Abstract
This paper provides an overview of autoimmune disorders of the central nervous system, specifically those caused by demyelination. We explore new research regarding potential therapeutic interventions, particularly those aimed at inducing remyelination. Remyelination is a detailed process, involving many cell types–oligodendrocyte precursor cells (OPCs), astrocytes, and microglia–and both the innate and adaptive immune systems. Our discussion of this process includes the differentiation potential of neural stem cells, the function of adult OPCs, and the impact of molecular mediators on myelin repair. Emerging therapies are also explored, with mechanisms of action including the induction of OPC differentiation, the transplantation of mesenchymal stem cells, and the use of molecular mediators. Further, we discuss current medical advancements in relation to many myelin-related disorders, including multiple sclerosis, optic neuritis, neuromyelitis optica spectrum disorder, myelin oligodendrocyte glycoprotein antibody-associated disease, transverse myelitis, and acute disseminated encephalomyelitis. Beyond these emerging systemic therapies, we also introduce the dimethyl fumarate/silk fibroin nerve conduit and its potential role in the treatment of peripheral nerve injuries. Despite these aforementioned scientific advancements, this paper maintains the need for ongoing research to deepen our understanding of demyelinating diseases and advance therapeutic strategies that enhance affected patients’ quality of life.
Keywords: Central nervous system disease, Autoimmune, Remyelination, Demyelination, Myelin, Oligodendrocyte, Emerging therapies, Multiple Sclerosis
Core Tip: Autoimmune disorders of the nervous system still pose a significant therapeutic challenge. Current treatments focus on symptom management but no cure exists. Since many of these disorders are caused by demyelination, it follows that remyelination may be key in finding a cure. Promising new research focuses on the use of endogenous cellular and inflammatory mediators to induce remyelination in patients with demyelinating diseases. These efforts may culminate in treatments such as stem cell transplantation and signaling pathway manipulation. In addition to these systemic therapies, nerve guide conduits have shown promise in aiding the recovery of peripheral nerve injuries.
INTRODUCTION
Autoimmune disorders constitute a diverse group of conditions where immune dysregulation causes damage to healthy tissue. This paper focuses on autoimmune disorders of the nervous system that are driven by demyelination, which we will refer to as demyelinating diseases. We explore the endogenous mechanism of remyelination in order to discuss emerging therapies aimed at promoting this process in patients with demyelinating diseases.
Myelin membranes originate from Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS). Myelin is an electrical insulator that allows nerve impulses to flow in a saltatory fashion, allowing for faster propagation of the impulse in myelinated axons when compared to unmyelinated axons[1]. Disruption of myelin can lead to axonal degeneration and thus neurological deficits.
This paper specifically discusses demyelinating diseases of the CNS. From local effects to diffuse abnormalities affecting multiple systems, there is a large range of clinical presentations for such diseases. Currently, most available therapies provide symptomatic relief by reducing inflammation, downregulating the immune system, or removing antibodies that induce damage to myelin[2]. Although there are a varied palliative care options, there is no current cure available for demyelinating diseases. Research is still ongoing to develop new therapies that induce remyelination in an effort to treat and potentially reverse demyelinating diseases. We will discuss the process of remyelination and the relevant emerging therapies throughout this paper.
BACKGROUND INFORMATION
Current models of inducing remyelination to reverse disease course
Broadly, the two main approaches proposed for the promotion of myelin repair include: (1) Transplanting cells with a repair-enhancing or myelinogenic capacity; and (2) Endogenously inducing remyelination processes[3]. The latter is a clinically popular approach that uses molecular targets to induce remyelination pharmacologically. To best discuss these emerging therapeutic approaches to inducing remyelination, we first need to understand the conditions in which remyelination occurs and the reasons this process fails.
Mechanism of remyelination
Remyelination occurs in four distinct steps: (1) Oligodendrocyte precursor cell (OPC) proliferation; (2) OPC migration toward demyelinated axons; (3) OPC differentiation, and (4) Premature oligodendrocyte interaction with the denuded axon. During the latter two steps, the newly differentiated oligodendrocytes gain the ability to myelinate the denuded axon and regenerate its myelin sheaths.
All four steps of remyelination are regulated by various signals, which we will discuss in the context of both cellular and inflammatory mediators. An understanding of this regulation is crucial, as emerging therapies can target these to induce remyelination in patients with demyelinating diseases.
Cellular mediators of remyelination
Recent research has explored the role of immune cells, namely astrocytes and microglial cells, in the regulation of remyelination in the CNS. Various cell processes and mediators determine whether the environment will foster remyelination or limit its processes and thus result in a loss of functionality of newly demyelinated neurons.
Astrocytes play a pivotal role in regulating remyelination processes in the CNS. One method by which astrocytes regulate remyelination is astrogliosis, a process in which glial scars form. The scarring response observed in the CNS may be due to the selective pressure to reseal the injury site and prevent additional tissue damage. These scars, however, act as physical barriers that impede OPC and axon entry into demyelinated plaques[4]. This halts the remyelination process in scarred areas and results in a loss of functionality of the demyelinated neurons. Although scarred areas have reduced remyelination capabilities, astrocytes do attempt to limit the extent of inflammation. This spares viable tissue from scarring and thus allows OPCs to enter these areas and restore myelin as usual[5]. Astrocytes also regulate remyelination through the expression of tumor necrosis factor-alpha (TNF-α), a cytokine that has implications in the pathogenesis of multiple sclerosis (MS) and has been positively correlated with demyelination and oligodendrocyte pathology[5]. In all, the role of astrocytes in the CNS seem to be rooted in modulating the inflammatory response by influencing its duration and extent, resulting in the spatial regulation of remyelination.
Moreover, microglia regulate remyelination in the CNS through cellular mediators and thus contribute to the pathogenesis of demyelinating diseases. Recent findings point to the development of distinct phenotypes of microglia, M1 and M2, under specific conditions[6]. The M1 phenotype releases destructive pro-inflammatory mediators, causing damage to healthy tissue; the M2 phenotype exhibits protective properties, promoting tissue remodeling and repair. The expression of the M1 vs. M2 phenotype may depend on the microenvironment and the extent of injury[6]. Notably, harmful properties include production of cytokines like TNF-α, as well as glutamate and reactive oxygen species which promote inflammation and inhibit remyelination[6]. Efficient removal of degenerated myelin by microglia remains a crucial step to facilitate remyelination. Microglial phagocytosis relies on the recognition of targets through various phagocytic receptors. One of the receptors expressed on myeloid cells is TREM-2 which plays a crucial role in facilitating repair in CNS murine models by promoting the clearing of debris during demyelination[7]. Furthermore, it was found that astrocytes and microglia are closely linked during remyelination. Astrocyte loss impairs microglial recruitment and clearance of damaged myelin, emphasizing the importance of maintaining astrocyte-microglia interactions in the demyelinating lesion for effective remyelination[5]. Table 1 summarizes the cell types discussed and their role during remyelination and demyelination.
Table 1.
Summary of different cell types and their function during remyelination and demyelination
|
Cell type
|
Role during remyelination
|
|
Roles during demyelination
|
| Microglial cell: M2 phenotype | Promotes repair and efficient removal of debris to enhance remyelination by expressing the TREM-2 receptor on the cell surface | Microglial cell: M1 phenotype | Produces pro-inflammatory cytokines, further activating demyelination by recruiting phagocytes to the area as well as generating reactive oxygen species |
| Oligodendrocyte progenitor cell | Precursor cell type found in the central nervous system that have the potential to differentiate into oligodendrocytes | ||
| Oligodendrocyte | Cell type in the central nervous system that produces myelin | ||
| Astrocytes | Generate a protein network to localize inflammation and sparing tissue | Can form glial scars, impeding OPC entry. Secretes TNF-α, which further promotes phagocytosis and demyelination |
OPC: Oligodendrocyte precursor cell; TNF-α: Tumor necrosis factor-alpha.
Additionally, astrocytes and microglia play crucial roles in the migration, proliferation, and differentiation of OPCs through the release of numerous neurotrophins, cytokines, and growth factors[5]. For instance, astrocytes and microglia are prolific producers of insulin-like growth factor-1 (IGF-1) and fibroblast growth factor 2 (FGF2). IGF-1 is crucial for promoting remyelination and is produced by both astrocytes and microglia[8]. These findings support the notion that IGF-1 plays a potential protective and regenerative role in the context of remyelination. Further, coordination of these factors is important for the regulation of the stages of lesion development. For one, microglial states shift to produce the specific, necessary factors for OPC proliferation vs. differentiation. The transition from the initial pro-inflammatory (iNOS+) to the subsequent anti-inflammatory (Arg-1+) microglial state aligns with the time when proliferating OPCs begin to differentiate into myelinating oligodendrocytes[9]. Consistent with this, factors promoting proliferation, such as pro-inflammatory cytokines like TNF-α, are released during the pro-inflammatory phase, while factors promoting differentiation, such as transforming growth factor beta (TGF-β), are more abundant during the anti-inflammatory phase[9]. On the other hand, the secretion of proteases when breaking down extracellular matrix during the remodeling phase has been shown to inhibit differentiation of oligodendrocytes, further emphasizing the notion that microglia cells influence remyelination[9]. All in all, it is the interplay of the chemokines that are being produced by astrocytes and microglia in the area of injury that are key factors in determining whether the environment fosters remyelination or if it results in scarring and effectively halts the process. Table 2 summarizes important chemokines discussed along with their function and the cell types that produce them.
Table 2.
Summary of important chemokines during demyelination and remyelination
|
Pro-inflammatory (demyelination) chemokines
|
Cell type that produces chemokine
|
Chemokine function
|
Anti-inflammatory (remyelination)
chemokines |
Cell type that produces chemokine
|
Chemokine function
|
| TNF-α | Astrocytes | Promotes demyelination and oligodendrocyte pathology | TGF-β | Microglia (M2) | Promotes differentiation of different cell types during repair |
| (iNOS+) | Microglia (M1) | Promotes the M1 phenotype, a pro-inflammatory state and induces phagocytosis | Insulin-like growth factor-1 | 1 Astrocytes; 2 Microglia (M2) | Regenerative role that promotes remyelination by promoting differentiation of different cell types such as oligodendrocytes |
| TREM-2 | Microglia (M2) | Cell surface receptor that clears myelin debris and promotes phagocytosis | Fibroblast growth factor 2 | 1 Astrocytes; 2 Microglia (M2) | Regenerative role that promotes remyelination by promoting differentiation of different cell types such as oligodendrocytes |
| Arg-1+ | Microglia (M2) | Promotes the M2 phenotype, an anti-inflammatory state |
TNF-α: Tumor necrosis factor-alpha; TGF-β: Transforming growth factor beta.
Overall, both astrocytes and microglia play crucial roles in regulating the remyelination of demyelinated lesions. While astrocytes prevent remyelination by creating scars, microglia have duality in their demyelinating M1 phenotype and repair-promoting M2 phenotype[5]. The coordination of signals from both astrocytes and microglia is also key in the promotion of effective remyelination over the formation of glial scars.
Inflammation and remyelination
Remyelination at its core continues to be a true regenerative process; therefore, the inflammatory response is intimately related. The initial stages of wound healing involves: (1) Local activation of the innate immune system, leading to the recruitment of leukocytes to the area, with early neutrophil entry and later monocyte infiltration; (2) Once the damaged site has been cleared of debris, innate immune cells initiate the repair phase, where factors are released to induce proliferation and guide the growth of new tissue; (3) The newly tissue will then go through remodeling which involves lesion contraction and cell differentiation; and (4) During the final phase of repair, growth factors, chemokines, and cytokines. Thus, the innate system plays a pivotal role in the outcome of the healing process.
Within the CNS, myelin disintegration due to damage may serve as a source for damage-associated molecular patterns when they are released extracellularly[10]. These signals are then recognized by immune cells and an inflammatory pathway is activated. One of the molecular patterns identified has been TREM2, as previously mentioned, which is present on myeloid cells, signaling the damage of myelin. Although this pro-inflammatory activation is crucial for the initiation of remyelination[11] as it allows for the clearance of debris, it is this prolonged immune response that is a major contributor to pathological scarring. Pathological scarring in the CNS can thus disrupt the blood-brain barrier as well can lead to antibodies entering the brain tissue, further exacerbating tissue injury[12].
The removal of myelin, as previously discussed, remains crucial for tissue remodeling and initiating repair. If myelin debris in the extracellular space is present, this inhibits OPC recruitment and differentiation and myelin repair is impeded. Thus, if myelin debris clearance is insufficient, this may lead to maladaptive inflammatory responses with prolonged pro-inflammatory signaling. A previous study demonstrated that the efflux pathway, a crucial biochemical pathway in clearing debris, if it failed to be activated in aged mice, this resulted in scar formation and aberrant remyelination. However, if therapeutic compounds were used to re-activate the pathway and restore debris clearance, it resulted in remyelination. Thus, this suggests a mechanistic link between debris clearance, resolution of innate inflammation, and myelin repair[13].
Overall, remyelination is a regenerative process tightly linked to the inflammatory response. It is through the activation of the innate immune system to clear debris, followed by tissue repair, and concluded with new tissue growth and remodeling that this process takes place. Failure during any of these steps results in pathogenic repair due to a prolonged inflammatory state which may result in scarring and failure of remodeling tissue back to its original functionality. Impaired debris clearing has thus shown itself to be a crucial step to properly remyelinate axons. It is this balance of promoting enough inflammation to promote the initiation of repair but not enough where repair is inhibited that is key to most effectively preserve functionality in damaged neurons.
Failure of remyelination
Current research on the treatment of demyelinating disease focuses on inducing remyelination in affected individuals[14]. Smith et al[15], determined that remyelination can successfully restore saltatory conduction in demyelinated lesions and thus regenerate normal nerve function in these areas. Consequently, therapies that enhance one’s ability to remyelinate are promising for treating and possibly curing demyelinating disease. To understand the significance of these emerging therapies, we must first delve into the endogenous mechanism of remyelination and the proposed explanations for its dormancy in patients with demyelinating diseases.
The process of remyelination is dependent on the recruitment and differentiation of OPCs. In response to demyelination, these cells can differentiate into mature oligodendrocytes and produce myelin to reinsulate damaged axons[16]. According to a meta-analysis by Keirstead and Blackmore[17], this process is initiated at the site of demyelination when OPCs are recruited from local areas of white matter. These progenitor cells, however, are finite in number, suggesting that individuals with repeated episodes of demyelination in the same location can exhaust their local reservoir of progenitor cells and lose the ability to restore nerve function through remyelination. Woodruff et al[18], examined this presumed importance of the number of available oligodendrocyte progenitor cells to the remyelination process. In this experiment, researchers artificially increased the density of OPCs in a group of mice and then induced demyelination in these mice as well as in a group of wild-type mice. By comparing the subsequent remyelination in these two groups of mice, Woodruff et al[18], found that the mice with increased densities of OPCs did not remyelinate faster or more effectively than the wild-type mice did. Hence, the availability of oligodendrocyte progenitor cells did not actually seem to be the limiting factor of remyelination as Keirstead and Blackmore[17], originally suggested, and the results of Woodruff et al[18], encouraged researchers to further explore the role of cell differentiation in remyelination. Most notably, Kuhlmann et al[19], determined that patients with MS–a demyelinating disease–have OPCs at the site of their demyelinated lesions regardless of their level of disease progression. The difference they found, however, was that patients in the chronic stage of the disease have fewer OPCs with the ability to mature into myelin-producing cells than those in the acute stage. The findings of Kuhlmann et al[19], therefore confirm that the differentiation of OPCs is central to successful remyelination and could therefore be a target of medical therapies for those with demyelinating disease.
As individuals with demyelinating disease age, they progressively lose the ability to remyelinate and their symptoms worsen as a result[20]. Researchers have used this correlation between age and worsening demyelinating disease to uncover the bodily conditions that affect remyelination. Age-related changes to both the innate immune system and epigenetic control have been identified as mechanisms that inhibit remyelination. Hinks and Franklin[21], explored the role of the aging innate immune system on remyelination and found that macrophages are responsible for the expression of IGF-1 and TGF-β1–two growth factors that are implicated in the maturation of OPCs into myelin-producing cells. Ruckh et al[22] built upon this discovery and demonstrated that remyelination can be restored in old mice through the transfer of youthful monocytes. In this experiment, old mice were connected to a young murine partner through parabiosis and used their partner’s youthful blood-derived monocytes to repair their own demyelinated lesions. Although these youthful monocytes successfully restored the ability of old mice to remyelinate, subsequent analysis revealed that this effect was not due to increased production of growth factors as one would expect based on the conclusions of Hinks and Franklin[21]. Rather, Ruckh et al[22], suggests that youthful monocytes are more proficient than aged monocytes at clearing inhibitory myelin debris from demyelinated lesions. Like these age-related changes to the immune system, changes to epigenetic control over time also diminish remyelination potential in older individuals. Shen et al[23], found that histone deacetylases play an essential role in preventing the transcription of genes that inhibit oligodendrocyte differentiation. Further, young brains with demyelinated lesions are better at recruiting these histone deacetylases to promoter regions than old brains. It follows that old brains with demyelinated lesions transcribe genes that inhibit oligodendrocyte differentiation despite the need for remyelination[23]. In all, age-related changes to both the immune system and epigenetic control help explain the time course of demyelinating disease and offer insight into the mechanisms that allow for the differentiation of oligodendrocyte progenitor cells during remyelination.
By discussing the scientific history of remyelination research, we can appreciate the current understanding of remyelination, as well as the need for further investigation into the factors that promote and inhibit this process. At this time, OPC differentiation and decreased age are the two main factors associated with improved remyelination capability. This knowledge may lend itself to the creation of therapies aimed at mimicking these factors to treat patients with demyelinating disease.
MECHANISMS OF EMERGING THERAPIES
While there is no cure for demyelinating diseases, there are emerging therapies that aim to halt disease progression. These diseases involve either damage to the myelin sheath or the cells that form them[24]. Thus, emerging therapies focus on inducing remyelination in order to regenerate myelin sheaths and, as a result, restore functionality to demyelinated axons. In this section, we will delve deeper into the research on remyelination, paying specific attention to the discovery of its underlying mechanisms which may be translated into future therapies.
Adult OPC differentiation
There is compelling yet indirect evidence that OPC differentiation plays a substantial role in the remyelination process. Most notably, retroviral and autoradiographic tracing shows that actively dividing cells in normal adult white matter (presumed to be adult OPCs) mature into remyelinating oligodendrocytes. Research has also shown that: (1) OPCs replenish CNS lesions before remyelinating oligodendrocytes appear; (2) Cells with markers of both OPCs and mature oligodendrocytes are present at the start of remyelination; and (3) Transplanted OPCs efficiently restore myelin in demyelinated regions[25-27].
Initially, neural stem cells give rise to OPCs, which are generated in specific locations within the neural tube during late embryonic development. One such location is the ventral ventricular zone of the spinal cord where OPCs respond to distinct molecular cues[28]. The molecular process governing the early development of OPCs entails initiation through neural patterning cues, such as sonic hedgehog (Shh), and inhibition by members of the bone morphogenetic protein family (BMP). While many OPCs establish contact with adjacent axons and differentiate into mature myelinating oligodendrocytes, a portion remains undifferentiated and is distributed throughout the gray and white matter of the mature CNS[29,30]. Composing approximately 5% of the total cell population in the adult CNS, adult OPCs are its primary cycling cell population and contribute to remyelination following CNS injury[25].
The differentiation of adult OPCs adheres to a general pattern involving activation and recruitment, differentiation, regulation of their differentiation, inflammation, remyelination, and removal of macrophages and myelin debris. OPCs first undergo a switch from a quiescent-like state into a regenerative phenotype, which involves changes in morphology and the upregulation of several genes[31]. OPCs are likely activated by microglia and astrocytes that sense acute injury-induced changes in the environment and subsequently release factors that trigger the rapid proliferation of OPCs in response to demyelinating injury. This proliferation is influenced by the levels of the cell cycle regulatory protein p27Kip1 and is promoted by growth factors like PDGF and FGF, among others[31].
The failure of adult OPCs to differentiate significantly affects myelin production in response to injury. In an effort to pinpoint the factors responsible for stimulating and sustaining adult OPC differentiation, numerous studies have explored the role of the inflammatory response in remyelination through experimental demyelination models. Such research has demonstrated that the depletion or inhibition of macrophages, as well as T cells, following toxin-induced demyelination inhibits remyelination. Conversely, the activation of macrophages has been shown to enhance remyelination by transplanted OPCs in the myelin-free retinal nerve fiber layer, suggesting the presence of unidentified regenerative factors produced by macrophages[31]. Furthermore, growth factor combinations that augment the expression of inflammatory cytokines and chemokines in cuprizone-induced demyelination models enhance remyelination, as well[31]. These findings suggest that targeting the factors involved in OPC differentiation, particularly by focusing on the immune response, could play a critical role in developing potential therapeutic strategies for demyelinating diseases.
Mesenchymal stem cells
Another potential approach to achieving remyelination and restoring neuronal function is through the transplantation of myelinating cells from an external source.
Myelinating cells such as Schwann cells, neural stem cells, oligodendrocytes, or stem cell-derived oligodendrocytes can be transplanted into an injured area in the PNS to encourage axonal regeneration via remyelination. Schwann cells are particularly noteworthy for their therapeutic potential in MS due to their capacity for clearing myelin debris and resistance to MS-related immune attacks. However, the clinical use of Schwann cells is limited by practical challenges such as the time required to generate sufficient quantities for transplantation and the sacrifice of functioning nerves during transplantation[32].
Mesenchymal stem cells (MSCs) offer an alternative source for Schwann cells, as they can differentiate into myelinating cells of the PNS under appropriate conditions. In one study comparing undifferentiated MSCs or transdifferentiated MSCs (Schwann cell-like) in PC12 cells, both Schwann cells and transdifferentiated MSCs effectively wrapped normally unmyelinated neurites, forming myelin membranes composed of several layers after 2 weeks[32]. Additionally, biogenic grafts, composed of devitalized muscle and transdifferentiated MSCs, supported sciatic nerve regeneration akin to Schwann cells[32].
The aforementioned study illustrates the ability to prompt MSCs to develop a myelinating capacity through transdifferentiation, which allows MSCs to morphologically resemble Schwann cells and express the same biochemical markers. With additional research, this could offer a therapeutic approach in which stem cells in cell-based therapies are manipulated to treat demyelinating diseases.
Molecular mediators of myelin repair
Improved treatment of myelin disorders is likely to be achieved through early intervention using combination therapies that target inflammation and other relevant processes, such as signaling pathways that support remyelination.
A myriad of signaling pathways govern the process of myelination, including those originating from the axonal membrane as one notable example. These pathways are facilitated by intricate protein and lipid complexes that facilitate the physical interaction between axons and myelinating glia. For instance, these complexes induce the arrangement of proteins within the axonal and glial membranes, as well as ensure close cell proximity, facilitating the transmission of signals that support myelination[33]. Besides the molecules found within the axolemma, there are secreted extracellular molecules that influence myelination, acting either independently or in conjunction with neuregulins or other axonal signals.
Ambiguity arises when considering the role of these same molecules in the context of remyelination. It remains premature to determine whether these molecules exhibit consistent behavior across both developmental myelination and myelin repair. Some molecules demonstrate exclusive roles in either myelination or remyelination, while others exhibit contrasting effects in these two processes[33]. Part of this differentiation may arise from the fact that there are different intrinsic mechanisms at play during the myelin formation of development vs after damage. The differing functions of signaling molecules may stem from the inhibitory properties of the demyelinating environment, specifically the presence of inflammatory cytokines that are not typically encountered during development[33]. Exploring the distinctions in signaling molecules between the processes of myelination and myelin repair holds promise as a potential therapeutic avenue for patients afflicted with myelinating disorders.
CURRENT ADVANCEMENTS IN THE TREATMENT OF MULTIPLE SCLEROSIS
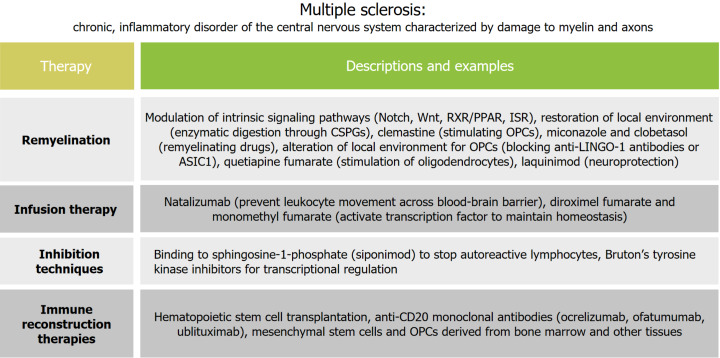
MS is a chronic, inflammatory disorder of the central nervous system characterized by damage to myelin and axons. There is a growing range of treatment options for multiple sclerosis, all with the goal of developing more effective and safer therapies.
Remyelination of MS
The promotion of regeneration of the myelin sheath through endogenous remyelination has emerged as one of the most promising therapies for MS, as there is potential to restore neuronal function and prevent further neuronal loss[14]. Remyelination processes are most effective in individuals aged less than 55 years and within the first 10 years of disease onset[3]. Researchers have advocated testing potential remyelinating agents in clinical trials with patients who have axons still present in their demyelinated lesions[3]. Additionally, due to the underlying heterogeneity in OPCs, there are less inhibitors of remyelination in the cortex, leading to anatomical variations in the effectiveness of remyelination of different lesions; for instance, subcortical lesions are more susceptible to remyelination than periventricular lesions[3]. In even the most effective of these myelination treatments, there remains a significant number of demyelinated axons and further potential for degeneration. This highlights the urgent clinical need to find remyelination therapies that have the potential to restore function and prevent axon degeneration[3].
There have been numerous strategies aimed at enhancing the remyelination capacity of endogenous OPCs. One such strategy is through the modulation of intrinsic signaling pathways. Because the manipulation of one pathway often induces alterations in another pathway, it is imperative to study the interaction of pathways in myelinating cells[14]. Several pathways that influence myelination have been explored: Notch, which impacts the spatial regulation of OPCs[34]; inhibition of Wnt, which promotes differentiation and myelination[35]; RXR/PPAR, which inhibits microglial activation and accelerated remyelination[36]; and ISR, which promotes stress resistance and protection[37].
Because the extracellular environment is altered in MS lesions, restoration of the local environment, such as clearance of myelin debris and the glial scar of astrocytes, can promote more effective remyelination[14]. To this end, enzymatic digestion through the use of chondroitin sulfate proteoglycans has been shown to be effective[38]. Additionally, providing metabolic support such as through lactate supplementation or reducing metabolic stress signaling could help to preserve neurons and oligodendroglia[39]. There are further interactions between glial cells and OPCs that must be incorporated into the exploration of remyelination therapy techniques; for example, reactive astrocytes that are typically found at sites of demyelination secrete inhibitors of remyelination such as Endothelin-1[3].
Clemastine, a first-generation anti-histamine, is capable of stimulating OPCs to differentiate and facilitate myelination[40]. Clinical trials revealed promising results for its therapeutic use in MS[41]. It has been proposed that the pro-myelinating effects of clemastine may be due to its antagonism of M1/M3 muscarinic acetylcholine receptors[42].
Miconazole, an antifungal agent, and clobetasol, a corticosteroid, act directly on oligodendrocytes as remyelinating drugs. Miconazole acts through MAPK signaling, while clobetasol acts through glucocorticoid receptor signaling. Because these drugs have a high safety profile, they are likely to be approved for phase 2 trials in the near future to establish efficacy in MS[43].
A promising target for promoting remyelination in MS is by altering the local environment to make it more favorable for endogenous OPCs. Phase 2 clinical trials showed that function-blocking anti-LINGO-1 antibodies enhanced OPC differentiation and myelination[44]. Blocking ASIC1, the acid-sensing ion channel, has shown positive effects in MS by decreasing the excessive intracellular accumulation of injurious Na+ and Ca2+[45]. Quetiapine fumarate, an atypical antipsychotic, appears to stimulate oligodendrocytes and has both remyelinating and neuroprotective properties for MS[46]. Laquinimod affects lymphocytes and reduces glial reactivity, and has indicated a neuroprotective effect in MS[47].
Infusion therapy
While there has been much promise to overcome the past failures of remyelination, there exists several efficacious therapies as well as newer emerging therapies being explored. Natalizumab was the first approved infusion therapy by inhibiting α4 integrins and preventing leukocyte movement across the blood-brain barrier, which reduces the inflammation of the CNS[48]. While there are some minor adverse effects such as headaches, fatigue, GI issues, and upper respiratory and urinary tract infections, natalizumab is generally well-tolerated and has shown efficacy in real-world, long-term studies. However, because of its mechanism of action, extended-interval dosing is preferred over standard-interval dosing; it also decreases the safety concern and risk of progressive multifocal leukoencephalopathy in patients exposed to John Cunningham Virus[48].
Fumarates act in multiple sclerosis by activating NRF2, a transcription factor that maintains redox homeostasis[48]. Dimethyl fumarate has demonstrated clinical efficacy; however, newer-generation fumarates, such as diroximel fumarate and monomethyl fumarate, have also shown clinical efficacy but with fewer gastrointestinal adverse effects compared to dimethyl fumarate[48].
Inhibition techniques
A key step in MS pathogenesis is the recirculation of autoreactive lymphocytes to the CNS. Inhibiting this step is possible by reducing circulating B and T lymphocytes by binding to sphingosine-1-phosphate (S1P)[48]. This also facilitates anti-inflammatory and neuroprotective effects by interacting with astrocytes and the S1P receptor on oligodendrocytes. Fingolimod, while a promising S1P receptor modulator, had many adverse effects, including cardiac effects and collar edema[48]. Some of these adverse effects were not seen in the use of Siponimod, a second-generation S1P receptor modulator[48]. Siponimod selectively binds and does not require activation through phosphorylation. It also has indicated neuroprotective mechanisms such as promotion of axonal remyelination through oligodendrocytes. Siponimod demonstrated reduced disability progression in patients with secondary progressive multiple sclerosis[48].
Recently, inhibition of Bruton’s tyrosine kinase (BTK) has been explored as a new treatment for multiple sclerosis. Expressed in hematopoietic cell lines, BTK is a cytoplasmic Tec family that serves an essential role in signaling for peripheral myeloid cells, B cells, and CNS microglia[41]. BTK inhibitors are currently an approved treatment for lymphoma and are being investigated for the treatment of autoimmune disorders. BTK activates B-cell receptors and results in downstream transcriptional regulation through intracellular signaling[41]. BTKs are relatively small molecules, which is an advantage for CNS penetration. CNS penetration is favorable because BTK inhibitors can directly interact with microglial cells. BTK inhibitors such as Evobrutinib, Tolebrutinib, Fenebrutinib, Reminbrutinib, and Orelabrutinib have shown therapeutic potential and have moved toward phase 3 clinical trials[41].
Immune reconstruction therapies
Additionally, immune reconstitution therapies (IRTs) have the potential to cause immune system renewal and durable disease remission in multiple sclerosis. IRTs deplete components of the immune system in order to facilitate it to renew itself[49]. IRTs include hematopoietic stem cell transplantation and monoclonal antibodies. Anti-CD20 monoclonal antibodies such as ocrelizumab and ofatumumab are high efficacy agents, and may have better safety than rituximab[48]. A glycoengineered antipCD20 monoclonal antibody called ublituximab has demonstrated efficacy in disease freedom, and has a shorter infusion time than other B-cell depleting therapies, which may promote greater accessibility for patients[48]. As with all monoclonal antibodies, therapies must be balanced with any potential safety risks. There has only been variable evidence regarding the ability of these IRTs to cause immune system renewal; however, there has been discussion on how IRTs can foster the expansion of cells that survive immunosuppression. It is through this expansion that new functional phenotypes can be acquired[49].
There have been experimental approaches to cell-based therapies, including mesenchymal stem cells and OPCs derived from bone marrow and other tissues. There remain many unresolved barriers to implementing the transplant-based approach to MS, such as the mode of delivery, tumor-facing potential, and requirements for immune suppression[50]. Ultimately, there is a need for more advanced clinical trials. The role of immunoablation and autologous hematopoietic stem cell transplantation is being investigated[51].
It is important to note that as new therapies emerge, the overall aim remains to improve the quality of life for individuals with demyelinating diseases. With numerous options for medications for multiple sclerosis, healthcare providers should aim to determine a specific treatment strategy with patients prior to medication selection. This allows for more focus to be taken on the subset of the therapeutic options, leading to more elaboration regarding the mechanisms of action, efficacy, and safety considerations of each therapeutic option[52]. Figure 1 summarizes the current advancements in the treatment of MS.
Figure 1.
Summary of current advancements in the treatment for multiple sclerosis.
CURRENT ADVANCEMENTS IN THE TREATMENT OF OTHER DEMYELINATING DISEASES
Optic neuritis
Optic neuritis (ON) is an inflammatory condition of the optic nerve that can be linked to autoimmune neurological disorders like multiple sclerosis, myelin-oligodendrocyte glycoprotein antibody-associated disease, and neuromyelitis optica spectrum disorder. Individuals with idiopathic or multiple sclerosis-related optic neuritis often regain high-contrast visual acuity but may face long-term challenges with contrast sensitivity, binocular vision, and motion perception. Conversely, those with antibodies to myelin oligodendrocyte glycoprotein, AQP4, and CRMP5 may experience poorer visual acuity recovery and more severe optic nerve atrophy than typical optic neuritis cases[53]. As such, myelination plays a significant role in this condition, affecting its clinical presentation and outcomes. The standard treatment for typical optic neuritis, based on the ONTT, includes either no treatment for mild cases or intravenous steroids to speed up visual recovery. High-dose oral corticosteroids are considered equally effective[54].
Following optic neuritis, ganglion cell layer complex loss may start within 8 days, and retinal nerve fiber layer thinning can begin within 1 month, with the potential for optic atrophy by month 6. MS relapse recovery entails remyelination of white matter and optic nerve lesions but is constrained by axonal degeneration and glial scarring[54]. There is evidence of a therapeutic gap as current therapies do not enhance remyelination. One potential emerging therapy is the sphingosine-1 phosphate receptor modulator fingolimod, which has shown effectiveness in promoting remyelination after acute optic neuritis. It exerts immune modulation and has neuroprotective and pro-regenerative effects on neurons, astrocytes, oligodendrocytes, and myeloid cells[54].
NMSOD
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune disease of the CNS, marked by significant demyelination and axonal damage that primarily impacts the optic nerves and the spinal cord. Most patients with this disease can be identified through testing for serum antibodies targeting the aquaporin-4 water channel (AQP4) expressed on the end feet of astrocytes[55].
Current treatments for NMOSD involve various drugs targeting different mechanisms and are categorized into first-line therapy (i.e. glucocorticoids, azathioprine, rituximab) and second-line therapy (i.e. methotrexate, mycophenolate mofetil, mitoxantrone)[56]. There has been significant progress through the development of new biological therapies targeting B cells (inebilizumab, ocrelizumab), anti-interleukin-6 receptor antibodies (tocilizumab, satralizumab), and complement inhibitor (eculizumab)[56]. However, it is important to note that to date, there is no approved curative treatment; current lines of therapy can prevent acute attacks or maintain remission. Standard treatments have been shown to be ineffective in patient populations who are AQP4-IgG seronegative or refractory[56].
When considering the pathogenesis of NMOSD, pathological findings typical for seropositive NMOSD reveal severe damage to astrocytes and oligodendrocytes, with preferential loss of AQP4, glial fibrillary associated proteins, and myelin associated glycoproteins, showing a vasculocentric pattern[57]. In cases where seronegativity is observed, other pathogenic antibodies are identified, targeting myelin oligodendrocyte glycoprotein. This element of the myelin sheath plays a crucial role in maintaining the integrity of myelin structure and its interactions with the immune system, particularly involving the complement activation pathway[57]. It's worth noting that there can be an overlap of myelin-associated parts affected in both these case subtypes, indicating complex interactions within NMOSD pathology. Thereby, efforts to repair demyelination damage have been explored, which are similar to the approaches used in multiple sclerosis. Stimulating remyelination involves promoting differentiation and proliferation of oligodendrocyte precursor cells, with opicinumab (LINGO 1 blocker) and clobetasol showing promising effects[57].
Myelin oligodendrocyte glycoprotein antibody-associated disease
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is the CNS's most recently defined inflammatory demyelinating disease. It presents with heterogeneous clinical manifestations, such as optic neuritis, myelitis, or acute disseminated encephalomyelitis (ADEM) around 50% of patients experience relapses[58]. Confirmation of MOGAD involves detecting MOG-IgG in serum (and sometimes CSF) in patients with compatible MRI findings[58].
MOGAD exacerbations are typically managed with high-dose corticosteroids, with the majority of patients showing a rapid response to this treatment[58]. Immunoglobulin therapy, primarily focusing on the use of intravenous Ig (IVIG), has been suggested as a maintenance option to prevent relapses[59]. Future research can focus on exploring the potential of remyelination-targeted therapies to identify effective treatments for both acute management and long-term care.
Transverse myelitis
Transverse myelitis is an inflammation of part of the spinal cord. While the exact cause of transverse myelitis is not known, it is prevalent in individuals with autoimmune disorders, including MS and MOGAD[60]. Depending on the level of spinal cord damage, the clinical manifestations of transverse myelitis may include sensorimotor deficits and autonomic dysfunction. Immunosuppression is often recommended for the immune-related diseases in which transverse myelitis has a higher risk of occurrence[60]. The current perspective on management of transverse myelitis is to emphasize preventative treatment, as well as exploring clinical trials that have been underway for the underlying autoimmune disorders relating to it[61]. However, the use of preventative treatment is challenging due to the uncertainty of whether the disease process is monophasic or relapsing[62].
ADEM
Acute disseminated encephalomyelitis (ADEM) is a rare demyelinating disease of the central nervous system that predominantly occurs in children. ADEM is a generally monophasic disease, with symptoms lasting between weeks and months[63]. Symptoms range from fever, nausea, and headaches to encephalopathy or brain lesions, which tends to present as drowsiness or confusion. It is imperative to properly differentiate ADEM from other demyelinating diseases, such as MS, in order to facilitate the correct treatment without delays.
In most cases, steroids and immunosuppressive drugs are used for treatment and are aimed at reducing inflammation[63]. The first-line of therapy involves high-dose intravenous corticosteroids, typically methylprednisolone, to dampen the inflammatory cytokine cascade, inhibit T cell activation, decrease movement of immune cells into the CNS, and facilitate apoptosis of the activated immune cells[64]. Many children respond favorably to this first-line therapy; however, if sequential therapy is needed, it is recommended to use therapeutic plasma exchange. Therapeutic plasma exchange as a second-line therapy involves removing circulating pathogenic immunoglobulins and other immune-related complexes[64]. Finally, IVIG is also utilized by decreasing the endogenous production of immunoglobulins[64]. In tandem, commonly used drugs for long-term immune therapy include rituximab and mycophenolate mofetil[64]. Long-term outcomes for individuals with ADEM are generally favorable, with long-term follow-up of pediatric patients being essential in order to mitigate any potential relapse of the condition. Figure 2 summarizes the characteristics and treatments for these diseases.
Figure 2.
Summary of characteristics and treatments for demyelinating diseases.
Peripheral nerve injuries
Peripheral nerve injuries are a common clinical condition associated with numerous autoimmune conditions. Many peripheral nerve injuries prove difficult to treat due to the resulting inflammatory microenvironment inhibiting the repair of Schwann cells and neurons. During injury, Schwann cells undergo pyroptosis and release numerous cytokines, mainly IL-18 and IL-1β. M1 Macrophages in the area also secrete TNF-α which also inhibits remyelination directly[65]. Recent advancements have been made using nerve guide conduits (NGCs), which are protein-based sheaths that are placed between damaged neurons and aid in their healing. One such conduit that has been researched is a dimethyl fumarate (DMF)/silk fibroin (RSF)/P: P conduit. It is a neuromechanical matched NGC composed of RSF loaded with poly (3,4-ethylenedioxythiophene): Poly(styrene sulfonate) (P: P) and DMF. This exhibits a matched elastic modulus (25.1 ± 3.5 MPa) to that of the peripheral nerve and has the highest 80% elongation at break, which is better than most protein-based conduits[65]. Nerve conduits need to be able to mechanically match the tissue and its regeneration because if the conduit is stiffer than human nerves, it can result in nerve entrapment and hinder regeneration. Furthermore, another property to consider in the conduits is that they should have a high elastic modulus resisting stretching to support PNRs; otherwise, they might collapse or break off, resulting in nerve compression by nearby muscles[65]. This specific modeled conduit resulted in reduced pyroptosis of Schwann cells. RT-PCR analysis showed decreased expression of IL-6 and IL-8 within the conduit compared to the controls, as well as reduced levels of NLRP3, N-GSDMD, and C-Casp1, which are markers for pyroptosis. Additionally, macrophage polarization shifted toward the M2 subtype, which promotes a regenerative environment in the peripheral nerve cells both in vitro and in vivo[65]. Arg1 is a cell marker for M2 macrophages and its expression was significantly higher compared to the control. In addition to these in vitro studies, rat models using a sciatic nerve crush injury and treatment were used to observe in vivo systems. After 12 weeks, SFI analysis was used to record footprints and this showed that the rats with the nerve conduit recovered faster than those without the specified conduit. Using H&E staining, the myelinated fibers of the recovered nerves showed round, dense, and uniform nerve fibers compared to those without the DMF/RSF conduit, which were loose, thin, and disorganized[65]. Overall, the DMF/RSF nerve conduit has promise in the future implementation of its use in peripheral nerve injury and offers alternative treatments to this patient population. It provides a new potential therapeutic approach to promote nerve repair in future clinical treatments.
CONCLUSION
Autoimmune disorders affecting the nervous system, particularly demyelinating diseases, present significant challenges to functional recovery. Current therapies primarily focus on mitigating symptoms and slowing disease progression but no cure has yet been made available. A deep understanding of remyelination is paramount in order to induce this state on neurons with the aim of reversing these conditions. Understanding the intricate interplay between various cell types, including astrocytes, microglia, and OPCs, as well as the roles of the innate and adaptive immune responses, provides insights into emerging therapeutic strategies. Ongoing research aims to enhance endogenous remyelination processes and address the complex factors influencing successful regeneration in the context of demyelinating diseases.
Thus far, this research has determined that the limiting factor of remyelination is the differentiation of oligodendrocyte progenitor cells. Studies have shown that both the innate immune system and epigenetics play a role in this process, and could therefore have applications for future therapies. Emerging therapies for demyelinating diseases focus on remyelination as a key strategy to restore the functionality of damaged axons within the CNS. Efforts to expand the population of OPCs and promote their differentiation into myelinating oligodendrocytes, alongside exploration of molecular mediators of myelin repair, offer promising avenues for therapeutic intervention by understanding intricate signaling pathways involved in remyelination. Conditions such as MS, ON, NMOSD, MOGAD, transverse myelitis, and ADEM underscore the clinical significance of myelination in neurological disorders and highlight the need for novel treatments that enhance remyelination to improve patient outcomes. Beyond systemic therapy, physical nerve conduits have recently begun to be developed as a potential option for patients experiencing peripheral nerve injury. Recent advancements have been made using NGCs which are protein-based sheathes that are placed between damaged neurons and aid in their healing by promoting less Schwann cell inflammation while enhancing M2 macrophage recruitment to the area of damage. Further research into stem cell transplantation, manipulation of signaling pathways, and targeted therapies holds great promise for the future management of demyelinating diseases.
Footnotes
Conflict-of-interest statement: All authors report no conflicts-of-interests.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Soldera J S-Editor: Liu JH L-Editor: A P-Editor: Wang WB
Contributor Information
Robert Medina, University of Florida College of Medicine, University of Florida, Gainesville, Fl 32610, United States. medina.robert@ufl.edu.
Ann-Marie Derias, University of Florida College of Medicine, University of Florida, Gainesville, Fl 32610, United States.
Maria Lakdawala, University of Florida College of Medicine, University of Florida, Gainesville, Fl 32610, United States.
Skye Speakman, University of Florida College of Medicine, University of Florida, Gainesville, Fl 32610, United States.
Brandon Lucke-Wold, Department of Neurosurgery, University of Florida, Gainesville, FL 32611, United States.
References
- 1.Morell P, Quarles RH. The Myelin Sheath. Nih.gov; Lippincott-Raven. 2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27954/
- 2.Mayo Clinic. (January 7, 2022). Multiple Sclerosis. Mayoclinic.org. Available from: https://www.mayoclinic.org/diseases-conditions/multiple-sclerosis/diagnosis-treatment/drc-20350274 .
- 3.Cunniffe N, Coles A. Promoting remyelination in multiple sclerosis. J Neurol. 2021;268:30–44. doi: 10.1007/s00415-019-09421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobel RA. The extracellular matrix in multiple sclerosis: an update. Braz J Med Biol Res. 2001;34:603–609. doi: 10.1590/s0100-879x2001000500007. [DOI] [PubMed] [Google Scholar]
- 5.Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 6.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth GA, Spada V, Hamill K, Bornstein MB. Insulin-like growth factor I increases myelination and inhibits demyelination in cultured organotypic nerve tissue. Brain Res Dev Brain Res. 1995;88:102–108. doi: 10.1016/0165-3806(95)00088-u. [DOI] [PubMed] [Google Scholar]
- 9.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, Ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 11.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell. 2017;169:1276–1290.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Crawford AH, Chambers C, Franklin RJ. Remyelination: the true regeneration of the central nervous system. J Comp Pathol. 2013;149:242–254. doi: 10.1016/j.jcpa.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Franklin RJM, Simons M. CNS remyelination and inflammation: From basic mechanisms to therapeutic opportunities. Neuron. 2022;110:3549–3565. doi: 10.1016/j.neuron.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Harlow DE, Honce JM, Miravalle AA. Remyelination Therapy in Multiple Sclerosis. Front Neurol. 2015;6:257. doi: 10.3389/fneur.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–396. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- 16.Punjani N, Senthilnathan V, Ahuja CS, Fehlings MG. Chapter 25 - Clinical trials: cellular regenerative approaches. In: Neural Repair and Regeneration After Spinal Cord Injury and Spine Trauma. Academic Press, 2022: 441-471. [Google Scholar]
- 17.Keirstead HS, Blakemore WF. The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination. Adv Exp Med Biol. 1999;468:183–197. doi: 10.1007/978-1-4615-4685-6_15. [DOI] [PubMed] [Google Scholar]
- 18.Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–262. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 20.Graves JS, Krysko KM, Hua LH, Absinta M, Franklin RJM, Segal BM. Ageing and multiple sclerosis. Lancet Neurol. 2023;22:66–77. doi: 10.1016/S1474-4422(22)00184-3. [DOI] [PubMed] [Google Scholar]
- 21.Hinks GL, Franklin RJ. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16:542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- 22.Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love S. Demyelinating diseases. J Clin Pathol. 2006;59:1151–1159. doi: 10.1136/jcp.2005.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford AH, Stockley JH, Tripathi RB, Richardson WD, Franklin RJ. Oligodendrocyte progenitors: adult stem cells of the central nervous system? Exp Neurol. 2014;260:50–55. doi: 10.1016/j.expneurol.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Brück W, Lucchinetti C, Lassmann H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 27.Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu-Rub M, Miller RH. Emerging Cellular and Molecular Strategies for Enhancing Central Nervous System (CNS) Remyelination. Brain Sci. 2018;8 doi: 10.3390/brainsci8060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RH, Dinsio K, Wang R, Geertman R, Maier CE, Hall AK. Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4. J Neurosci Res. 2004;76:9–19. doi: 10.1002/jnr.20047. [DOI] [PubMed] [Google Scholar]
- 30.Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–2429. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- 31.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 32.Keilhoff G, Stang F, Goihl A, Wolf G, Fansa H. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol. 2006;26:1235–1252. doi: 10.1007/s10571-006-9029-9. [DOI] [PubMed] [Google Scholar]
- 33.Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol. 2010;6:276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HC, Appel B. Delta-Notch signaling regulates oligodendrocyte specification. Development. 2003;130:3747–3755. doi: 10.1242/dev.00576. [DOI] [PubMed] [Google Scholar]
- 35.Games Collaborative Group. Ban M, Booth D, Heard R, Stewart G, Goris A, Vandenbroeck K, Dubois B, Laaksonen M, Ilonen J, Alizadeh M, Edan G, Babron MC, Brassat D, Clanet M, Cournu-Rebeix I, Fontaine B, Semana G, Goedde R, Epplen J, Weber A, Infante-Duarte C, Zipp F, Rajda C, Bencsik K, Vécsei L, Heggarty S, Graham C, Hawkins S, Liguori M, Momigliano-Richiardi P, Caputo D, Grimaldi LM, Leone M, Massacesi L, Milanese C, Salvetti M, Savettieri G, Trojano M, Bielecki B, Mycko MP, Selmaj K, Santos M, Maciel P, Pereira C, Silva A, Silva BM, Coraddu F, Marrosu MG, Akesson E, Hillert J, Datta P, Oturai A, Harbo HF, Spurkland A, Goertsches R, Villoslada P, Eraksoy M, Hensiek A, Compston A, Setakis E, Gray J, Yeo TW, Sawcer S. Linkage disequilibrium screening for multiple sclerosis implicates JAG1 and POU2AF1 as susceptibility genes in Europeans. J Neuroimmunol. 2006;179:108–116. doi: 10.1016/j.jneuroim.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- 37.Lin W, Lin Y, Li J, Fenstermaker AG, Way SW, Clayton B, Jamison S, Harding HP, Ron D, Popko B. Oligodendrocyte-specific activation of PERK signaling protects mice against experimental autoimmune encephalomyelitis. J Neurosci. 2013;33:5980–5991. doi: 10.1523/JNEUROSCI.1636-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starkey ML, Bartus K, Barritt AW, Bradbury EJ. Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice. Eur J Neurosci. 2012;36:3665–3678. doi: 10.1111/ejn.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi DC, Zhang CL, Lin TM, Gusain A, Harris MG, Tree E, Yin Y, Wu C, Sheng ZH, Dempsey RJ, Fabry Z, Chiu SY. Deletion of mitochondrial anchoring protects dysmyelinating shiverer: implications for progressive MS. J Neurosci. 2015;35:5293–5306. doi: 10.1523/JNEUROSCI.3859-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, Inman J, Arnow S, Devereux M, Abounasr A, Nobuta H, Zhu A, Friessen M, Gerona R, von Büdingen HC, Henry RG, Hauser SL, Chan JR. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390:2481–2489. doi: 10.1016/S0140-6736(17)32346-2. [DOI] [PubMed] [Google Scholar]
- 41.Cree BAC, Hartung HP, Barnett M. New drugs for multiple sclerosis: new treatment algorithms. Curr Opin Neurol. 2022;35:262–270. doi: 10.1097/WCO.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 42.De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev Neurobiol. 2012;72:713–728. doi: 10.1002/dneu.20976. [DOI] [PubMed] [Google Scholar]
- 43.Najm FJ, Madhavan M, Zaremba A, Shick E, Karl RT, Factor DC, Miller TE, Nevin ZS, Kantor C, Sargent A, Quick KL, Schlatzer DM, Tang H, Papoian R, Brimacombe KR, Shen M, Boxer MB, Jadhav A, Robinson AP, Podojil JR, Miller SD, Miller RH, Tesar PJ. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522:216–220. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 45.Arun T, Tomassini V, Sbardella E, de Ruiter MB, Matthews L, Leite MI, Gelineau-Morel R, Cavey A, Vergo S, Craner M, Fugger L, Rovira A, Jenkinson M, Palace J. Targeting ASIC1 in primary progressive multiple sclerosis: evidence of neuroprotection with amiloride. Brain. 2013;136:106–115. doi: 10.1093/brain/aws325. [DOI] [PubMed] [Google Scholar]
- 46.Zhornitsky S, Wee Yong V, Koch MW, Mackie A, Potvin S, Patten SB, Metz LM. Quetiapine fumarate for the treatment of multiple sclerosis: focus on myelin repair. CNS Neurosci Ther. 2013;19:737–744. doi: 10.1111/cns.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filippi M, Rocca MA, Pagani E, De Stefano N, Jeffery D, Kappos L, Montalban X, Boyko AN, Comi G ALLEGRO Study Group. Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J Neurol Neurosurg Psychiatry. 2014;85:851–858. doi: 10.1136/jnnp-2013-306132. [DOI] [PubMed] [Google Scholar]
- 48.Amin M, Hersh CM. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegener Dis Manag. 2023;13:47–70. doi: 10.2217/nmt-2021-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16:56–62. doi: 10.1038/s41582-019-0268-z. [DOI] [PubMed] [Google Scholar]
- 50.Scolding NJ, Pasquini M, Reingold SC, Cohen JA International Conference on Cell-Based Therapies for Multiple Sclerosis; International Conference on Cell-Based Therapies for Multiple Sclerosis; International Conference on Cell-Based Therapies for Multiple Sclerosis. Cell-based therapeutic strategies for multiple sclerosis. Brain. 2017;140:2776–2796. doi: 10.1093/brain/awx154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldschmidt C, McGinley MP. Advances in the Treatment of Multiple Sclerosis. Neurol Clin. 2021;39:21–33. doi: 10.1016/j.ncl.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cross A, Riley C. Treatment of Multiple Sclerosis. Continuum (Minneap Minn) 2022;28:1025–1051. doi: 10.1212/CON.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 53.Bennett JL, Costello F, Chen JJ, Petzold A, Biousse V, Newman NJ, Galetta SL. Optic neuritis and autoimmune optic neuropathies: advances in diagnosis and treatment. Lancet Neurol. 2023;22:89–100. doi: 10.1016/S1474-4422(22)00187-9. [DOI] [PubMed] [Google Scholar]
- 54.Saitakis G, Chwalisz BK. Treatment and Relapse Prevention of Typical and Atypical Optic Neuritis. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23179769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy M, Fujihara K, Palace J. New therapies for neuromyelitis optica spectrum disorder. Lancet Neurol. 2021;20:60–67. doi: 10.1016/S1474-4422(20)30392-6. [DOI] [PubMed] [Google Scholar]
- 56.Shi M, Chu F, Jin T, Zhu J. Progress in treatment of neuromyelitis optica spectrum disorders (NMOSD): Novel insights into therapeutic possibilities in NMOSD. CNS Neurosci Ther. 2022;28:981–991. doi: 10.1111/cns.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waliszewska-Prosół M, Chojdak-Łukasiewicz J, Budrewicz S, Pokryszko-Dragan A. Neuromyelitis Optica Spectrum Disorder Treatment-Current and Future Prospects. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22062801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sechi E, Cacciaguerra L, Chen JJ, Mariotto S, Fadda G, Dinoto A, Lopez-Chiriboga AS, Pittock SJ, Flanagan EP. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD): A Review of Clinical and MRI Features, Diagnosis, and Management. Front Neurol. 2022;13:885218. doi: 10.3389/fneur.2022.885218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sotirchos ES, Vasileiou ES, Salky R, Huda S, Mariotto S, Chen JJ, Levy M. Treatment of myelin oligodendrocyte glycoprotein antibody associated disease with subcutaneous immune globulin. Mult Scler Relat Disord. 2022;57:103462. doi: 10.1016/j.msard.2021.103462. [DOI] [PubMed] [Google Scholar]
- 60.Grasso EA, Pozzilli V, Tomassini V. Transverse myelitis in children and adults. Handb Clin Neurol. 2023;196:101–117. doi: 10.1016/B978-0-323-98817-9.00020-X. [DOI] [PubMed] [Google Scholar]
- 61.Tisavipat N, Flanagan EP. Current perspectives on the diagnosis and management of acute transverse myelitis. Expert Rev Neurother. 2023;23:389–411. doi: 10.1080/14737175.2023.2195095. [DOI] [PubMed] [Google Scholar]
- 62.Perez-Giraldo G, Caldito NG, Grebenciucova E. Transverse myelitis in myelin oligodendrocyte glycoprotein antibody-associated disease. Front Neurol. 2023;14:1210972. doi: 10.3389/fneur.2023.1210972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolska-Krawczyk M. [Acute disseminated encephalomyelitis] Radiologe. 2022;62:316–321. doi: 10.1007/s00117-022-00982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang CX. Assessment and Management of Acute Disseminated Encephalomyelitis (ADEM) in the Pediatric Patient. Paediatr Drugs. 2021;23:213–221. doi: 10.1007/s40272-021-00441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang JY, Yuan Y, Zhang SY, Lu SY, Han GJ, Bian MX, Huang L, Meng DH, Su DH, Xiao L, Xiao Y, Zhang J, Gong NJ, Jiang LB. Remodeling of the Intra-Conduit Inflammatory Microenvironment to Improve Peripheral Nerve Regeneration with a Neuromechanical Matching Protein-Based Conduit. Adv Sci (Weinh) 2024;11:e2302988. doi: 10.1002/advs.202302988. [DOI] [PMC free article] [PubMed] [Google Scholar]