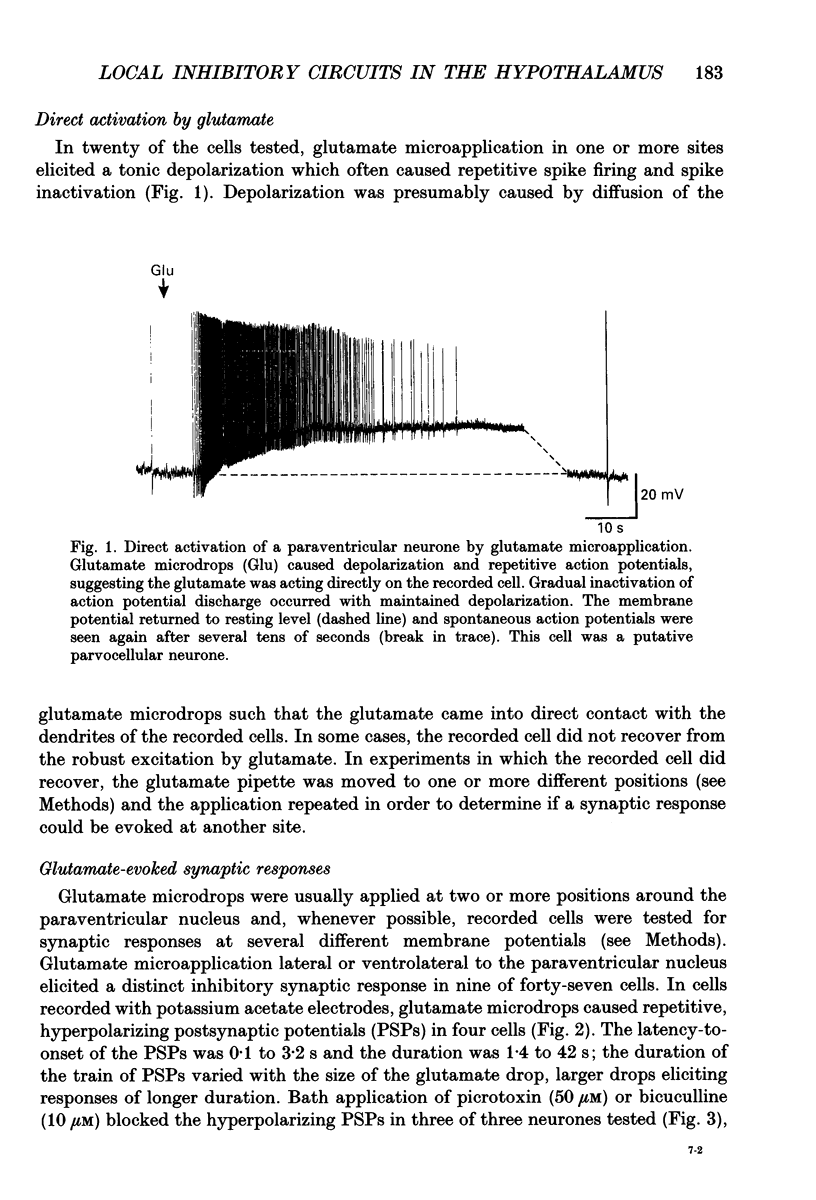
Abstract
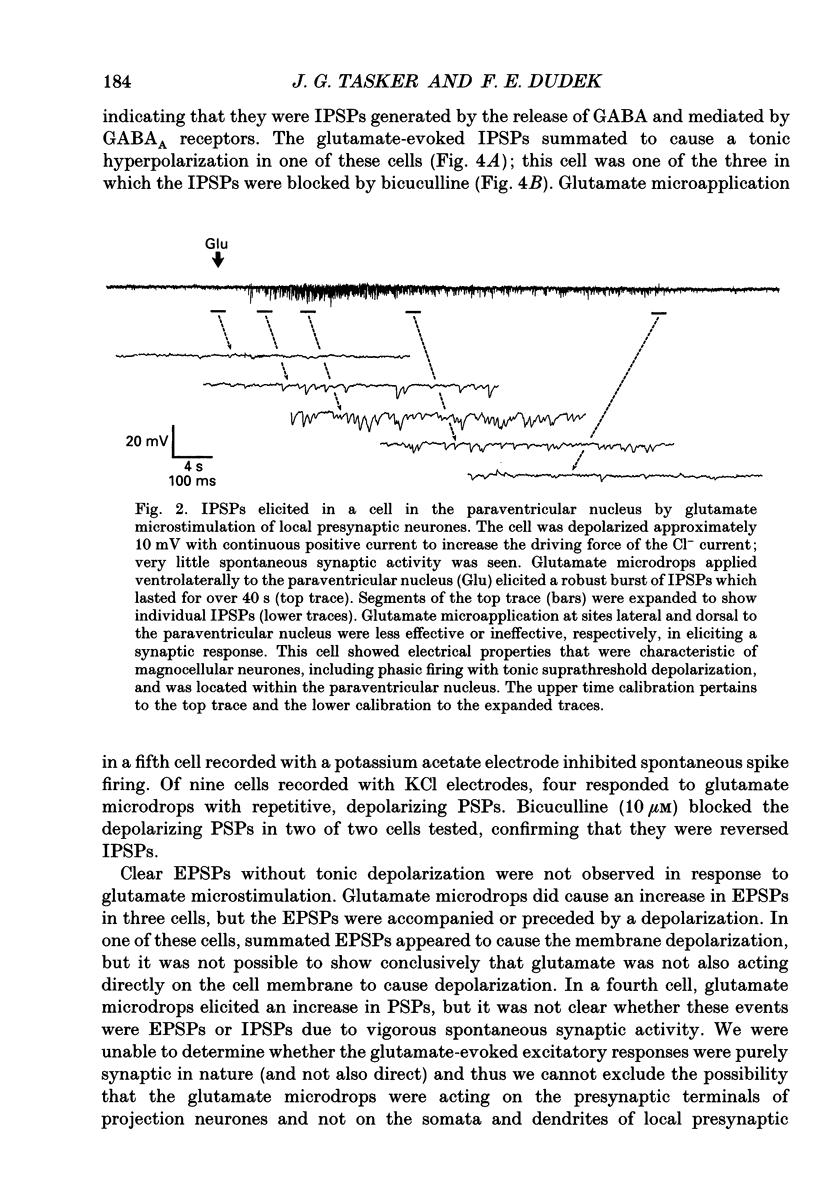
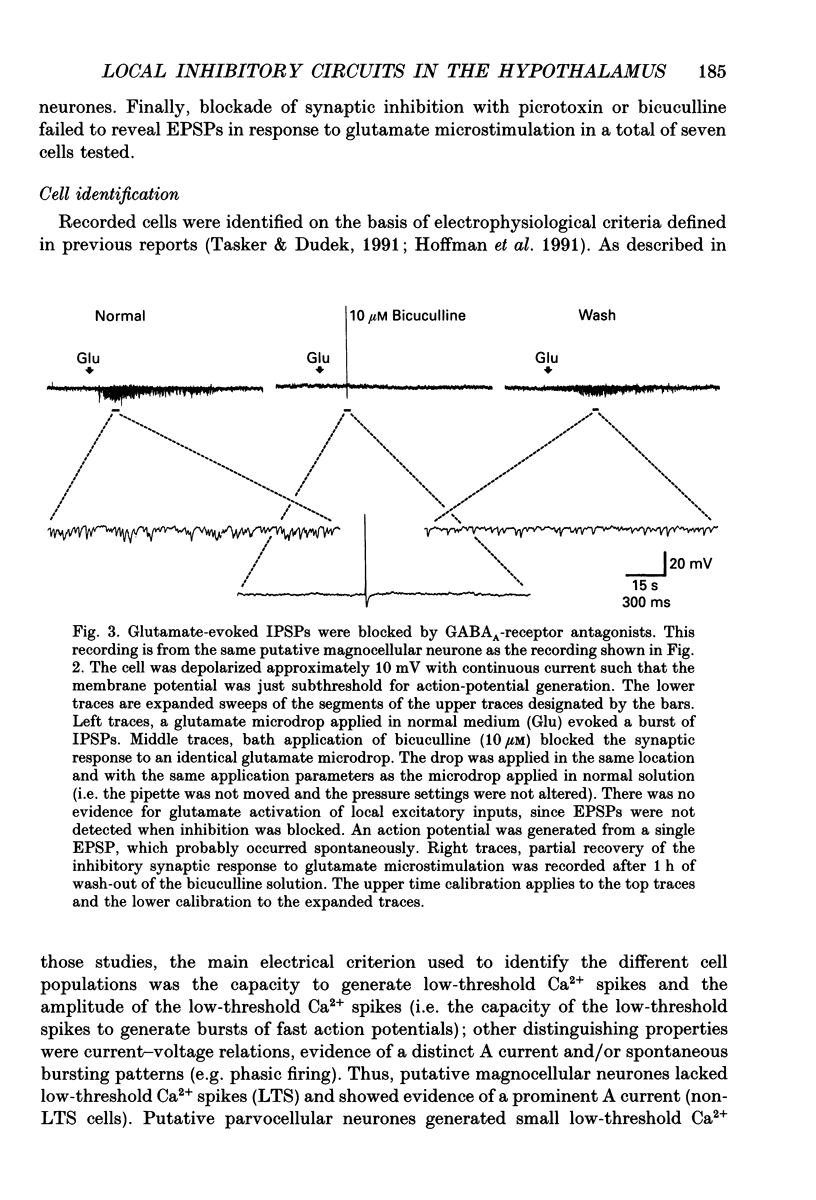
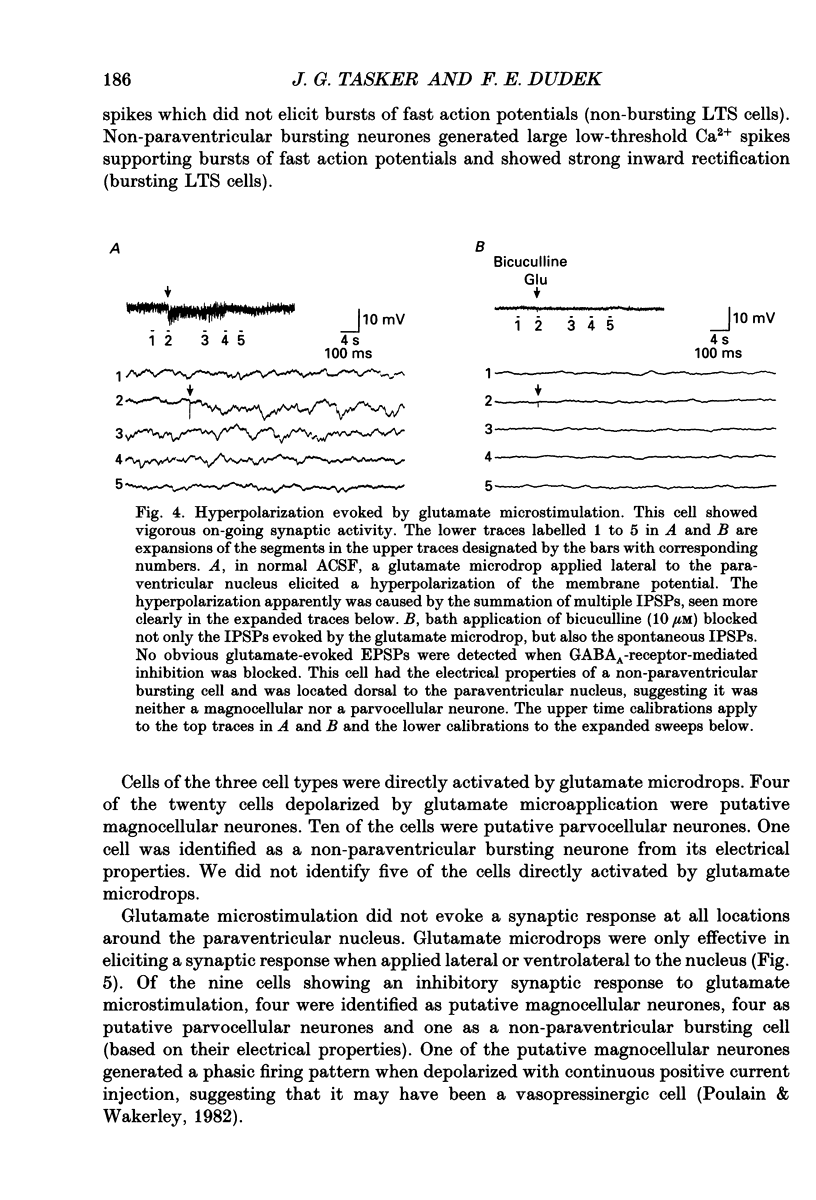
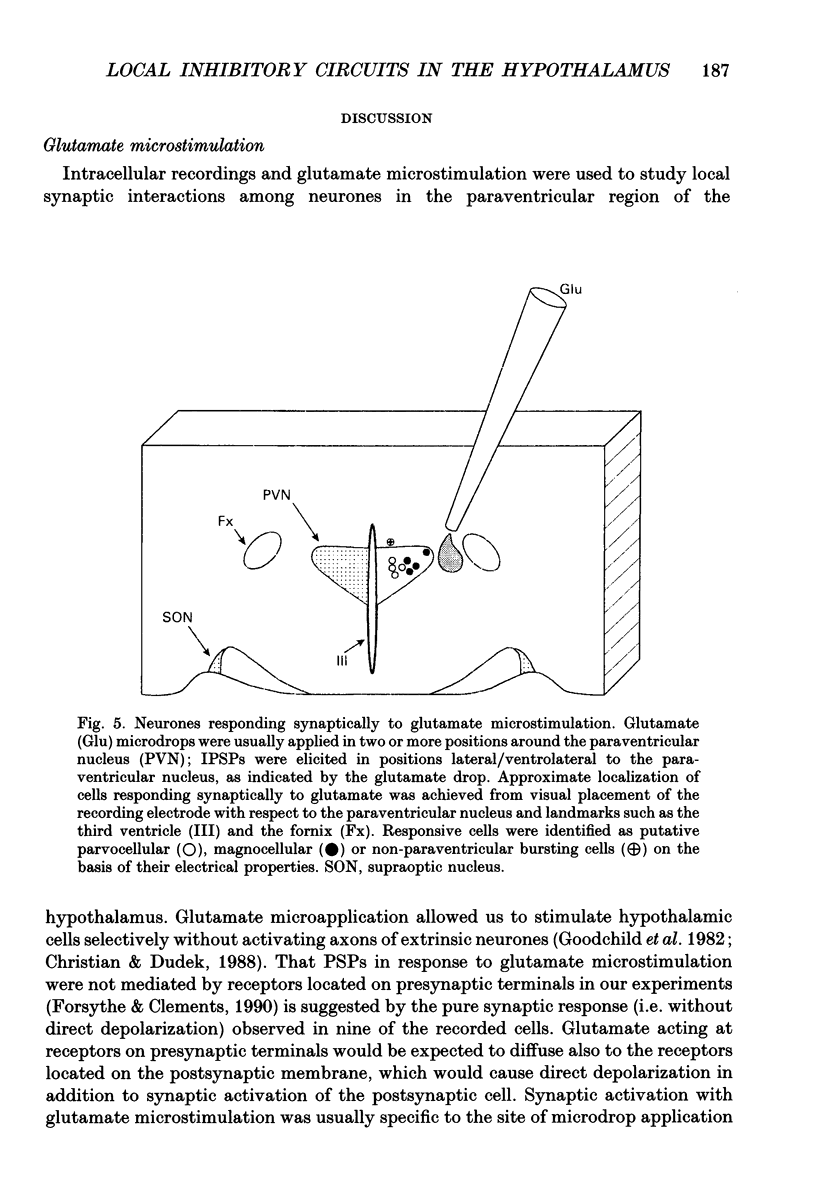
1. Intracellular recordings were obtained from neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. Glutamate microdrops were applied to the surface of the slices at sites dorsal, lateral and ventral to the paraventricular nucleus to selectively activate local presynaptic neurones. The gamma-aminobutyric acidA (GABAA)-receptor antagonists picrotoxin or bicuculline were bath-applied to block synaptic inhibition. 2. Glutamate microapplication caused a tonic depolarization and often repetitive action potentials in twenty of forty-seven recorded cells. This was probably caused by the direct exposure of the dendrites of the recorded cells to the glutamate microdrops. 3. Glutamate microstimulation elicited inhibitory synaptic responses in nine of forty-seven neurones tested. Glutamate microdrops caused discrete, hyperpolarizing postsynaptic potentials (PSPs) in four cells recorded with microelectrodes containing potassium acetate and evoked depolarizing PSPs in four cells recorded with KCl-filled microelectrodes. Glutamate microapplication inhibited spontaneous spike firing in another cell recorded with a potassium acetate microelectrode. 4. Bath application of GABAA-receptor antagonists completely blocked the hyperpolarizing PSPs elicited by glutamate microstimulation in three of three cells recorded with potassium acetate electrodes and the depolarizing PSPs in two of two cells recorded with KCl electrodes, indicating they were inhibitory PSPs caused by the release of GABA. Suppression of GABAA-mediated synaptic inhibition did not reveal any glutamate-evoked excitatory PSPs. 5. Recorded cells were identified as magnocellular, parvocellular or non-paraventricular bursting neurones on the basis of their electrophysiological properties. Direct depolarization and local inhibitory synaptic responses were observed in all three cell types. 6. Several conclusions can be drawn from these data: (1) functional glutamate receptors are distributed throughout neuronal populations in the paraventricular region of the hypothalamus, confirming and extending previous observations; (2) local synaptic inputs to neurones in the paraventricular nucleus are primarily inhibitory, supplied by perinuclear GABAergic neurones; (3) both magnocellular and parvocellular subpopulations receive local inhibitory synaptic inputs. The possibility that these local GABAergic circuits mediate inhibitory inputs to paraventricular neurones from limbic structures is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong W. E., Warach S., Hatton G. I., McNeill T. H. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5(11):1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- Bettler B., Boulter J., Hermans-Borgmeyer I., O'Shea-Greenfield A., Deneris E. S., Moll C., Borgmeyer U., Hollmann M., Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990 Nov;5(5):583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bioulac B., Gaffori O., Harris M., Vincent J. D. Effects of acetylcholine, sodium glutamate and GABA on the discharge of supraoptic neurons in the rat. Brain Res. 1978 Oct 6;154(1):159–162. doi: 10.1016/0006-8993(78)91064-8. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Dudek F. E. Characteristics of local excitatory circuits studied with glutamate microapplication in the CA3 area of rat hippocampal slices. J Neurophysiol. 1988 Jan;59(1):90–109. doi: 10.1152/jn.1988.59.1.90. [DOI] [PubMed] [Google Scholar]
- Decavel C., Dubourg P., Leon-Henri B., Geffard M., Calas A. Simultaneous immunogold labeling of GABAergic terminals and vasopressin-containing neurons in the rat paraventricular nucleus. Cell Tissue Res. 1989 Jan;255(1):77–80. doi: 10.1007/BF00229068. [DOI] [PubMed] [Google Scholar]
- Decavel C., Van den Pol A. N. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990 Dec 22;302(4):1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Ferreyra H., Kannan H., Koizumi K. Influences of the limbic system on hypothalamo-neurohypophysial system. Brain Res. 1983 Mar 28;264(1):31–45. doi: 10.1016/0006-8993(83)91118-6. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Clements J. D. Presynaptic glutamate receptors depress excitatory monosynaptic transmission between mouse hippocampal neurones. J Physiol. 1990 Oct;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild A. K., Dampney R. A., Bandler R. A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of the central nervous system. J Neurosci Methods. 1982 Nov;6(4):351–363. doi: 10.1016/0165-0270(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Gribkoff V. K., Dudek F. E. Effects of excitatory amino acid antagonists on synaptic responses of supraoptic neurons in slices of rat hypothalamus. J Neurophysiol. 1990 Jan;63(1):60–71. doi: 10.1152/jn.1990.63.1.60. [DOI] [PubMed] [Google Scholar]
- Hoffman N. W., Tasker J. G., Dudek F. E. Immunohistochemical differentiation of electrophysiologically defined neuronal populations in the region of the rat hypothalamic paraventricular nucleus. J Comp Neurol. 1991 May 15;307(3):405–416. doi: 10.1002/cne.903070306. [DOI] [PubMed] [Google Scholar]
- Meister B., Hökfelt T., Geffard M., Oertel W. Glutamic acid decarboxylase- and gamma-aminobutyric acid-like immunoreactivities in corticotropin-releasing factor-containing parvocellular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1988 Nov;48(5):516–526. doi: 10.1159/000125058. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J Physiol. 1989 Jan;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Urban I., Cross B. A. Microelectrophoresis of cholinergic and aminergic drugs on paraventricular neurons. Am J Physiol. 1972 Aug;223(2):310–318. doi: 10.1152/ajplegacy.1972.223.2.310. [DOI] [PubMed] [Google Scholar]
- Oldfield B. J., Hou-Yu A., Silverman A. J. A combined electron microscopic HRP and immunocytochemical study of the limbic projections to rat hypothalamic nuclei containing vasopressin and oxytocin neurons. J Comp Neurol. 1985 Jan 8;231(2):221–231. doi: 10.1002/cne.902310209. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Blume H. W., Renaud L. P. Connections of the hypothalamic paraventricular nucleus with the neurohypophysis, median eminence, amygdala, lateral septum and midbrain periaqueductal gray: an electrophysiological study in the rat. Brain Res. 1981 Jun 29;215(1-2):15–28. doi: 10.1016/0006-8993(81)90488-1. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Poulain P., Carette B. Low-threshold calcium spikes in hypothalamic neurons recorded near the paraventricular nucleus in vitro. Brain Res Bull. 1987 Oct;19(4):453–460. doi: 10.1016/0361-9230(87)90149-3. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Characterization of spontaneous and evoked inhibitory postsynaptic potentials in rat supraoptic neurosecretory neurons in vitro. J Neurophysiol. 1986 Dec;56(6):1703–1717. doi: 10.1152/jn.1986.56.6.1703. [DOI] [PubMed] [Google Scholar]
- Sakaue M., Saito N., Taniguchi H., Baba S., Tanaka C. Immunohistochemical localization of gamma-aminobutyric acid in the rat pituitary gland and related hypothalamic regions. Brain Res. 1988 Apr 19;446(2):343–353. doi: 10.1016/0006-8993(88)90893-1. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982 Mar 1;205(3):260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983 Aug 1;218(2):121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Oldfield B. J. Synaptic input to vasopressin neurons of the paraventricular nucleus (PVN). Peptides. 1984;5 (Suppl 1):139–150. doi: 10.1016/0196-9781(84)90272-9. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Kuypers H. G. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980 Dec 1;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tasker J. G., Dudek F. E. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991 Mar;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker J. G., Peacock W. J., Dudek F. E. Local synaptic circuits and epileptiform activity in slices of neocortex from children with intractable epilepsy. J Neurophysiol. 1992 Mar;67(3):496–507. doi: 10.1152/jn.1992.67.3.496. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T. Oxytocin-immunoreactive terminals synapse on oxytocin neurones in the supraoptic nucleus. Nature. 1985 Feb 21;313(6004):682–684. doi: 10.1038/313682a0. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Paut L., Tappaz M. L. Immunocytochemical analysis of the GABAergic innervation of oxytocin- and vasopressin-secreting neurons in the rat supraoptic nucleus. Neuroscience. 1986 Sep;19(1):207–222. doi: 10.1016/0306-4522(86)90016-3. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Poulain D. A. Neuronal-glial and synaptic plasticity in the adult rat paraventricular nucleus. Brain Res. 1989 Apr 10;484(1-2):361–366. doi: 10.1016/0006-8993(89)90382-x. [DOI] [PubMed] [Google Scholar]
- Wuarin J. P., Dudek F. E. Excitatory amino acid antagonists inhibit synaptic responses in the guinea pig hypothalamic paraventricular nucleus. J Neurophysiol. 1991 Apr;65(4):946–951. doi: 10.1152/jn.1991.65.4.946. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N. The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. J Comp Neurol. 1982 Apr 20;206(4):317–345. doi: 10.1002/cne.902060402. [DOI] [PubMed] [Google Scholar]


