Abstract
BACKGROUND
The incidence of hypertriglyceridemia (HTG)-induced acute pancreatitis (AP) is steadily increasing in China, becoming the second leading cause of AP. Clinical complications and outcomes associated with HTG-AP are generally more severe than those seen in AP caused by other etiologies. HTG-AP is closely linked to metabolic dysfunction and frequently coexists with metabolic syndrome or its components. However, the impact of metabolic syndrome components on HTG-AP clinical outcomes remains unclear.
AIM
To investigate the impact of metabolic syndrome component burden on clinical outcomes in HTG-AP.
METHODS
In this retrospective study of 255 patients diagnosed with HTG-AP at the First Affiliated Hospital of Guangxi Medical University, we collected data on patient demographics, clinical scores, complications, and clinical outcomes. Subsequently, we analyzed the influence of the presence and number of individual metabolic syndrome components, including obesity, hyperglycemia, hypertension, and low high-density lipoprotein cholesterol (HDL-C), on the aforementioned parameters in HTG-AP patients.
RESULTS
This study found that metabolic syndrome components were associated with an increased risk of various complications in HTG-AP, with low HDL-C being the most significant risk factor for clinical outcomes. The risk of complications increased with the number of metabolic syndrome components. Adjusted for age and sex, patients with high-component metabolic syndrome had significantly higher risks of renal failure [odds ratio (OR) = 3.02, 95%CI: 1.12-8.11)], SAP (OR = 5.05, 95%CI: 2.04-12.49), and intensive care unit admission (OR = 6.41, 95%CI: 2.42-16.97) compared to those without metabolic syndrome.
CONCLUSION
The coexistence of multiple metabolic syndrome components can synergistically worsen the clinical course of HTG-AP, making it crucial to monitor these components for effective disease management.
Keywords: Hypertriglyceridemia-induced acute pancreatitis, Metabolic syndrome, High density lipoprotein cholesterol, Obesity, Hyperglycemia, Hypertension, Clinical outcomes
Core Tip: This retrospective study, the first to systematically investigate the association between the metabolic syndrome component burden and clinical outcomes in patients with hypertriglyceridemia-induced acute pancreatitis, found that individual metabolic syndrome components, especially low high density lipoprotein cholesterol, increased the risk of complications. The number of metabolic syndrome components was positively correlated with the incidence of these complications. Patients with high-component metabolic syndrome had an elevated risk of developing renal failure, severe acute pancreatitis, and requiring intensive care unit admission.
INTRODUCTION
Acute pancreatitis (AP) is an acute inflammatory disease characterized by a variable clinical course, which can lead to a series of complications, potentially even resulting in death[1]. The common etiologies of AP include gallstones, alcohol consumption, and hyperlipidemia, among other causes[2]. Hypertriglyceridemia-induced AP (HTG-AP), a specific form of AP caused by elevated triglyceride levels, accounts for 4%-10% of AP cases globally[3]. Recent research indicates a concerning trend in China, with the prevalence of HTG-AP increasing approximately 2.6-fold between 2012 and 2021[4]. This surge has positioned HTG-AP as the second leading cause of AP within the Chinese population[5].The rising trend of HTG-AP in China is likely attributed to the increasing caloric intake associated with lifestyle changes and the growing prevalence of metabolic syndrome among the Chinese population[6]. Patients with HTG-AP generally experience more severe clinical outcomes compared to those with AP from other etiologies, characterized by a higher incidence of complications, prolonged hospital stays, and increased mortality[7]. Furthermore, a multicenter study demonstrated that patients with HTG-AP had a significantly higher incidence of potentially fatal complications, including acute kidney injury, acute respiratory distress syndrome (ARDS), and multiple organ dysfunction syndrome (MODS), compared to those with biliary AP[8].
Metabolic syndrome is a cluster of metabolic disorders characterized by hypertriglyceridemia, low high-density lipoprotein cholesterol (HDL-C), hyperglycemia, hypertension, and obesity[9]. Patients with metabolic syndrome exhibit poorer overall metabolic health. In addition, metabolic syndrome, characterized by poorer overall metabolic health, can accelerate the progression of other primary diseases and increase the risk of developing complications when coexisting with them[10]. A substantial body of evidence has confirmed the contribution of metabolic syndrome and its individual components to the progression of various systemic diseases, including cardiovascular disease, chronic kidney disease, and numerous types of cancer[9]. Furthermore, research has shown that metabolic syndrome not only increases susceptibility to AP but also raises the risk of AP patients developing moderate severe AP (MSAP), severe AP (SAP), and increased risk of mortality[11,12].
Hypertriglyceridemia is a component of metabolic syndrome and closely interacts with other components, exerting mutual influence[13]. Given their hypertriglyceridemic state, patients with HTG-AP often present with metabolic syndrome or its individual components. While prior research suggests that HTG-AP patients with co-existing metabolic disturbances, such as high body mass index (BMI) or diabetes, experience worse outcomes, however, the comprehensive influence of metabolic syndrome on the clinical outcomes of HTG-AP remains underexplored[14,15]. Therefore, this study will systematically analyze the impact of individual metabolic syndrome components, and their overall burden, on clinical outcomes in HTG-AP. Clarifying this relationship is crucial for the early identification of patients at risk of severe clinical outcomes, playing a pivotal role in the effective management of this disease.
MATERIALS AND METHODS
Study design and patients
This retrospective study included patients diagnosed with HTG-AP at the First Affiliated Hospital of Guangxi Medical University between January 2012 and February 2023. Patients were included if they met the established criteria for HTG-AP and excluded if they met any of the following criteria: (1) Age under 18 years; (2) Pre-existing chronic pancreatitis; (3) Pregnancy; (4) Any malignancy; or (5) Lack of complete clinical data, such as abdominal CT scans or other examinations necessary to assess for the presence of complications. This study employed strict inclusion and exclusion criteria to minimize selection bias and relied on medical records to reduce recall bias. However, the retrospective design makes it impossible to fully eliminate these biases. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (2024-E278-01). Participants were stratified into subgroups based on the presence or absence of individual metabolic syndrome components, including low HDL-C, obesity, and hypertension. Hypertriglyceridemia was not included as a stratifying factor because all HTG-AP patients inherently exhibit hypertriglyceridemia. Further stratification was conducted according to the total number of coexisting metabolic syndrome components.
Definition
AP was diagnosed based on the presence of at least two of the following three criteria: (1) Acute onset of persistent abdominal pain; (2) Serum lipase or amylase level exceeding three times the upper limit of normal; and (3) Abdominal imaging findings consistent with AP[16]. HTG-AP was diagnosed when all of the following criteria were met: (1) Established AP diagnosis; (2) Serum triglyceride level greater than 11.3 mmol/L (or 5.65-11.3 mmol/L with lactescent serum); and (3) Exclusion of other AP etiologies[4]. AP severity was classified according to the Revised Atlanta Classification as follows: Mild AP (MAP), characterized by the absence of organ failure and local or systemic complications; moderately SAP (MSAP), characterized by the presence of transient organ failure (< 48 hours) and/or local or systemic complications without persistent organ failure; and SAP (SAP), characterized by persistent organ failure (> 48 hours)[16]. Organ failure was defined as a modified Marshall score ≥ 2 in the respiratory, cardiovascular, or renal systems. Local complications included acute peripancreatic fluid collection (APFC), acute necrotic collection (ANC), pancreatic pseudocyst, walled-off necrosis (WON), and infected pancreatic necrosis. Systemic complications encompassed organ failure, systemic inflammatory response syndrome (SIRS), and abdominal compartment syndrome.
The components of metabolic syndrome were defined according to the diagnostic criteria for the Chinese population: Hyperglycemia (fasting plasma glucose ≥ 6.1 mmol/L and/or a previous diabetes diagnosis); hypertension (blood pressure ≥ 130/85 mmHg and/or a previous hypertension diagnosis); hypertriglyceridemia (triglyceride level > 1.7 mmol/L); low HDL-C (HDL-C level < 1 mmol/L); and obesity, which was defined in this study as BMI ≥ 28 kg/m2 instead of waist circumference. Metabolic syndrome was diagnosed in the presence of three or more of these components[17].
Data collection
To ensure data accuracy, two trained extractors independently collected demographic, clinical, and laboratory data from electronic medical records using standardized data extraction forms. All extracted data were then reviewed by clinical experts in gastroenterology. These data included age, sex, BMI, medical history (hypertension, diabetes), and the presence of pleural effusion and peritoneal effusion. Additionally, information regarding disease severity and clinical outcomes was collected, encompassing localized and systemic complications, ARDS, MODS, severity of AP, length of hospital stays, intensive care unit (ICU) admission, and mortality. Clinical and radiological parameters required for the Bedside Index of Severity in AP (BISAP)[18], the modified computed tomography severity index (MCTSI)[19], and the Ranson score[20] were recorded, and the corresponding scores were calculated. Laboratory indicators were measured within 24 hours of admission. The Ranson score was calculated using laboratory values obtained within the initial 48 hours. Imaging examinations were conducted within 5 days of admission.
Statistical analysis
Continuous variables were presented as mean ± SD when normally distributed, and assessed using Student’s t-test. Non-normally distributed variables were reported as median (interquartile range) and compared using the Mann-Whitney U test or Kruskal-Wallis test as appropriate. Categorical variables were presented as counts (percentages) and analyzed using the χ2 test or Fisher’s exact test, as appropriate. Kolmogorov-Smirnov test was used to assess the normality of continuous variables. The missing values in this study were confirmed to be missing completely at random (MCAR) using Little's MCAR test performed in SPSS. The expectation maximization algorithm was then used to impute the missing values. A Mantel-Haenszel χ2 test was employed to assess for linear trends across groups. To identify independent risk factors associated with different clinical courses of HTG-AP, logistic regression analysis was performed, with results reported as odds ratios (OR) and 95%CI. Statistical analyses were performed using SPSS (version 26.0), and P values < 0.05 were considered statistically significant. Bar charts and box plots were generated using GraphPad Prism (version 8).
RESULTS
Demographic and clinical characteristics of patients with HTG-AP
This retrospective study included 255 patients diagnosed with HTG-AP. Detailed baseline characteristics of the patients are summarized in Table 1. The study population consisted of 195 males (76.5%) and 60 females (23.5%). The mean age of the patients was 40.2 ± 8.9 years, and the mean BMI was 26.7 ± 3.9 kg/m². A history of hypertension was present in 16.5% (n = 42) of patients, and a history of diabetes was present in 23.5% (n = 60). Among these patients, 28.2% (n = 72) had MAP, 49.4% (n = 126) had MSAP, and 22.4% (n = 57) had SAP. Given the small sample size of patients with WON and limited data on mortality, further analysis of these parameters was not performed in this study.
Table 1.
Patient characteristics at baseline, n (%)
|
Characteristic
|
All (n = 255)
|
| Age (years), mean ± SD | 40.2 ± 8.9 |
| Sex | |
| Male | 195 (76.5) |
| Female | 60 (23.5) |
| BMI (kg/m2), mean ± SD | 26.7 ± 3.9 |
| History of hypertension | 42 (16.5) |
| History of diabetes | 60 (23.5) |
| Pleural effusion | 129 (50.6) |
| Peritoneal effusion | 78 (30.6) |
| Localized complications | 183 (71.8) |
| APFC | 62 (24.3) |
| ANC | 93 (36.5) |
| PPC | 17 (6.7) |
| WON | 3 (1.2) |
| IPN | 8 (3.1) |
| Systemic complications | 135 (52.9) |
| Respiratory failure | 53 (20.8) |
| Renal failure | 39 (15.3) |
| Circulatory failure | 16 (6.3) |
| SIRS | 123 (48.2) |
| Sepsis | 17 (6.7) |
| ACS | 22 (8.6) |
| ARDS | 16 (6.3) |
| MODS | 32 (12.5) |
| Grades of severity | |
| MAP | 72 (28.2) |
| MSAP | 126 (49.4) |
| SAP | 57 (22.4) |
| ICU admission | 54 (21.2) |
| Length of hospital stays (days), median (IQR) | 10 (6, 14) |
| Total costs (thousand CNY), median (IQR) | 19.7 (11.0, 37.8) |
| Mortality | 2 (0.78) |
IQR: Interquartile range; BMI: Body mass index; APFC: Acute peripancreatic fluid collection; ANC: Acute necrotic collection; PPC: Pancreatic pseudocyst; WON: Walled-off necrosis; IPN: Infected pancreatic necrosis; SIRS: Systemic inflammatory response syndrome; ACS: Abdominal compartment syndrome; ARDS: Acute respiratory distress syndrome; MODS: Multiple organ dysfunction syndrome; MAP: Mild acute pancreatitis; MSAP: Moderately severe acute pancreatitis; SAP: Severe acute pancreatitis; CNY: China yuan; ICU: Intensive care unit.
Associations of metabolic syndrome components with clinical scores and hospitalization characteristics in patients with HTG-AP
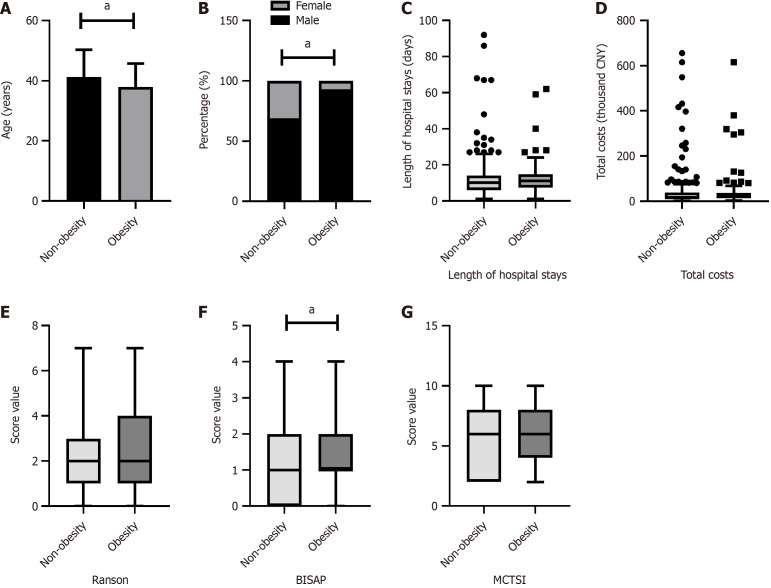
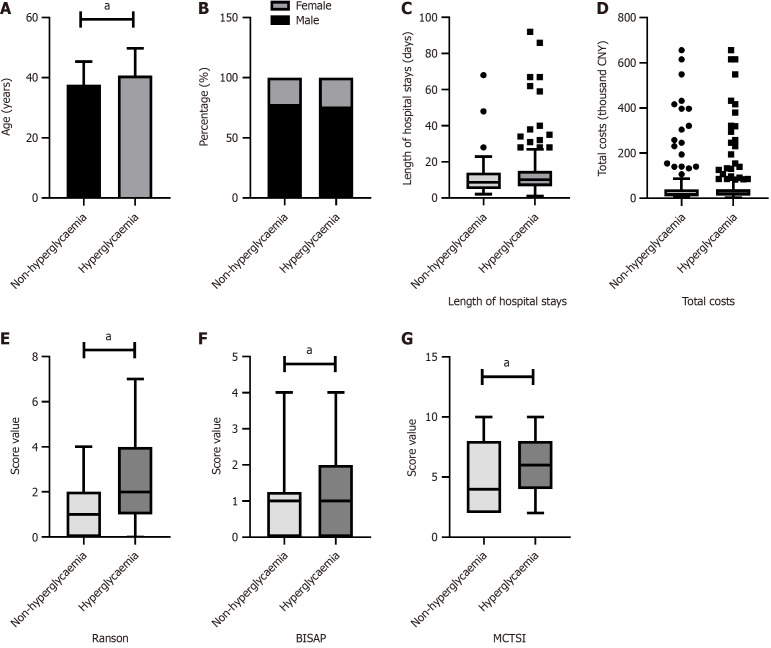
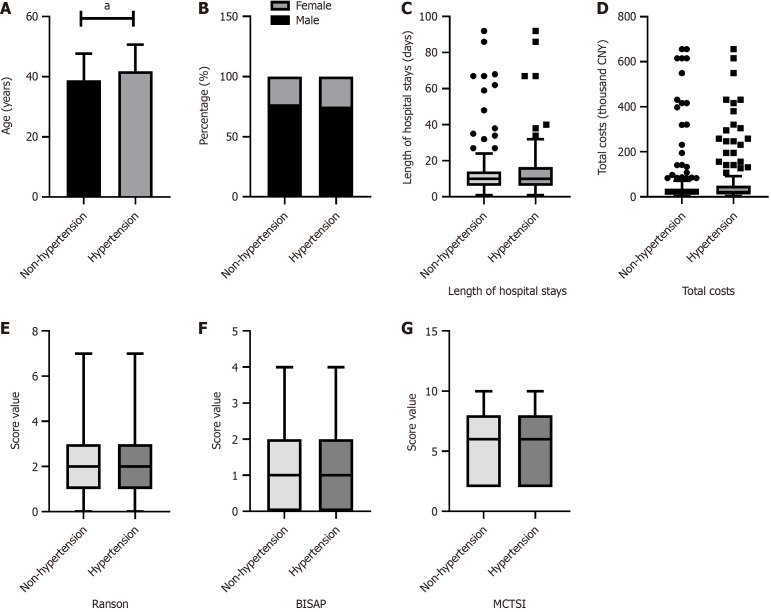
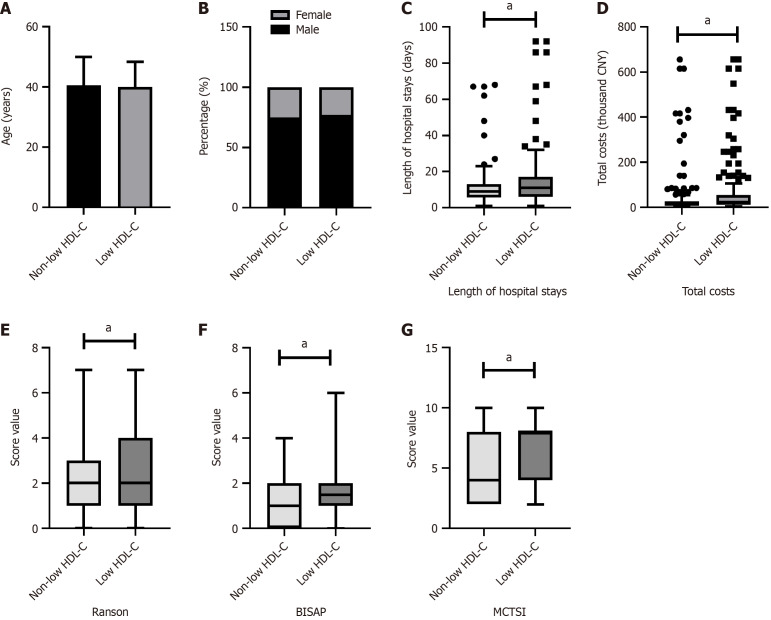
As shown in Figures 1, 2, 3 and 4, patients were categorized into two groups based on the presence or absence of obesity, hyperglycemia, hypertension, or low HDL-C. Compared to non-obese patients, obese patients were younger (P = 0.005), had a higher proportion of males (P < 0.001), and exhibited a higher BISAP score. In contrast to those without hyperglycemia, hyperglycemic patients were older (P = 0.029) and had significantly higher Ranson scores (P < 0.001), BISAP scores (P = 0.005), and MCTSI scores (P = 0.030). Furthermore, patients with hypertension were older than those without hypertension (P = 0.008). Patients with low HDL-C, compared to those without, had significantly higher Ranson scores (P = 0.035), BISAP scores (P < 0.001), and MCTSI scores (P < 0.001). Only patients with low HDL-C had significantly longer hospital stays (P = 0.001) and higher total hospital costs (P < 0.001), while no significant differences in these parameters were observed between patients with and without obesity, hyperglycemia, or hypertension. Detailed data are presented in Supplementary Tables 1-4.
Figure 1.
Associations of obesity with clinical scores and hospitalization characteristics in hypertriglyceridemia-acute pancreatitis. Comparison between patients with and without obesity. A: Age; B: Sex; C: Hospital stays; D: Hospital costs; E: Ranson scores; F: Bedside index of severity in acute pancreatitis scores; G: Modified computed tomography severity index scores. aP < 0.05; CNY: China yuan; BISAP: Bedside index of severity in acute pancreatitis; MCTSI: Modified computed tomography severity index.
Figure 2.
Associations of hyperglycaemia with clinical scores and hospitalization characteristics in hypertriglyceridemia-acute pancreatitis. Comparison between patients with and without hyperglycaemia. A: Age; B: Sex; C: Hospital stays; D: Hospital costs; E: Ranson scores; F: Bedside index of severity in acute pancreatitis scores; G: Modified computed tomography severity index scores. aP < 0.05; CNY: China yuan; BISAP: Bedside Index of Severity in acute pancreatitis; MCTSI: Modified computed tomography severity index.
Figure 3.
Associations of hypertension with clinical scores and hospitalization characteristics in hypertriglyceridemia-acute pancreatitis. Comparison between patients with and without hypertension. A: Age; B: Sex; C: Hospital stays; D: Hospital costs; E: Ranson scores; F: Bedside index of severity in acute pancreatitis scores; G: Modified computed tomography severity index scores. aP < 0.05; CNY: China yuan; BISAP: Bedside Index of Severity in acute pancreatitis; MCTSI: Modified computed tomography severity index.
Figure 4.
Associations of low high-density lipoprotein cholesterol with clinical scores and hospitalization characteristics in hypertriglyceridemia-acute pancreatitis. Comparison between patients with and without low high-density lipoprotein cholesterol. A: Age; B: Sex; C: Hospital stays; D: Hospital costs; E: Ranson scores; F: Bedside index of severity in acute pancreatitis scores; G: Modified computed tomography severity index scores. aP < 0.05; CNY: China yuan; HDL-C: High-density lipoprotein cholesterol; BISAP: Bedside Index of Severity in acute pancreatitis; MCTSI: Modified computed tomography severity index.
The impact of individual components of metabolic syndrome on clinical outcomes in patients with HTG-AP
Univariate logistic regression analysis was performed to investigate the influence of obesity, hyperglycemia, hypertension, and low HDL-C on clinical outcomes in HTG-AP patients. Table 2 reveals that obesity increased the risk of localized complications (OR = 2.07, 95%CI: 1.07-4.00), APFC (OR = 2.73, 95%CI: 1.50-4.96), systemic complications (OR = 1.81, 95%CI: 1.05-3.14), respiratory failure (OR = 2.16, 95%CI: 1.15-4.04), and renal failure (OR = 2.06, 95%CI: 1.02-4.14). Hyperglycemia increased the risk of localized complications (OR = 3.04, 95%CI: 1.53-6.06), systemic complications (OR = 2.86, 95%CI: 1.40-5.82), and SIRS (OR = 3.04, 95%CI: 1.53-6.06). Hypertension increased the risk of systemic complications (OR = 1.81, 95%CI: 1.09-2.98) and SIRS (OR = 1.84, 95%CI: 1.12-3.03). Low HDL-C was associated with an increased risk of pleural effusion (OR = 2.57, 95%CI: 1.55-4.27), peritoneal effusion (OR = 3.02, 95%CI: 1.70-5.36), localized complications (OR = 2.06, 95%CI: 1.18-3.59), ANC (OR = 2.32, 95%CI: 1.37-3.94), systemic complications (OR = 2.38, 95%CI: 1.37-3.93), respiratory failure (OR = 4.94, 95%CI: 2.35-10.37), renal failure (OR = 3.40, 95%CI: 1.54-7.49), SIRS (OR = 1.76, 95%CI: 1.07-2.90), ARDS (OR = 4.02, 95%CI: 1.12-14.47), MODS (OR = 3.54, 95%CI: 1.47-8.52), SAP (OR = 4.32, 95%CI: 2.16-8.66), and ICU admission (OR = 3.91, 95%CI: 1.94-7.85).
Table 2.
Impact of individual metabolic syndrome components on adverse clinical outcomes in hypertriglyceridemia-acute pancreatitis
| Outcome |
Obesity
|
Hyperglycaemia
|
Hypertension
|
Low HDL-C
|
||||
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
|
| Pleural effusion | 1.52 (0.89-2.61) | 0.129 | 1.75 (0.88-3.46) | 0.109 | 1.12 (0.69-1.84) | 0.644 | 2.57 (1.55-4.27) | < 0.001a |
| Peritoneal effusion | 0.98 (0.55-1.75) | 0.941 | 1.44 (0.67-3.11) | 0.349 | 1.20 (0.70-2.05) | 0.505 | 3.02 (1.70-5.36) | < 0.001a |
| Localized complications | 2.07 (1.07-4.00) | 0.031a | 3.04 (1.53-6.06) | 0.002a | 0.82 (0.47-1.42) | 0.476 | 2.06 (1.18-3.59) | 0.011a |
| APFC | 2.73 (1.50-4.96) | 0.001a | 2.61 (0.98-6.99) | 0.055 | 0.96 (0.54-1.71) | 0.889 | 0.82 (0.46-1.46) | 0.499 |
| ANC | 1.02 (0.59-1.79) | 0.963 | 1.47 (0.71-3.05) | 0.298 | 0.75 (0.45-1.25) | 0.271 | 2.32 (1.37-3.94) | 0.002a |
| PPC | 0.48 (0.13-1.74) | 0.484 | 0.89 (0.24-3.24) | 0.855 | 1.45 (0.54-3.89) | 0.461 | 0.58 (0.21-1.58) | 0.288 |
| Systemic complications | 1.81 (1.05-3.14) | 0.034a | 2.86 (1.40-5.82) | 0.004a | 1.81 (1.09-2.98) | 0.021a | 2.38 (1.43-3.93) | 0.001a |
| Respiratory failure | 2.16 (1.15-4.04) | 0.016a | 2.08 (0.77-5.60) | 0.146 | 1.40 (0.76-2.57) | 0.276 | 4.94 (2.35-10.37) | < 0.001a |
| Renal failure | 2.06 (1.02-4.14) | 0.044a | 2.56 (0.75-8.75) | 0.133 | 0.85 (0.43-1.71) | 0.654 | 3.40 (1.54-7.49) | 0.002a |
| Circulatory failure | 1.08 (0.36-3.21) | 0.896 | 3.02 (0.39-23.48) | 0.292 | 0.55 (0.19-1.64) | 0.283 | 2.74 (0.86-8.73) | 0.089 |
| SIRS | 1.39 (0.81-2.37) | 0.235 | 3.04 (1.53-6.06) | 0.002a | 1.84 (1.12-3.03) | 0.017 | 1.76 (1.07-2.90) | 0.026a |
| Sepsis | 0.71 (0.22-2.25) | 0.560 | 1.47 (0.32-6.69) | 0.618 | 1.13 (0.42-3.02) | 0.814 | 2.99 (0.95-9.43) | 0.062 |
| ACS | 1.11 (0.43-2.84) | 0.829 | 1.23 (0.35-4.38) | 0.745 | 1.57 (0.65-3.78) | 0.315 | 2.47 (0.93-6.53) | 0.069 |
| ARDS | 1.92 (0.69-5.35) | 0.214 | 1.37 (0.30-6.25) | 0.688 | 1.28 (0.46-3.51) | 0.637 | 4.02 (1.12-14.47) | 0.033a |
| MODS | 1.74 (0.81-3.73) | 0.156 | 3.18 (0.73-13.86) | 0.124 | 0.84 (0.40-1.79) | 0.654 | 3.54 (1.47-8.52) | 0.005a |
| SAP | 1.85 (1.00-3.43) | 0.050 | 1.83 (0.73-4.59) | 0.201 | 1.17 (0.65-2.12) | 0.598 | 4.32 (2.16-8.66) | < 0.001a |
| ICU admission | 1.69 (0.90-3.17) | 0.102 | 2.14 (0.80-5.74) | 0.132 | 1.47 (0.80-2.69) | 0.211 | 3.91 (1.94-7.85) | < 0.001a |
P < 0.05.
Adjusted for age and sex. OR: Odds ratio; HDL-C: High-density lipoprotein cholesterol; APFC: Acute peripancreatic fluid collection; ANC: Acute necrotic collection; PPC: Pancreatic pseudocyst; SIRS: Systemic inflammatory response syndrome; ACS: Abdominal compartment syndrome; ARDS: Acute respiratory distress syndrome; MODS: Multiple organ dysfunction syndrome; SAP: Severe acute pancreatitis; ICU: Intensive care unit.
The association of the number of metabolic syndrome components with clinical scores and hospitalization characteristics in patients with HTG-AP
To assess the impact of the number of metabolic syndrome components on HTG-AP clinical course, patients were stratified into five groups based on the number of metabolic syndrome components (1 to 5) present. Table 3 demonstrates that patients exhibiting a greater number of metabolic syndrome components had significantly higher Ranson (P < 0.001), BISAP (P < 0.001), and MCTSI (P = 0.005) scores, as well as increased hospital costs (P = 0.014) and prolonged hospital stays (P < 0.001).
Table 3.
Association of metabolic syndrome component count with clinical severity scores and hospitalization characteristics in hypertriglyceridemia-acute pancreatitis, n (%)
| Characteristic |
Number of metabolic syndrome components
|
P value | ||||
|
1 (n = 6)
|
2 (n = 61)
|
3 (n = 102)
|
4 (n = 69)
|
5 (n = 17)
|
||
| Age (years) | 35.8 ± 8.8 | 39.8 ± 9.6 | 40.9 ± 8.2 | 39.5 ± 9.9 | 40.2 ± 8.9 | 0.545 |
| Sex | ||||||
| Male | 5 (2.6) | 42 (21.5) | 76 (39.0) | 57 (29.2) | 15 (7.7) | 0.290 |
| Female | 1 (1.7) | 19 (31.7) | 26 (43.3) | 12 (20.0) | 2 (3.3) | |
| Scoring systems | ||||||
| Ranson | 0.5 (0, 1) | 1 (1, 3) | 2 (1, 3) | 2 (1, 4) | 4 (2, 5) | < 0.001a |
| BISAP | 0 (0, 0.5) | 0 (0, 1) | 1 (0, 2) | 2 (1, 2) | 2 (1, 2.5) | < 0.001a |
| MCTSI | 4 (2, 6.5) | 4 (2, 8) | 6 (2, 8) | 8 (4, 8) | 8 (4, 8) | 0.005a |
| Length of hospital stays (days) | 6 (4.3, 9.8) | 8 (5, 12.5) | 10 (7, 14.3) | 11 (6, 17) | 19 (13, 19.5) | 0.014a |
| Total costs (thousand CNY) | 8.6 (5.9, 17.1) | 14.0 (6.8, 24.6) | 17.9 (11.4, 37.7) | 26.1 (14.7, 61.1) | 25.8 (16.7, 81.3) | < 0.001a |
P < 0.05.
CNY: China yuan; BISAP: Bedside Index of Severity in acute pancreatitis; MCTSI: Modified computed tomography severity index.
Metabolic syndrome components exhibit an additive effect on adverse clinical outcomes in HTG-AP
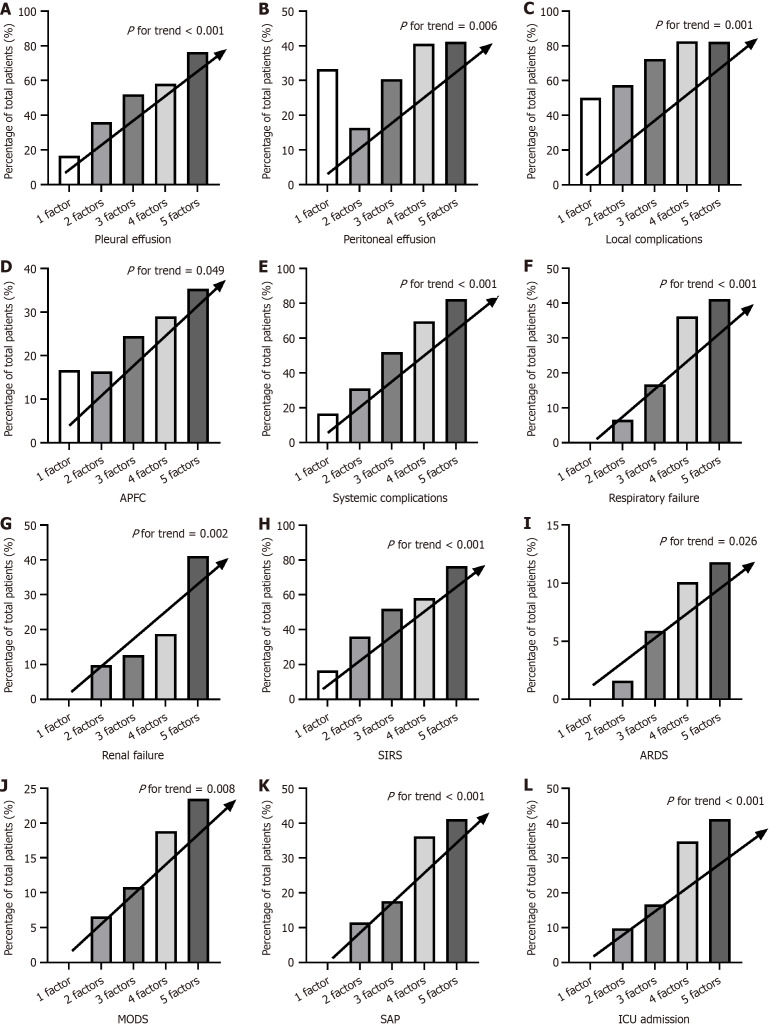
To further assess whether the incidence of adverse clinical outcomes demonstrates a linear trend with increasing numbers of metabolic syndrome components, a Mantel-Haenszel χ2 test was conducted. As shown in Figure 5, the incidence of pleural effusion (P < 0.001), peritoneal effusion (P = 0.006), local complications (P = 0.001), APFC (P = 0.049), systemic complications (P < 0.001), respiratory failure (P < 0.001), renal failure (P = 0.002), SIRS (P < 0.001), ARDS (P = 0.026), MODS (P = 0.008), SAP (P < 0.001), and ICU admission (P < 0.001) demonstrated a significant linear increase with an increasing number of metabolic syndrome components. Detailed data, including complications that did not demonstrate a statistically significant association with the number of metabolic syndrome components, are presented in Supplementary Table 5.
Figure 5.
The additive effect of metabolic syndrome components on adverse clinical outcomes in hypertriglyceridemia-acute pancreatitis. The impact of different numbers of metabolic syndrome components (1-5) on the incidence. A: Pleural effusion; B: Peritoneal effusion; C: Local complications; D: Acute peripancreatic fluid collection; E: Systemic complications; F: Respiratory failure; G: Renal failure; H: Systemic inflammatory response syndrome; I: Acute respiratory distress syndrome; J: Multiple organ dysfunction syndrome; K: Severe acute pancreatitis; L: Intensive care unit admission. APFC: Acute peripancreatic fluid collection; SIRS: Systemic inflammatory response syndrome; ARDS: Acute respiratory distress syndrome; MODS: Multiple organ dysfunction syndrome; SAP: Severe acute pancreatitis; ICU: Intensive care unit.
Metabolic syndrome increases the risk of multiple adverse clinical outcomes in patients with HTG-AP
To assess the impact of metabolic syndrome on the clinical outcomes of HTG-AP, patients were categorized into three groups based on the number of metabolic syndrome components present: Non-metabolic syndrome group (< 3 components), low-component metabolic syndrome group (3 components), and high-component metabolic syndrome group (≥ 4 components). As shown in Table 4, multivariate logistic regression analysis, adjusted for age and sex, revealed that patients with low-component metabolic syndrome had a significantly higher risk of pleural effusion (OR = 2.16, 95%CI: 1.13-4.14), peritoneal effusion (OR = 2.27, 95%CI: 1.04-4.92), localized complications (OR = 2.14, 95%CI: 1.11-4.13), systemic complications (OR = 2.67, 95%CI: 1.38-5.16), respiratory failure (OR = 3.24, 95%CI: 1.03-10.16), and SIRS (OR = 2.54, 95%CI: 1.30-4.93) compared with patients without metabolic syndrome. Patients with high-component metabolic syndrome exhibited a significantly higher risk of pleural effusion (OR = 3.43, 95%CI: 1.73-6.80), peritoneal effusion (OR = 3.78, 95%CI: 1.72-8.34), localized complications (OR = 3.63, 95%CI: 1.73-7.65), systemic complications (OR = 6.49, 95%CI: 3.17-13.30), respiratory failure (OR = 9.32, 95%CI: 3.07-28.25), renal failure (OR = 3.02, 95%CI: 1.12-8.11), SIRS (OR = 4.54, 95%CI: 2.25-9.14), SAP (OR = 5.05, 95%CI: 2.04-12.49), and ICU admission (OR = 6.41, 95%CI: 2.42-16.97) compared to patients without metabolic syndrome. Notably, renal failure (OR = 3.02, 95%CI: 1.12-8.11), SAP (OR = 5.05, 95%CI: 2.04-12.49) and ICU admission (OR = 6.41, 95%CI: 2.42-16.97) were only significantly increased in patients with high-component metabolic syndrome. suggesting a greater impact of a higher number of metabolic syndrome components on these specific complications.
Table 4.
Relationship between metabolic syndrome and clinical outcomes in hypertriglyceridemia-acute pancreatitis
| Outcome parameter |
Group
|
|||||
|
Non-metabolic syndrome
|
Low-component metabolic Syndrome
|
High-component metabolic syndrome
|
||||
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
|
| Pleural effusion | Reference | - | 2.16 (1.13-4.14) | 0.019a | 3.43 (1.73-6.80) | < 0.001a |
| Peritoneal effusion | Reference | - | 2.27 (1.04-4.92) | 0.039a | 3.78 (1.72-8.34) | 0.001a |
| Localized complications | Reference | - | 2.14 (1.11-4.13) | 0.023a | 3.63 (1.73-7.65) | 0.001a |
| APFC | Reference | - | 1.61 (0.73-3.55) | 0.240 | 2.09 (0.94-4.65) | 0.071 |
| ANC | Reference | - | 0.99 (0.52-1.93) | 0.992 | 1.64 (0.84-3.20) | 0.149 |
| PPC | Reference | - | 1.10 (0.33-3.62) | 0.876 | 0.72 (0.18-2.89) | 0.638 |
| Systemic complications | Reference | - | 2.67 (1.38-5.16) | 0.004a | 6.49 (3.17-13.30) | < 0.001a |
| Respiratory failure | Reference | - | 3.24 (1.03-10.16) | 0.044a | 9.32 (3.07-28.25) | < 0.001a |
| Renal failure | Reference | - | 1.54 (0.55-4.30) | 0.414 | 3.02 (1.12-8.11) | 0.028a |
| Circulatory failure | Reference | - | 2.86 (0.56-14.73) | 0.207 | 3.14 (0.60-16.26) | 0.173 |
| SIRS | Reference | 2.54 (1.30-4.93) | 0.006a | 4.54 (2.25-9.14) | < 0.001a | |
| SAP | Reference | - | 1.88 (0.74-4.82) | 0.187 | 5.05 (2.04-12.49) | < 0.001a |
| ICU admission | Reference | - | 2.25 (0.83-6.13) | 0.112 | 6.41 (2.42-16.97) | < 0.001a |
P < 0.05.
OR: Odds ratio; APFC: Acute peripancreatic fluid collection; ANC: Acute necrotic collection; PPC: Pancreatic pseudocyst; SIRS: Systemic inflammatory response syndrome; SAP: Severe acute pancreatitis; ICU: Intensive care unit.
DISCUSSION
While the pathogenesis of HTG-AP remains incompletely understood, the prevailing hypothesis suggests that high triglyceride levels are hydrolyzed by pancreatic lipase, generating excessive free fatty acids (FFAs) that damage pancreatic acinar and vascular endothelial cells, leading to pancreatic edema, necrosis, and systemic inflammation[21]. Concurrently, elevated concentrations of chylomicrons contribute to increased pancreatic capillary viscosity, further exacerbating pancreatic ischemia and injury[22]. Therefore, metabolic disturbances, including individual components and the overall burden of metabolic syndrome, may negatively impact the progression of HTG-AP. This study systematically analyzes the association between individual components and the number of metabolic syndrome components with key clinical outcomes, including severity, complications, ICU admission, and hospitalization costs, in patients with HTG-AP.
Dysregulation of HDL-C metabolism is a hallmark of metabolic syndrome. Evidence suggests that serum HDL-C levels are inversely correlated with the SAP and can serve as an effective prognostic marker for persistent organ failure in AP patients[23,24]. A case-control study of 1127 AP patients revealed that, after adjusting for confounders, HDL-C was found to have a protective effect against SAP development and demonstrated superior predictive value for SAP compared to other lipid parameters[25]. However, the influence of low levels of HDL-C on the progression of HTG-AP still unclear. This study found that patients with low HDL-C experienced significantly higher Ranson, BISAP, and MCTSI scores, prolonged hospital stays, and increased total medical expenses compared to those with normal HDL-C levels. Furthermore, low HDL-C was associated with an increased risk of various local and systemic complications, including ANC, respiratory and renal failure, SIRS, ARDS, MODS, as well as SAP and ICU admission. Evidence suggests that HDL-C exerts potent anti-inflammatory, antioxidant, and endotoxin-neutralizing effects, playing a central role in the clearance of FFAs[26,27]. However, the development of SIRS disrupts the balance of HDL-C, contributing to AP progression towards organ failure and death by inhibiting its synthesis and accelerating its degradation through inflammatory cytokines such as TNF-α and IL-6[23,28]. Furthermore, inflammation can alter HDL-C structure and composition, leading to the formation of dysfunctional HDL-C[29]. These alterations may explain the observed association between low HDL-C levels and poor clinical outcomes in HTG-AP.
According to the World Health Organization definition, obesity is characterized by abnormal or excessive fat accumulation[30]. Obesity is associated with a variety of chronic diseases, including cardiovascular disease, type 2 diabetes, and certain cancers[31]. Furthermore, evidence suggests that obesity influences the course of AP, increasing the likelihood of early shock, respiratory failure, renal failure, and prolonged hospital stays, leading to more severe outcomes[32]. Yang et al[33] found that abdominal obesity is an independent risk factor for respiratory failure and local complications in AP patients, with those exhibiting both obesity and hypertriglyceridemia having a higher incidence of SAP, organ failure, and local complications compared to those with obesity alone. BMI is widely employed as a measure for assessing obesity because it is easily obtainable in clinical practice. A retrospective study demonstrated that patients with AP and a BMI within the Class I obesity range (25-29.9 kg/m2) exhibited a significantly higher incidence of organ failure and local complications compared to those with normal weigh[34]. HTG-AP often occurs in younger patients with a high BMI[7]. Our study demonstrates that obesity (BMI ≥ 28) significantly increases the risk of complications in patients with HTG-AP, including local complications such as APFC and systemic complications like respiratory and renal failure. However, these findings are not entirely consistent with those of another retrospective study of 96 HTG-AP patients. While that study found a positive correlation between higher BMI and an increased risk of respiratory failure, it did not identify an association with local complications or renal dysfunction[14]. These discrepancies may be attributed to differences in patient characteristics, specifically a larger sample size and a higher prevalence of renal dysfunction and local complications in our cohort. These factors may have contributed to statistically significant findings. Obesity exacerbates clinical outcomes in HTG-AP, driven by a complex interplay of factors. As BMI increases, so too does peripancreatic and intrapancreatic fat deposition, making the pancreas and surrounding adipose tissue more susceptible to extensive necrosis during HTG-AP episodes[35]. This increased necrosis leads to a surge in FFA release, triggering a cascade of inflammatory responses that can ultimately contribute to multi-organ failure[36]. Furthermore, obesity can elevate the diaphragm and impair pulmonary ventilation, increasing the risk of respiratory complications associated with HTG-AP[32].
Hyperglycemia is closely associated with the development and progression of many diseases, and its role in the advancement of AP has been increasingly studied. A prospective international cohort study revealed a dose-dependent relationship between both admission and peak in-hospital blood glucose levels and AP severity, mortality, length of hospital stays, and systemic and local complication rates. Notably, patients with peak blood glucose levels exceeding 7 mmol/L exhibited a 15-fold increased risk of developing SAP and a 5-fold increased risk of mortality[37]. In another retrospective study, AP patients with comorbid diabetes mellitus (DM) experienced higher rates of SAP, necrotizing pancreatitis, and local complications, as well as prolonged hospital stays, compared to non-diabetic patients[38]. Likewise, a meta-analysis has revealed that patients with AP and comorbid DM experience a significantly elevated risk of local and systemic complications, ICU admission, and mortality compared to those with AP without DM[39]. The positive correlation between triglyceride levels and the severity of hyperglycemia often leads to coexisting hyperglycemia in patients with HTG-AP[40]. Liao et al[15] found that patients with HTG-AP and coexisting DM presented with a significantly older age at onset, a heightened risk of ischemic heart disease, and elevated Acute Physiology and Chronic Health Evaluation II scores compared to their non-diabetic counterparts. Our study also demonstrated that patients with hyperglycemia in the context of HTG-AP demonstrated significantly higher Ranson, BISAP, and MCTSI scores, as well as a significantly older age, compared to those without hyperglycemia. Furthermore, we found that hyperglycemia increased the risk of SIRS, local complications, and systemic complications in HTG-AP patients. This heightened risk may be attributed to the exacerbating effects of hyperglycemia on the inflammatory cascade within the pancreas. Elevated blood glucose levels can increase intracellular reactive oxygen species production, leading to mitochondrial dysfunction, promoting inflammation, and inducing cell death[41]. Consequently, these patients have a reduced physiological reserve to respond to acute illnesses, making them more susceptible to severe complications when faced with the stress of AP[42]. However, our study found that hyperglycemia did not significantly increase the risk of SAP in HTG-AP patients, a finding that diverges from previous research on the impact of DM on AP. This discrepancy may be attributed to differences in inclusion criteria. While previous studies primarily focused on patients with pre-existing diabetes, our study defined hyperglycemia based on metabolic syndrome diagnostic criteria, encompassing individuals with both diagnosed diabetes and elevated fasting blood glucose.
Despite hypertension is a systemic disease that can affect multiple organ systems and cause chronic damage to organs and tissues, research exploring the link between hypertension and AP remains limited[43]. A study by Szentesi et al[44] revealed hypertension to be an independent risk factor associated with the occurrence of SAP, systemic complications, and renal failure. Additionally, another study has demonstrated that hypertension serves as an independent predictor of 30-day readmission among patients with HTG-AP[45]. Our study revealed that hypertension in patients with HTG-AP is associated with an increased risk of developing systemic complications and SIRS. This association may be driven by hypertension-induced immune dysregulation and endothelial dysfunction, leading to increased susceptibility to infections and a potential progression to SIRS[46]. However, our study found no significant association between hypertension and HTG-AP severity, a finding that contrasts with previous research on the impact of hypertension on AP of other etiologies. This discrepancy may be attributed to the unique pathogenesis of HTG-AP, where hypertension plays a less prominent role in disease severity.
Our study found that individual components of metabolic syndrome were associated with an increased risk of adverse clinical outcomes in HTG-AP. Notably, low HDL-C significantly impacted a broader range of complications, making it the most significant risk factor influencing clinical outcomes. This suggests that individual components of metabolic syndrome, particularly low HDL-C, could potentially serve as predictors of adverse clinical outcomes in HTG-AP.
The interplay and synergistic effects among the various components of metabolic syndrome is crucial in influencing the clinical outcomes of AP, and should not be solely attributed to any single component. A previous prospective cross-sectional study demonstrated that AP patients with comorbid metabolic syndrome had a significantly higher incidence of complications and longer hospital stays compared to patients without metabolic syndrome[47]. Furthermore, a study reported that the incidence of adverse outcomes in AP progressively increased with the number of metabolic syndrome components, rising by 9.5%, 24.1%, and 66.7% when two, three, and four components were present, respectively[44]. However, these studies primarily focused on the impact of metabolic syndrome on AP of all etiologies, with limited research specifically addressing its influence on HTG-AP. Our study is the first to demonstrate a linear increase in the incidence of various adverse clinical complications and outcomes, including local complications, systemic complications, ARDS, SIRS, MODS, SAP, and ICU admission, as the number of metabolic syndrome components progressively increased. Furthermore, a higher number of metabolic syndrome components was associated with elevated clinical severity scores, prolonged hospital stays, and increased hospitalization costs. These findings suggest a cumulative effect of multiple metabolic syndrome components on adverse clinical outcomes in HTG-AP. For further analysis, both the low-component and high-component metabolic syndrome groups exhibited a significantly increased risk of pleural effusion, peritoneal effusion, local complications, systemic complications, respiratory failure, and SIRS compared to the non-metabolic syndrome group. Interestingly, only the high-component metabolic syndrome group demonstrated an increased risk of renal failure, SAP, and ICU admission. These findings suggest that a greater number of metabolic syndrome components indicates a progressively worse metabolic state. Consequently, the presence of metabolic syndrome, particularly the high-component metabolic syndrome, significantly increases the risk of adverse clinical outcomes in patients with HTG-AP.
This study's strength lies in systematically analyzing the relationship between the number of metabolic syndrome components and adverse clinical outcomes in HTG-AP patients, rather than simply classifying them as having or not having metabolic syndrome. However, this study still has several limitations. First, the low number of observed in-hospital deaths precluded its inclusion in the analysis. This may be partially attributed to some critically ill patients choosing to leave the hospital to die at home, driven by personal or cultural beliefs. Second, the study employed a BMI ≥ 28 kg/m2 to define obesity, aligning with the metabolic characteristics of the Chinese population but not fully adhering to the metabolic syndrome criteria for abdominal obesity. Third, the relatively small sample size may have limited the statistical power to identify true associations. Finally, this single-center retrospective study is inherently susceptible to biases, including information bias, recall bias, and selection bias. Patient recall may be inaccurate, and medical records might be incomplete or contain errors. Furthermore, the selection of patients solely from tertiary hospitals introduces limitations, as these patients tend to have more complex conditions and higher rates of comorbidities, potentially limiting the generalizability of the findings. Moreover, due to the limited availability of accurate data on smoking and alcohol consumption in medical records, this study did not adjust for these potential confounders, which may have introduced bias into the study findings.
CONCLUSION
The presence of individual metabolic syndrome components can increase the risk of certain adverse outcomes in patients with HTG-AP. Crucially, the coexistence of multiple metabolic syndrome components can exert synergistic effects, further worsening the clinical course of HTG-AP. These findings highlight the importance of vigilant monitoring and tailored interventions for HTG-AP patients with multiple metabolic syndrome components to prevent severe outcomes. Further validation through multicenter prospective studies is needed to confirm the findings.
Footnotes
Institutional review board statement: This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 2024-E278-01).
Informed consent statement: The need for patient consent was waived due to the retrospective nature of the study.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B
Novelty: Grade A, Grade C
Creativity or Innovation: Grade A, Grade C
Scientific Significance: Grade A, Grade C
P-Reviewer: Shikha D; Yang XY S-Editor: Li L L-Editor: A P-Editor: Zheng XM
Contributor Information
Zhen-Hua Fu, Department of Gastroenterology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Zi-Yue Zhao, Department of Gastroenterology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Yao-Bing Liang, Department of Gastroenterology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Dong-Yu Cheng, Department of Gastroenterology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Jian-Ming Luo, Department of Gastroenterology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Hai-Xing Jiang, Department of Gastroenterology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Shan-Yu Qin, Department of Gastroenterology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China. qinshanyu@gxmu.edu.cn.
Data sharing statement
No additional data are available.
References
- 1.Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096–1101. doi: 10.1053/j.gastro.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 2.Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726–734. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 3.de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6:649–655. doi: 10.1177/2050640618755002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin XY, Zeng Y, Zhang ZC, Lin ZH, Chen LC, Ye ZS. Incidence and clinical characteristics of hypertriglyceridemic acute pancreatitis: A retrospective single-center study. World J Gastroenterol. 2022;28:3946–3959. doi: 10.3748/wjg.v28.i29.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin G, Cang X, Yu G, Hu G, Ni J, Xiong J, Hu Y, Xing M, Chen C, Huang Y, Tang M, Zhao Y, Cheng G, Wan R, Wang S, Wang X. Different Clinical Presentations of Hyperlipidemic Acute Pancreatitis: A Retrospective Study. Pancreas. 2015;44:1105–1110. doi: 10.1097/MPA.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 6.Jin M, Bai X, Chen X, Zhang H, Lu B, Li Y, Lai Y, Qian J, Yang H. A 16-year trend of etiology in acute pancreatitis: The increasing proportion of hypertriglyceridemia-associated acute pancreatitis and its adverse effect on prognosis. J Clin Lipidol. 2019;13:947–953.e1. doi: 10.1016/j.jacl.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Shafiq S, Patil M, Gowda V, Devarbhavi H. Hypertriglyceridemia-Induced Acute Pancreatitis - Course, Outcome, and Comparison with Non-Hypertriglyceridemia Associated Pancreatitis. Indian J Endocrinol Metab. 2022;26:459–464. doi: 10.4103/ijem.ijem_206_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Ke L, Dong J, Ye B, Meng L, Mao W, Yang Q, Li W, Li J. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: a retrospective study of 730 patients from a tertiary center. BMC Gastroenterol. 2018;18:89. doi: 10.1186/s12876-018-0821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung SY, Shim HS, Hah YM, Kim SH, Yeo SG. Association of Metabolic Syndrome With Sudden Sensorineural Hearing Loss. JAMA Otolaryngol Head Neck Surg. 2018;144:308–314. doi: 10.1001/jamaoto.2017.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Z, Wang X, Zhen Z, Wang Y, Sun P. Metabolic syndrome components and acute pancreatitis: a case-control study in China. BMC Gastroenterol. 2021;21:17. doi: 10.1186/s12876-020-01579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikolasevic I, Milic S, Orlic L, Poropat G, Jakopcic I, Franjic N, Klanac A, Kristo N, Stimac D. Metabolic syndrome and acute pancreatitis. Eur J Intern Med. 2016;32:79–83. doi: 10.1016/j.ejim.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Valdivielso P, Sánchez-Chaparro MA, Calvo-Bonacho E, Cabrera-Sierra M, Sainz-Gutiérrez JC, Fernández-Labandera C, Fernández-Meseguer A, Quevedo-Aguado L, Moraga MR, Gálvez-Moraleda A, González-Quintela A, Roman-Garcia J ICARIA (Ibermutuamur CArdiovascular RIsk Assesment) study group. Association of moderate and severe hypertriglyceridemia with obesity, diabetes mellitus and vascular disease in the Spanish working population: results of the ICARIA study. Atherosclerosis. 2009;207:573–578. doi: 10.1016/j.atherosclerosis.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Liu J, Xing Y, Du L, Chen J, Liu X, Hao J. Correlation of Body Mass Index and Waist-Hip Ratio with Severity and Complications of Hyperlipidemic Acute Pancreatitis in Chinese Patients. Gastroenterol Res Pract. 2017;2017:6757805. doi: 10.1155/2017/6757805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao W, Niu X, Zhang W, Liu X. Comparison of the Development and Prognosis in Patients of Hypertriglyceridemic Pancreatitis with and without Diabetes. Gastroenterol Res Pract. 2021;2021:8895268. doi: 10.1155/2021/8895268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 17.Joint committee for guideline revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15:1–29. doi: 10.11909/j.issn.1671-5411.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arif A, Jaleel F, Rashid K. Accuracy of BISAP score in prediction of severe acute pancreatitis. Pak J Med Sci. 2019;35:1008–1012. doi: 10.12669/pjms.35.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Q, He WH, Li TM, Lai C, Yu L, Xia LY, Luo Y, Zhu P, Liu H, Zeng Y, Zhu NH, Lyu N. [Evaluation of severity and prognosis of acute pancreatitis by CT severity index and modified CT severity index] Zhonghua Yi Xue Za Zhi. 2022;102:2011–2017. doi: 10.3760/cma.j.cn112137-20220424-00914. [DOI] [PubMed] [Google Scholar]
- 20.Ong Y, Shelat VG. Ranson score to stratify severity in Acute Pancreatitis remains valid - Old is gold. Expert Rev Gastroenterol Hepatol. 2021;15:865–877. doi: 10.1080/17474124.2021.1924058. [DOI] [PubMed] [Google Scholar]
- 21.Guo YY, Li HX, Zhang Y, He WH. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discov Med. 2019;27:101–109. [PubMed] [Google Scholar]
- 22.Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology. 2020;20:795–800. doi: 10.1016/j.pan.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhou CL, Zhang CH, Zhao XY, Chen SH, Liang HJ, Hu CL, Chen NW. Early prediction of persistent organ failure by serum apolipoprotein A-I and high-density lipoprotein cholesterol in patients with acute pancreatitis. Clin Chim Acta. 2018;476:139–145. doi: 10.1016/j.cca.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Peng YS, Chen YC, Tian YC, Yang CW, Lien JM, Fang JT, Wu CS, Hung CF, Hwang TL, Tsai YH, Lee MS, Tsai MH. Serum levels of apolipoprotein A-I and high-density lipoprotein can predict organ failure in acute pancreatitis. Crit Care. 2015;19:88. doi: 10.1186/s13054-015-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Zheng R, Gao F, Wang L, Feng S, Li J, Huang Z. Association between high-density lipoprotein cholesterol and apolipoprotein A-I and severe acute pancreatitis: a case-control study. Eur J Gastroenterol Hepatol. 2021;33:1517–1523. doi: 10.1097/MEG.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 26.Bugdaci MS, Sokmen M, Zuhur SS, Altuntas Y. Lipid profile changes and importance of low serum α-lipoprotein fraction (high-density lipoprotein) in cases with acute pancreatitis. Pancreas. 2011;40:1241–1244. doi: 10.1097/MPA.0b013e3182211bbf. [DOI] [PubMed] [Google Scholar]
- 27.Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D, Chin-Dusting JP. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- 28.Sharma D, Jakkampudi A, Reddy R, Reddy PB, Patil A, Murthy HVV, Rao GV, Reddy DN, Talukdar R. Association of Systemic Inflammatory and Anti-inflammatory Responses with Adverse Outcomes in Acute Pancreatitis: Preliminary Results of an Ongoing Study. Dig Dis Sci. 2017;62:3468–3478. doi: 10.1007/s10620-017-4813-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Jin S, Pan J, Lin Q, Yang S, Lu Y, Qiu M, Ambe PC, Basharat Z, Zimmer V, Wang W, Hong W. Relationship between Cholesterol-Related Lipids and Severe Acute Pancreatitis: From Bench to Bedside. J Clin Med. 2023;12 doi: 10.3390/jcm12051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tejera C, Porca C, Rodriguez-Carnero G, Andújar P, Casanueva FF, Bellido D, Crujeiras AB. Reducing Metabolic Syndrome through a Group Educational Intervention Program in Adults with Obesity: IGOBE Program. Nutrients. 2022;14 doi: 10.3390/nu14051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahrai MS, Huybrechts I, Biessy C, Rinaldi S, Ferrari P, Wasiq AW, Gunter MJ, Dossus L. Determinants of Obesity and Metabolic Health in the Afghan Population: Protocol, Methodology, and Preliminary Results. J Epidemiol Glob Health. 2022;12:113–123. doi: 10.1007/s44197-021-00026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Guo X, Ji H, Niu J, Gao P. Relationships between Metabolic Comorbidities and Occurrence, Severity, and Outcomes in Patients with Acute Pancreatitis: A Narrative Review. Biomed Res Int. 2019;2019:2645926. doi: 10.1155/2019/2645926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, He J, Ma S, Wang T, Zhu Q, Cao F, Li Y, Yang C, Chen C, Lu G, Hu L, Liu J, Chen W. The role of comorbid hypertriglyceridemia and abdominal obesity in the severity of acute pancreatitis: a retrospective study. Lipids Health Dis. 2021;20:171. doi: 10.1186/s12944-021-01597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y, Gong G, Aierken R, Liu X, Cheng W, Guan J, Jiang Z. A retrospective study investigating the clinical significance of body mass index in acute pancreatitis. PeerJ. 2024;12:e16854. doi: 10.7717/peerj.16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakker OJ, van Santvoort H, Besselink MG, Boermeester MA, van Eijck C, Dejong K, van Goor H, Hofker S, Ahmed Ali U, Gooszen HG, Bollen TL Dutch Pancreatitis Study Group. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62:1475–1480. doi: 10.1136/gutjnl-2012-302870. [DOI] [PubMed] [Google Scholar]
- 36.Sun Q, Ren Q, Du L, Chen S, Wu S, Zhang B, Wang B. Cardiometabolic Index (CMI), Lipid Accumulation Products (LAP), Waist Triglyceride Index (WTI) and the risk of acute pancreatitis: a prospective study in adults of North China. Lipids Health Dis. 2023;22:190. doi: 10.1186/s12944-023-01948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagy A, Juhász MF, Görbe A, Váradi A, Izbéki F, Vincze Á, Sarlós P, Czimmer J, Szepes Z, Takács T, Papp M, Fehér E, Hamvas J, Kárász K, Török I, Stimac D, Poropat G, Ince AT, Erőss B, Márta K, Pécsi D, Illés D, Váncsa S, Földi M, Faluhelyi N, Farkas O, Nagy T, Kanizsai P, Márton Z, Szentesi A, Hegyi P, Párniczky A. Glucose levels show independent and dose-dependent association with worsening acute pancreatitis outcomes: Post-hoc analysis of a prospective, international cohort of 2250 acute pancreatitis cases. Pancreatology. 2021;21:1237–1246. doi: 10.1016/j.pan.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Durmuş ET, Akdağ İ, Yıldız M. Diabetes is an independent predictor of severe acute pancreatitis. Postgrad Med. 2022;134:711–716. doi: 10.1080/00325481.2022.2105613. [DOI] [PubMed] [Google Scholar]
- 39.Mikó A, Farkas N, Garami A, Szabó I, Vincze Á, Veres G, Bajor J, Alizadeh H, Rakonczay Z Jr, Vigh É, Márta K, Kiss Z, Hegyi P, Czakó L. Preexisting Diabetes Elevates Risk of Local and Systemic Complications in Acute Pancreatitis: Systematic Review and Meta-analysis. Pancreas. 2018;47:917–923. doi: 10.1097/MPA.0000000000001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shemesh E, Zafrir B. Hypertriglyceridemia-Related Pancreatitis In Patients With Type 2 Diabetes: Links And Risks. Diabetes Metab Syndr Obes. 2019;12:2041–2052. doi: 10.2147/DMSO.S188856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15:4137–4142. doi: 10.3748/wjg.15.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nawaz H, O'Connell M, Papachristou GI, Yadav D. Severity and natural history of acute pancreatitis in diabetic patients. Pancreatology. 2015;15:247–252. doi: 10.1016/j.pan.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Rizzoni D, Agabiti-Rosei C, De Ciuceis C, Boari GEM. Subclinical Hypertension-Mediated Organ Damage (HMOD) in Hypertension: Atherosclerotic Cardiovascular Disease (ASCVD) and Calcium Score. High Blood Press Cardiovasc Prev. 2023;30:17–27. doi: 10.1007/s40292-022-00551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szentesi A, Párniczky A, Vincze Á, Bajor J, Gódi S, Sarlós P, Gede N, Izbéki F, Halász A, Márta K, Dobszai D, Török I, Farkas H, Papp M, Varga M, Hamvas J, Novák J, Mickevicius A, Maldonado ER, Sallinen V, Illés D, Kui B, Erőss B, Czakó L, Takács T, Hegyi P. Multiple Hits in Acute Pancreatitis: Components of Metabolic Syndrome Synergize Each Other's Deteriorating Effects. Front Physiol. 2019;10:1202. doi: 10.3389/fphys.2019.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kichloo A, El-Amir Z, Aucar M, Dahiya DS, Al-Haddad M, Pisipati S, Beiz H, Singh G, Gandhi D, Singh J, Pathappillil P, Mohideen H, Shaka H. Clinical Outcomes and Predictors of Thirty-Day Readmissions of Hypertriglyceridemia-Induced Acute Pancreatitis. Gastroenterology Res. 2022;15:19–25. doi: 10.14740/gr1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson JA, Banerjee A, Smeeth L, McDonald HI, Grint D, Herrett E, Forbes H, Pebody R, Warren-Gash C. Risk of acute respiratory infection and acute cardiovascular events following acute respiratory infection among adults with increased cardiovascular risk in England between 2008 and 2018: a retrospective, population-based cohort study. Lancet Digit Health. 2021;3:e773–e783. doi: 10.1016/S2589-7500(21)00203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niknam R, Moradi J, Jahanshahi KA, Mahmoudi L, Ejtehadi F. Association Between Metabolic Syndrome and Its Components with Severity of Acute Pancreatitis. Diabetes Metab Syndr Obes. 2020;13:1289–1296. doi: 10.2147/DMSO.S249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.