Abstract
Nowadays, a pathological increase in the permeability of the intestinal barrier (the so-called leaky gut) is increasingly being diagnosed. This condition can be caused by various factors, mainly from the external environment. Damage to the intestinal barrier entails a number of adverse phenomena: dysbiosis, translocation of microorganisms deep into the intestinal tissue, immune response, development of chronic inflammation. These phenomena can ultimately lead to a vicious cycle that promotes the development of inflammation and further damage to the barrier. Activated immune cells in mucosal tissues with broken barriers can migrate to other organs and negatively affect their functioning. Damaged intestinal barrier can facilitate the development of local diseases such as irritable bowel disease, inflammatory bowel disease or celiac disease, but also the development of systemic inflammatory diseases such as rheumatoid arthritis, ankylosing spondylitis, hepatitis, and lupus erythematosus, neurodegenerative or psychiatric conditions, or metabolic diseases such as diabetes or obesity. However, it must be emphasized that the causal links between a leaky gut barrier and the onset of certain diseases often remain unclear and require in-depth research. In light of recent research, it becomes crucial to prevent damage to the intestinal barrier, as well as to develop therapies for the barrier when it is damaged. This paper presents the current state of knowledge on the causes, health consequences and attempts to treat excessive permeability of the intestinal barrier.
Keywords: Intestinal barrier, Gut permeability, Gut dysbiosis, Zonulin, Leaky gut diseases
Introduction
The incidence of certain allergic, autoimmune and metabolic diseases has been increasing in recent decades and has reached almost epidemic dimensions. There is a significant increase in the incidence of allergic and autoimmune diseases, such as asthma, atopic dermatitis, allergic rhinitis, chronic sinusitis, food allergies, celiac disease and inflammatory bowel disease. Systemic and metabolic conditions such as diabetes, obesity, multiple sclerosis, rheumatoid arthritis, lupus erythematosus, ankylosing spondylitis, as well as Alzheimer’s disease, Parkinson’s disease, chronic depression and autism spectrum disorders also become a growing health problem [1–6].
One of various possible hypotheses for the rapid increase in the incidence of such conditions is the damage to the intestinal and/or respiratory epithelial/ skin integrity observed in their course and the associated disruption of the epithelial barrier, causing it to leak and increase permeability. Conditions such as asthma and atopic dermatitis are characterized by systemic type 2 immune responses. The migration of activated T lymphocytes, where they cause disease, has been shown for food allergen-specific and skin-homing T lymphocytes that are primed in the inflamed gut and migrate to the skin to cause atopic dermatitis. The mucosal barrier is crucial to protect the body from exogenous harmful biological and chemical agents, such as microorganisms and environmental pollutants. As such, what could have caused the weakening of the epithelial barrier in recent decades? Is it possible that, as a result of industrial development and environmental pollution, damage to the epithelial barrier has become so widespread that it has become one of the factors in increased incidence of so many diseases? Or is this phenomenon more of an effect of already developing disease than a cause? [1]. This paper presents the current state of knowledge on the role of intestinal barrier in the development of some diseases and attempts to treat them by repairing the epithelial barrier. A literature review was performed using the PubMed and Google School databases. We used the following descriptors and their combinations in our research: “intestinal/gut barrier/permeability”, “leaky gut/intestine”, “zonulin”, “gut dysbiosis”, “disease”, “cause”, “diagnosis”, “treatment”, “therapy”, “physical activity”, “exercises”. Only articles in English were selected and these fit the best to our research area.
Intestinal barrier
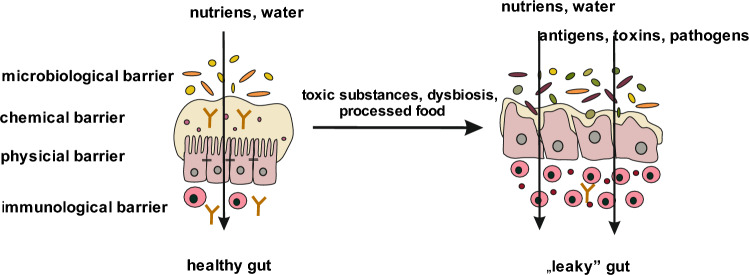
The intestinal epithelium is the largest contact site between the external environment and the internal milieu. The function of gastrointestinal epithelial barrier is to protect against the entry of foreign antigens and microorganisms, while allowing the absorption of essential nutrients, water and electrolytes [2, 7].
The intestinal barrier consists of four components: microbial barrier, biochemical barrier, physical barrier and immune barrier [8].
The microbial barrier is the intestinal microbiota, located in the lumen of an intestine. The microbiota produces many metabolically active compounds that show antimicrobial activity and affect the function of the entire intestinal barrier. Commensal bacteria digest certain food components and also compete with pathogens for nutrients [8–11].
The biochemical barrier is mucus, which contains about 98% water [8] and, among others, mucins, glycoproteins, IgA antibodies, antimicrobial substances, produced by microorganisms—bacteria, viruses, fungi and intestinal cells. Mucus coats epithelial cells and protects them from the harmful effects of pathogenic microorganisms and toxic substances [8, 9, 12, 13].
The physical (epithelial) barrier is an essential component of the entire intestinal barrier. It consists of a single layer of specialized cells: enterocytes, goblet cells (produce mucins), Paneth cells (produce antimicrobial peptides and proteins), enteroendocrine cells, M cells and intestinal stem cells. These cells undergo renewal every 3–5 days. Epithelial cells have a variety of functions and are closely interconnected [8, 9, 14, 15].
The immune barrier is associated with the presence of lymphoid tissue in the intestines known as gut-associated lymphoid tissue (GALT). The GALT system is located in the mucosa and submucosa of the intestines, directly beneath the epithelial cells. This system consists of intraepithelial lymphocytes (IELs), Peyer’s patches, which are clustered lymphoid papules, and lymphocyte clusters. The GALT system has also been found to contain antigen-presenting cells (APCs), T lymphocytes, B lymphocytes, plasma cells, as well as macrophages, mast cells and granulocytes. The secretory IgA antibody (sIgA) is synthesized in the intestine in particular [8, 9, 16, 17].
Most dietary proteins undergo endocytosis by intestinal epithelial cells. Lysosomal degradation leads to the breakdown of proteins into smaller peptides, thus avoiding activation of the immune system. Fluids and solutes are transported between cells. This transport is regulated by tight junctions (TJ) [18]. The intestinal barrier is a selective barrier—its function is to allow the transport of digested food essential for the body’s function, but at the same time to keep harmful substances and microorganisms in the intestinal lumen, which requires strict regulation of the barrier’s permeability [9, 19]. The TJ between enterocytes play a key role in providing an intestinal barrier. These junctions are composed of proteins, including occludin, claudin and junctional adhesion molecules (JAMs). TJ-building proteins bind to actin proteins, which are part of the cell’s cytoskeleton, via special zonula occludens proteins (ZO). These proteins are the target element for molecules that regulate TJ function, and are therefore responsible for the regulation of permeability of the intestinal barrier [18, 19] (Fig. 1).
Fig. 1.
Gut barrier. The intestinal barrier consists of 4 layers: microbiological, chemical, physical and immunological. Under the influence of exogenous or endogenous factors, the intestinal barrier is destroyed. This phenomenon is called "leaky gut"
Factors that can damage the intestinal barrier
The external environment, as a result of urbanization and globalization, has changed significantly in recent decades. Pollution and climate change, chemical compounds commonly used in industry and households, ecosystem changes, unhealthy diet, and stimulants, mainly alcohol, tobacco and e-cigarettes, may disrupt the epithelial barriers of the skin and mucosal surfaces. Air, water and food pollution, microplastic particles, nanoparticles, household chemicals and tobacco smoke are the most common epithelial barrier disrupting factors. Complex interactions between all these factors affect organisms, the end result of which depends on the combination of these factors and the body’s susceptibility [2].
It is well known that in recent decades the contamination with chemicals of the food we eat and the water we drink has increased significantly, if only by widely used food additives, pesticides or additives added to livestock feed. Recently, there has been increasing reports of food contamination with microplastics and nanoparticles. Such substances can easily penetrate tissues and interact with cellular structures [2, 20, 21]. Microplastics cause changes in protein structure [2], interact with cell membrane lipids, induce transcription of inflammatory genes and increase production of pro-inflammatory cytokines, lead to dysfunction of the endoplasmic reticulum, mitochondria and induce cell death due to oxidative stress. Nanoparticles also cause changes in protein structure and interact with cell membrane lipids [2, 22, 23]. Nanoplastics have been shown to induce transcription of inflammatory genes, increase levels of pro-inflammatory cytokines, and alter the expression of certain proteins [2, 24]. Microplastics have a high rate of absorption in the gastrointestinal tract and accumulate in the external environment and living organisms [2, 21]. Experiments on mice have revealed that polystyrene microplastics damage the intestinal barrier and reduce intestinal mucus production [2, 25]. Table 1 shows the main factors considered to have a deleterious effect on the intestinal barrier status: microplastic [20–25], nanoparticles [26–29], detergents and emulsifiers [30–36], the state of the intestinal microbiota [37–45] and diet [46–51].
Table 1.
The main factors damaging the intestinal barrier include environmental factors, microbiological factors and dietary factors. Each of these groups exerts broad and diverse biological effects on the state of intestinal barrier
| Factor | Definition | Biological effects |
|---|---|---|
| Environmental substances | ||
| Microplastic | Plastic waste with a diameter of less than 5 mm can be an environmental pollutant or accumulate in living organisms | Interaction with cell structures, cell membranes, proteins and changes in their function increased gene expression of pro-inflammatory and pro-apoptotic factors induction of apoptosis [20–25] |
| Nanoparticles | A fragment of matter with a dimension not exceeding 100 nm (nm) can be formed naturally in the environment or used in industry, such as cosmetics | Stimulation of collagen production and its deposition in the extracellular matrix, which can lead to fibrosis destabilization of mitochondrial and lysosome function disruption of the integrity of phospholipid and lysosomal membranes disruption of intercellular connections and increase in cell permeability induction of apoptosis [26–29] |
| Detergents and emulsifiers | Detergents and emulsifiers are surfactants detergents are used for cleaning, emulsifiers are mainly used in the food industry | TJ damage increased production of certain interleukins changes in microbiota composition, disruption of mucus-bacteria interactions in the intestines [30–36] |
| Status of intestinal microbiota | ||
| Medications (especially antibiotics), stress, low physical activity, alcohol, Dietary changes (low fiber, meals high in simple sugars) | Dysbiosis—an imbalance of microbiology, involving a change in the composition of normal microbiota | Loss of biodiversity decrease in the number of commensals increase in opportunistic pathogens [37–45] |
| Diet | ||
| Unhealthy diet | Processed food—preservatives, emulsifiers, artificial colors, enzymes, surfactants, high amount of saturated fatty acids, incorrect amount and ratio of omega-3 and omega-6 fatty acids | Increase in the incidence of food allergies weakening of the intestinal barrier [46–51] |
Dysbiosis vs intestinal tightness
Exposure to environmental factors can directly weaken the integrity of the intestinal epithelial barrier and alter the microbiome structure. “Leaky” epithelium and intestinal dysbiosis, often concomitant phenomena, cause the development of inflammation. This is also due to the direct proximity of immune cells, located under the layer of intestinal epithelial cells [1, 2]. Under the influence of a leaky epithelial barrier, the immune system is locally activated and inflammation develops, which can be transmitted via immune cells and the cytokines they secrete to other systems and organs. This is the reason why diseases, the development of which, at least in part, attempts to link to a leaky epithelial barrier, can have such different localizations [1]. Often after damage to the intestinal epithelium, colonization with opportunistic pathogens occurs, and commensal abundance and biodiversity begins to decline [40, 41, 43–45, 52]. Translocation of microorganisms into the intercellular compartments and further into the deeper tissue layers causes microinflammation. Exposure to various chemical compounds can also induce epigenetic changes that modulate the immune system and result in the facilitated development of certain diseases [1, 2, 37].
It is widely accepted that the microbiome plays a key role in the intestinal epithelial well-being. As mentioned above, a normal microbiota regulates various aspects of epithelial barrier function, such as TJ expression, angiogenesis, vascular permeability and immunomodulation [2, 37]. In the case of a leaky epithelial barrier, commensals and opportunistic pathogens migrate below the epithelial cell layer. This results in the development of inflammation, which is associated with the development of certain diseases. It has been postulated that the increase in allergic diseases may be due to bacterial dysbiosis and reduced biodiversity of the healthy microbiota [1, 2, 39].
In turn, the state of intestinal microbiota is influenced by a wide range of factors, with the main ones we can include diet, medications used, especially antibiotics, psychotropic drugs, proton pump inhibitors (PPIs) and genetic predisposition. There are speculations that non-pathogenic commensal microorganisms, which co-evolved with humans, have been a source of immunomodulatory signals for the human body (the “old friends” hypothesis), and now prevent the development of immune-mediated chronic diseases (the “hygiene hypothesis”), with the proper composition of the microbiome promoting the maintenance of the body’s immune balance (the “biodiversity hypothesis”) [1, 39, 53, 54]. In general, these hypotheses present the assumption that microbiota of proper composition regulates epithelial barrier function, including by regulating barrier permeability and TJ, influencing vascular permeability and angiogenesis, and affecting local inflammation and GALT tolerance [1, 55, 56]. Dysbiosis, through the lack of appropriate immunoregulatory and barrier factors, such as, e.g., short-chain fatty acids, causes inappropriate activation of the immune system, resulting in epithelial inflammation, epithelial barrier damage and disease development [55, 57–61]. This activation is characterized by a predominance of T helper 2 (TH2) lymphocytes, innate lymphoid cells type 2 (ILC2) and eosinophils. Mast cells, macrophages and lymphocytes may also be involved in this reaction. A vicious cycle emerges: dysbiosis—epithelial damage—chronic inflammation, which results in the epithelium not regenerating [1].
Inflammation, smoldering in the damaged intestinal epithelium, activates immune cells that can migrate and cause inflammation to develop in other organs [62, 63]. The relationship between disruption of the epithelial barrier in the intestine and the development of diseases in other organs is now widely studied [1] (Fig. 1).
The detailed interrelationships and interdependencies between substances that damage the epithelial barrier, the intestinal microbiota, the immune system, the development of inflammation, changes in cell function and ultimately the development of diseases, often located distantly from the intestine, remain unclear.
Role of zonulin in the regulation of TJ function
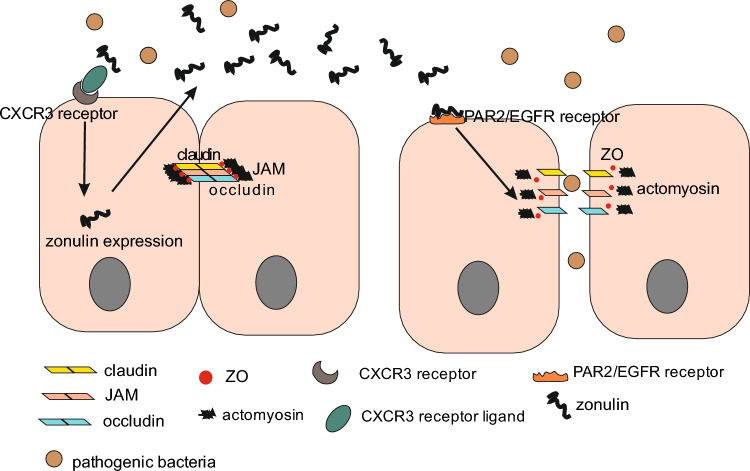
Zonulin is a paracrine protein with a molecular weight of 47 kDa. This protein is produced by several cells in the body, including epithelial cells of the small intestine. Zonulin has the ability to reversibly regulate the function of TJs [9, 16, 18, 64–66]. It is likely that zonulin release is initiated by dysbiosis and gliadin by a similar mechanism. On enterocytes and monocytes, gliadin binds to the CXCR3 chemokine receptor, which provides a signal for the cell to increase zonulin synthesis. Zonulin is then secreted into the intestinal lumen activates the epidermal growth factor receptor (EGFR) via protease-activated receptor 2 (PAR2). The activation of an intracellular cascade of biochemical reactions results in the phosphorylation of zona occludens (ZO) protein and myosin, as well as actin polymerization. These processes result in the detachment of, among others, the ZO protein from the TJ complex and impair integrity of the TJ [16, 18, 66–69].
Increased zonulin production has been observed under the presence of certain bacteria and food components, such as gluten. Excessive release of zonulin results in weakening of TJ and consequent passage of antigens into the vicinity of immune cells and into the circulatory system. As a result, local inflammation develops, and activated immune cells and cytokines can affect other organs or trigger immune-related diseases, e.g., autoimmunity. Excessive secretion of zonulin causes prolongation of the opening time of TJ, which can prolong the abnormal activation of the immune system over time [16, 18, 70–72]. Elevated zonulin levels have been found in patients with rheumatoid arthritis, multiple sclerosis or ankylosing spondylitis [1, 73–75].
There are reports that indicate that the increase in intestinal permeability in response to bacterial exposure is abolished by administration of a synthetic inhibitor of peptide binding to the zonulin receptor [18]. The release of zonulin is a physiological mechanism that regulates microbial colonization in the small intestine [18, 66, 76]. Zonulin transgenic mice showed resistance to “normalization” of the microbiota by transferring the normal microbiota. Therefore, a dysregulated zonulin system may also be the cause of intestinal dysbiosis [68, 77] (Fig. 2).
Fig. 2.
Mechanism of zonulin secretion. In the course of inflammation, CXCR3 receptors are activated, leading to the expression of zonulin protein in intestinal epithelial cells. Zonulin is secreted into the extracellular space and then activates PAR2/EGFR receptors, which triggers a biochemical cascade inside the cell and ultimately causes TJ relaxation
Relationship between disrupted intestinal barrier and disease incidence
Three basic elements are interdependent: altered microbiota, disrupted intestinal barrier, disease. However, the directionality of this relationship is controversial. Is it the abnormal intestinal microbiota that causes increased intestinal permeability and translocation of bacteria as well as their products and the development of disease, or is it the disease that causes a systemic inflammatory response resulting in disruption of the intestinal barrier, subsequent translocation of bacteria and further damage to the organ? Therefore, is leaky gut a cause or an effect of disease? [17, 78–80]. There are also reports that rupture of the respiratory or intestinal epithelial barrier and weakening of other barriers, for example, the blood–brain barrier or the vascular endothelial barrier, may facilitate the development of metabolic and autoimmune diseases [55, 81–84].
For example, a growing number of studies indicate that dysbiosis may promote the development of hypertension, and hypertension may affect the composition of the intestinal microbiota. Proper microbiota status is a factor that promotes normalization of blood pressure, and proper blood pressure promotes normalization of microbiota status. A correlation is apparent between these factors, but it is difficult to clearly determine its direction. Results indicate that the prevalence of intestinal barrier dysfunction is higher in patients with hypertension than in those without hypertension [45, 85–88]. Perhaps intestinal barrier damage may be linked to the development of hypertension [89–91]. Impaired barrier function does not simply and directly lead to disease in animal models of disease. It is also uncertain whether improvement of barrier function can, and to what extent, alter the course of disease [92–94]. A dysfunctional intestinal barrier can thus have many possible causes and produce symptoms that can range from gastrointestinal disorders such as bloating, cramping, and food intolerance to inflammatory bowel diseases (IBD), systemic, neurological, and psychiatric diseases [17].
Of particular interest are the findings on the link between the leaky gut barrier and disease incidence in humans. Inflammatory bowel diseases, which include ulcerative colitis and Crohn’s disease, are chronic diseases with an incompletely understood etiology [95, 96]. Intestinal leakage in IBD patients is associated with dysbiosis, inflammatory response and TJ abnormalities. The intestinal microbiota of IBD patients is characterized by an increase in pro-inflammatory bacteria and altered expression of multiple cytokines [95, 97, 98]. Altered intestinal permeability has been shown to occur in asymptomatic patients before the onset of clinical symptoms [95, 99, 100].
The intestines and liver are anatomically and functionally interconnected, forming the so-called gut liver axis [101–105]. Increased dysbiosis, impaired intestinal barrier, bacterial translocation and pro-inflammatory response play an important role in the development of chronic liver disease. Inflammation worsens liver damage and promotes the development of cirrhosis and its complications [79, 80, 103, 104]. Research data indicate that damage to both the intestinal epithelium and vascular barrier is necessary for the development of non-alcoholic steatohepatitis, which increases the role of intestinal dysbiosis in the process. Alcoholic liver disease is also associated with intestinal dysbiosis and disruption of the intestinal barrier, as both alcohol and its metabolites are toxic agents for intestinal cells. Impaired not only the intestinal epithelial barrier, but also the vascular barrier is responsible for damage to intestinal epithelial function in alcoholic liver disease [106–108].
Elevated serum zonulin levels, as well as bacterial dysbiosis and a leaky gut barrier, have been observed in patients with rheumatoid arthritis, while subclinical intestinal inflammation has been observed in patients with ankylosing spondylitis [74, 75]. Activation of Paneth cells in response to intestinal dysbiosis may be responsible for early symptoms of this disease, confirming the existence of an intestinal-articular axis. It has been observed that patients affected by systemic lupus erythematosus are colonized by a more homogeneous intestinal microbiota [1, 74, 75, 95].
In Parkinson’s disease, patients complain of gastrointestinal symptoms years prior to diagnosis [95, 109]. α-Synuclein, a protein typical of Parkinson’s disease, is synthesized in the intestines and then carried to the central nervous system via the vagus nerve [95, 110, 111]. In contrast, children with autism spectrum disorders develop intestinal symptoms, such as constipation, abdominal pain and diarrhea [95, 112]. Studies on the intestinal microbiota have shown an inverse relationship between microbiota diversity and neurological disorders, a reduction in Bacteroidetes, and reduced expression of TJ proteins in the intestinal mucosa [95, 113–115].
Other studies have shown that changes in intestinal permeability occur before the onset of type 1 diabetes symptoms, and a change in TJ and dysbiosis associated specifically with a decrease in butyrate-producing bacteria have also been observed [1, 95]. Changes in the composition of intestinal microbiota in patients with type 2 diabetes worsen the abnormal functioning of intestinal barrier and increase the pro-inflammatory state. This leads to increased insulin resistance and impaired beta cell function [1, 95, 116]. Moreover, in patients with type 2 diabetes, hyperglycemia promotes the maintenance of pro-inflammatory state [95, 117, 118]. Obesity, in turn, is associated with chronic low-grade inflammation. It is associated with pro-inflammatory macrophage activity in adipose tissue. Dysbiosis is also more common in obesity [95, 119] (Table 2).
Table 2.
Diseases postulated to have a leaky intestinal barrier, their etiology, part of the body affected by the disease and possible therapy for the permeable intestinal barrier, mainly in relation to IBD
| Disease [95, 120] | Proposed etiology-often multifactorial and/ or unknown [95, 120] | Part of the body affected by the disease [95, 120] | Possible therapy for the permeable intestinal barrier, mainly in relation to IBD [121–123] |
|---|---|---|---|
| Immune diseases |
Pharmacological treatments: typical drug therapy: aminosalicylates, corticosteroids (CS), immunomodulators, and biologics drugs, (anti-TNF therapy, anti-IL-12/23 therapy, anti-integrin therapy) other medicines: robiotics, prebiotics glutamine vitamin D, A metformin Non-pharmacological treatments: healthy diet medical herbs phenolic compounds physical activity fecal microbiota transplantation (FMT) More information about these compounds is included in section “Possibilities for diagnosis and regeneration of damaged intestinal barrier” |
||
| Inflammatory bowel diseases (IBD), such as ulcerative colitis (UC) and Crohn’s disease (CD) | Multifactorial; also immunological | Intestine | |
| Irritable bowel syndrome | Multifactorial; also immunological | Intestine | |
| Celiac disease | Factors: mainly genetic, autoimmunological | Intestine | |
| Rheumatoid arthritis | Multifactorial; autoimmunological | Joints | |
| Ankylosing spondylitis | Multifactorial; autoimmunological | Spine joints | |
| Systemic lupus erythematosus | Multifactorial; autoimmunological | Various tissues | |
| Type 1 diabetes | Autoimmunological | Damage to pancreatic beta cells | |
| Liver diseases | |||
| Liver cirrhosis | Alcohol, hepatitis B, C or D | Liver | |
| Non-alcoholic fatty liver disease (NAFLD) | Unhealthy diet, obesity, lack of physical activity | Liver | |
| Alcoholic liver disease (ALD) | Alcohol | Liver | |
| Metabolic diseases | |||
| Type 2 diabetes | Unhealthy diet, obesity, lack of physical activity | Tissue insulin resistance | |
| Obesity | Unhealthy diet, lack of physical activity | Adipose tissue | |
| Neurological diseases | |||
| Alzheimer’s disease | Multifactorial | Brain | |
| Parkinson’s disease | Unknown | Brain | |
| Major depression disorder | Factors: genetic, psychological stress | Brain | |
| Autism spectrum disorders | Multifactorial | Brain |
Possibilities for diagnosis and regeneration of damaged intestinal barrier
Evaluation of the level of intestinal barrier permeability is possible during indirect or direct diagnostic tests [124].
Indirect tests involve oral administration of test substances, followed by measurement of their concentration in blood or urine. The most commonly administered substance in indirect tests are sugars. The lactulose/mannitol test (L/M test), the most popular sugar test, assesses small intestinal permeability by measuring the excretion of these substances in the urine. Lactulose is a large oligosaccharide that is adsorbed only when the intercellular junctions leak; mannitol is a smaller molecule that can freely penetrate the intestinal barrier. The L/M test is non-invasive and has high sensitivity. It is also possible to administer other sugars and multi-sugar tests [124].
In addition, there are also possibilities to measure the level of substances of endogenous origin in the blood. Such biomarkers include zonulin, fatty acid binding proteins (FABP), citrulline, glucagon-like peptide (GLP)-2, LPS, LPS-binding protein (LBP) or fecal α1 antitrypsin (AAT), for example. However, these tests are not as sensitive as sugar tests [120, 125].
New imaging techniques, particularly confocal laser endomicroscopy, allow in vivo evaluation of the integrity of intestinal barrier after intravenous administration of fluorescein as a contrast agent. Fluorescein does not reveal paracellular transport. Confocal laser endomicroscopy is currently used in the diagnosis of gastrointestinal cancers, as well as in irritable bowel syndrome or celiac disease. Magnification of up to a thousand times allows detection of pathological changes within the intestinal epithelium [124].
Conventional treatments for IBS alleviate disease symptoms with pharmacotherapy. The most commonly used drugs are aminosalicylates, corticosteroids (CS), immunomodulators (for example, thiopurines (TPs), methotrexate (MTX), calcineurin inhibitors, Janus kinase (JAK) inhibitors) and biologics drugs. Biological therapies mainly include inhibitors of pro-inflammatory cytokines and integrin antagonists: anti-TNF therapy, anti-IL-12/23 therapy, anti-integrin therapy. Biological therapies, especially anti-TNF therapy are effective treatments, but the primary lack of response to TNF inhibitors or the secondary loss of response among some patients requires the search for new therapeutic solutions [121–123]. Importantly, the inflammatory environment may negatively affect the function of epithelial stem cells, and thus the reconstitution of healthy epithelium may be hindered [126–129]. Leaky gut treatment should begin with an attempt to determine the causes of intestinal barrier damage and then eliminate this factor. Until the intestinal barrier is restored, it seems reasonable to use drugs that alleviate inflammation in the intestine.
One method of restoring normal intestinal microbiota is fecal microbiota transplantation (FMT). This therapy involves the transfer of gastrointestinal microbiota obtained from a healthy donor to the gastrointestinal tract of a recipient, i.e., a person with known dysbiosis [86].
The fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet is a diet based on short-chain carbohydrates and polyols. These compounds are poorly absorbed and rapidly ferment, and due to their osmotic properties cause increased water content in the intestinal lumen [130–132]. Consumption of FODMAPs has broad effects on the digestive system. A FODMAP diet has a beneficial effect on the intestinal microbiota composition and function. And as we know, proper microbiota helps maintain the tightness of the intestinal barrier. However, patients with irritable bowel syndrome or IBD are better off with a low-FODMAP diet, as it can exacerbate disease symptoms due to gas production and stretching of the intestinal lumen [8, 130–132].
Probiotics are live microorganisms that have beneficial effects on the host’s health. Probiotic administration reduces intestinal leakage by, among others, affecting intestinal immunoregulation and anti-inflammatory effects (for example, an increase in sIgA production), anti-inflammatory effects, strengthening the epithelial barrier (for example, an increase in the synthesis of mucin and short-chain fatty acids (SCFAs), and the production of bacteriocins, which limit the growth of pathogenic microorganisms (for example, β defensin) [8, 133, 134]. Probiotics also increase the synthesis of proteins that constitute the TJ. The main probiotics showing protective effects on the state of the intestinal barrier include Lactobacillus rhamnosus GG, Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterim infantis, E. coli Nissle 1917 and Bifidobacterium animalis lactis BB-12 [8, 133–136].
Research results indicate that vitamins A and D affect the intestinal barrier in an indirect and multidirectional manner [137, 138]. In humans, these vitamins increased the diversity of microbiota compared to their deficiency [8, 139], improved TJ [8, 140, 141], inhibited the production of IFN-γ by T cells [8, 142] and inhibited Th17 cells and promoted the synthesis of IL-10 and FOXP3 protein [8, 143]. Retinoic acid stimulates the synthesis of antimicrobial factors [8, 144]. However, there is a small number of studies on this issue [8].
Another substance that has a positive effect on the state of intestinal barrier is dietary fiber. Fermentation of dietary fiber in the gastrointestinal tract produces SCFAs, such as butyrate, propionate and acetate. Fermentation occurs with the participation of beneficial bacteria, mainly Lactobacillus and Bifidobacterium [8, 145]. Research results indicate that butyrate affects mucin levels and TJ status. Short-chain fatty acids affect immune cell function, leading to changes in the levels of released cytokines and reactive oxygen species. Deficiency of fiber and short-chain acids can lead to increased intestinal permeability [8, 145–147].
Glutamine is considered a key amino acid capable of regulating the expression of TJ proteins. Findings indicate that glutamine has anti-inflammatory effects and reduces intestinal mucosal permeability, especially when combined with probiotics. However, the results of preliminary studies indicate that detailed studies are needed [148, 149]. Similarly, the suggested beneficial effect of arginine on the state of intestinal barrier requires further research. The beneficial effects of polyphenols on the state of intestinal epithelium are associated with beneficial effects on the state of TJs and an increase in mucus production, as well as effects on the activity of several protein kinases. Polyphenols also increase the activity of antioxidant enzymes. Research results suggest that a diet rich in polyphenols reduces the risk of intestinal barrier dysfunction [8, 150].
Medicinal herbs are used to treat autoimmune diseases associated with leaky gut, such as ulcerative colitis [8, 151]. The mechanism of medicinal plants is broad, and includes regulation of intestinal microbiota composition and intestinal permeability, increased expression of both mRNA and claudin-1 protein, and anti-inflammatory effects (Chinese tea, hibiscus, liquorice, marshmallow, ginger root, peppermint, plantain, curcumin). The mechanisms of action and efficacy of herbs remain inconclusive [8, 151–153].
Mushrooms are a source of bioactive compounds, such as vitamin D and phenolic compounds and also a source of prebiotics, as they contain various polysaccharides. Thus, they modulate the intestinal microbiota by stimulating the production of catecholamines and their metabolites as well as affecting the inflammatory response [8, 154, 155].
It is also well known that physical activity can increase the diversity of intestinal microbiota, enhance SCFA production and stimulate anti-inflammatory mechanisms, mainly aerobic exercises, such as running, cycling, fitness exercises [86, 156, 157].
Research results indicate that physical activity regulates the level of intestinal barrier permeability in a manner that is dependent on exercise intensity. Regular, moderate-intensity physical exercise has a positive effect on intestinal epithelial status and intestinal barrier integrity. Exercise increases the diversity of intestinal microbiome, which, as mentioned above, contributes to maintaining the integrity of the intestinal barrier, and also shows anti-inflammatory and antioxidant effects. Moderate physical activity can be an adjunctive factor in the treatment of leaky gut [158, 159]. High-intensity physical activity, typical of competitive or extreme sports, is correlated with a high incidence of gastrointestinal disorders and symptoms of increased intestinal permeability. This is a stressor for the body, causing hypoxia of intestinal cells due to redistribution of blood in the body, hyperthermia and dehydration [158, 159].
Therapy for leaky gut syndrome should include dietary modification and supplementation with probiotics and prebiotics and, perhaps the aforementioned compounds. Treatment of minor disorders can be achieved with a non-pharmacological dietary approach. Treatment with a zonulin antagonist inhibited the development of arthritis in mouse models of rheumatoid arthritis [1, 17]. Epigenetic changes may also underlie epithelial barrier leakage. Some experiments have shown that inhibition of histone deacetylase can restore barrier tightness [128, 160]. This issue still requires further research.
Conclusion
Contemporary recommendations regarding the described risks associated with a leaky gut barrier include, first and foremost, the prevention of epithelial barrier damage. This can be done by beginning with examining substances for which there are reports of their potential toxicity to the intestinal barrier and determining the degree of toxicity, monitoring the concentrations in the environment and various products of compounds toxic to the intestinal barrier, as well as limiting contact with substances that may cause intestinal leakage to doses that could be considered safe. An alternative is to develop substances that are safer for the epithelial barrier and are substitutes for commonly used, dangerous compounds [1].
The relationship between barrier function and clinical symptoms of diseases is often unclear and anecdotal, and this issue requires particularly thorough research. However, regardless of the degree of involvement of the leaky gut barrier in the development of certain diseases, the persistent inflammation that accompanies damage to the intestinal barrier negatively affects the functioning of gastrointestinal tract and the whole body. It is necessary to continue molecular research on the epithelial barrier in order to develop preventive guidelines for maintaining its tightness, to develop new methods for evaluation of its functionality and therapy in case of diagnosed damage.
The study on the relationship between the intestinal microbiota and certain diseases may help to understand the broader etiology of these conditions, including the influence of microbiota on their development. Understanding these mechanisms is very important in the prevention and treatment of many diseases. Combining traditional forms of treatment, mainly pharmacotherapy for conditions, with normalization of the intestinal barrier and the state of microbiota can greatly enhance the efficacy and effectiveness of many therapies.
Author contributions
BM and MS substantial contributions to the conception of the work, BM and AK drafting the work, MS revising it critically for important intellectual content, BM, AK and MS final approval of the version to be published, BM, MS and AK agreement to be accountable for all aspects of the work.
Funding
This work was not supported by any funding.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–51. 10.1038/s41577-021-00538-7. [DOI] [PubMed] [Google Scholar]
- 2.Celebi Sozener Z, Ozdel Ozturk B, Cerci P, Turk M, Gorgulu Akin B, Akdis M, Altiner S, Ozbey U, Ogulur I, Mitamura Y, Yilmaz I, Nadeau K, Ozdemir C, Mungan D, Akdis CA. Epithelial barrier hypothesis: effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy. 2022;77(5):1418–49. 10.1111/all.15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecchi L, D’Amato G, Annesi-Maesano I. External exposome and allergic respiratory and skin diseases. J Allergy Clin Immunol. 2018;141(3):846–57. 10.1016/j.jaci.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Sözener ZC, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145(6):1517–28. 10.1016/j.jaci.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML. Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol. 2017;140(1):1–12. 10.1016/j.jaci.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sbihi H, Boutin RC, Cutler C, Suen M, Finlay BB, Turvey SE. Thinking bigger: how early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy. 2019;74(11):2103–15. 10.1111/all.13812. [DOI] [PubMed] [Google Scholar]
- 7.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20. 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleman RS, Moncada M, Aryana KJ. Leaky gut and the ingredients that help treat it: a review. Molecules. 2023;28(2):619. 10.3390/molecules28020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandl C, Bucci L, Schett G, Zaiss MM. Crossing the barriers: Revisiting the gut feeling in rheumatoid arthritis. Eur J Immunol. 2021;51(4):798–810. 10.1002/eji.202048876. [DOI] [PubMed] [Google Scholar]
- 10.Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013;28(Suppl 4):9–17. 10.1111/jgh.12294. [DOI] [PubMed] [Google Scholar]
- 11.Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535(7610):85–93. 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5(10):1465–83. 10.1002/emmm.201201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2012;15(1):57–62. 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerbe F, Legraverend C, Jay P. The intestinal epithelium tuft cells: specification and function. Cell Mol Life Sci. 2012;69(17):2907–17. 10.1007/s00018-012-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1(2):a002584. 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drąg J, Goździalska A, Knapik-Czajka M, Matuła A, Jaśkiewicz J. Nieszczelność jelit w chorobach autoimmunologicznych. Państwo i Społeczeństwo. 2017;17(4):133–46. [Google Scholar]
- 17.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516–26. 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood Heickman LK, DeBoer MD, Fasano A. Zonulin as a potential putative biomarker of risk for shared type 1 diabetes and celiac disease autoimmunity. Diabetes Metab Res Rev. 2020;36(5):e3309. 10.1002/dmrr.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50(8):1–9. 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environ Sci Technol. 2017;51(12):6634–47. 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 21.Yee M-L, Hii L-W, Looi CK, et al. Impact of microplastics and nanoplastics on human health. Nanomaterials. 2021;11(2):496. 10.3390/nano11020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holloczki O, Gehrke S. Can nanoplastics alter cell membranes? ChemPhysChem. 2020;21(1):9–12. 10.1002/cphc.201900481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holloczki O, Gehrke S. Nanoplastics can change the secondary structure of proteins. Sci Rep. 2019;9(1):16013. 10.1038/s41598-019-52495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu M, Halimu G, Zhang Q, Song Y, Fu X, Li Y, Li Y, Zhang H. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ. 2019;694:133794. 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]
- 25.Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308–17. 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- 26.Vietti G, Lison D, van den Brule S. Mechanisms of lung fibrosis induced by carbon nanotubes: towards an adverse outcome pathway (AOP). Part Fibre Toxicol. 2016;13:11. 10.1186/s12989-016-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty S, Castranova V, Perez MK, Piedimonte G. Nanoparticles-induced apoptosis of human airway epithelium is mediated by proNGF/p75(NTR) signaling. J Toxicol Environ Health A. 2017;80(1):53–68. 10.1080/15287394.2016.1238329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbančič I, Garvas M, Kokot B, Majaron H, Umek P, Cassidy H, Škarabot M, Schneider F, Galiani S, Arsov Z, Koklic T, Matallanas D, Čeh M, Muševič I, Eggeling C, Štrancar J. Nanoparticles can wrap epithelial cell membranes and relocate them across the epithelial cell layer. Nano Lett. 2018;18(8):5294–305. 10.1021/acs.nanolett.8b02291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim SL, Ng CT, Zou L, Lu Y, Chen J, Bay BH, Shen HM, Ong CN. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology. 2019;13(8):1117–32. 10.1080/17435390.2019.1640913. [DOI] [PubMed] [Google Scholar]
- 30.Kogawa AC, Cernic BG, do Couto LGD, Salgado HRN. Synthetic detergents: 100 years of history. Saudi Pharm J. 2017;25(6):934–8. 10.1016/j.jsps.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M, Tan G, Eljaszewicz A, Meng Y, Wawrzyniak P, Acharya S, Altunbulakli C, Westermann P, Dreher A, Yan L, Wang C, Akdis M, Zhang L, Nadeau KC, Akdis CA. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. 2019;143(5):1892–903. 10.1016/j.jaci.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Xian M, Wawrzyniak P, Rückert B, Duan S, Meng Y, Sokolowska M, Globinska A, Zhang L, Akdis M, Akdis CA. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol. 2016;138(3):890-893.e9. 10.1016/j.jaci.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Viennois E, Chassaing B. First victim, later aggressor: How the intestinal microbiota drives the pro-inflammatory effects of dietary emulsifiers? Gut Microbes. 2018;9(3):1–4. 10.1080/19490976.2017.1421885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pressman P, Clemens R, Hayes W, Reddy C. Food additive safety: a review of toxicologic and regulatory issues. Toxicol Res Appl. 2017;1:1–22. [Google Scholar]
- 35.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–6. 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krempski JW, Dant C, Nadeau KC. The origins of allergy from a systems approach. Ann Allergy Asthma Immunol. 2020;125(5):507–16. 10.1016/j.anai.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Fiuza BSD, Fonseca HF, Meirelles PM, Marques CR, da Silva TM, Figueiredo CA. Understanding asthma and allergies by the lens of biodiversity and epigenetic changes. Front Immunol. 2021;12:623737. 10.3389/fimmu.2021.623737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O’Mahony L. Recent developments and highlights in mechanisms of allergic diseases: microbiome. Allergy. 2018;73(12):2314–27. 10.1111/all.13634. [DOI] [PubMed] [Google Scholar]
- 39.Haahtela T, Holgate S, Pawankar R, Akdis CA, Benjaponpitak S, Caraballo L, Demain J, Portnoy J, von Hertzen L, WAO Special Committee on Climate Change and Biodiversity. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. 2013;6(1):3. 10.1186/1939-4551-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altunbulakli C, Reiger M, Neumann AU, Garzorz-Stark N, Fleming M, Huelpuesch C, Castro-Giner F, Eyerich K, Akdis CA, Traidl-Hoffmann C. Relations between epidermal barrier dysregulation and Staphylococcus species-dominated microbiome dysbiosis in patients with atopic dermatitis. J Allergy Clin Immunol. 2018;142(5):1643-1647.e12. 10.1016/j.jaci.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Altunbulakli C, Costa R, Lan F, Zhang N, Akdis M, Bachert C, Akdis CA. Staphylococcus aureus enhances the tight junction barrier integrity in healthy nasal tissue, but not in nasal polyps. J Allergy Clin Immunol. 2018;142(2):665-668.e8. 10.1016/j.jaci.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 42.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71(6):1216–28. 10.1016/j.jhep.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YC, Won HK, Lee JW, Sohn KH, Kim MH, Kim TB, Chang YS, Lee BJ, Cho SH, Bachert C, Song WJ. Staphylococcus aureus nasal colonization and asthma in adults: systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2019;7(2):606-615.e9. 10.1016/j.jaip.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Kim JW, Kwok SK, Choe JY, Park SH. Recent advances in our understanding of the link between the intestinal microbiota and systemic lupus erythematosus. Int J Mol Sci. 2019;20(19):4871. 10.3390/ijms20194871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018;132(6):701–18. 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Søyland E, Funk J, Rajka G, Sandberg M, Thune P, Rustad L, Helland S, Middelfart K, Odu S, Falk ES, et al. Dietary supplementation with very long-chain n-3 fatty acids in patients with atopic dermatitis: a double-blind, multicentre study. Br J Dermatol. 1994;130(6):757–64. 10.1111/j.1365-2133.1994.tb03414.x. [DOI] [PubMed] [Google Scholar]
- 47.Hussain M, Bonilla-Rosso G, Kwong Chung CKC, Bäriswyl L, Rodriguez MP, Kim BS, Engel P, Noti M. High dietary fat intake induces a microbiota signature that promotes food allergy. J Allergy Clin Immunol. 2019;144(1):157-170.e8. 10.1016/j.jaci.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 48.Yamashita H, Matsuhara H, Miotani S, Sako Y, Matsui T, Tanaka H, Inagaki N. Artificial sweeteners and mixture of food additives cause to break oral tolerance and induce food allergy in murine oral tolerance model for food allergy. Clin Exp Allergy. 2017;47(9):1204–13. 10.1111/cea.12928. [DOI] [PubMed] [Google Scholar]
- 49.Venter C, Meyer RW, Nwaru BI, Roduit C, Untersmayr E, Adel-Patient K, Agache I, Agostoni C, Akdis CA, Bischoff SC, du Toit G, Feeney M, Frei R, Garn H, Greenhawt M, Hoffmann-Sommergruber K, Lunjani N, Maslin K, Mills C, Muraro A, Pali-Schöll I, Poulson LK, Reese I, Renz H, Roberts GC, Smith P, Smolinska S, Sokolowska M, Stanton C, Vlieg-Boerstra B, O’Mahony L. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy. 2019;74(8):1429–44. 10.1111/all.13764. [DOI] [PubMed] [Google Scholar]
- 50.Miles EA, Calder PC. Omega-6 and omega-3 polyunsaturated fatty acids and allergic diseases in infancy and childhood. Curr Pharm Des. 2014;20(6):946–53. 10.2174/138161282006140220125732. [DOI] [PubMed] [Google Scholar]
- 51.Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–8. 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Dainichi T, Kitoh A, Otsuka A, Nakajima S, Nomura T, Kaplan DH, Kabashima K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol. 2018;19(12):1286–98. 10.1038/s41590-018-0256-2. [DOI] [PubMed] [Google Scholar]
- 53.Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol. 2004;25(3–4):237–55. 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- 54.Panelli S, Epis S, Cococcioni L, Perini M, Paroni M, Bandi C, Drago L, Zuccotti GV. Inflammatory bowel diseases, the hygiene hypothesis and the other side of the microbiota: parasites and fungi. Pharmacol Res. 2020;159:104962. 10.1016/j.phrs.2020.104962. [DOI] [PubMed] [Google Scholar]
- 55.Mu Q, Kirby J, Reilly CM, Luo XM. Leaky gut as a danger signal for autoimmune diseases. Front Immunol. 2017;8:598. 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soderholm AT, Pedicord VA. Intestinal epithelial cells: at the interface of the microbiota and mucosal immunity. Immunology. 2019;158(4):267–80. 10.1111/imm.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng X, Zhou HY, Shen HH, Lufumpa E, Li XM, Guo B, Li BZ. Microbe-metabolite-host axis, two-way action in the pathogenesis and treatment of human autoimmunity. Autoimmun Rev. 2019;18(5):455–75. 10.1016/j.autrev.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207–15. 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 59.Dutta SK, Verma S, Jain V, Surapaneni BK, Vinayek R, Phillips L, Nair PP. Parkinson’s disease: the emerging role of gut dysbiosis, antibiotics, probiotics, and fecal microbiota transplantation. J Neurogastroenterol Motil. 2019;25(3):363–76. 10.5056/jnm19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol. 2018;136(3):345–61. 10.1007/s00401-018-1856-5. [DOI] [PubMed] [Google Scholar]
- 61.Tanoue T, Umesaki Y, Honda K. Immune responses to gut microbiota-commensals and pathogens. Gut Microbes. 2010;1(4):224–33. 10.4161/gmic.1.4.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chunxi L, Haiyue L, Yanxia L, Jianbing P, Jin S. The gut microbiota and respiratory diseases: new evidence. J Immunol Res. 2020;2020:2340670. 10.1155/2020/2340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ventura MT, Polimeno L, Amoruso AC, Gatti F, Annoscia E, Marinaro M, Di Leo E, Matino MG, Buquicchio R, Bonini S, Tursi A, Francavilla A. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006;38(10):732–6. 10.1016/j.dld.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106(39):16799–804. 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, Saieva L, Cypers H, Stampone T, Di Benedetto P, Gabrielli A, Fasano A, Elewaut D, Triolo G. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76(6):1123–32. 10.1136/annrheumdis-2016-210000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. (2002) Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123(5):1607–15. 10.1053/gast.2002.36578. Erratum in: 2003, Gastroenterology124(1):275. [DOI] [PubMed]
- 67.Lammers KM, Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135(1):194-204.e3. 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4(4):e1251384. 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sturgeon C, Lan J, Fasano A. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Ann NY Acad Sci. 2017;1397(1):130–42. 10.1111/nyas.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero-Figueroa MDS, Ramírez-Durán N, Montiel-Jarquín AJ, Horta-Baas G. Gut-joint axis: Gut dysbiosis can contribute to the onset of rheumatoid arthritis via multiple pathways. Front Cell Infect Microbiol. 2023;13:1092118. 10.3389/fcimb.2023.1092118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu X, Wang M, Wang Z, Chen Q, Chen X, Xu Y, Dai M, Wu B, Li Y. The bridge of the gut-joint axis: Gut microbial metabolites in rheumatoid arthritis. Front Immunol. 2022;13:1007610. 10.3389/fimmu.2022.1007610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Audo R, Sanchez P, Rivière B, Mielle J, Tan J, Lukas C, Macia L, Morel J, Immediato Daien C. Rheumatoid arthritis is associated with increased gut permeability and bacterial translocation which are reversed by inflammation control. Rheumatology. 2022. 10.1093/rheumatology/keac454. [DOI] [PubMed] [Google Scholar]
- 73.Tajik N, Frech M, Schulz O, Schälter F, Lucas S, Azizov V, Dürholz K, Steffen F, Omata Y, Rings A, Bertog M, Rizzo A, Iljazovic A, Basic M, Kleyer A, Culemann S, Krönke G, Luo Y, Überla K, Gaipl US, Frey B, Strowig T, Sarter K, Bischoff SC, Wirtz S, Cañete JD, Ciccia F, Schett G, Zaiss MM. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11(1):1995. 10.1038/s41467-020-15831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ciccia F, Rizzo A, Triolo G. Subclinical gut inflammation in ankylosing spondylitis. Curr Opin Rheumatol. 2016;28(1):89–96. 10.1097/BOR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 75.Ciccia CF, Ferrante A, Triolo G. Intestinal dysbiosis and innate immune responses in axial spondyloarthritis. Curr Opin Rheumatol. 2016;28(4):352–8. 10.1097/BOR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 76.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91(1):151–75. 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 77.Miranda-Ribera A, Miranda-Ribera A, Ennamorati M, Serena G, Cetinbas M, Lan J, Sadreyev RI, Jain N, Fasano A, Fiorentino M. Exploiting the zonulin mouse model to establish the role of primary impaired gut barrier function on microbiota composition and immune profiles. Front Immunol. 2019;10:2233. 10.3389/fimmu.2019.02233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, Motola DL, Luther S, Bohr S, Jeoung SW, Deshpande V, Singh G, Turner JR, Yarmush ML, Chung RT, Patel SJ. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell Mol Gastroenterol Hepatol. 2015;1(2):222–32. 10.1016/j.jcmgh.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ponziani FR, Zocco MA, Cerrito L, Gasbarrini A, Pompili M. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol. 2018;12(7):641–56. 10.1080/17474124.2018.1481747. [DOI] [PubMed] [Google Scholar]
- 80.Ponziani FR, Nicoletti A, Gasbarrini A, Pompili M. Diagnostic and therapeutic potential of the gut microbiota in patients with early hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919848184. 10.1177/1758835919848184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. 2017;17(12):761–73. 10.1038/nri.2017.100. [DOI] [PubMed] [Google Scholar]
- 82.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–53. 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 83.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–60. 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Acharya NK, Qi X, Goldwaser EL, Godsey GA, Wu H, Kosciuk MC, Freeman TA, Macphee CH, Wilensky RL, Venkataraman V, Nagele RG. Retinal pathology is associated with increased blood-retina barrier permeability in a diabetic and hypercholesterolaemic pig model: Beneficial effects of the LpPLA2 inhibitor Darapladib. Diab Vasc Dis Res. 2017;14(3):200–13. 10.1177/1479164116683149. [DOI] [PubMed] [Google Scholar]
- 85.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, Zhou J, Guan X, Ding Y, Wang S, Khan M, Xin Y, Li S, Ma Y. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017;7:381. 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Z, Wang Q, Liu Y, Wang L, Ge Z, Li Z, Feng S, Wu C. Gut microbiota and hypertension: association, mechanisms and treatment. Clin Exp Hypertens. 2023;45(1):2195135. 10.1080/10641963.2023.2195135. [DOI] [PubMed] [Google Scholar]
- 87.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin D. Assocation between hypertension and impairment of intestinal barrier function. Guangzhong: Guangdong Pharmaceutical University; 2019. [Google Scholar]
- 89.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120(2):312–23. 10.1161/CIRCRESAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manner IW, Baekken M, Kvale D, Oektedalen O, Pedersen M, Nielsen SD, Nowak P, Os I, Troseid M. Markers of microbial translocation predict hypertension in HIV-infected individuals. HIV Med. 2013;14(6):354–61. 10.1111/hiv.12015. [DOI] [PubMed] [Google Scholar]
- 91.Jaworska K, Huc T, Samborowska E, Dobrowolski L, Bielinska K, Gawlak M, Ufnal M, Chamaillard M. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS ONE. 2017;12(12):e0189310. 10.1371/journal.pone.0189310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quigley EM. Leaky gut: concept or clinical entity? Curr Opin Gastroenterol. 2016;32(2):74–9. 10.1097/MOG.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 93.Turner JR, Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14(1):9–21. 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michielan A, Michielan A, Dincà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015. 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Tommaso N, Gasbarrini A, Ponziani FR. Intestinal barrier in human health and disease. Int J Environ Res Public Health. 2021;18(23):12836. 10.3390/ijerph182312836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):91–9. 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Mattos BR, Garcia MP, Nogueira JB, Paiatto LN, Albuquerque CG, Souza CL, Fernandes LG, Tamashiro WM, Simioni PU. Inflammatory bowel disease: an overview of immune mechanisms and biological treatments. Mediators Inflamm. 2015;2015:493012. 10.1155/2015/493012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11(1):1–10. 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 99.Mehandru S, Colombel JF. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat Rev Gastroenterol Hepatol. 2021;18(2):83–4. 10.1038/s41575-020-00399-w. [DOI] [PubMed] [Google Scholar]
- 100.Turpin W, Lee SH, Raygoza Garay JA, Madsen KL, Meddings JB, Bedrani L, Power N, Espin-Garcia O, Xu W, Smith MI, Griffiths AM, Moayyedi P, Turner D, Seidman EG, Steinhart AH, Marshall JK, Jacobson K, Mack D, Huynh H, Bernstein CN, Paterson AD, Croitoru K, Crohn’s and Colitis Canada Genetic Environmental Microbial Project Research Consortium; CCC GEM Project recruitment site directors include Maria Abreu. Increased intestinal permeability is associated with later development of crohn’s disease. Gastroenterology. 2020;159(6):2092-2100.e5. 10.1053/j.gastro.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 101.Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28(6):737–44. 10.1159/000324281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–77. 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R (2017) Targeting the gut-liver axis in liver disease. J Hepatol. Nov;67(5):1084–1103. 10.1016/j.jhep.2017.05.007. Erratum in: J Hepatol. 2018. [DOI] [PubMed]
- 104.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60(1):197–209. 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 105.J Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R (2018) The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 15(7):397–411. 10.1038/s41575-018-0011-z. Erratum in: Nat Rev Gastroenterol Hepatol. 2018. [DOI] [PMC free article] [PubMed]
- 106.Bishehsari F, Magno E, Swanson G, Desai V, Voigt RM, Forsyth CB, Keshavarzian A. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38(2):163–71. [PMC free article] [PubMed] [Google Scholar]
- 107.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94(1):200–7. 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 108.Maccioni L, Gao B, Leclercq S, Pirlot B, Horsmans Y, De Timary P, Leclercq I, Fouts D, Schnabl B, Stärkel P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes. 2020;12(1):1782157. 10.1080/19490976.2020.1782157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savica R, Carlin JM, Grossardt BR, Bower JH, Ahlskog JE, Maraganore DM, Bharucha AE, Rocca WA. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. 2009;73(21):1752–8. 10.1212/WNL.0b013e3181c34af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27(6):709–15. 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 111.Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models parkinson’s disease. Neuron. 2019;103(4):627-641.e7. 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buie T, Campbell DB, Fuchs GJ 3rd, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, Carr EG, Gershon MD, Hyman SL, Jirapinyo P, Jyonouchi H, Kooros K, Kushak R, Levitt P, Levy SE, Lewis JD, Murray KF, Natowicz MR, Sabra A, Wershil BK, Weston SC, Zeltzer L, Winter H. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1-18. 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 113.Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE. 2013;8(7):e68322. 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N, Fasano A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7:49. 10.1186/s13229-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabrò A, De Filippo C. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5(1):24. 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, Herrema H. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. 2020;11:571731. 10.3389/fimmu.2020.571731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Massier L, Chakaroun R, Tabei S, Crane A, Didt KD, Fallmann J, von Bergen M, Haange SB, Heyne H, Stumvoll M, Gericke M, Dietrich A, Blüher M, Musat N, Kovacs P. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut. 2020;69(10):1796–806. 10.1136/gutjnl-2019-320118. [DOI] [PubMed] [Google Scholar]
- 118.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 119.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O, MetaHIT consortium. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 120.Schoultz I, Keita ÅV. The intestinal barrier and current techniques for the assessment of gut permeability. Cells. 2020;9(8):1909. 10.3390/cells9081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cai Z, Wang S, Li. Treatment of inflammatory bowel disease: a comprehensive review. Front Med (Lausanne). 2021;8:765474. 10.3389/fmed.2021.765474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang S, Murphy M, Malter L. A review of available medical therapies to treat moderate-to-severe inflammatory bowel disease. Am J Gastroenterol. 2024;119(1):55–80. 10.14309/ajg.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 123.TriantafillidisJK ZCG, Konstadoulakis MM, Papalois AE. Combination treatment of inflammatory bowel disease: present status and future perspectives. World J Gastroenterol. 2024;30(15):2068–80. 10.3748/wjg.v30.i15.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Michielan A, D’Incà R. intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015. 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perez-Diaz-Del-Campo N, Castelnuovo G, Ribaldone DG, Caviglia GP. Fecal and circulating biomarkers for the non-invasive assessment of intestinal permeability. Diagnostics (Basel). 2023;13(11):1976. 10.3390/diagnostics13111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, Cremin C, Sones J, Djukanović R, Howarth PH, Collins JE, Holgate ST, Monk P, Davies DE. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128(3):549-56.e1. 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 127.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T, Boguniewicz M, Hata T, Schneider LC, Hanifin JM, Gallo RL, Novak N, Weidinger S, Beaty TH, Leung DY, Barnes KC, Beck LA. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127(3):773-86.e1. 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Rückert B, Globinska A, Jakiela B, Kast JI, Idzko M, Akdis M, Sanak M, Akdis CA. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139(1):93–103. 10.1016/j.jaci.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 129.Kubo T, Wawrzyniak P, Morita H, Sugita K, Wanke K, Kast JI, Altunbulakli C, Rückert B, Jakiela B, Sanak M, Akdis M, Akdis CA. CpG-DNA enhances the tight junction integrity of the bronchial epithelial cell barrier. J Allergy Clin Immunol. 2015. 10.1016/j.jaci.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 130.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1-63. 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 131.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106(10):1631–9. 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 132.Tuck CJ, Muir JG, Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: role in irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2014;8(7):819–34. 10.1586/17474124.2014.917956. [DOI] [PubMed] [Google Scholar]
- 133.Zakostelska Z, Kverka M, Klimesova K, Rossmann P, Mrazek J, Kopecny J, Hornova M, Srutkova D, Hudcovic T, Ridl J, Tlaskalova-Hogenova H. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE. 2011;6(11):e27961. 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lindström C, Patel A, Prajapati JB, Holst O. Probiotic properties of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented foods. Int J Ferment Foods. 2012;1(1):87–101. 10.1016/j.fbio.2013.10.002. [Google Scholar]
- 135.Persborn M, Gerritsen J, Wallon C, Carlsson A, Akkermans LM, Söderholm JD. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38(7):772–83. 10.1111/apt.12451. [DOI] [PubMed] [Google Scholar]
- 136.Tsai YL, Lin TL, Chang CJ, Tsung-Ru W, Lai WF, Chia-Chen L, Lai HC. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019. 10.1186/s12929-018-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437(3):357–72. 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cantorna MT, Snyder L, Arora J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit Rev Biochem Mol Biol. 2019;54(2):184–92. 10.1080/10409238.2019.1611734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lv Z, Wang Y, Yang T, Zhan X, Li Z, Hu H, Li T, Chen J. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J Clin Biochem Nutr. 2016;59(2):113–21. 10.3164/jcbn.15-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G208–16. 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 141.Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, Meddings J, O’Sullivan M. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: Results from a randomised double-blind placebo-controlled study. United Eur Gastroenterol J. 2015;3(3):294–302. 10.1177/2050640615572176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152(4):1515–22. [PubMed] [Google Scholar]
- 143.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111(3):1013–20. 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, Misiak A, Dungan LS, Sutton CE, Streubel G, Bracken AP, Mills KH. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med. 2013;210(6):1117–24. 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Van Immerseel F, Ducatelle R, De Vos M, Boon N, Van De Wiele T, Verbeke K, Rutgeerts P, Sas B, Louis P, Flint HJ. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J Med Microbiol. 2010;59(Pt 2):141–3. 10.1099/jmm.0.017541-0. [DOI] [PubMed] [Google Scholar]
- 146.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–25. 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 148.Kretzmann NA, Fillmann H, Mauriz JL, Marroni CA, Marroni N, González-Gallego J, Tuñón MJ. Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm Bowel Dis. 2008;14(11):1504–13. 10.1002/ibd.20543. [DOI] [PubMed] [Google Scholar]
- 149.Coëffier M, Marion R, Ducrotté P, Déchelotte P. Modulating effect of glutamine on IL-1beta-induced cytokine production by human gut. Clin Nutr. 2003;22(4):407–13. 10.1016/s0261-5614(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 150.Chapman JC, Liu Y, Zhu L, Rhoads JM. Arginine and citrulline protect intestinal cell monolayer tight junctions from hypoxia-induced injury in piglets. Pediatr Res. 2012;72(6):576–82. 10.1038/pr.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Valussi M. Functional foods with digestion-enhancing properties. Int J Food Sci Nutr. 2012;63(Suppl 1):82–9. 10.3109/09637486.2011.627841. [DOI] [PubMed] [Google Scholar]
- 152.Park J, Choi TJ, Kang KS, Choi SH. The interrelationships between intestinal permeability and phlegm syndrome and therapeutic potential of some medicinal herbs. Biomolecules. 2021;11(2):284. 10.3390/biom11020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ozaka S, Sonoda A, Ariki S, Minata M, Kamiyama N, Hidano S, Sachi N, Ito K, Kudo Y, Dewayani A, Chalalai T, Ozaki T, Soga Y, Fukuda C, Mizukami K, Ishizawa S, Nishiyama M, Fujitsuka N, Mogami S, Kubota K, Murakami K, Kobayashi T. Saireito, a Japanese herbal medicine, alleviates leaky gut associated with antibiotic-induced dysbiosis in mice. PLoS ONE. 2022;17(6):e0269698. 10.1371/journal.pone.0269698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang X, Nie S, Xie M. Interaction between gut immunity and polysaccharides. Crit Rev Food Sci Nutr. 2017;57(14):2943–55. 10.1080/10408398.2015.1079165. [DOI] [PubMed] [Google Scholar]
- 155.You S, Hoskin R, Komarnytsky S, Moncada M. Mushrooms as functional and nutritious food ingredients for multiple applications. ACS Food Sci Technol. 2022;2:1184–95. 10.1021/acsfoodscitech.2c00107. [Google Scholar]
- 156.Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O’Reilly M, Jeffery IB, Wood-Martin R, Kerins DM, Quigley E, Ross RP, O’Toole PW, Molloy MG, Falvey E, Shanahan F, Cotter PD. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–20. 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 157.Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, Shanahan F, Cotter PD, O’sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625–33. 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 158.Dmytriv TR, Storey KB, Lushchak VI. Intestinal barrier permeability: the influence of gut microbiota, nutrition, and exercise. Front Physiol. 2024;15:1380713. 10.3389/fphys.2024.1380713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Keirns BH, Koemel NA, Sciarrillo CM, Anderson KL, Emerson SR. Exercise and intestinal permeability: another form of exercise-induced hormesis? Am J Physiol Gastrointest Liver Physiol. 2020;319(4):G512–8. 10.1152/ajpgi.00232.2020. [DOI] [PubMed] [Google Scholar]
- 160.Steelant B, Wawrzyniak P, Martens K, Jonckheere AC, Pugin B, Schrijvers R, Bullens DM, Vanoirbeek JA, Krawczyk K, Dreher A, Akdis CA, Hellings PW. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J Allergy Clin Immunol. 2019;144(5):1242-1253.e7. 10.1016/j.jaci.2019.04.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.