Abstract
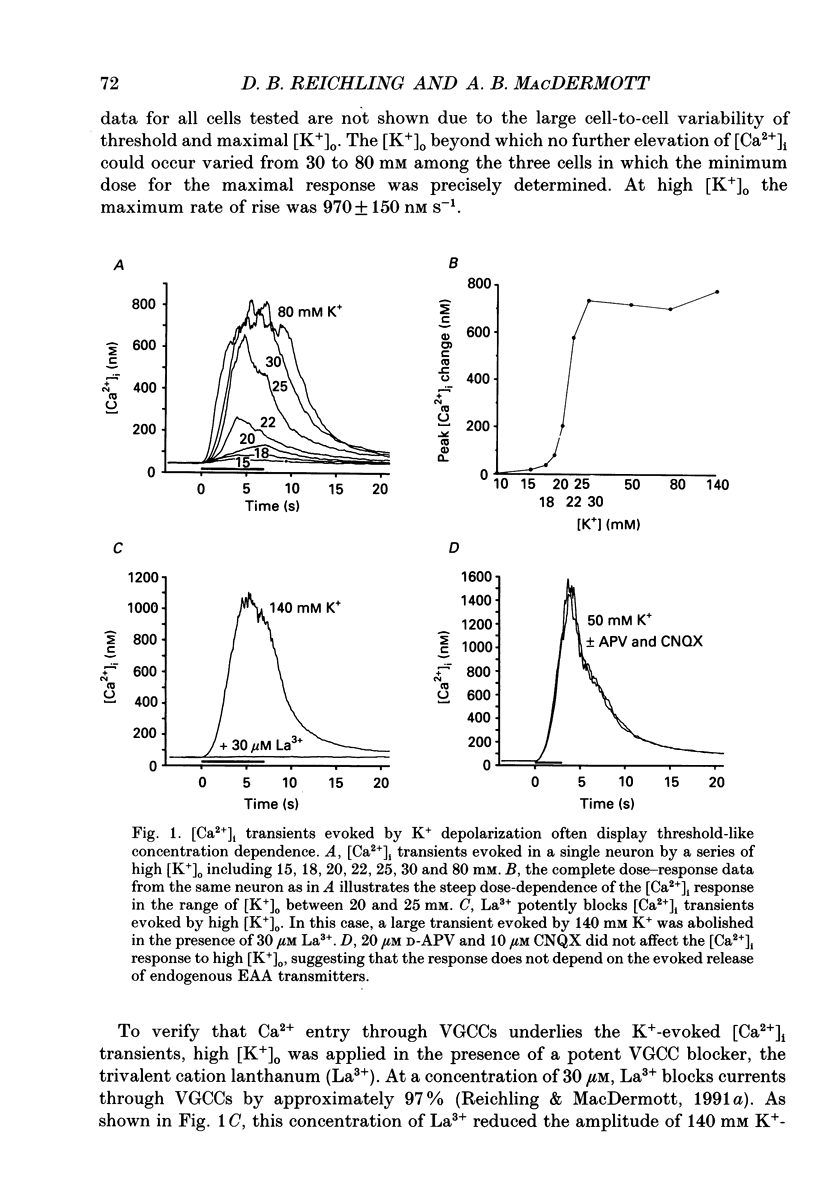
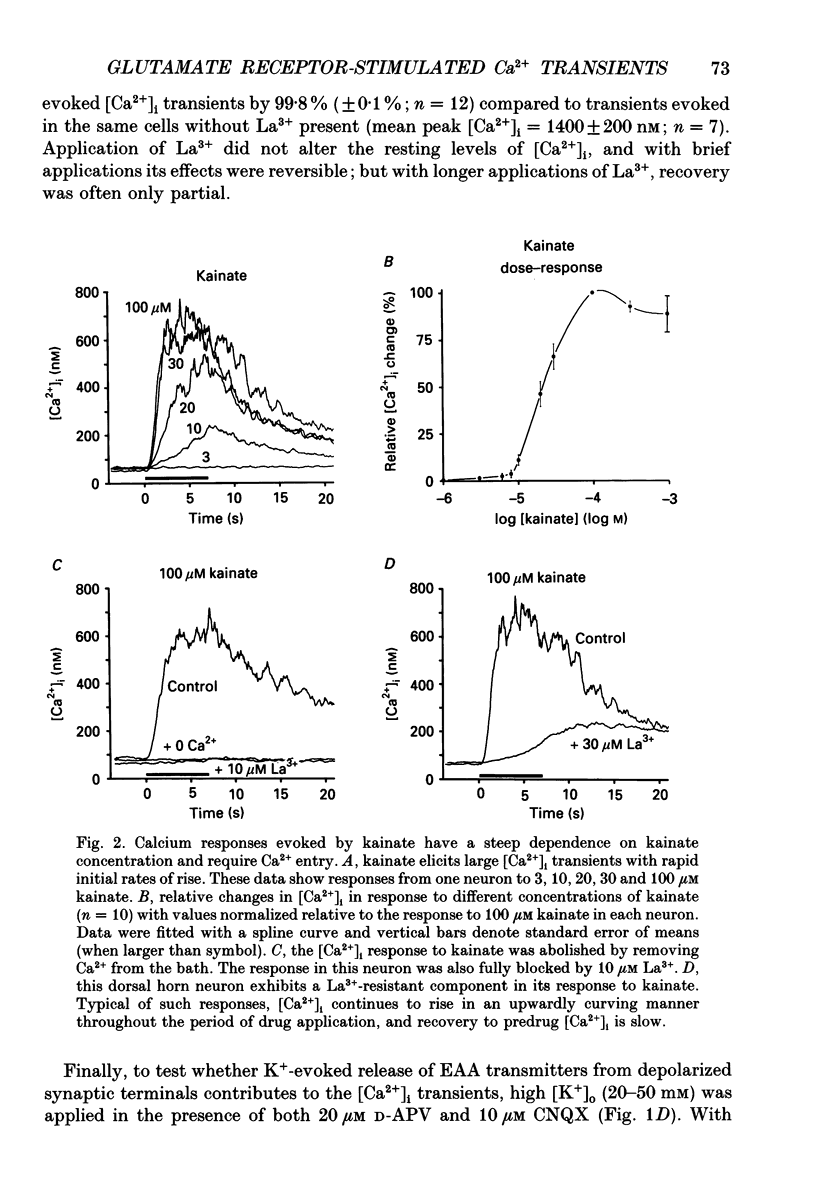
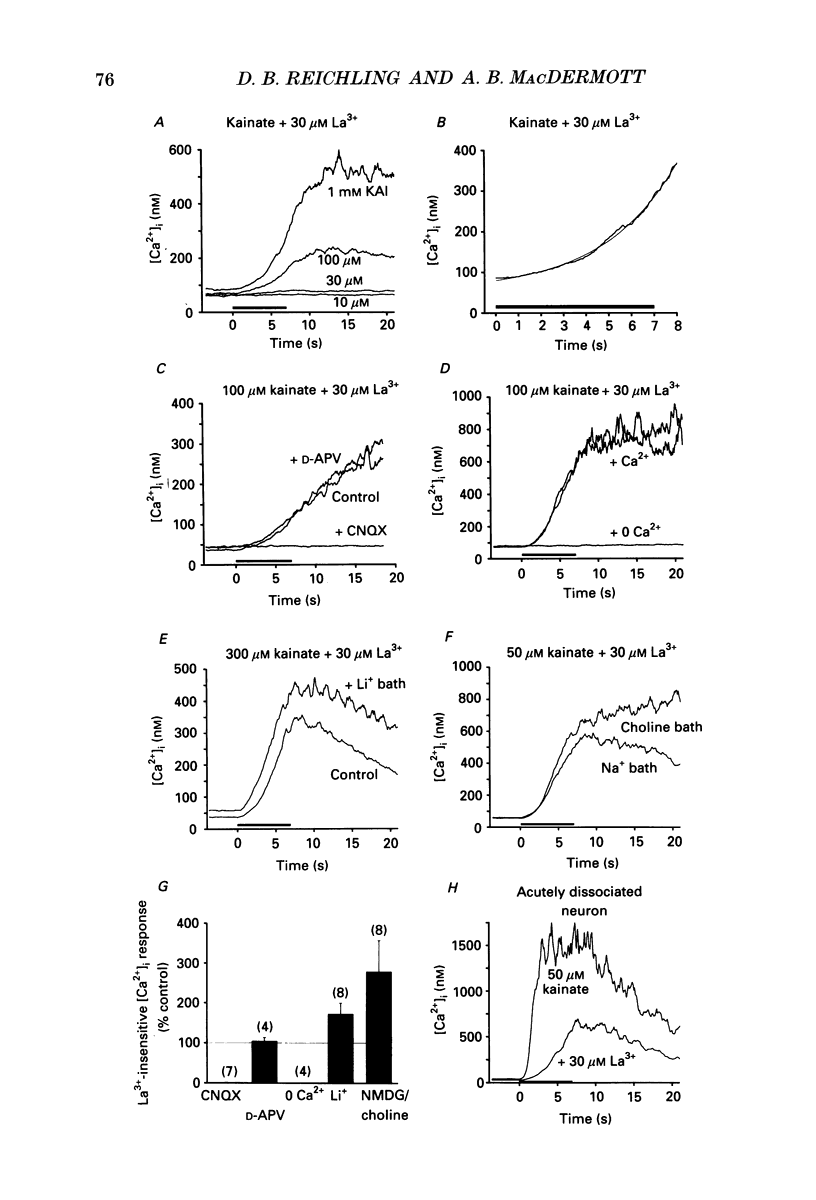
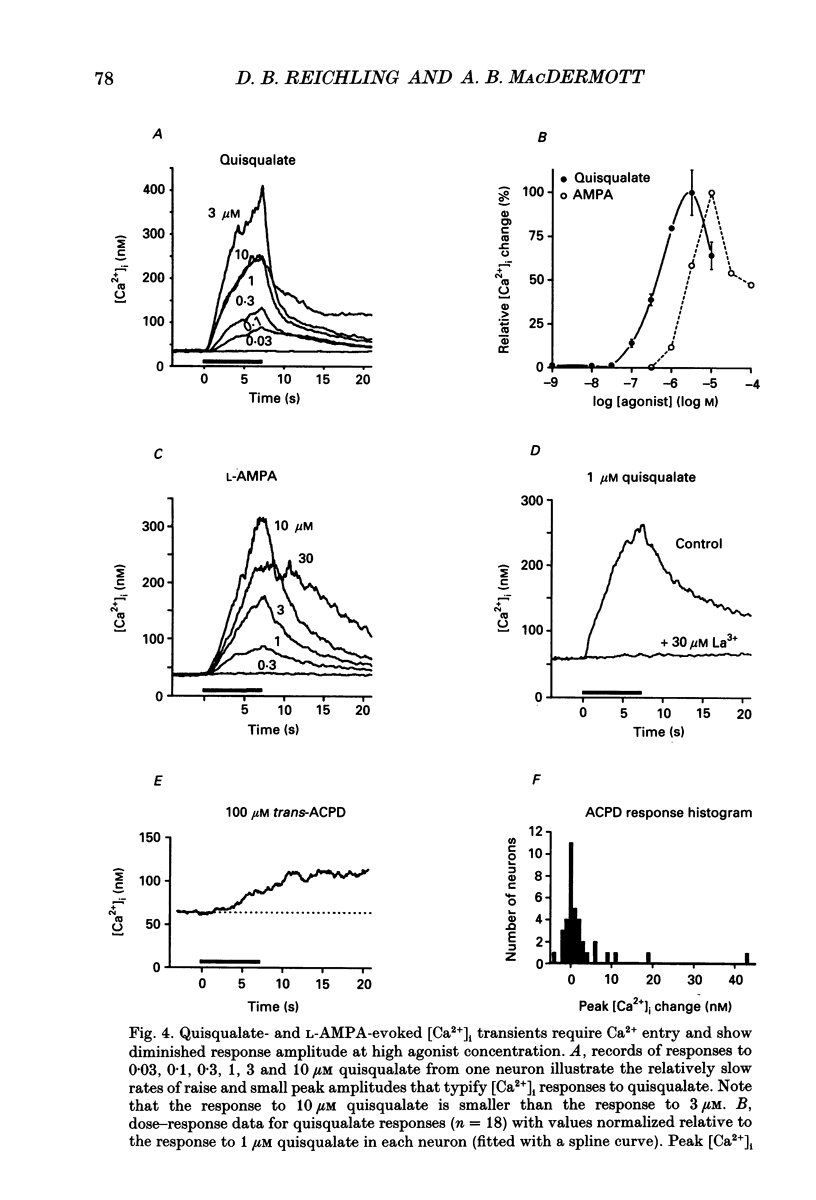
1. The calcium indicator dye, indo-1, was used to analyse the receptor-specific mechanisms of intracellular calcium ion ([Ca2+]i) responses evoked by excitatory amino acid (EAA) stimulation of dorsal horn neurons. Measurements of somal changes in [Ca2+]i were made on a subsecond time scale under conditions designed to allow membrane potential to mediate interactions between agonist-gated channels and voltage-gated calcium channels (VGCCs). 2. Voltage-gated calcium channels were activated in a receptor-independent manner using elevated extracellular [K+]. The concentration-dependence of K(+)-evoked [Ca2+]i transients was steep and variable among cells, with a mean maximal [Ca2+]i response of 1400 nM and a rapid maximal rate of rise. These data indicate that VGCCs provide a high-capacity route for Ca2+ entry that is very sensitive to small changes in membrane potential. 3. Stimulation of non-NMDA receptors using the non-desensitizing agonist kainate also evoked large [Ca2+]i responses (mean, 840 nM) that were predominantly due to indirect activation of VGCCs. However, in 60% of neurons tested, a component of the [Ca2+]i transient evoked by kainate at concentrations above 10 microM was not blocked by the potent VGCC blocker, lanthanum (La3+). The La(3+)-resistant [Ca2+]i responses to kainate rose exponentially, required extracellular Ca2+, and were caused neither by evoked release of EAA transmitters nor by reversal of Na(+)-Ca2+ exchange. These responses may be mediated by a Ca(2+)-permeable conformation of non-NMDA receptors and can also be evoked by quisqualate, (S)-alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and glutamate. 4. Non-NMDA receptors were activated in a desensitizing manner using quisqualate or AMPA. Quisqualate evoked small [Ca2+]i transients (210 nM) with a slow rate of rise. Typically, above 3 microM quisqualate, the size of the responses decreased, reflecting desensitization of the receptor. Responses to quisqualate were blocked by removal of extracellular Ca2+ indicating that mobilization of intracellular Ca2+ stores does not occur in the majority of dorsal horn neurons. However, trans-(+-)-1-amino-1,3-cyclopentane dicarboxylic acid (trans-ACPD) was occasionally able to evoke modest Ca2+ release. 5. Activation of the Ca(2+)-permeable NMDA receptors evoked [Ca2+]i transients that were large (780 nM), with a moderate rate of rise, and that generally achieved a maximum amplitude at NMDA concentrations around 300 microM. 6. Glutamate was used to examine [Ca2+]i responses to the activation of mixed EAA receptor subtypes by an endogenous ligand.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
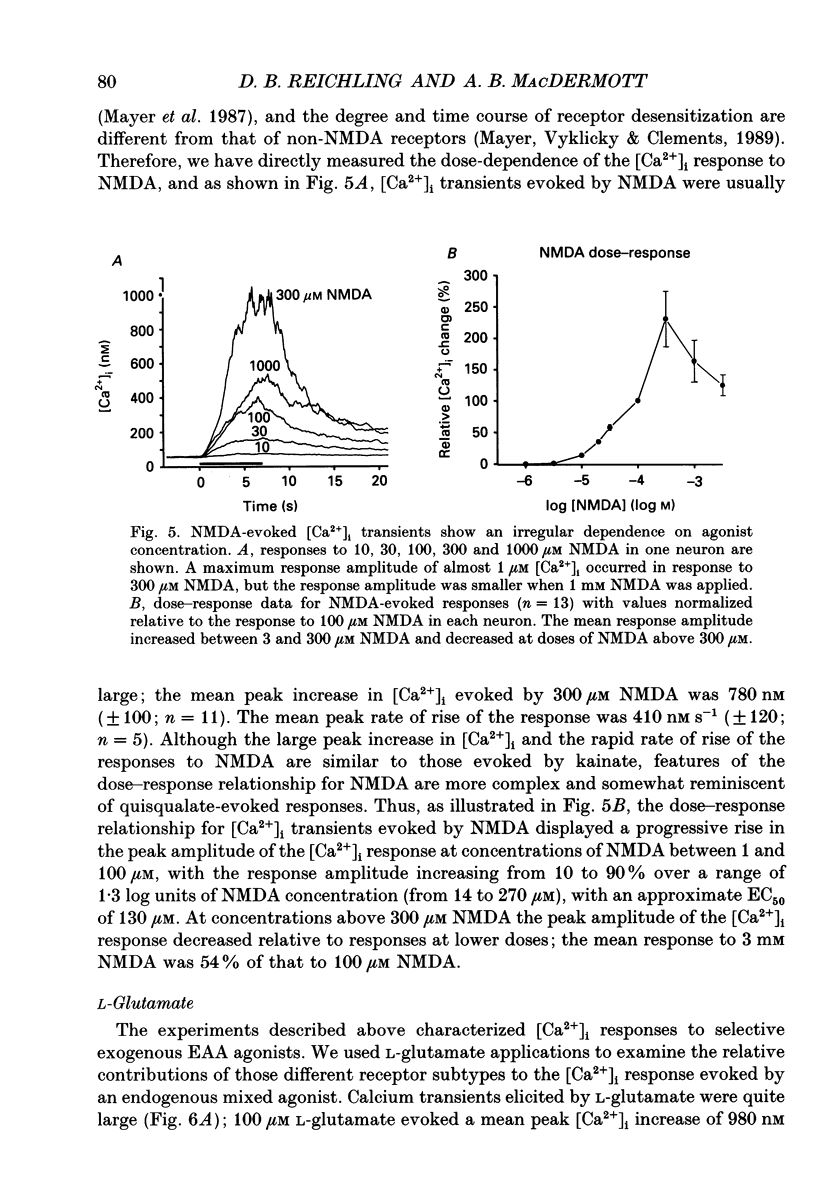
These references are in PubMed. This may not be the complete list of references from this article.
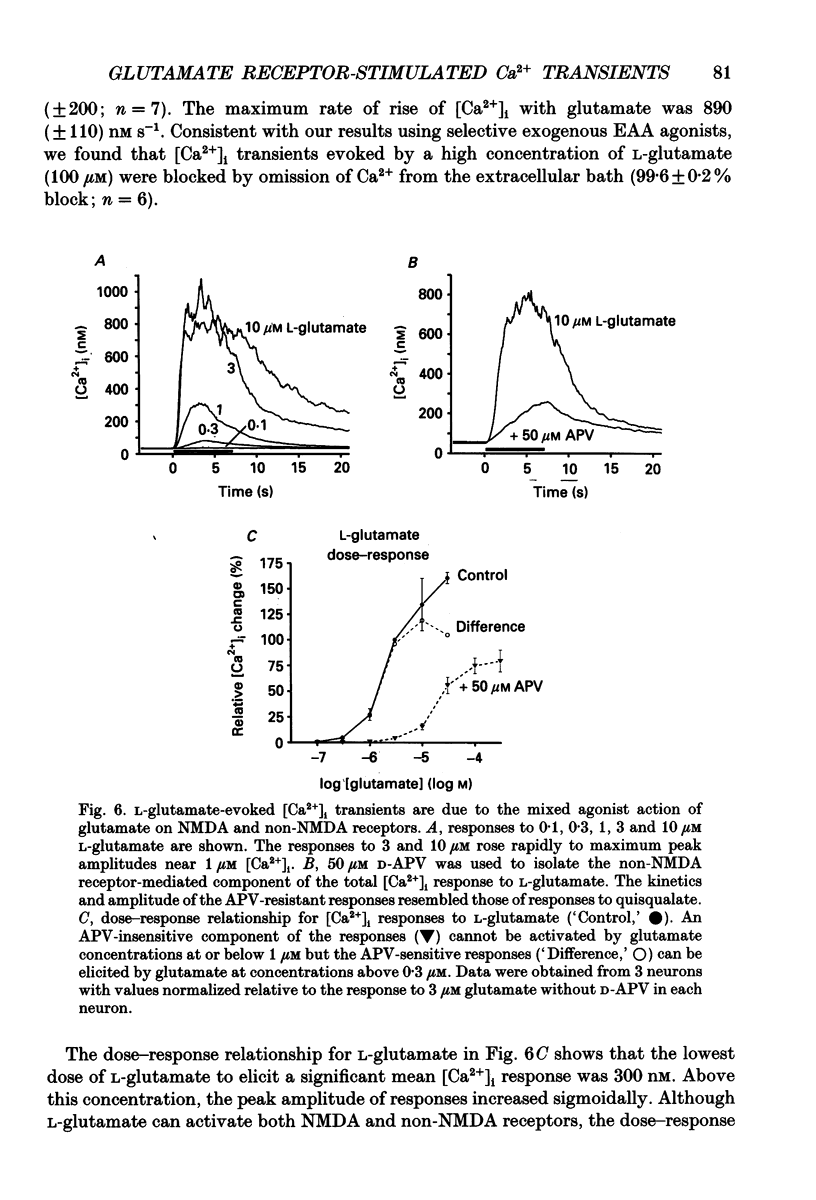
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bixby J. L., Spitzer N. C. Early differentiation of vertebrate spinal neurons in the absence of voltage-dependent Ca2+ and Na+ influx. Dev Biol. 1984 Nov;106(1):89–96. doi: 10.1016/0012-1606(84)90065-4. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H. A cure for wind up: NMDA receptor antagonists as potential analgesics. Trends Pharmacol Sci. 1990 Aug;11(8):307–309. doi: 10.1016/0165-6147(90)90228-z. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Bettler B., Hermans-Borgmeyer I., Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991 Jun 27;351(6329):745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Faden A. I., Simon R. P. A potential role for excitotoxins in the pathophysiology of spinal cord injury. Ann Neurol. 1988 Jun;23(6):623–626. doi: 10.1002/ana.410230618. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Holopainen I., Enkvist M. O., Akerman K. E. Glutamate receptor agonists increase intracellular Ca2+ independently of voltage-gated Ca2+ channels in rat cerebellar granule cells. Neurosci Lett. 1989 Mar 13;98(1):57–62. doi: 10.1016/0304-3940(89)90373-x. [DOI] [PubMed] [Google Scholar]
- Holopainen I., Louve M., Enkvist M. O., Akerman K. E. Coupling of glutamatergic receptors to changes in intracellular Ca2+ in rat cerebellar granule cells in primary culture. J Neurosci Res. 1990 Feb;25(2):187–193. doi: 10.1002/jnr.490250206. [DOI] [PubMed] [Google Scholar]
- Huang L. Y. Calcium channels in isolated rat dorsal horn neurones, including labelled spinothalamic and trigeminothalamic cells. J Physiol. 1989 Apr;411:161–177. doi: 10.1113/jphysiol.1989.sp017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Dingledine R., Heinemann S. F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991 Aug 30;253(5023):1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Llano I., Dreessen J., Kano M., Konnerth A. Intradendritic release of calcium induced by glutamate in cerebellar Purkinje cells. Neuron. 1991 Oct;7(4):577–583. doi: 10.1016/0896-6273(91)90370-f. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillian M., Pritchard G. A., Miller L. G. Characterization of Ca2(+)-mobilizing excitatory amino acid receptors in cultured chick cortical cells. Eur J Pharmacol. 1990 Oct 30;189(4-5):253–266. doi: 10.1016/0922-4106(90)90118-h. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. A glutamate receptor regulates Ca2+ mobilization in hippocampal neurons. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8737–8741. doi: 10.1073/pnas.85.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. Regulation of Ca++ influx into striatal neurons by kainic acid. J Pharmacol Exp Ther. 1989 Apr;249(1):184–193. [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. Two distinct quisqualate receptors regulate Ca2+ homeostasis in hippocampal neurons in vitro. Mol Pharmacol. 1989 May;35(5):671–680. [PubMed] [Google Scholar]
- Murphy S. N., Thayer S. A., Miller R. J. The effects of excitatory amino acids on intracellular calcium in single mouse striatal neurons in vitro. J Neurosci. 1987 Dec;7(12):4145–4158. doi: 10.1523/JNEUROSCI.07-12-04145.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A., Akita K., Kudo Y. Non-NMDA receptor mediates cytoplasmic Ca2+ elevation in cultured hippocampal neurones. Neurosci Res. 1990 Nov;9(2):103–113. doi: 10.1016/0168-0102(90)90026-b. [DOI] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neurosci. 1990 Jul;10(7):2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling D. B., MacDermott A. B. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J Physiol. 1991 Sep;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosper T. L., Philipson K. D. Effects of divalent and trivalent cations on Na+-Ca2+ exchange in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1983 May 26;731(1):63–68. doi: 10.1016/0005-2736(83)90398-x. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Weiss S., Kemp D. E., Bauce L., Tse F. W. Kainate receptors coupled to the evoked release of [3H]-gamma-aminobutyric acid from striatal neurons in primary culture: potentiation by lithium ions. Mol Pharmacol. 1990 Aug;38(2):229–236. [PubMed] [Google Scholar]
- Werner P., Voigt M., Keinänen K., Wisden W., Seeburg P. H. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991 Jun 27;351(6329):742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990 Nov;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R., Katz L. C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991 Mar;6(3):333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]


