Abstract
Dental disease remains the most common non-communicable disease worldwide. It predisposes patients to significant morbidities following bone modifying agents or radiation therapy to the head and neck. Preventative dental regimes effectively reduce the risk of both medication-related osteonecrosis of the jaw (MRONJ) and osteoradionecrosis (ORN) in these patients. Co-ordination of routine dental care as a component of mainstream oncology treatment optimises long term outcomes for oncology patients. This case series offers insights into patient, institutional and social difficulties that challenge the dental-oncology interface. These obstacles and subsequent resolutions experienced whilst establishing a dental-oncology service in a cancer centre highlight the importance of effective multidisciplinary lead care for oncology patients. It reinforces the need for structured, supported dental pathways for these oncology patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-024-08872-x.
Keywords: Dental-oncology service, Bone modifying agents, Head and neck radiation therapy, Multidisciplinary
Introduction
In dentistry, the term bridging refers to providing a connection or contact between two different subjects or matter [1]. The term also resonates in interdisciplinary care where bridges are created based on patient needs. Globally, dental disease has been the most common, non-communicable disease worldwide for over 3 decades [2, 3]. For oncologists, the dental-oncology interface is most evident in the conditions of medication-related osteonecrosis of the jaw (MRONJ) and osteoradionecrosis (ORN). These well-recognised conditions have devastating quality of survival implications for patients [4]. Improved cancer outcomes have led to increased number of at risk patients and consequently a requirement for greater integration of dentistry into cancer care. Traditionally, this care relied upon non-structured, individual patient constructed bridges. In this paper, we outline the benefits of establishing formal bridging pathways in cancer care.
The growing significance of the dental-oncology interface results from the widespread integration of bone modifying agents (BMAs) to combat skeletal-related events (SREs), bone metastases and hypercalcemia. SREs in cancer patients are associated with loss of mobility, reduced quality of life and healthcare expenditure [5–7]. BMAs are commonly prescribed to patients with prostate (85%), breast (70%), lung (40%), kidney (40%) cancer and multiple myeloma (95%) [8–10]. Their utilisation is integrated into guideline-based cancer care [9]. BMAs are also given to patients in the curative setting such as early breast cancer to reduce recurrence rates and improve survival [9]. MRONJ is the most concerning side effect of BMAs, a side effect compounded by the lack of dental integration into oncology practice. It follows a clinical path of exposed bone in the maxillofacial region, present for at least 8 weeks in a patient exposed to a BMA (anti-resorptive/anti-angiogenic) and excluding radiation therapy or metastatic deposit to the jaws [11]. The prevalence of MRONJ in cancer patients ranges from 0 to 12% [11][12, 13]. RANK-ligand inhibitors have been used as alternatives to bisphosphonates, and their prevalence of MRONJ ranges from 0.7 to 6.7% [14, 15]. Incorporation of bisphosphonates into the curative anti-cancer setting poses a long-term risk of MRONJ due to the long half-life of the agents with a MRONJ rate of 0.00 to 1.8% in clinical trials [16–18]. As outlined in case reports/series and cohort studies, MRONJ is also associated with targeted therapies (sunitinib, bevacizumab and imatinib), immunomodulators (infliximab, rituximab and everolimus), immunotherapies (ipilimumab, pembrolizumab and nivolumab), cytotoxic agents (5-fluorouracil, paclitaxel and azacytidine) and folate antagonist (methotrexate) [19–25].
Radiation therapy is used in almost 75% of patients with a head and neck squamous cell carcinoma as either primary or adjuvant therapy following primary resection or concurrent chemoradiotherapy for local advanced cancer and organ preservation [26, 27]. Radiation toxicity is implicated in hyposalivation, mucositis, dermatitis, dysphagia, dysgeusia and hoarseness. Chronic implications and late toxicity events of radiation therapy include osteoradionecrosis, a fibro-atrophic osseous change seen in 5 to 15% of patients who have been exposed to radiation therapy to the jaws. This usually develops following a dentoalveolar procedure such as a dental extraction [28]. The incidence of ORN increases with radiation doses above 60 Gy and the risk of development increases with time [29, 30]. Prophylactic extraction of unfavourable teeth in the field of radiation therapy is recommended prior to radiation exposure [31, 32]. Cancer care is time sensitive, placing added emphasis on timely dental management [32].
Dental disease is a mitigating factor for the development of MRONJ and ORN [13, 28]. Poorly controlled, severe periodontal disease and dentoalveolar surgery such as a tooth extraction elevate the risk of MRONJ and ORN [33]. Dental disease increases the prevalence of invasive dental procedures in patients following radiation therapy and BMAs [13, 34, 35]. The influence of preventative dental strategies has proven efficacious in the prevention of MRONJ and ORN [29, 36]. Elimination of over-reliance on patients to implement aspects of care such as long-term dental care and formal integration of preventative dental services prior to BMA and radiation therapy remains beneficial [37].
To date, the integration of dental care into oncology specialities is unstructured [38]. Improving the oral health status and reducing treatment needs in oncology patients will reduce morbidities of treatment-implicated consequences [37]. This article provides a cautionary message for clinicians regarding dental risks in these groups [16, 18, 39–42]. Integrating a dental-oncology service into cancer care may help optimise patient care as a component of the multidisciplinary care model [43]. In this paper, we use a case series derived from both at-risk patients for MRONJ and ORN development to highlight the needs and benefits of an integrative dental service.
Materials and methods
In Ireland, the community dental care service is operated by the Health Service Executive (HSE) Dental Service. Primarily, this is based in the primary care setting which is comprised of predominantly privately funded treatment with general dental practitioners. There is a limited availability of HSE operated, state-funded dental schemes. Such treatments are means tested based on weekly income, savings, investments and property, including spousal incomes. This community-based service offers a limited amount of dental treatment for head and neck oncology patients, and a limited availability of dental practitioners compounds its impact.
For head and neck cancer patients, dental treatment prior to radiation therapy is provided by the HSE to ensure patients are dentally suitable to commence radiation therapy to the head and/or neck. Following radiation therapy, means testing and limited state-funded dental care for oncology patients renders access to dental care challenging. Consequently, patients face many barriers to care including access issues and financial constraints while trying to navigate complex dental pathways. Currently, dental services for oncology patients operate as a reflex dental service rather than a pre-emptive one. Dental oncology guidance advocates 3 monthly recall reviews for oncology patients at risk of MRONJ and ORN [44, 45]. There is no structured pathway for cancer patients prior to BMAs [38].
In 2022, a pilot dental oncology program was established using grant funding (CiSA, College of Medicine and Health interdisciplinary Seeds Award). Oncology patients planned for BMA therapy were referred from various oncology services in Cork University Hospital (CUH). The CUH is a level 5 hospital with 800 beds; 25,000 in-patient admissions; 27,000-day cases and 58,000 emergency cases (A&E) every year and employs over 4000 staff. It is 1 of 8 adult National Cancer Control Programme (NCCP)-affiliated Cancer Centres and 1 of 54 acute hospitals in Ireland run by the HSE [46]. The CUH is collocated with the Cork University Dental School and Hospital (CUDSH), which is governed by the University College Cork and structured to facilitate pre-dental assessments for head and neck cancer patients under the HSE Cancer Service Level Agreement.
Regional head and neck cancer services based in the South Infirmary Victoria University Hospital (SIVUH) and CUH refer head and neck cancer patients for pre-radiation dental treatment in the CUDSH. We have included a radiation therapy case to highlight some challenges in this cohort. The case summaries are highlighted in Table 1.
Table 1.
Clinical details of six dental-oncology cases
| Case number | Cancer diagnosis | Age | Stage | Planned BMA | Treatment |
|---|---|---|---|---|---|
| 1 | De novo metastatic, castrate sensitive prostate cancer | 65 years | 4 | Zoledronic acid | First line: docetaxel, abiraterone and androgen deprivation therapy (ADT) |
| 2 | Hormone sensitive, HER2 negative breast cancer | 55 years | 2 | Zoledronic acid |
Surgery: mastectomy and sentinel lymph node biopsy Adjuvant: doxorubicin, cyclophosphamide and paclitaxel (AC-T), radiation therapy, hormone treatment |
| 3 | Hormone sensitive, HER2 negative breast cancer | 43 years | 2 | Zoledronic acid |
Neoadjuvant: AC-T (in Ukraine) Surgery: mastectomy and axillary lymph node clearance (in Ukraine) Adjuvant: tamoxifen, zoladex (2 years previously) |
| 4 | De novo metastatic, castrate sensitive prostate cancer | 61 years | 4 | Zoledronic acid | First line: docetaxel, abiraterone, androgen deprivation therapy (ADT) |
| 5 | Metastatic castrate resistant prostate cancer | 81 years | 4 | Denosumab | Not currently on treatment. Multiple prior lines including ADT, chemotherapy, abiraterone and radium-223 |
| 6 | Head and neck cancer | 59 years | 4a | Radiation therapy | Primary surgery, reconstruction and adjuvant radiation therapy |
Case-based scenarios — patient cohort
Case 1
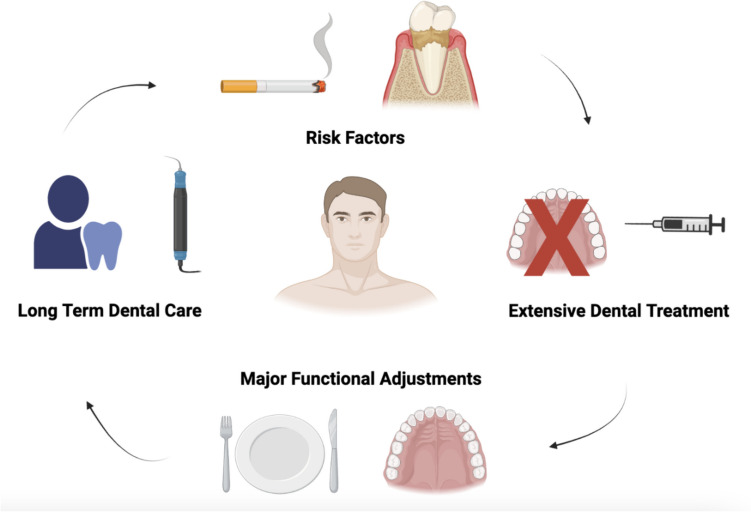
A 65-year-old male with de novo metastatic prostate cancer and bone-dominant disease was referred for a dental assessment. He had a longstanding history of dental neglect, uncontrolled periodontal disease (stage 3, grade B, British Society of Periodontology classification system [47]) and periapical infections. He had a 20-pack year smoking history, had not seen a dentist in over 7 years, nor was he registered with one. The treatment plan included 9 dental extractions, intensive periodontal therapy, provision of partial dentures, integration into the community care dental services and co-ordination with oncology throughout his dental care visits in tandem with his oncology chemotherapy treatment. He had a high dental treatment need and transitioning the patient into a partial dentition with dentures was challenging. See Fig. 1 which highlights the challenges experienced in case 1.
Fig. 1.
Summary of pre-existing MRONJ exacerbation factors (smoking and severe periodontal disease), and a high dental treatment need
Case 2
A 55-year-old post-menopausal female with early stage breast cancer presented for a dental assessment. She was scheduled for adjuvant chemotherapy, hormone treatment and BMA therapy. She complained of recurrent dental pain and swelling associated with a lower right wisdom tooth. The patient lived alone and could not drive. Risk factors for dental complications included chemotherapy treatment, smoking (20 pack years) and recurrent wisdom tooth infections. Extraction of her lower right wisdom tooth would place the patient at high risk of lower lip and chin paraesthesia due to the intimate nature of her wisdom tooth and inferior alveolar nerve. As a consequence of this relationship, staged oral surgery procedures were required to initially surgically section the crown off the impacted wisdom tooth, actively monitor for 3 months and then surgically remove the roots after they dissociated themselves from the inferior alveolar nerve. This staged, surgical approach was necessary to alleviate her pain, reduce the risk of permanent numbness to the lower lip and chin and eliminate the source of infection prior to BMA therapy. As a result of post-operative infection, her treatment plan also included 3 months of intense aftercare and wound debridement following decoronation of the wisdom tooth. Treatment was supported by meticulous wound-care in the oral surgery department throughout chemotherapy, antibiotic therapy and at-home oral hygiene regimes. We reinforced the importance of smoking cessation and effective multidisciplinary communication throughout chemotherapy, alongside the co-ordination of BMA therapy, following surgical removal of the wisdom tooth roots. She was integrated into the community-based dental service with 3 monthly dental reviews following administration of her BMA.
Case 3
A 43-year-old pre-menopausal Ukrainian refugee receiving adjuvant hormone treatment for early stage breast cancer was referred prior to BMA treatment. Her oncology treatment was initially commenced in the Ukraine; however, conflict there led to her seeking refugee status in Ireland. The patient was a poor dental attender in Ukraine for the previous 7 years. She did not speak English, did not possess a mobile phone and did not have a means of transport to the dental hospital. The patient failed to attend 3 dental hospital appointments over an 8-week period prior to the initial dental consultation. This impacted on the timely commencement of her BMA treatment. The reason for poor attendance was attributed to her movement between refugee centres, lack of direct means of contact and difficulties in accessing the patient directly in refugee homes. When she attended, treatment was also delayed due to access barriers to translation services. This case highlighted the lack of service familiarity, consequences of displacement, cost implications and co-ordination challenges between patient and translator. The translator service was also redundant on multiple occasions as the patient failed to attend appointments while the translator was present. The challenges involved both social and demographic prerequisites to language barriers, social instability, communication and poor access to routine health care services and have persisted during integration with a community dental service.
Case 4
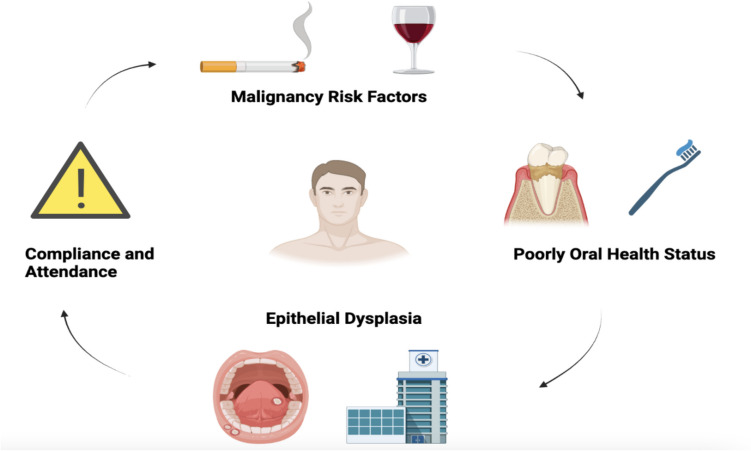
A 61-year-old male with de novo metastatic prostate cancer requiring BMA treatment was referred for a dental assessment. He complained of long-standing dental pain and mobility of some posterior teeth. He had a 20-year-pack history of smoking both cigarettes and cannabis and consumed 45 units of alcohol per week. The patient had not attended a dentist in the previous 5 years and was not registered with a general dental practitioner. Routine examination revealed uncontrolled periodontal disease (stage 3, grade B, British Society of Periodontology classification system [47]), 3 unrestorable molar teeth and two hyperplastic, heterogeneous plaques along the palatoglossal folds, concurrent with smoking-related mucosal changes. The patient received a course of periodontal debridement, extraction of the 3 unrestorable teeth and incisional biopsies of the heterogeneous soft tissue plaques. Both incisional biopsies revealed pre-cancerous, mucosal changes (moderate dysplasia) following histopathological examination. Routine dental examinations not only assess dental disease but also oral cancer screening which is a vital component of all dental examinations. The resolution for this patient incorporated an educational strategy to combat the social risk factors of oral cancer; smoking and alcohol. Invasive dental procedures such as extractions and biopsies were completed over multiple sessions to acclimatise the patient to dental work under local anaesthetic. Review regimes remained challenging due to the patients’ poor attendance and long-standing history of dental neglect and avoidance. We liaised with oral medicine colleagues who now actively monitor the moderate dysplasia on a 3–6 monthly review in a designated dysplasia clinic. Long-term integration into community dental services for long-term dental care remains challenging due to the patients’ poor compliance with oral health regimes. See Fig. 2 for a summary of the patients’ onco-dental issues.
Fig. 2.
A summary of the social and dental factors is depicted in Case 5. Routine dental examinations prior to BMA treatment are important to identify dental disease alongside mucosal examination and oral cancer screening
Case 5
An 81-year-old male with metastatic castrate resistant prostate cancer requiring both hormone and BMA treatment presented for dental assessment. He was previously assessed by the dental service 12 months prior, where he did not achieve dental fitness. The patient had severe, uncontrolled periodontitis (stage 3, Grade C, British Society of Periodontology [47]). He required multiple dental extractions due to their poor prognosis, extensive mobility and associated periodontal disease. The patient was also a native French Canadian speaker with poor English and required a translator. He returned for 2 further consultations to rediscuss his dental status and the proposed treatment plan including long-term periodontal root debridement therapy and multiple dental extractions. The patient had an inherent reluctance to proceed with the proposed plan due to an apprehension about his functional and chewing capacity thereafter. He refused all forms of invasive dental treatment such as extractions. We proceeded with the periodontal root debridement therapy; however, the patient subsequently decided to refrain from all dental treatments after the initial phase of periodontal therapy. There was a 14-month delay in BMA treatment in this case due to the patients’ reluctance to proceed with invasive dental treatment. Long-term dental care was not accepted in this case, and the patient proceeded with BMA treatment. He remains a high risk for MRONJ.
Case 6
A 59-year-old male patient with head and neck cancer presented following primary resection of his intraoral tumour, neck dissection and reconstruction with a fibula free flap, was planned for adjuvant radiation therapy. The patient was 2 weeks post-surgery and had a high level of post-operative care demands. He was on an intense analgesic regime and had airway challenges due to the recent decannulation of his tracheostomy. The surgical sites included the tracheostomy site, the neck dissection, the lower lip split/segmental mandibulectomy, free flap site in the left mandible and floor of mouth, donor site from the fibular free flap and abdomen skin graft donor site. His mouth opening was limited at 25 mm, and he currently was taking a direct oral anti-coagulant (apixaban) for atrial fibrillation. The patient was transferred with nursing staff to the CUDSH for a dental assessment prior to radiation therapy. The patient had an impacted, symptomatic, lower left wisdom tooth (Class 2, level B[48]). There was evidence of long-standing pericoronitis associated with the lower left wisdom tooth. Operative difficulties included limited access due to trismus, extensive cancer-related intra and extraoral healing surgical sites and anti-coagulant considerations for the surgical removal of the lower wisdom tooth. The patient also had a low mood and poor engagement with medical advice, stemming from his recent, intensive surgery and difficult initial post-operative recovery phase. He required the surgical removal of his impacted wisdom tooth by a specialist oral surgeon. Careful holistic planning at cancer diagnosis should integrate necessary surgical dental procedures with the concurrent cancer-directed surgery. The patient was seen regularly during his radiation therapy for supportive, preventative dental measures and introduced back to his private dental practitioner following the radiation therapy.
Table 2 below summarises the dental and oncology challenges that arose in these 6 clinical cases. The oncology cohorts have a high burden of care and challenges following cancer treatment. The resolutions of their onco-dental challenges are also highlighted in Table 2 which may serve as a template for future integration of dental services into oncology care.
Table 2.
Summary of dental issues, oncology challenges and resolution for each clinical-based scenario
| Case number | Dental issue | Oncology challenge | Resolution |
|---|---|---|---|
| Case 1 |
Poor pre-existing dental health Extraction of 9 teeth — functional adjustment to dental prosthesis |
Burden of treatment, co-ordination of invasive dental care Rural living and transport barriers to frequent health care |
Access to dental care Multidisciplinary co-ordination Transition to partial dentition |
| Case 2 |
Dental infection on presentation High risk of morbidity following surgical extraction/neural disturbance High risk of post-operative infection |
Management of chemotherapy and post-operative infection interface Access to care — transport barriers |
Multidisciplinary co-ordination Patient education and support throughout oral surgery recovery |
| Case 3 |
Chronic periodontitis Refugee Service and language barrier Infrequent dental attender (7 years ago) |
Social challenges Inconsistencies in oncology treatment between countries Transport and technological barriers |
Provision of translation and communication support Multidisciplinary support and access to dental service Co-ordination of transport |
| Case 4 |
Dental phobia Dental neglect and unreliable attender Significant cigarette smoker Uncontrolled dental disease Pre-malignant oral mucosal changes (moderate dysplasia) |
Intense treatment regime Social and educational barriers |
Smoking cessation Pre-malignant mucosal monitoring Regular mucosal review regime in tertiary setting |
| Case 5 |
Uncontrolled severe periodontitis Poor dental care compliance Resilience to engage with necessary pre-BMA dental intervention |
Delayed commencement of BMA therapy High risk of MRONJ |
Time for patient education, support and communication Engagement with periodontal maintenance programme after 12 months |
| Case 6 | Symptomatic, impacted lower wisdom teeth | Delayed commencement of radiation therapy due to surgical removal and awaiting mucosal coverage | Proposition for invasive dental extractions at the time of primary resection |
Summary of the challenges encountered whilst establishing a dental-oncology service
Discussion
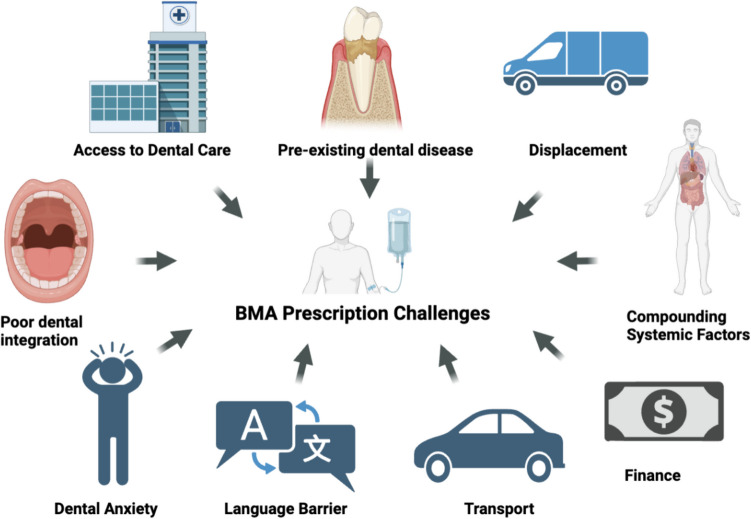
In this report, the multifaceted challenges to providing a dental oncology service are outlined. See Fig. 3 which provides an overview of the multifaceted nature of onco-dental challenges. The challenges predominantly include social, demographic, medical, cultural and financial restraints. These cases demonstrate inherent social and dental challenges such as dental phobia and dental neglect which are endemic in a cohort with pre-existing dental disease. Disruption of oncology treatment plans are evident alongside financial, health literacy, language and transport restraints. Oral healthcare is isolated from mainstream oncology. As a result, patients are responsible for accessing dental services and ensuring their dental needs are met. Reducing the burden of dental care and self-reliance on patients encourages the development of dental incorporation and services into oncology [49]. Barriers to dental care provision such as timing or insufficient time prior to commencing BMA or radiation therapy, lack of patient education regarding oral complications of BMAs and radiation therapy, co-ordination of healthcare specialities and lack of dentist confidence to treat this cohort are all modifiable factors for a service to address [50, 51]. Designated dental services have the potential to eliminate barriers to care.
Fig. 3.
Illustration highlighting the range of challenges that face patients and dental-oncology teams for patients receiving BMAs. The diagram below depicts a flow chart for patients at risk of MRONJ and the barriers to care
Managing care pathways pose challenges; however, identifying vulnerable patients requires vigilance from all healthcare professionals [52]. Since 2009, dental oncology integration has been shown to reduce MRONJ rates [53]. When referrer barriers are eliminated, it can lead to a 12-fold decrease in MRONJ in patients following their pre-medication dental examination [15]. Preventative dental strategies have a positive impact and help reduce the rate of MRONJ development [53–55]. Recognition of MRONJ in the early stages has a better prognosis, and lesser invasive therapies are required [56, 57]. The early incorporation of dental services for the head and neck oncology cohort has also proven beneficial. The inclusion of dental professionals into multidisciplinary team (MDT) meetings for head and neck oncology patients has allowed prompt assessment of the oral health status, and treatment of active dental disease prior to radiation exposure [58]. Additional benefits include reduction of potential radiation treatment timeframe delays, reduction in the burden of post radiation dental treatment needs, improved oral health-related quality of life and increased patient and provider education [58, 59]. Dental-oncology integration in an MDT setting with BMA therapy however remains sparsely documented in the literature and suggests suboptimal levels of integration. A recent review by Lim et al. in 2024 assessed the prevalence of dental assessments in 15,357 metastatic breast and prostate cancer patients, where 11.1% underwent dental check-ups prior to BMA treatment [60]. This contrasts with several guideline-based documents advocating such integration. The Royal College of Physicians 2019 document offers a collaborative approach to reduce the risk of MRONJ and integrate dental preventative service into oncology regimes [61]. In the USA, MASCC/ISOO/ASCO Clinical Practice Guideline on Medication-Related Osteonecrosis of the Jaw published in 2019 has also provided clinical guidance for oncologists on best practice to prevent MRONJ in oncology [13]. Introduction of a standardised pathway for oncology patients and considerations of real-time challenges is an important step towards the optimal standard of care for this cohort [62]. Both American and European collaborations are published in the literature to encourage pre-emptive integration of efficient dental services in oncology [11, 13, 36, 63–65]. Clinical guidance influences service provision and integration in local oncology communities to ensure cohesive dental care in the oncology setting. The National Cancer Strategy in 2017–2026, published by the National Cancer Control Programme (NCCP) in Ireland, has included a note on the importance of regular oral cancer screenings with a dentist and the inclusion of pre-radiation dental assessments prior to head and neck radiation therapy. Recommendations regarding access to care, dental care as a component of BMA therapy and post-treatment dental regimes were not included [38].
A cost analysis of state-funded dental care for head and neck oncology patients in Sweden revealed dental care consumptions increased significantly with cancer diagnosis, with statistically significant increase in dental costs and number of procedures [66]. These figures declined 2 years following cancer diagnosis but remained persistently higher compared to non-irradiated paired controls [66]. Patients were covered under a state-funded dental insurance scheme which compensated them for extensive dental rehabilitation. A special care allowance for radiation-related disease such as hyposalivation included an additional allowance to ensure maintenance of oral care in vulnerable cohorts [66]. A cost analysis for irradiated patient in this study was 900 euro compared to 210 euro in non-irradiated patients [66]. Cost effectiveness strategies and analyses of claims data reported the impact a dental service can have on an oropharyngeal service by reducing the cost of treatment and length of days required for treatment compared to reduced dental support [32, 66]. Acute oral complications were more expensive (1672 USD) when treated without dental integration. Average treatment times were 74 days shorter with immediate involvement of a dentist in an oropharyngeal cancer service, and dental decay was reduced [67]. In the present study oncology patients will benefit from direct involvement with dental services designated for pre-BMA and pre-radiation treatment alongside integration into community dentistry for long-term care [68].
A growing additional facet of dental oncology is the care of the displaced cancer patient. Based on Data from the United Nations High Commissioner for Refugees (UNHCR), 110 million people had been forcibly displaced, and 36.4 million people were refugees in mid-2023 [69]. Since Russia’s military aggression on Ukraine in February 2022, Europe has received the largest influx of people fleeing war since World War II. Oncology services must begin to accept the increased prevalence of refugees and the necessity to facilitate healthcare for this cohort of patients [70]. Ireland remains prominent in the promotion of inclusive policies to facilitate “intercultural healthcare” specifically targeting migrants’ health [70–72]. Irish society has become increasingly diverse related to immigrants, people seeking asylum and the impact of war in neighbouring continents [73]. Translation, transport and communication challenge healthcare provision to refugees. In Ireland, major oncology centres have access to two dental hospitals in Dublin and Cork. Relationships and co-located dental-oncology services require the uptake of facilities, finance, structural and organisational initiatives to foster improvements in healthcare [49]. However, global studies have highlighted substandard access particularly in mental health and dental care services within migrant populations [74]. Oral health tends to be poorer in refugee populations. A study of 12-year-old migrants in Austria demonstrated 42% higher rate of decay and more affected by gingivitis in migrant children compared to children with no migrant background [74, 75]. Language barriers significantly impact the quality of care in refugees. Cross-cultural consultations can be hindered by lack of healthcare staff awareness of resources, societal racism, ethnocentrism, engagement of senior HSE and governmental offices and the financial concerns regarding access to professional interpreting services were shown to hinder service provision [72, 76]. Barriers to care have deleterious consequences for cancer outcomes in migrant populations [77–79]. Migrants’ vulnerabilities include poorer psychological and health-related quality of life outcomes [80]. Dental workforce planning, professional interpreters, funding, research and awareness can facilitate communication and integration of healthcare for a multicultural nation.
De-escalation of BMAs in patients with both metastatic and early disease in breast cancer is advocated due to adverse symptomatic skeletal events, quality of life and healthcare expenditure [9, 81–84]. The literature has demonstrated the equivalent benefit of de-escalated regimens in patients with bone metastases, irrespective of completing 1 year of standard BMA treatment [85, 85]. The long-term implications of dental disease, dental interventions and risk of MRONJ persist for at least the half-life of the BMA therapy (11.2 years for intravenous zoledronic acid). This is also reiterated for the patients at risk of ORN, which increases in risk over time [30]. Long-term integration of dental services, patient awareness and proactivity to maintain healthy oral health statuses remain challenging [86, 87].
The challenges highlighted in this case series will increase in coming decades. The Irish Longitudinal Study on Ageing (TILDA) have highlighted a national population shift using a microsimulation which projected a growing cohort of elderly patients nationally with increased health and related outcomes in the years 2018–2040 [88]. This increasing aging population will place significant demands on the long-term effects of chronic conditions and legacy of oncological treatment, such as MRONJ and ORN. The dental demands of the elderly including aging dentition and oral physiological changes such as hyposalivation, medication-induced mucosal changes, reduced oral hygiene abilities and dexterity, mental impairment and reduced calorific nutrition will compound vulnerabilities to MRONJ and ORN in the growing cohort of cancer survivors [89]. Preventative strategies play a pivotal role in these populations. This population will see an increase in healthcare utilisation, functional limitations and burden of chronic illness impacting our service provision in the future [88].
Conclusion
The prevention of MRONJ and ORN is documented worldwide from an array of both medical and dental organisations. Dental care is a subsidiary of oncology care for patients with both early and late-stage cancer on BMA regimes and receiving head and neck radiotherapy. Dental integration into multidisciplinary cancer care can have significant quality-of-life implications for patients treated in both the curative and palliative settings. Historically such integration has been patient dependent compounding the impact of pre-existing dental disease — the world’s most prevalent non-communicable illness. The changes of dental oncology integration have grown with age as Ireland’s population becomes more ethnically diverse. Addressing these challenges will require more formulated cohesion between cancer centres and dental practitioners, analogous to those seen from integration of psycho-oncology services. In response to this, a pilot dental oncology service has been launched in 2022 in our centre integrating dental oncology into routine cancer care [68]. This paper raises some real-world challenges that can occur for both service and patient experience. Valuable lessons can be taken from this experience.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the production of the manuscript. Harriet Byrne and Claire Curtin collected and analysed data. Harriet Byrne complied the figures and tables.
Funding
This project was funded in part by the College of Medicine and Health Interdisciplinary Seeds Award (CiSA), University College Cork.
Data Availability
Data is available as supplementary information.
Declarations
Ethics approval
The ethical approval application was submitted to the Clinical Research Ethics Committee (CREC), University College Cork (UCC under the title “Oral Health Status and Dental Treatment Needs of Oncology Patients Receiving Bone Modifying Agents”. The research team included Dr Harriet Byrne (oral surgery speciality registrar), Professor Seamus O Reilly (consultant in medical oncology) and Professor Richeal Ní Riordáin (consultant in oral medicine). The stakeholders included University College Cork (UCC) and Health Service Executive (HSE), and the research project was conducted in the Oral Surgery Department, Cork University Dental School and Hospital, Wilton, Co Cork. The study was conducted in accordance with the requirements of the WMA Declaration of Helsinki (2008). Written informed consent will be obtained from all the participants.
Consent to participate and publish
The authors affirm that human research participants provided informed consent for publication of the data and qualitative findings within this paper.
Conflict of interest
Authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Artificial intelligence
This paper was completed unassisted by artificial intelligence.
Compilation
Each author contributed to the concept and writing of the manuscript.
The data was collected by Dr Harriet Byrne and Dr Claire Curtin.
References
- 1.bridge_1 noun - Definition, pictures, pronunciation and usage notes | Oxford Advanced American Dictionary at OxfordLearnersDictionaries.com. [Online]. Available: https://www.oxfordlearnersdictionaries.com/definition/american_english/bridge_1. Accessed 27 Dec 2023
- 2.WHO Discussion Paper: draft global strategy on oral health, dental health foundation. [Online]. Available: https://www.dentalhealth.ie/news/who-discussion-paper-draft-global-strategy-on-oral-health/. Accessed 28 Jun 2023
- 3.Eke PI, Borgnakke WS, Genco RJ (2020) Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000 82(1):257–267. 10.1111/prd.12323 [DOI] [PubMed] [Google Scholar]
- 4.Caminha R-D-G, Alcantara P-L, Carvalho C-G, Reia V-C-B, Capelozza A-L-A, Santos P-SS (2020) The impact of medication-related osteonecrosis of the jaws on the quality of life in cancer patients. J Clin Exp Dent 12(8):e725–e729. 10.4317/jced.56307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinfurt KP, Castel LD, Li Y, Timbie JW, Glendenning GA, Schulman KA (2004) Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med Care 42(2):164–175. 10.1097/01.mlr.0000108746.69256.45 [DOI] [PubMed] [Google Scholar]
- 6.Weinfurt KP, Li Y, Schulman KA et al. (2005) The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 16(4):579–584. 10.1093/annonc/mdi122 [DOI] [PubMed] [Google Scholar]
- 7.OCarrigan B, Wong MH, Willson ML, Stockler MR, Pavlakis N, A, (2017) Goodwin, Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 10(10):CD003474. 10.1002/14651858.CD003474.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman RE, McCloskey EV (2011) Bisphosphonates in oncology. Bone 49(1):71–76. 10.1016/j.bone.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 9.Coleman R, Hadji P, Body JJ, SAntini D, Chow E, Terpos E, et al. (2020) Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol 31(12):1650–1663. 10.1016/j.annonc.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 10.Berenson JR, Rosen LS, Howell A, Poerter L, Coleman RE, Morley W, et al. (2001) Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 91(7):1191–1200. 10.1002/1097-0142(20010401)91:7%3c1191::aid-cncr1119%3e3.0.co;2-0 [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D (2022) American Association of Oral and Maxillofacial Surgeons Position Paper on medication-related osteonecrosis of the jaws-2022 update. J Oral Maxillofac Surg 80(5):920–943. 10.1016/j.joms.2022.02.008 [DOI] [PubMed] [Google Scholar]
- 12.S Yao, X Ding, G Rong, J Zhou, B. Zhang, Association between malignant diseases and medication-related osteonecrosis of the jaw (MRONJ): a systematic review and meta-analysis. J Craniofac Surg 10.1097/SCS.0000000000009033 [DOI] [PubMed]
- 13.Yarom N, Shapiro CL, Peterson DE, Van Posnak CH, Bohlke K, Ruggiero SL et al. (2019) Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. JCO 37(25):2270–2290. 10.1200/JCO.19.01186 [DOI] [PubMed] [Google Scholar]
- 14.Bacci C, Cerrato A, Bardhi E, Frigo AC, Djaballah SA, Sivolella S (2022) A retrospective study on the incidence of medication-related osteonecrosis of the jaws (MRONJ) associated with different preventive dental care modalities. Support Care Cancer 30(2):1723–1729. 10.1007/s00520-021-06587-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owosho AA, Liang STY, Sax AZ, Wu K, Yom SK, Huryn JM et al. (2018) Medication-related osteonecrosis of the jaw (MRONJ): an update on the Memorial Sloan Kettering Cancer Center (MSKCC) experience and the role of pre-medication dental evaluation in the prevention of MRONJ. Oral Surg Oral Med Oral Pathol Oral Radiol 125(5):440–445. 10.1016/j.oooo.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kizub DA, Miao J, Schubert MM, Paterson AHG, Clemons M, Dees EC et al. (2021) Risk factors for bisphosphonate-associated osteonecrosis of the jaw in the prospective randomized trial of adjuvant bisphosphonates for early-stage breast cancer (SWOG 0307). Support Care Cancer 29(5):2509–2517. 10.1007/s00520-020-05748-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman R, Cameron D, Dodwell D, Bell R, Wilson C, Rathbone E et al. (2014) Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol 15(9):997–1006. 10.1016/S1470-2045(14)70302-X [DOI] [PubMed] [Google Scholar]
- 18.Gnant M, Hadji P (2010) Prevention of bone metastases and management of bone health in early breast cancer. Breast Cancer Res 12(6):216. 10.1186/bcr2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guida A, Perri F, Ionna F, Ascierto PA, Grimaldi AM (2020) New-generation anticancer drugs and medication-related osteonecrosis of the jaw (MRONJ): late onset 3 years after ipilimumab endovenous administration with a possible role of target therapy. Clin Case Rep 9(1):61–66. 10.1002/ccr3.3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.M. Y, Is bevacizumab a direct cause of osteonecrosis of the jaw like bisphosphonate?, AJBSR, vol. 9, no. 1, pp. 71–72, Jun. 2020, 10.34297/AJBSR.2020.09.001354.
- 21.Brijs K, Miclotte I, Vermeire S, Darche V, Politis C (2020) Osteonecrosis of the jaw in patients with inflammatory bowel disease treated with tumour necrosis factor alpha inhibitors. Int J Oral Maxillofac Surg 49(3):317–324. 10.1016/j.ijom.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 22.Henien M, Carey B, Hullah E, Sproat C, Patel V (2017) Methotrexate-associated osteonecrosis of the jaw: a report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol 124(6):e283–e287. 10.1016/j.oooo.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 23.Nicolatou-Galitis O, Galiti S, Moschogianni M, Sachanas S, Edwards BJ, Migliorati CA et al. (2016) Osteonecrosis of the jaw in a patient with acute myeloid leukemia, who received azacitidine. Journal of Cancer Metastasis and Treatment 2:220–223. 10.20517/2394-4722.2016.06 [Google Scholar]
- 24.Yamamoto D, Tsubota Y, Utsunomiya T, Sueoka N, Udea A, Endo K et al. (2017) Osteonecrosis of the jaw associated with everolimus: a case report. Mol Clin Oncol 6(2):255–257. 10.3892/mco.2016.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallina C, Ramírez L, Torres J, Casañas E, Hernández G, López-Pintor R-M (2019) Osteonecrosis of the jaws produced by sunitinib: a systematic review. Med Oral Patol Oral Cir Bucal 24(3):e326–e338. 10.4317/medoral.22858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saloura V, Langerman A, Rudra S, Chin R, Cohen EEW (2013) Multidisciplinary care of the patient with head and neck cancer. Surg Oncol Clin N Am 22(2):179–215. 10.1016/j.soc.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 27.Marur S, Forastiere AA (2016) Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc 91(3):386–396. 10.1016/j.mayocp.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 28.Kojima Y, Otsuru M, Hasegawa T, Ueda N, Kirita T, Yamada S ichi et al. (2022) Risk factors for osteoradionecrosis of the jaw in patients with oral or oropharyngeal cancer: verification of the effect of tooth extraction before radiotherapy using propensity score matching analysis. J Dent Sci 17(2):1024–1029. 10.1016/j.jds.2021.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kufta K, Forman M, Swisher-McClure S, Sollecito TP, Panchal N (2018) Pre-radiation dental considerations and management for head and neck cancer patients. Oral Oncol 76:42–51. 10.1016/j.oraloncology.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 30.Boromand G, Haugen-Cange H, Asparusova M, Ekestubbe A, Kjeller G (2024) Long-term follow-up of osteoradionecrosis of the mandible. Acta Odontol Scand 82(1):48–54. 10.1080/00016357.2023.2257316 [DOI] [PubMed] [Google Scholar]
- 31.Ben-David MA, Diamante M, Radawski JD, Vinebery KA, Stroup C, Murdoch-Kinch CA et al. (2007) Lack of osteoradionecrosis of the mandible after intensity-modulated radiotherapy for head and neck cancer: likely contributions of both dental care and improved dose distributions. Int J Radiat Oncol Biol Phys 68(2):396–402. 10.1016/j.ijrobp.2006.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward MC, Petersen CM, Noll J, Bernard MS, Kuremsky JG, Patel A et al. (2024) Planned dental extractions after radiation therapy. JAMA Otolaryngology-Head Neck Surg. 10.1001/jamaoto.2024.2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.P. Boffano, Agnone AM, Neirotti F, Bonfiglio R, Brucoli M, Ruslin M et al. (2024) Epidemiology, etiopathogenesis, and management of MRONJ: a European multicenter study. J Stomatol Oral Maxillofac Surg 101931. 10.1016/j.jormas.2024.101931 [DOI] [PubMed]
- 34.Thumbigere-Math V, Michalowicz BS, Hodges JS, Tsai ML, Swenson KK, Rockwell L et al. (2014) Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw. J Periodontol 85(2):226–233. 10.1902/jop.2013.130017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.J. G. Thomas and A. Ouanounou (2023) Medication-related osteonecrosis of the jaw: a narrative review of risk factors, diagnosis, and management. Front Oral Maxillofacial Med 5 0 10.21037/fomm-21-106.
- 36.Campisi G, Mauceri R, Bertoldo F, Bettini G, Biasotto M, Colella G et al. (2020) Medication-related osteonecrosis of jaws (MRONJ) prevention and diagnosis: Italian Consensus Update 2020. Int J Environ Res Public Health 17(16):16. 10.3390/ijerph17165998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coropciuc R, Moreno-Rabié C, De Vos W, Van de Casteele E, Marks L, Lenaerts V et al. (2024) Navigating the complexities and controversies of medication-related osteonecrosis of the jaw (MRONJ): a critical update and consensus statement. Acta Chir Belg 124(1):1–11. 10.1080/00015458.2023.2291295 [DOI] [PubMed] [Google Scholar]
- 38.National Cancer Strategy 2017 - 2026. Accessed: Jan. 08, 2024. [Online]. Available: https://www.gov.ie/en/publication/a89819-national-cancer-strategy-2017-2026/
- 39.Vidula N, Greenberg S, Petrillo L, Hwang J, Melisko M, Goga A et al. (2021) Evaluation of disseminated tumor cells and circulating tumor cells in patients with breast cancer receiving adjuvant zoledronic acid. NPJ Breast Cancer 7(1):113. 10.1038/s41523-021-00323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paterson AHG, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM et al. (2012) Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol 13(7):734–742. 10.1016/S1470-2045(12)70226-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman R, de Boer R, Eidtmann H, Llombart A, Davidso N, Neven P et al. (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 24(2):398–405. 10.1093/annonc/mds277 [DOI] [PubMed] [Google Scholar]
- 42.von Minckwitz G, Möbus V, Scneeweiss A, Huober J, Thomssen C, Untch M et al. (2013) German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol 31(28):3531–3539. 10.1200/JCO.2012.47.2167 [DOI] [PubMed] [Google Scholar]
- 43.He L, Sun X, Liu Z, Qiu Y, Niu Y (2020) Pathogenesis and multidisciplinary management of medication-related osteonecrosis of the jaw. Int J Oral Sci 12(1):1. 10.1038/s41368-020-00093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beth-Tasdogan NH, Mayer B, Hussein H, Zolk O, Peter J-U (2022) Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev 7(7):CD012432. 10.1002/14651858.CD012432.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goh EZ, Beech N, Johnson NR, Batstone M (2023) The dental management of patients irradiated for head and neck cancer. Br Dent J 234(11):11. 10.1038/s41415-023-5864-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.PricewaterhouseCoopers (PwC), Conti cyber attack on the HSE: independent post incident review, Health Service Executive (HSE), Report, Dec. 2021. Accessed: Dec. 06, 2023. [Online]. Available: https://www.lenus.ie/handle/10147/631006
- 47.Dietrich T, Ower P, Tank M, West NX, Walte C, Needleman I et al. (2019) Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions - implementation in clinical practice. Br Dent J 226(1):16–22. 10.1038/sj.bdj.2019.3 [DOI] [PubMed] [Google Scholar]
- 48.Yuasa H, Kawai T, Sugiura M (2002) Classification of surgical difficulty in extracting impacted third molars. Br J Oral Maxillofac Surg 40(1):26–31. 10.1054/bjom.2001.0684 [DOI] [PubMed] [Google Scholar]
- 49.Bledsaw K, Prudowsky ZD, Yang E, Harriehausen CX, Robis J, DeJeanJ et al. (2023) A novel oncodental collaborative team: integrating expertise for central line-associated bloodstream infection prevention in pediatric oncology patients. JCO Oncol Pract 19(1):1. 10.1200/OP.22.00302 [DOI] [PubMed] [Google Scholar]
- 50.Dang RR, Brar B, Pasco JM, Rebhun C, Sohn W, Salama A (2022) Dental practice patterns for oral care in medical oncology patients—a survey-based assessment of Massachusetts dentists. J Canc Educ 37(3):555–560. 10.1007/s13187-020-01845-8 [DOI] [PubMed] [Google Scholar]
- 51.Taichman LS, Gomez G, Inglehart MR (2015) Oral health-related complications of breast cancer treatment: assessing dental hygienists knowledge and professional practice. J Dent Hyg 89(Suppl 2):22–37 [PMC free article] [PubMed] [Google Scholar]
- 52.Drudge-Coates L, Van den Wyngaert T, Schiødt M, van Muilekom HAM, Demonty G, Otto S (2020) Preventing, identifying, and managing medication-related osteonecrosis of the jaw: a practical guide for nurses and other allied healthcare professionals. Support Care Cancer 28(9):4019–4029. 10.1007/s00520-020-05440-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dimopoulos MA, Kastritis E,Bamia C, Melakopoulos I, Gika D, Roussou M et al. (2009) Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol 20(1):117–120. 10.1093/annonc/mdn554 [DOI] [PubMed] [Google Scholar]
- 54.Bonacina R, Mariani U, Villa F, Villa A (2011) Preventive strategies and clinical implications for bisphosphonate-related osteonecrosis of the jaw: a review of 282 patients. J Can Dent Assoc 77:b147 [PubMed] [Google Scholar]
- 55.Saad F, Brown JE,Van Poznak C,Ibrahim T, Stemmer SM, Stopek AT et al. (2012) Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol 23(5):1341–1347. 10.1093/annonc/mdr435 [DOI] [PubMed] [Google Scholar]
- 56.Varoni EM, Lombardi N, Villa G, Pispero A, Sardella A, Lodi G (2021) Conservative management of medication-related osteonecrosis of the jaws (MRONJ): a retrospective cohort study. Antibiotics (Basel) 10(2):195. 10.3390/antibiotics10020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcianò A, Rubino E, Peditto M, Mauceri R, Oteri G (2020) Oral surgical management of bone and soft tissues in MRONJ treatment: a decisional tree. Life (Basel) 10(7):99. 10.3390/life10070099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bertl K, Savvidis P, Kukla EB, Schneide S, Zauz K, Bruckmann C et al. (2022) Including dental professionals in the multidisciplinary treatment team of head and neck cancer patients improves long-term oral health status. Clin Oral Investig 26(3):2937–2948. 10.1007/s00784-021-04276-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morais MO, Elias MRA, Leles CR, DouradoPinezi JC, Mendonça EF (2016) The effect of preventive oral care on treatment outcomes of a cohort of oral cancer patients. Support Care Cancer 24(4):1663–1670. 10.1007/s00520-015-2956-6 [DOI] [PubMed] [Google Scholar]
- 60.Lim AR, Park W, Moon SJ, Kim MS, Lee S (2024) The trend of dental check-up and prevalence of dental complications following the use of bone modifying agents in patients with metastatic breast and prostate cancer: analysis of data from the Korean National Health Insurance Service. BMC Health Serv Res 24:412. 10.1186/s12913-024-10859-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.UKCB medication-related osteonecrosis of the jaw (MRONJ) guidance_Dec 2019, BOPA.[Online]. Available: https://www.bopa.org.uk/resources/ukcb-medication-related-osteonecrosis-of-the-jaw-mronj-guidance_dec-2019/. Accessed 09 Jul 2023
- 62.Kuchuk I, Mazzarello S, Butterfield K, Appleton A, Addison CL, Clemons M (2013) Oral care and the use of bone-targeted agents in patients with metastatic cancers: a practical guide for dental surgeons and oncologists. J Bone Oncol 2(1):38–46. 10.1016/j.jbo.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medication-related osteonecrosis of the jaw | Scottish Denta. [Online]. Available: https://www.sdcep.org.uk/published-guidance/medication-related-osteonecrosis-of-the-jaw/. Accessed 28 Sep 2022
- 64.Bedogni A, Maucei R,Fusco V,Betoldo F, Bettini G, Fede OD et al. (2023) Italian Position Paper (SIPMO-SICMF) on medication-related osteonecrosis of the jaw (MRONJ). Qeios. 10.32388/PBUJ6Z [DOI] [PubMed] [Google Scholar]
- 65.Schiodt M, Otto S, Fedele S,Bedogni A, Nicolatou-Galitis O,Guggenberge R et al. (2019) Workshop of European task force on medication-related osteonecrosis of the jaw—current challenges. Oral Dis 25(7):1815–1821. 10.1111/odi.13160 [DOI] [PubMed] [Google Scholar]
- 66.Lexomboon D, Karlsson P, Adolfsso J, Ekbo A, Naimi-Akbar A,Bahmanyar S et al.(2017) Consumption and direct costs of dental care for patients with head and neck cancer: a 16-year cohort study. PLoS ONE 12(8):e0182877. 10.1371/journal.pone.0182877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi SE, Choudhary A, Sonis S, Villa A (Nov.2021) Benefits of the involvement of dentists in managing oral complications among patients with oral cavity and oropharyngeal cancer: an analysis of claims data. JCO Oncol Pract 17(11):e1668–e1677. 10.1200/OP.20.00892 [DOI] [PubMed] [Google Scholar]
- 68.ESMO Congress 2023. [Online]. Available: https://oncologypro.esmo.org/meeting-resources/esmo-congress?event_resources_filter_form%5Bsearch%5D=harriet%20byrne. Accessed 08 Jan 2024
- 69.https://www.unhcr.org/external/component/header, UNHCR. Accessed: Dec. 06, 2023. [Online]. Available: https://www.unhcr.org/external/component/header
- 70.Ledoux C, Pilot E, Diaz E, Krafft T (Jun.2018) Migrants access to healthcare services within the European Union: a content analysis of policy documents in Ireland. Portugal Spain, Global Health 14(1):57. 10.1186/s12992-018-0373-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Villarroel N, Hannigan A, Severoni S, Puthoopparambil S, MacFarlane A (2019) Migrant health research in the Republic of Ireland: a scoping review. BMC Public Health 19(1):324. 10.1186/s12889-019-6651-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacFarlane A, Puthoopparmbil SJ,Waagensn E,Sisti LG,Costanzo G,Kayi I et al. (2023) Framework for refugee and migrant health research in the WHO European Region. Trop Med Int Health 28(2):90–97. 10.1111/tmi.13845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arrivals from Ukraine in Ireland Series 10 - CSO - Central Statistics Office. [Online]. Available: https://www.cso.ie/en/releasesandpublications/fp/p-aui/arrivalsfromukraineinirelandseries10/. Accessed 15 Sep 2023
- 74.Lebano A, Hame S, Braby H, Gil-Salmerón A, Durá-Ferrandis E, Garcés-Fere J et al. (2020) Migrants and refugees health status and healthcare in Europe: a scoping literature review. BMC Public Health 20(1):1039. 10.1186/s12889-020-08749-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banihashem Rad SA, Esteves Oliveira M, Maklennan A, Castiglia P, Campus G (2023) Higher prevalence of dental caries and periodontal problems among refugees: a scoping review. J Glob Health 13:04111. 10.7189/jogh.13.04111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puthoopparambil SJ, Phelan M, MacFarlane A (2021) Migrant health and language barriers: uncovering macro level influences on the implementation of trained interpreters in healthcare settings. Health Policy 125(8):1085–1091. 10.1016/j.healthpol.2021.05.018 [DOI] [PubMed] [Google Scholar]
- 77.von Au A, Weile U,Stefanovic S,Wallwiene M,Heil J,Golatta M et al. (2016) Breast cancer presentation and therapy in migrant versus native German patients: contrasting and convergent data of a retrospective monocentric study. Arch Gynecol Obstet 294(1):145–152. 10.1007/s00404-015-3938-0 [DOI] [PubMed] [Google Scholar]
- 78.Van Hemelrijck WMJ, De Schutter H, de Valk HAG, Silversmit G, Rosskamp M, Vandenheede H (2020) Breast cancer by migrant background in Belgium: lower risk, but worse survival in women of non-European origin. Int J Cancer 147(2):350–360. 10.1002/ijc.32726 [DOI] [PubMed] [Google Scholar]
- 79.Ikram UZ, Mackenbach JP, Harding S, Rey G, Bhopal RS, Regidor E et al. (2016) All-cause and cause-specific mortality of different migrant populations in Europe. Eur J Epidemiol 31(7):655–665. 10.1007/s10654-015-0083-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sze M, Butow P, Bell M, Vaccaro L, Dog S, Eisenbruch M et al. (2015) Migrant health in cancer: outcome disparities and the determinant role of migrant-specific variables. Oncologist 20(5):523–531. 10.1634/theoncologist.2014-0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu C, Wang L, Liu L, Zhuang J, Tang S, Zhang T et al. (2018) Efficacy and safety of de-escalation bone- modifying agents for cancer patients with bone metastases: a systematic review and meta-analysis. Cancer Manag Res 10:3809–3823. 10.2147/CMAR.S176811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cremers SCLM, Papapoulos SE, Geldeblom H, Seynaeve C, den Hartigh J, Vemij P et al. (2005) Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res 20(9):1543–1547. 10.1359/JBMR.050522 [DOI] [PubMed] [Google Scholar]
- 83.Doshi S, Sutjandra L, Zheng J, Sohn W, Peterson M, Jang G et al. (2012) Denosumab dose selection for patients with bone metastases from solid tumors. Clin Cancer Res 18(9):2648–2657. 10.1158/1078-0432.CCR-11-2944 [DOI] [PubMed] [Google Scholar]
- 84.Bouganim N, Dranitsaris G, Amir E, Clemons M (2011) Optimising the use of bone-targeted agents in patients with metastatic cancers: a practical guide for medical oncologists. Support Care Cancer 19(11):1687–1696. 10.1007/s00520-011-1230-9 [DOI] [PubMed] [Google Scholar]
- 85.Treatment by cancer type, NCCN. Accessed: Sep. 13, 2023. [Online]. Available: https://www.nccn.org/guidelines/category_1
- 86.Amadori D, Aglietta M, Alessi B, Gianni L, Ibrahim T, Farina G et al. (2013) Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol 14(7):663–670. 10.1016/S1470-2045(13)70174-8 [DOI] [PubMed] [Google Scholar]
- 87.Hortobagyi GN, Van Poznak C, Karker WG, Gradishar WJ, Chew H, Dakhil Sr et al. (2017) Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. JAMA Oncol 3(7):906–912. 10.1001/jamaoncol.2016.6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.May P, Normand C, Matthews S, Kenny RA, Romero-Ortuno R, Tysinger B (2022) Projecting future health and service use among older people in Ireland: an overview of a dynamic microsimulation model in The Irish Longitudinal Study on Ageing (TILDA). HRB Open Res 5:21. 10.12688/hrbopenres.13525.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Razak PA, Richard KMJ, Thankachan RP, Hafiz KAA, Kumar KN, Sameer KM (2014) Geriatric oral health: a review article. J Int Oral Health 6(6):110–116 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available as supplementary information.