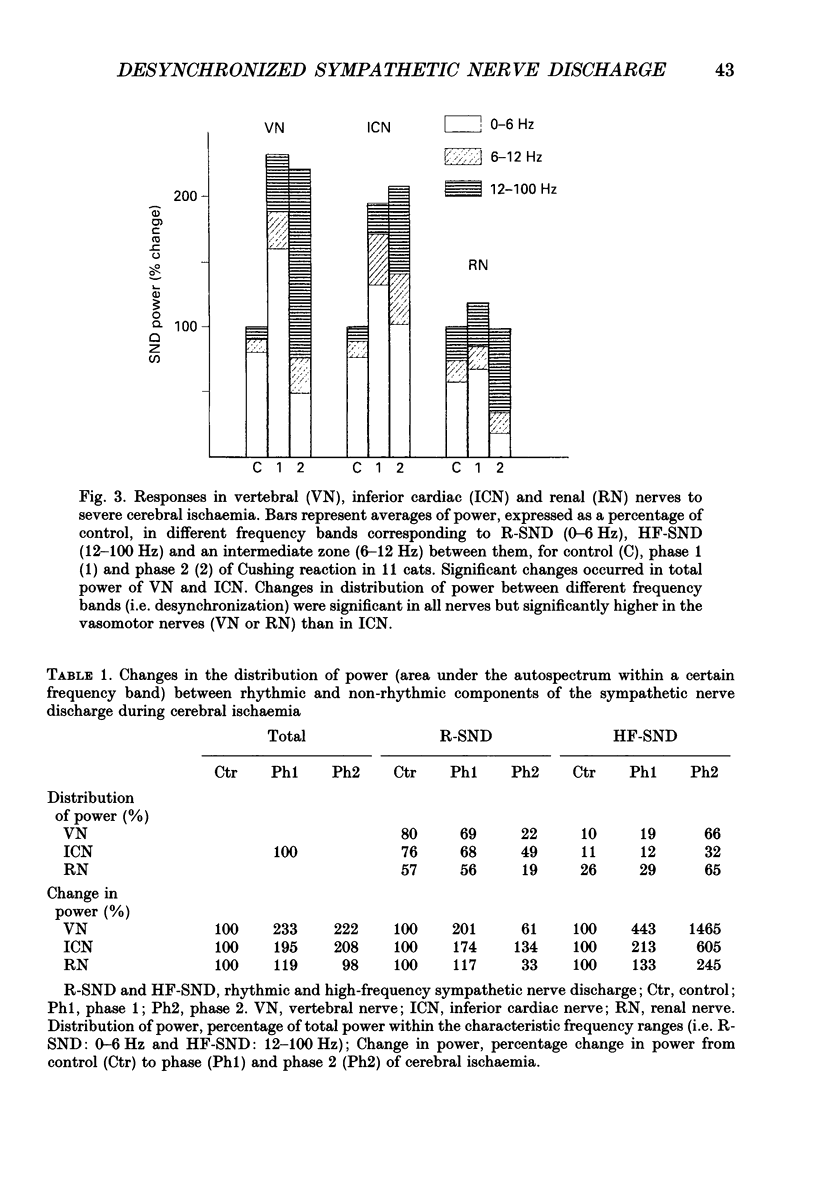
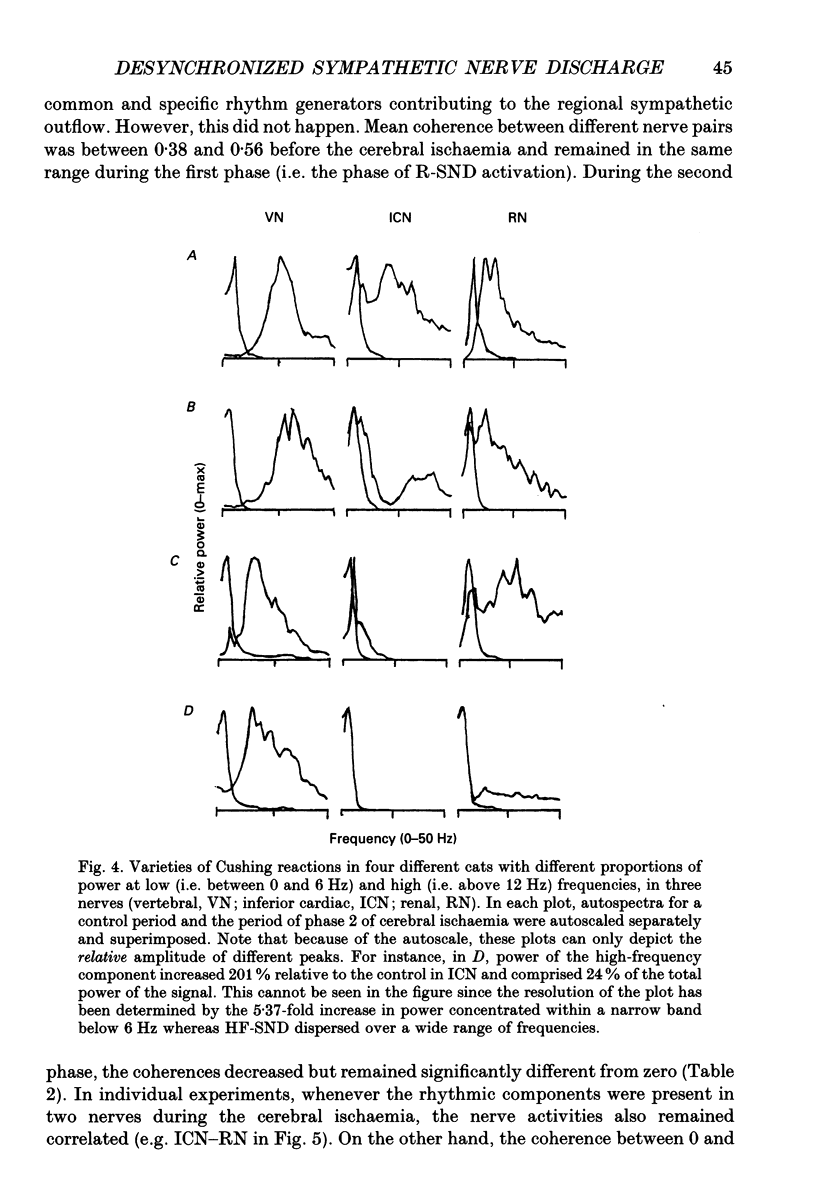
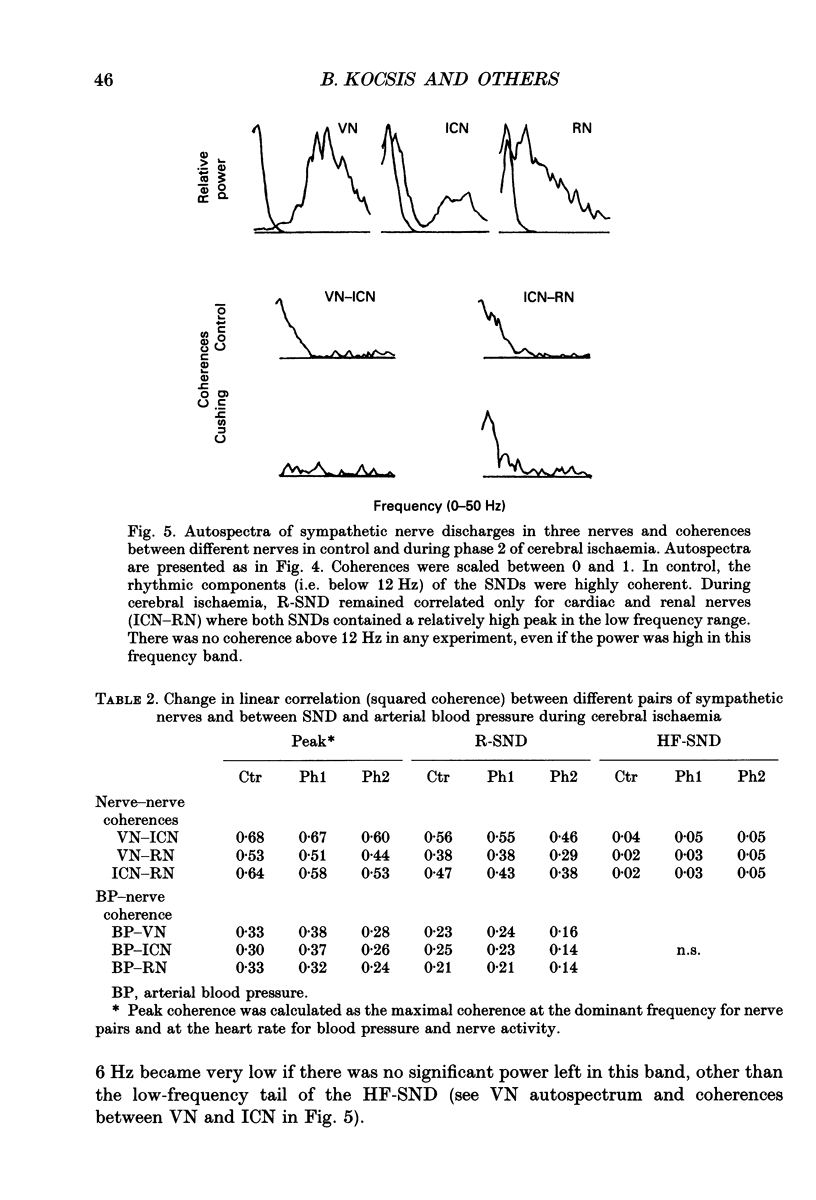
1. Sympathetic nerve discharge (SND) of three postganglionic nerves with different functions and anatomical locations was simultaneously recorded at rest and during severe cerebral ischaemia (Cushing reaction). The three nerves, controlling the heart (inferior cardiac nerve), visceral (renal nerve) and skeletal muscle circulation (vertebral nerve), were selected with the assumption that their activity pattern will represent the differential central autonomic command to the major players of the circulatory response to cerebral ischaemia. 2. Changes in the power density spectra of the nerve signals, and in the pairwise coherence functions, elicited by the cerebral ischaemia, were evaluated separately for the rhythmic (R-SND, i.e. between 0 and 6 Hz) and high-frequency (HF-SND, i.e. between 12 and 100 Hz) components of the nerve signals. 3. The sympathetic nerve response to cerebral ischaemia developed in two phases. Phase 1 was a massive R-SND reaction and phase 2 was characterized by SND desynchronization and by the emergence of HF-SND. The power of HF-SND occupied a wide band between 12 and 80 Hz with maximum between 20 and 30 Hz. All three nerves were involved in the Cushing response but the magnitude and character of the reactions were specific for each nerve. In the cardiac nerve, the power of the rhythmic component of the discharge increased almost twice the control and remained dominant during the whole reaction, strongly modulating HF-SND during the second phase. In the vasomotor nerves, R-SND was suppressed during phase 2 and HF-SND occupied 65% of the total power of the signal. Near equal R- to HF-SND proportions, however, were reached on different activity levels in renal and vertebral nerves. Whereas total renal SND did not change, the power of the vertebral SND increased more than twice. In addition, desynchronization in the vertebral SND was preceded by a massive R-SND reaction during phase 1, which was missing in the renal nerve. 4. For all possible nerve pairs, R-SND was highly coherent before the reaction and remained so during intracranial pressure elevation, regardless of the direction and magnitude of the changes in absolute and/or relative power of this component in different nerves. On the other hand, HF-SND never correlated between any of the nerve pairs indicating that this component in each nerve originated from specific sources of regional sympathetic activity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Bronk D. W., Phillips G. Discharges in mammalian sympathetic nerves. J Physiol. 1932 Feb 8;74(2):115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN F. K. Cardiovascular effects of acutely raised intracranial pressure. Am J Physiol. 1956 Jun;185(3):510–514. doi: 10.1152/ajplegacy.1956.185.3.510. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Gootman P. M. Periodicities in efferent discharge of splanchnic nerve of the cat. Am J Physiol. 1970 Apr;218(4):1092–1101. doi: 10.1152/ajplegacy.1970.218.4.1092. [DOI] [PubMed] [Google Scholar]
- Dampney R. A., Kumada M., Reis D. J. Central neural mechanisms of the cerebral ischemic response. Characterization, effect of brainstem and cranial nerve transections, and simulation by electrical stimulation of restricted regions of medulla oblongata in rabbit. Circ Res. 1979 Jul;45(1):48–62. doi: 10.1161/01.res.45.1.48. [DOI] [PubMed] [Google Scholar]
- Dean C., Coote J. H. Discharge patterns in postganglionic neurones to skeletal muscle and kidney during activation of the hypothalamic and midbrain defence areas in the cat. Brain Res. 1986 Jul 9;377(2):271–278. doi: 10.1016/0006-8993(86)90868-1. [DOI] [PubMed] [Google Scholar]
- Gebber G. L. Central oscillators responsible for sympathetic nerve discharge. Am J Physiol. 1980 Aug;239(2):H143–H155. doi: 10.1152/ajpheart.1980.239.2.H143. [DOI] [PubMed] [Google Scholar]
- Gootman P. M., Cohen M. I. Efferent splanchnic activity and systemic arterial pressure. Am J Physiol. 1970 Oct;219(4):897–903. doi: 10.1152/ajplegacy.1970.219.4.897. [DOI] [PubMed] [Google Scholar]
- Gootman P. M., Cohen M. I. Periodic modulation (cardiac and respiratory) of spontaneous and evoked sympathetic discharge. Acta Physiol Pol. 1973 Jan-Feb;24(1):97–109. [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Rev Physiol Biochem Pharmacol. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Kocsis B., Fedina L., Pasztor E. Effect of preexisting brain ischemia on sympathetic nerve response to intracranial hypertension. J Appl Physiol (1985) 1991 May;70(5):2181–2187. doi: 10.1152/jappl.1991.70.5.2181. [DOI] [PubMed] [Google Scholar]
- Kocsis B., Fedina L., Pasztor E. Two-phase change of sympathetic rhythms in brain ischemia, Cushing reaction, and asphyxia. Am J Physiol. 1989 Jan;256(1 Pt 2):R120–R132. doi: 10.1152/ajpregu.1989.256.1.R120. [DOI] [PubMed] [Google Scholar]
- Kocsis B., Gebber G. L., Barman S. M., Kenney M. J. Relationships between activity of sympathetic nerve pairs: phase and coherence. Am J Physiol. 1990 Sep;259(3 Pt 2):R549–R560. doi: 10.1152/ajpregu.1990.259.3.R549. [DOI] [PubMed] [Google Scholar]
- Kocsis B., Lenkei Z. Coordination between cardiovascular and respiratory control systems during and after cerebral ischemia. J Appl Physiol (1985) 1992 Apr;72(4):1595–1603. doi: 10.1152/jappl.1992.72.4.1595. [DOI] [PubMed] [Google Scholar]
- Kocsis B. Whether there are differential relationships among discharges of postganglionic sympathetic nerves remains an open question. Am J Physiol. 1992 Apr;262(4 Pt 2):R717–R720. doi: 10.1152/ajpregu.1992.262.4.R717. [DOI] [PubMed] [Google Scholar]
- Kollai M., Fedina L., Kovách A. G. Effect of bleeding, cooling and asphyxia on the activity of vertebral and cardiac sympathetic nerves. Acta Physiol Acad Sci Hung. 1973;44(2):145–155. [PubMed] [Google Scholar]
- Kollai M., Koizumi K., Brooks C. M. Nature of differential sympathetic discharges in chemoreceptor reflexes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5239–5243. doi: 10.1073/pnas.75.10.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa K., Ninomiya I. Changes in renal sympathetic nerve activity, heart rate and arterial blood pressure associated with eating in cats. J Physiol. 1987 Sep;390:229–242. doi: 10.1113/jphysiol.1987.sp016696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S., Sakamoto H., Hayashida Y., Kuno M. Efferent discharges of sympathetic and parasympathetic nerve fibers during increased intracranial pressure in anesthetized cats in the absence and presence of pressor response. Brain Res. 1984 Jul 9;305(2):291–301. doi: 10.1016/0006-8993(84)90435-9. [DOI] [PubMed] [Google Scholar]
- Ninomiya I., Nishiura N., Matsukawa K., Akiyama T. Fundamental rhythm of cardiac sympathetic nerve activity in awake cats at rest and during body movement. Jpn J Physiol. 1989;39(5):743–753. doi: 10.2170/jjphysiol.39.743. [DOI] [PubMed] [Google Scholar]
- Ninomiya I., Nisimaru N., Irisawa H. Sympathetic nerve activity to the spleen, kidney, and heart in response to baroceptor input. Am J Physiol. 1971 Nov;221(5):1346–1351. doi: 10.1152/ajplegacy.1971.221.5.1346. [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R., Mitra J., Van de Graaff W., Haxhiu M. A., Cherniack N. S. Effect of focal cooling of central chemosensitive areas on cerebral ischemic response. Am J Physiol. 1986 Aug;251(2 Pt 2):R295–R302. doi: 10.1152/ajpregu.1986.251.2.R295. [DOI] [PubMed] [Google Scholar]
- Pásztor E., Fedina L., Kocsis B., Berta Z. Activity of peripheral sympathetic efferent nerves in experimental subarachnoid haemorrhage. Part I: Observations at the time of intracranial hypertension. Acta Neurochir (Wien) 1986;79(2-4):125–131. doi: 10.1007/BF01407456. [DOI] [PubMed] [Google Scholar]
- van Wylen D. G., D'Alecy L. G. Regional blood flow distribution during the Cushing response: alterations with adrenergic blockade. Am J Physiol. 1985 Jan;248(1 Pt 2):H98–108. doi: 10.1152/ajpheart.1985.248.1.H98. [DOI] [PubMed] [Google Scholar]


