Abstract
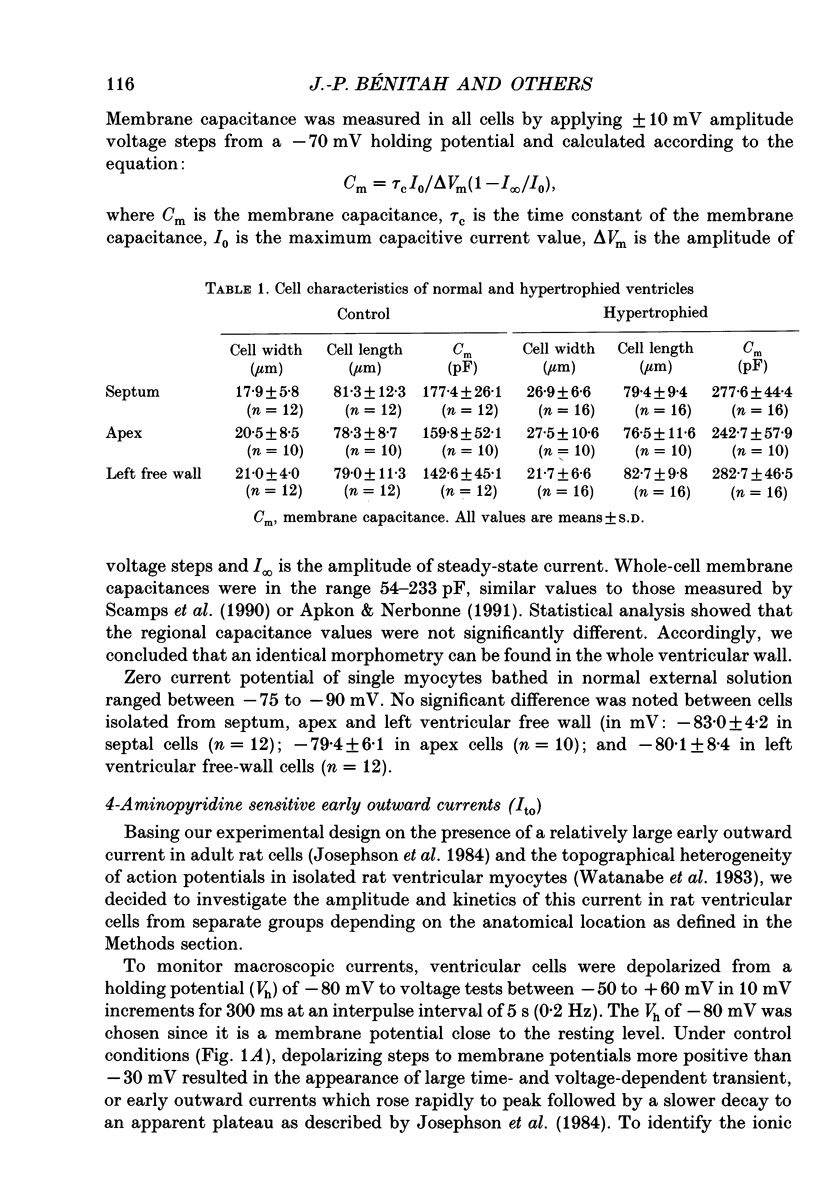
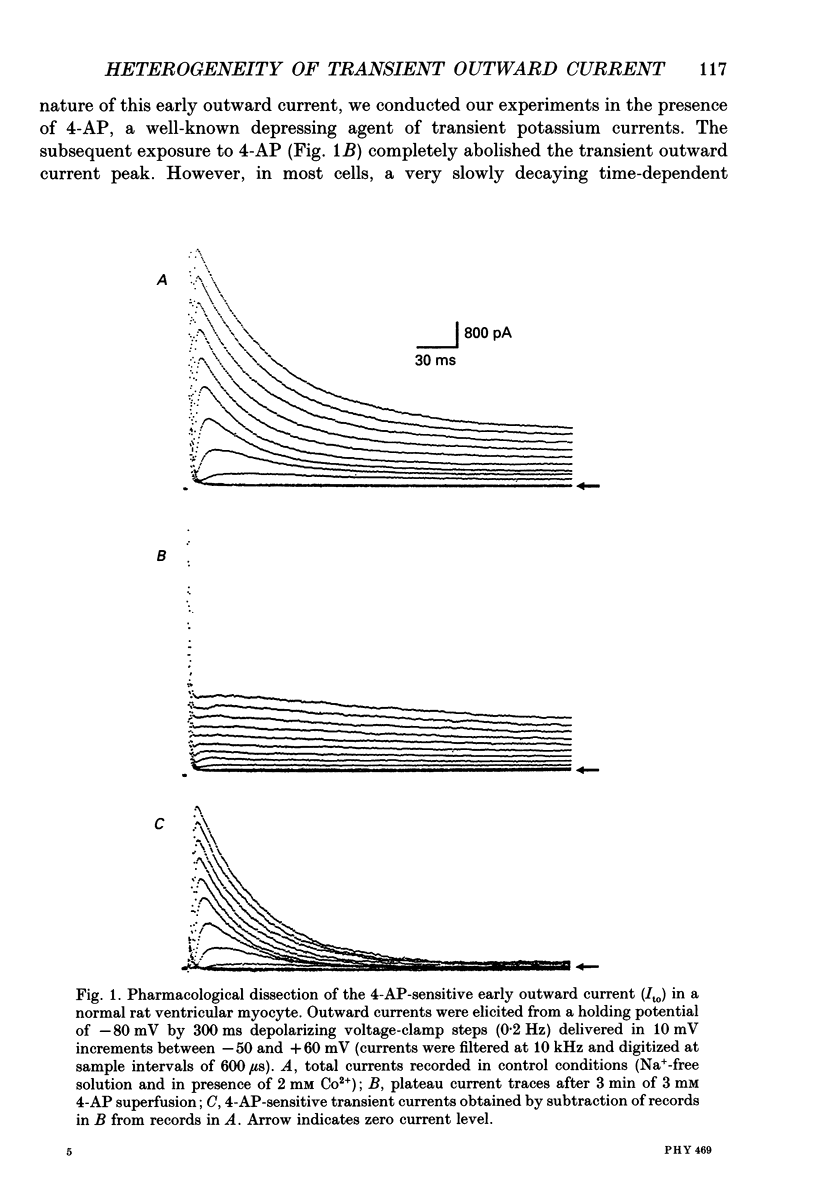
1. The nature, magnitude and kinetics of the 4-aminopyridine-sensitive early outward current (Ito) were analysed in isolated ventricular myocytes from the septum, the apex and the left ventricular free wall of rat ventricles using the whole-cell voltage clamp method. The modulatory effect of pressure overload-induced cardiac hypertrophy on the regional variations of Ito was assessed in each topographical class of cells. 2. Voltage clamp experiments were performed at room temperature (20-25 degrees C) in the absence of Na+ on both sides of the membrane and in the presence of 3 mM CoCl2. Ito was studied from a holding potential of -80 mV and determined by subtraction of total outward currents elicited by the same protocols in the presence of 3 mM 4-aminopyridine (4-AP) from those obtained in its absence. 3. In normal hearts, membrane passive properties were very similar in each topographical class of cells. Our results confirmed that the predominant early outward current in rat ventricular cells was 4-AP-sensitive, time and voltage dependent, and demonstrated that the magnitude of the current varied on a regional basis: current density of Ito in left ventricular free wall cells (30.1 +/- 9.2 pA/pF at +60 mV) was larger than in apex cells (20.2 +/- 1.7 pA/pF) or in septum cells (11.9 +/- 3.3 pA/pF). We noticed a larger variability in data from left ventricular free wall compared with other regions. 4. No shift in steady-state voltage dependence of Ito activation and inactivation was found. However, the maximal computed chord conductances were (in microS/pF): 0.18 +/- 0.07 for left ventricular free wall cells, 0.13 +/- 0.02 for apex cells, and 0.08 +/- 0.02 for septum cells. These findings might reflect a differential distribution in functional channel densities. 5. No difference in voltage-dependent Ito activation kinetics was present with respect to topography. However, inactivation time constants in septum were longer than those of both other groups. 6. Left ventricular hypertrophy was induced by abdominal aortic constriction and its effects compared to the findings from normal rats. Hypertrophied cells had similar resting potentials but higher capacitance values than normal cells. Although Ito magnitude appeared not to be modified, the current density-voltage curves were slightly shifted to more positive potentials and significantly decreased as compared to normal cells (in pA/pF, at +60 mV): 8.4 +/- 5.0 in the left free wall group, 11.6 +/- 2.0 in the apex group, and 3.8 +/- 1.5 in the septum group.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




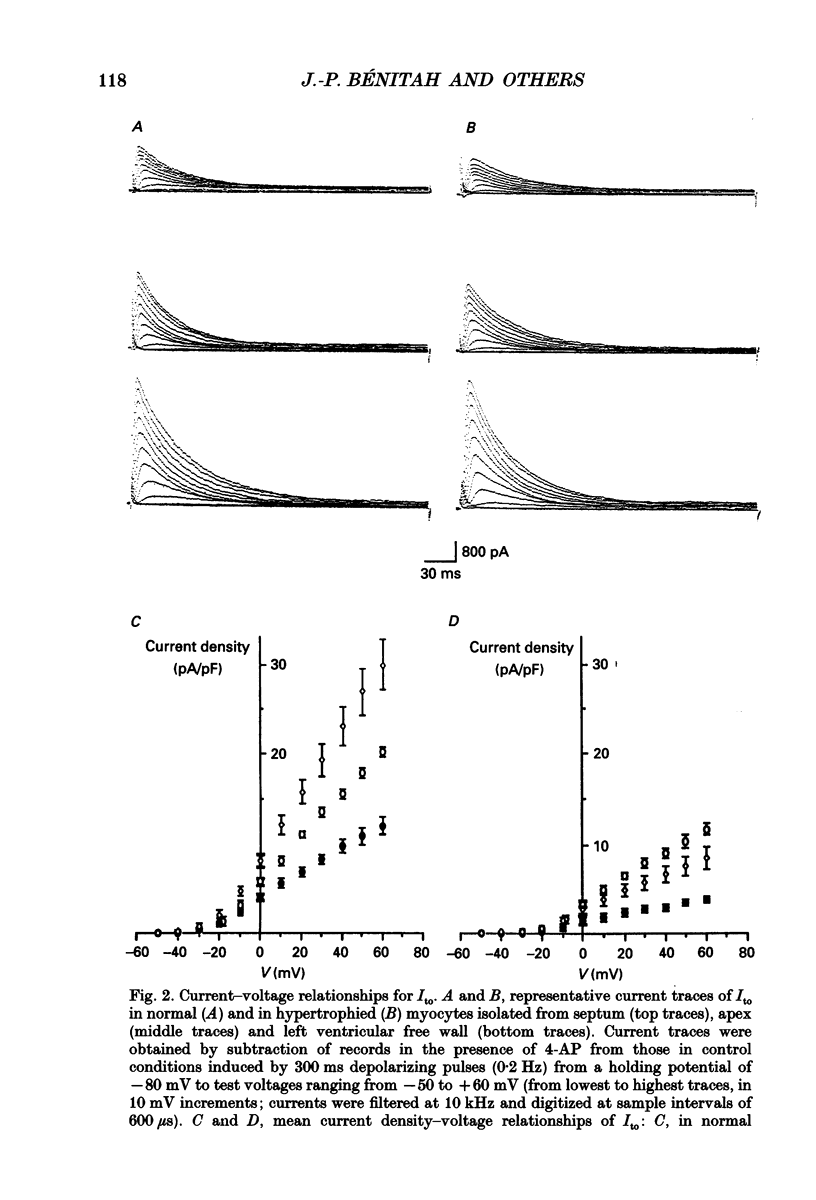



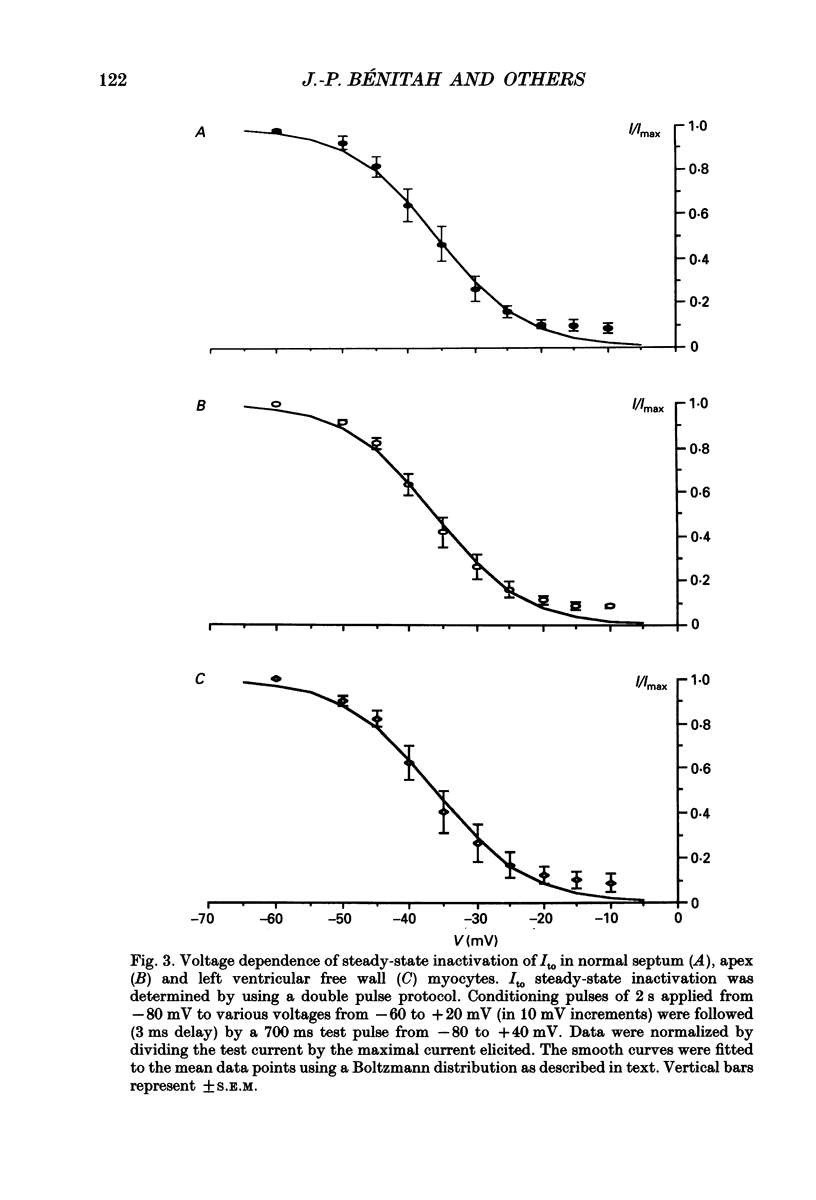
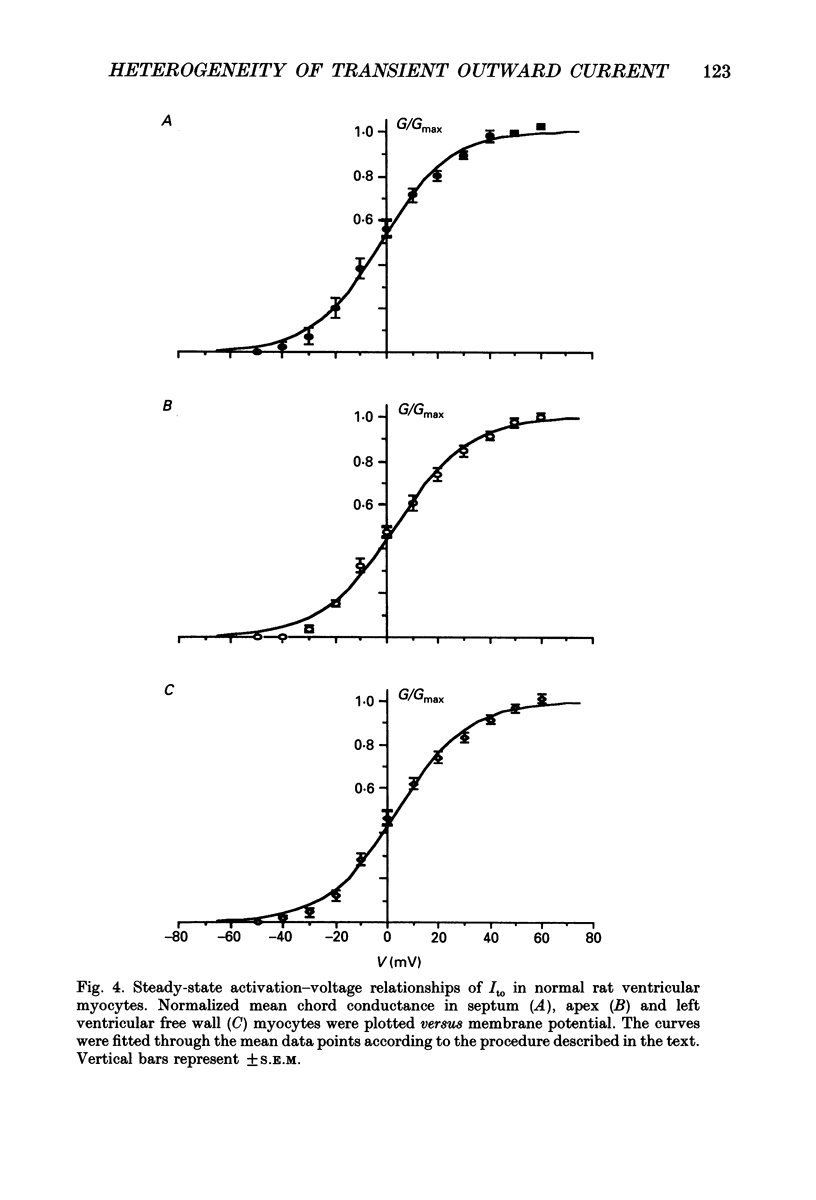

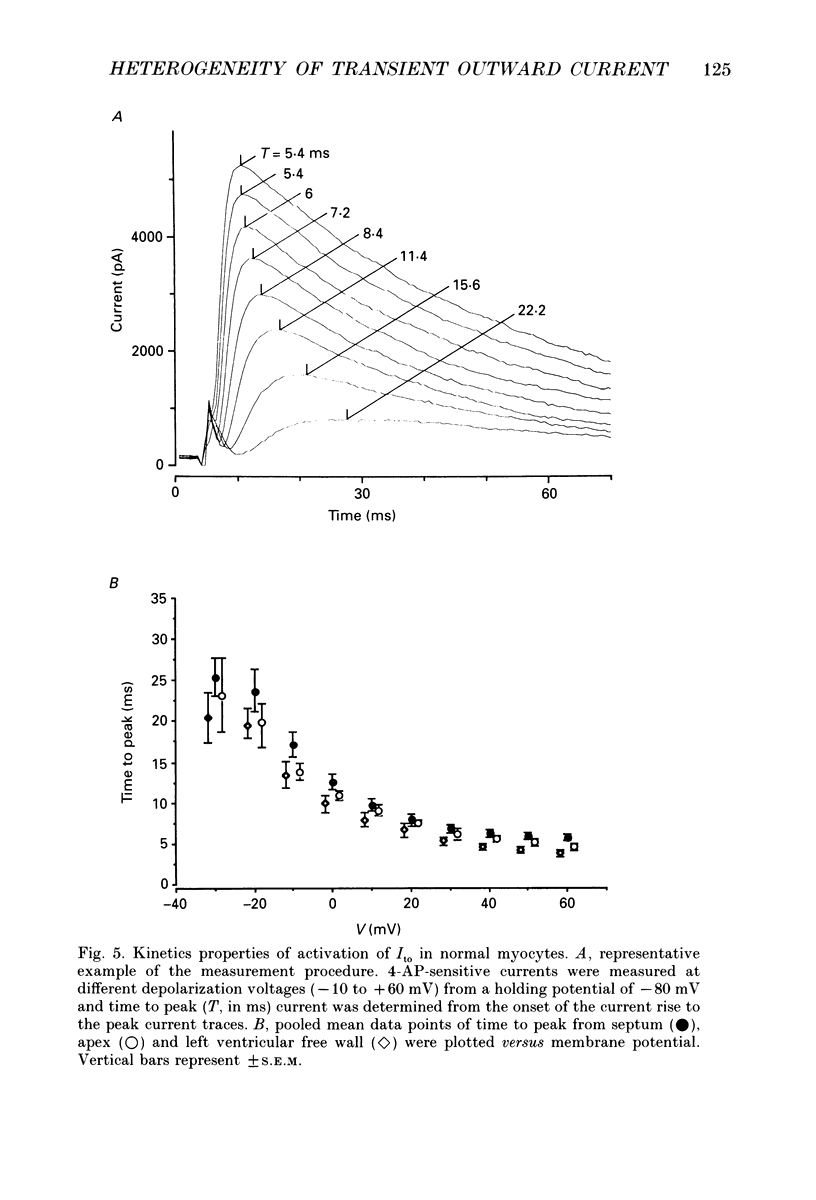

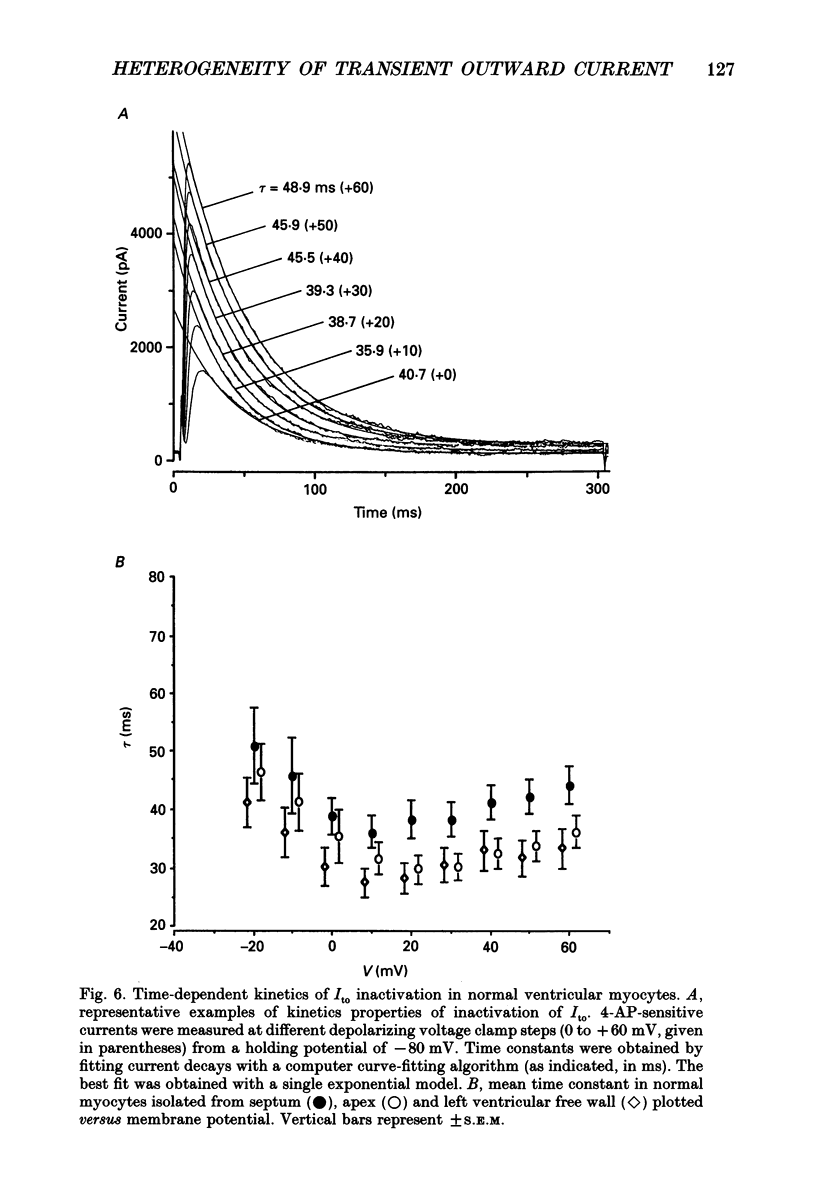


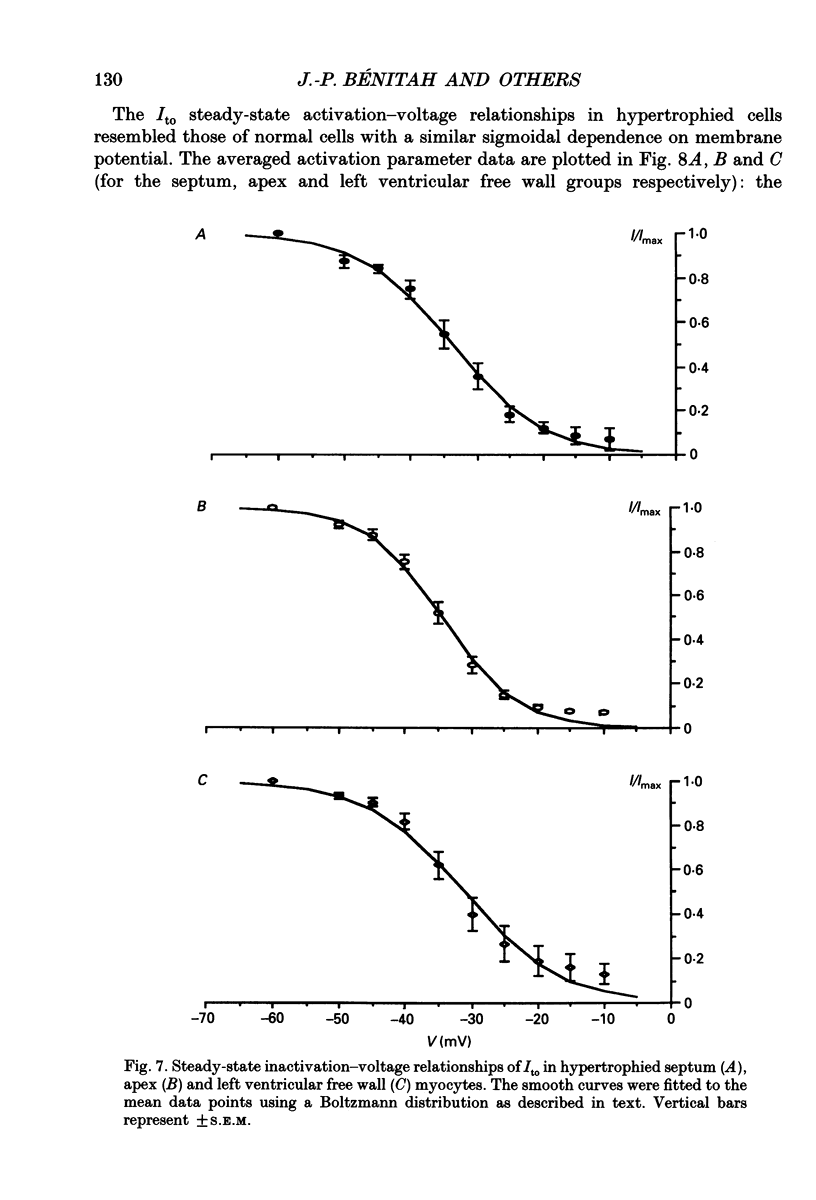
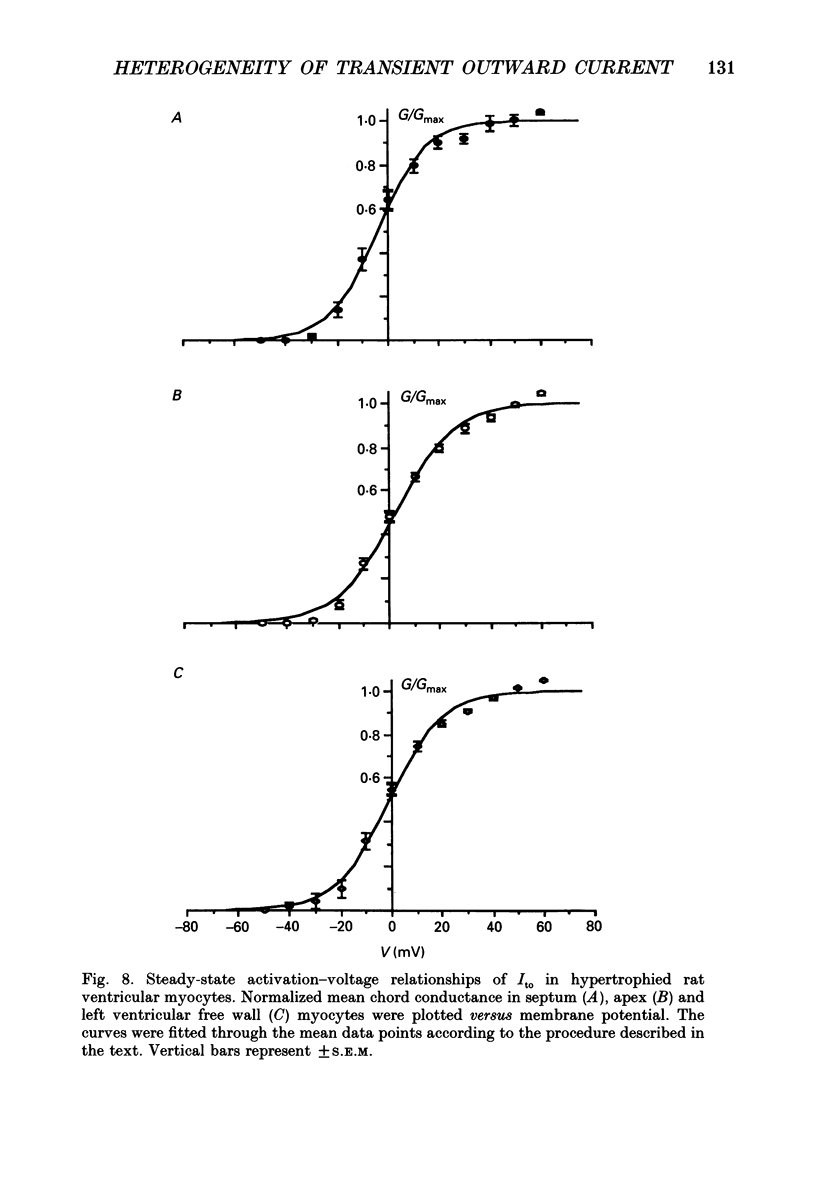

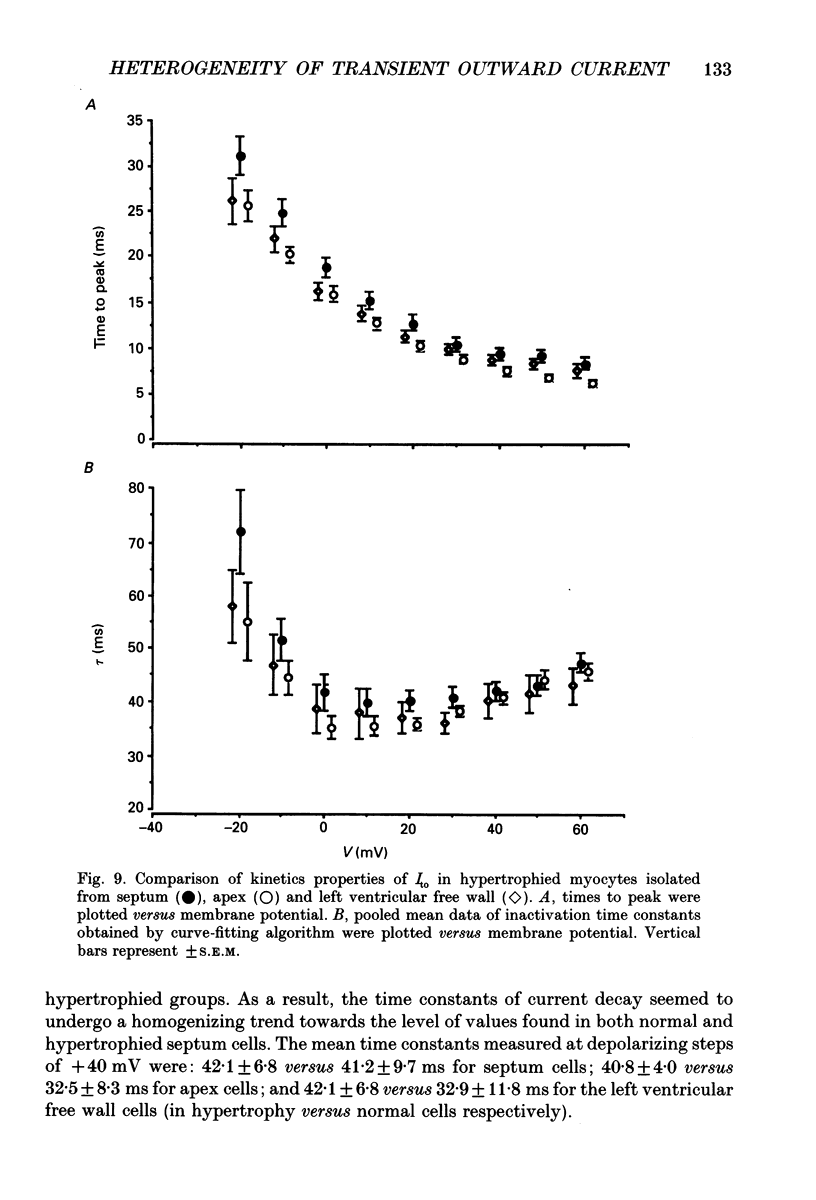





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Dukes I. D., Morad M. Divalent cations modulate the transient outward current in rat ventricular myocytes. Am J Physiol. 1991 Aug;261(2 Pt 1):C310–C318. doi: 10.1152/ajpcell.1991.261.2.C310. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C., Sicouri S., Litovsky S. H., Lukas A., Krishnan S. C., Di Diego J. M., Gintant G. A., Liu D. W. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991 Dec;69(6):1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- Anversa P., Loud A. V., Giacomelli F., Wiener J. Absolute morphometric study of myocardial hypertrophy in experimental hypertension. II. Ultrastructure of myocytes and interstitium. Lab Invest. 1978 May;38(5):597–609. [PubMed] [Google Scholar]
- Apkon M., Nerbonne J. M. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J Gen Physiol. 1991 May;97(5):973–1011. doi: 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson R. S., Nordin C. Electrophysiologic properties of hypertrophied myocytes isolated from rats with renal hypertension. Eur Heart J. 1984 Dec;5 (Suppl F):339–345. doi: 10.1093/eurheartj/5.suppl_f.339. [DOI] [PubMed] [Google Scholar]
- Bénitah J. P., Bailly P., D'Agrosa M. C., Da Ponte J. P., Delgado C., Lorente P. Slow inward current in single cells isolated from adult human ventricles. Pflugers Arch. 1992 Jun;421(2-3):176–187. doi: 10.1007/BF00374825. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Giles W. R., Imaizumi Y. Properties of the transient outward current in rabbit atrial cells. J Physiol. 1988 Nov;405:147–168. doi: 10.1113/jphysiol.1988.sp017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- DECK K. A., TRAUTWEIN W. IONIC CURRENTS IN CARDIAC EXCITATION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:63–80. doi: 10.1007/BF00412616. [DOI] [PubMed] [Google Scholar]
- Dukes I. D., Morad M. The transient K+ current in rat ventricular myocytes: evaluation of its Ca2+ and Na+ dependence. J Physiol. 1991 Apr;435:395–420. doi: 10.1113/jphysiol.1991.sp018516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande D., Coulombe A., Faivre J. F., Deroubaix E., Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- Escande D., Loisance D., Planche C., Coraboeuf E. Age-related changes of action potential plateau shape in isolated human atrial fibers. Am J Physiol. 1985 Oct;249(4 Pt 2):H843–H850. doi: 10.1152/ajpheart.1985.249.4.H843. [DOI] [PubMed] [Google Scholar]
- Fedida D., Giles W. R. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. J Physiol. 1991 Oct;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Hiraoka M. The positive dynamic current and its inactivation properties in cardiac Purkinje fibres. J Physiol. 1973 Nov;234(3):569–586. doi: 10.1113/jphysiol.1973.sp010361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Myerburg R. J., Furukawa N., Bassett A. L., Kimura S. Differences in transient outward currents of feline endocardial and epicardial myocytes. Circ Res. 1990 Nov;67(5):1287–1291. doi: 10.1161/01.res.67.5.1287. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol. 1989 Mar;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung E. C., Aronson R. S. Non-uniform electrophysiological properties and electrotonic interaction in hypertrophied rat myocardium. Circ Res. 1981 Jul;49(1):150–158. doi: 10.1161/01.res.49.1.150. [DOI] [PubMed] [Google Scholar]
- Keung E. C. Calcium current is increased in isolated adult myocytes from hypertrophied rat myocardium. Circ Res. 1989 Apr;64(4):753–763. doi: 10.1161/01.res.64.4.753. [DOI] [PubMed] [Google Scholar]
- Kilborn M. J., Fedida D. A study of the developmental changes in outward currents of rat ventricular myocytes. J Physiol. 1990 Nov;430:37–60. doi: 10.1113/jphysiol.1990.sp018280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman R. B., Houser S. R. Calcium currents in normal and hypertrophied isolated feline ventricular myocytes. Am J Physiol. 1988 Dec;255(6 Pt 2):H1434–H1442. doi: 10.1152/ajpheart.1988.255.6.H1434. [DOI] [PubMed] [Google Scholar]
- Lefevre I. A., Coulombe A., Coraboeuf E. The calcium antagonist D600 inhibits calcium-independent transient outward current in isolated rat ventricular myocytes. J Physiol. 1991 Jan;432:65–80. doi: 10.1113/jphysiol.1991.sp018376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988 Jan;62(1):116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- Mayoux E., Callens F., Swynghedauw B., Charlemagne D. Adaptational process of the cardiac Ca2+ channels to pressure overload: biochemical and physiological properties of the dihydropyridine receptors in normal and hypertrophied rat hearts. J Cardiovasc Pharmacol. 1988 Oct;12(4):390–396. doi: 10.1097/00005344-198810000-00003. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Cavalié A., Trautwein W., Pelzer D. Voltage-dependent properties of macroscopic and elementary calcium channel currents in guinea pig ventricular myocytes. Pflugers Arch. 1986 May;406(5):437–448. doi: 10.1007/BF00583365. [DOI] [PubMed] [Google Scholar]
- Nordin C., Siri F., Aronson R. S. Electrophysiologic characteristics of single myocytes isolated from hypertrophied guinea-pig hearts. J Mol Cell Cardiol. 1989 Jul;21(7):729–739. doi: 10.1016/0022-2828(89)90614-7. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F., Mayoux E., Charlemagne D., Vassort G. Calcium current in single cells isolated from normal and hypertrophied rat heart. Effects of beta-adrenergic stimulation. Circ Res. 1990 Jul;67(1):199–208. doi: 10.1161/01.res.67.1.199. [DOI] [PubMed] [Google Scholar]
- Severs N. J., Slade A. M., Powell T., Twist V. W., Warren R. L. Correlation of ultrastructure and function in calcium-tolerant myocytes isolated from the adult rat heart. J Ultrastruct Res. 1982 Nov;81(2):222–239. doi: 10.1016/s0022-5320(82)90078-8. [DOI] [PubMed] [Google Scholar]
- Tritthart H., Luedcke H., Bayer R., Stierle H., Kaufmann R. Right ventricular hypertrophy in the cat--an electrophysiological and anatomical study. J Mol Cell Cardiol. 1975 Mar;7(3):163–174. doi: 10.1016/0022-2828(75)90155-8. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Hoffman B. F. Two components of transient outward current in canine ventricular myocytes. Circ Res. 1989 Apr;64(4):633–647. doi: 10.1161/01.res.64.4.633. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Robinson R. B., Hoffman B. F. Passive properties and membrane currents of canine ventricular myocytes. J Gen Physiol. 1987 Nov;90(5):671–701. doi: 10.1085/jgp.90.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Delbridge L. M., Bustamante J. O., McDonald T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983 Mar;52(3):280–290. doi: 10.1161/01.res.52.3.280. [DOI] [PubMed] [Google Scholar]


