Abstract
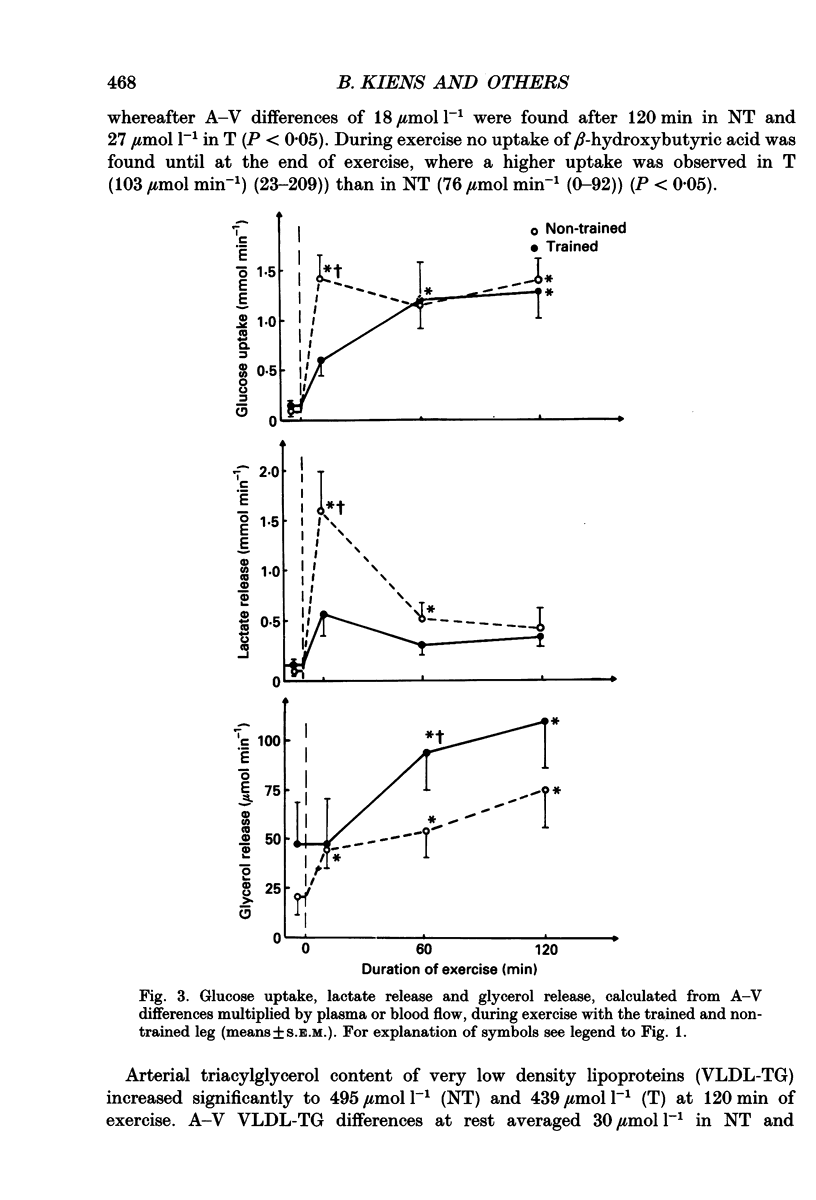
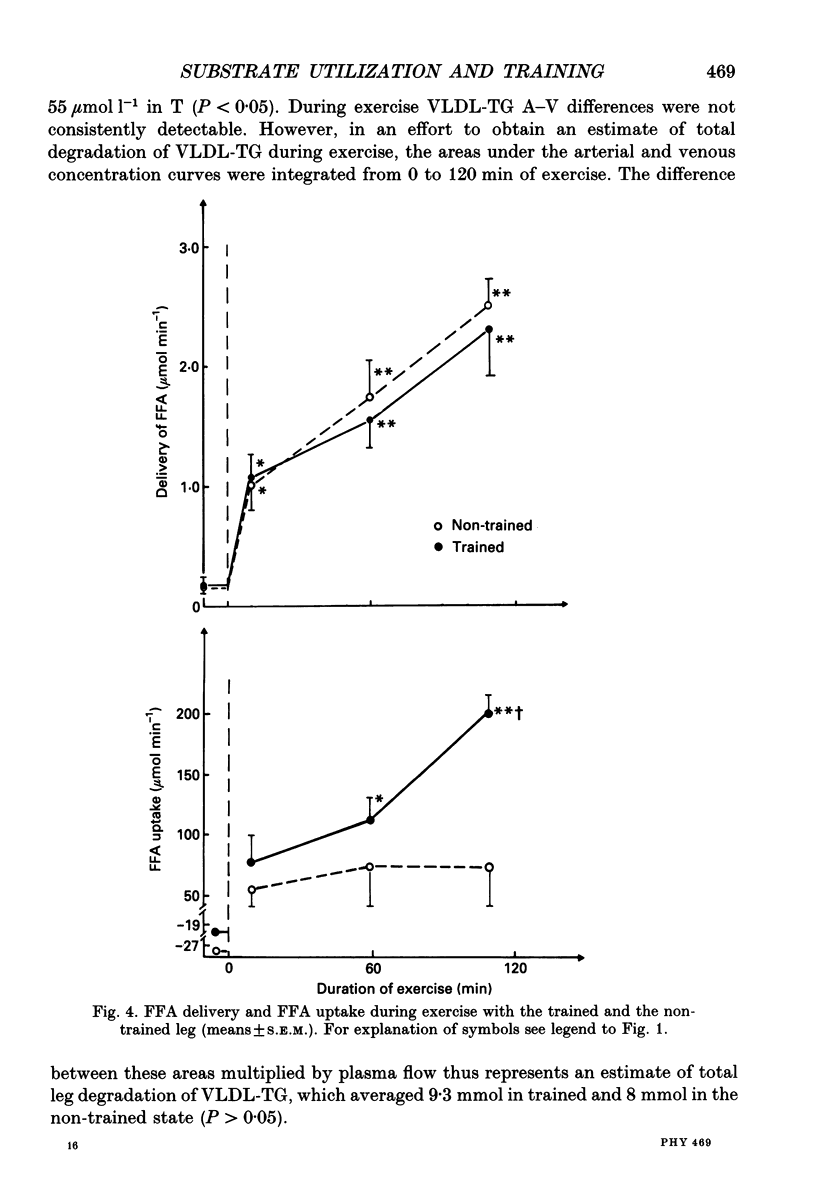
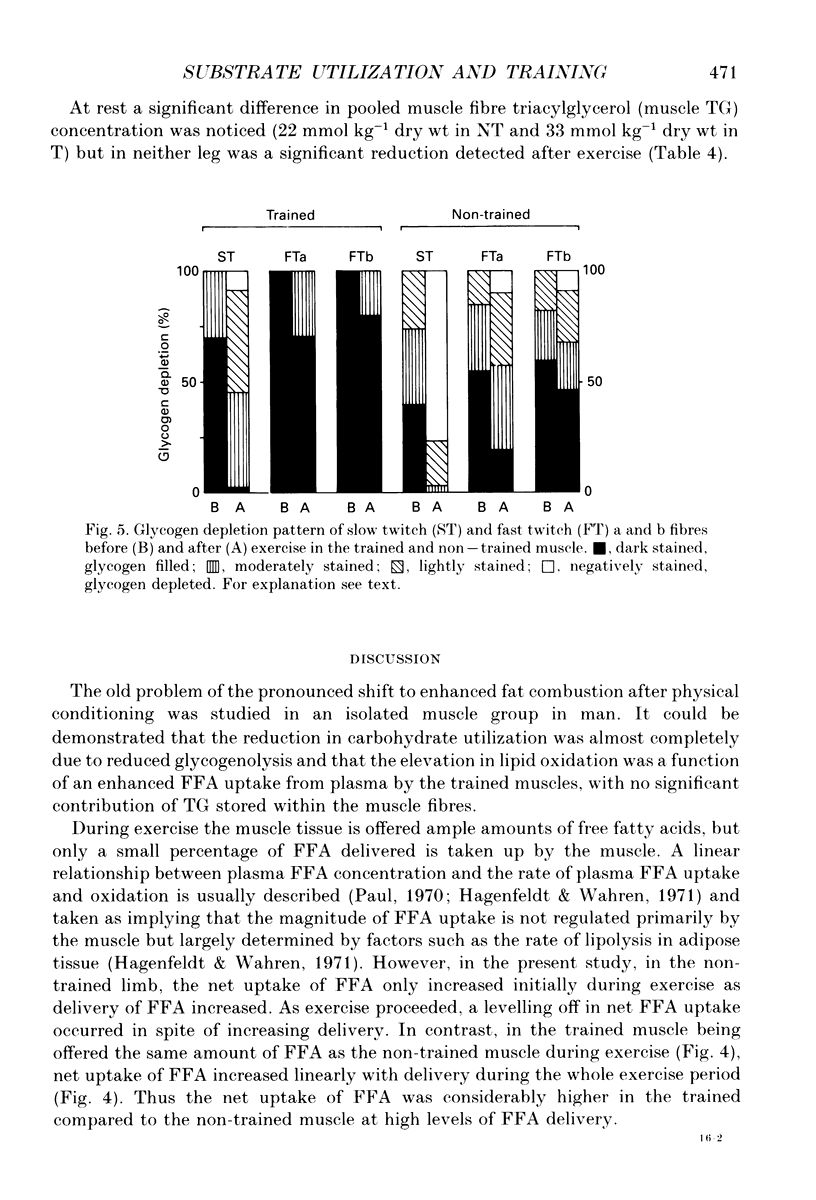
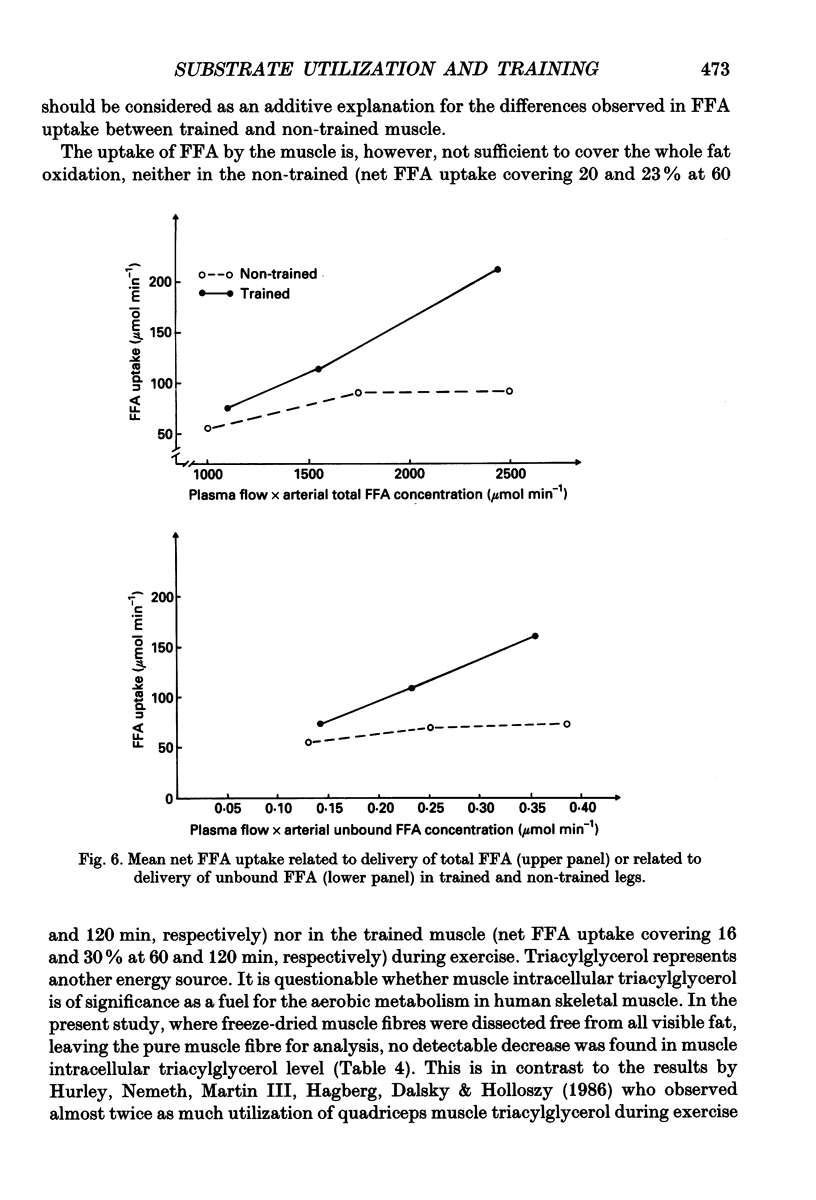
1. The influence of training-induced adaptations in skeletal muscle tissue on the choice between carbohydrates (CHO) and lipids as well as the extra- vs. intracellular substrate utilization was investigated in seven healthy male subjects performing one-legged knee-extension exercise. In each subject one of the knee extensors was endurance trained for eight weeks, whereafter the trained (T) and non-trained (NT) thighs were investigated a week apart. 2. The activity of beta-hydroxy-acyl-coenzyme A dehydrogenase (HAD) and capillary density in the knee extensors were significantly larger in T than in NT. 3. During dynamic knee-extension exercise, performed at the same absolute intensity for 2 h, femoral venous blood flow was lower in T than in NT (P < 0.05), but oxygen uptake was similar. 4. Respiratory quotient (RQ) values over the exercising thigh, averaging 0.81 (T) vs. 0.91 (NT; P < 0.05) indicated that a shift towards a larger fat combustion occurred with endurance training. 5. Both free fatty acids (FFA) and serum triacylglycerol contributed to the utilization of fat in NT and T muscles with no significant contribution from muscle fibre triacylglycerol. 6. At high plasma FFA concentrations net uptake of FFA plateaued in NT but not in T muscles. 7. The findings suggest that FFA uptake in exercising muscle is a saturable process and that the transport capacity is enhanced by training. The lower CHO utilization in the T leg was mainly a function of the glycogenolysis of the muscle being reduced. Hormones such as insulin, noradrenaline and adrenaline are unlikely to play a role in this shift as differences in plasma levels during T and NT leg exercise were small and insignificant, implying that local structural and functional adaptations of the training muscle are crucial for the observed shifts in the metabolic response to exercise.
Full text
PDF




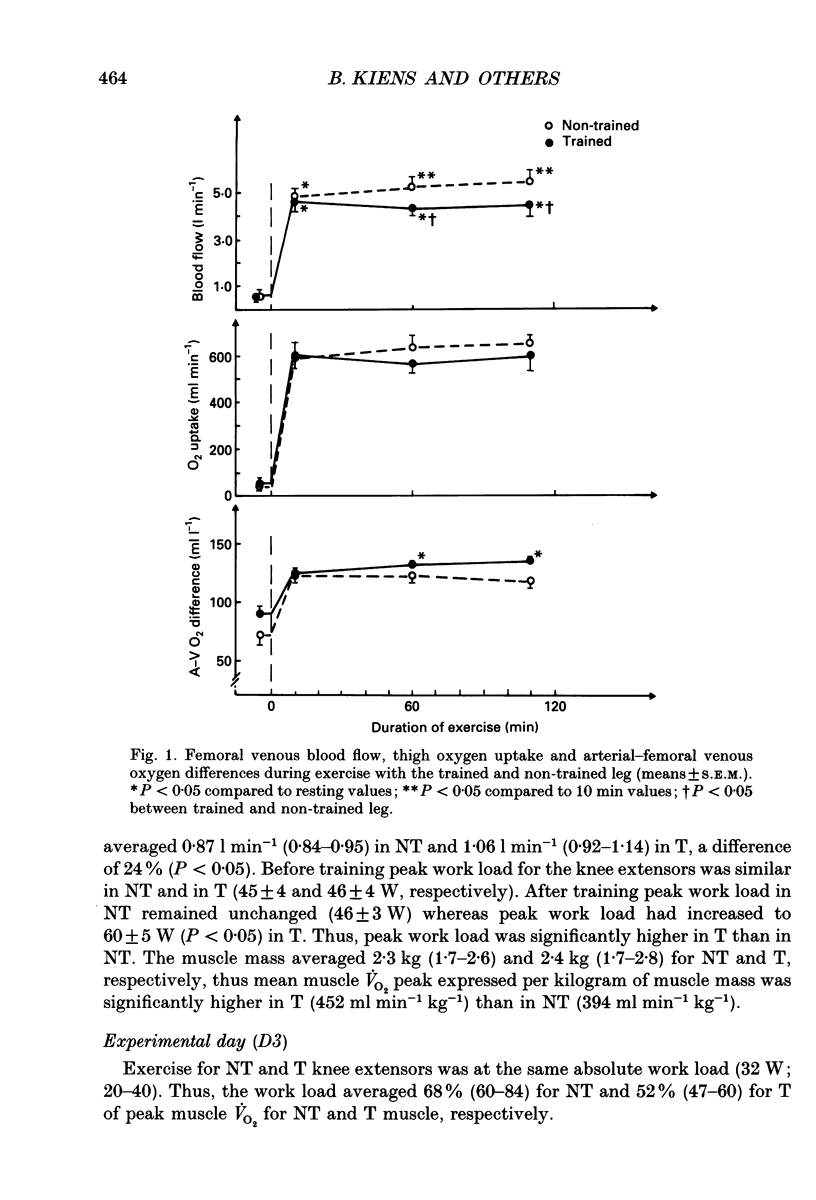
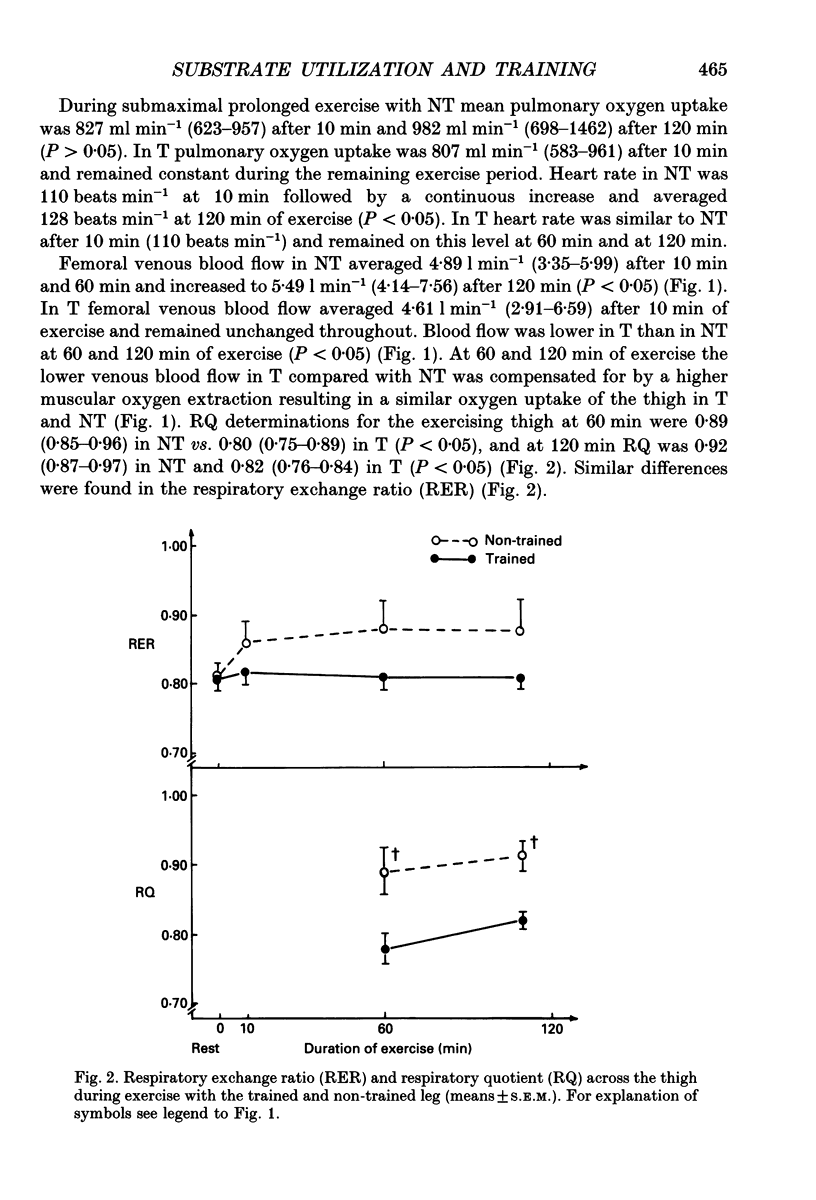






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Andersen P., Adams R. P., Sjøgaard G., Thorboe A., Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 1985 Nov;59(5):1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand. 1975 Oct;95(2):203–205. doi: 10.1111/j.1748-1716.1975.tb10043.x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985 Sep;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970 Sep;18(9):670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Christensen N. J., Vestergaard P., Sørensen T., Rafaelsen O. J. Cerebrospinal fluid adrenaline and noradrenaline in depressed patients. Acta Psychiatr Scand. 1980 Feb;61(2):178–182. doi: 10.1111/j.1600-0447.1980.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Cleroux J., Van Nguyen P., Taylor A. W., Leenen F. H. Effects of beta 1- vs. beta 1 + beta 2-blockade on exercise endurance and muscle metabolism in humans. J Appl Physiol (1985) 1989 Feb;66(2):548–554. doi: 10.1152/jappl.1989.66.2.548. [DOI] [PubMed] [Google Scholar]
- DeGrella R. F., Light R. J. Uptake and metabolism of fatty acids by dispersed adult rat heart myocytes. I. Kinetics of homologous fatty acids. J Biol Chem. 1980 Oct 25;255(20):9731–9738. [PubMed] [Google Scholar]
- Essén B. Studies on the regulation of metabolism in human skeletal muscle using intermittent exercise as an experimental model. Acta Physiol Scand Suppl. 1978;454:1–32. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Gaffney F. A., Sjøgaard G., Saltin B. Cardiovascular and metabolic responses to static contraction in man. Acta Physiol Scand. 1990 Mar;138(3):249–258. doi: 10.1111/j.1748-1716.1990.tb08844.x. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974 Aug;241(1):45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P. D., Saltin B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin Physiol. 1982 Feb;2(1):1–12. doi: 10.1111/j.1475-097x.1982.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Goresky C. A., Daly D. S., Mishkin S., Arias I. M. Uptake of labeled palmitate by the intact liver: role of intracellular binding sites. Am J Physiol. 1978 Jun;234(6):E542–E553. doi: 10.1152/ajpendo.1978.234.6.E542. [DOI] [PubMed] [Google Scholar]
- Graham T. E., Kiens B., Hargreaves M., Richter E. A. Influence of fatty acids on ammonia and amino acid flux from active human muscle. Am J Physiol. 1991 Aug;261(2 Pt 1):E168–E176. doi: 10.1152/ajpendo.1991.261.2.E168. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Simultaneous uptake and release of individual free fatty acids in human forearm muscle during exercise. Life Sci. 1966 Feb;5(4):357–364. doi: 10.1016/0024-3205(66)90021-x. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Pernow B., Jones N. L. Uptake and release of free fatty acids and other metabolites in the legs of exercising men. J Appl Physiol. 1967 Jul;23(1):90–99. doi: 10.1152/jappl.1967.23.1.90. [DOI] [PubMed] [Google Scholar]
- Henriksson J. Training induced adaptation of skeletal muscle and metabolism during submaximal exercise. J Physiol. 1977 Sep;270(3):661–675. doi: 10.1113/jphysiol.1977.sp011974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist N., Secher N. H., Sander-Jensen K., Knigge U., Warberg J., Schwartz T. W. Sympathoadrenal and parasympathetic responses to exercise. J Sports Sci. 1986 Autumn;4(2):123–128. doi: 10.1080/02640418608732108. [DOI] [PubMed] [Google Scholar]
- Hurley B. F., Nemeth P. M., Martin W. H., 3rd, Hagberg J. M., Dalsky G. P., Holloszy J. O. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol (1985) 1986 Feb;60(2):562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- Jansson E., Kaijser L. Substrate utilization and enzymes in skeletal muscle of extremely endurance-trained men. J Appl Physiol (1985) 1987 Mar;62(3):999–1005. doi: 10.1152/jappl.1987.62.3.999. [DOI] [PubMed] [Google Scholar]
- Jones P. R., Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969 Oct;204(2):63P–66P. [PubMed] [Google Scholar]
- Paul P. FFA metabolism of normal dogs during steady-state exercise at different work loads. J Appl Physiol. 1970 Feb;28(2):127–132. doi: 10.1152/jappl.1970.28.2.127. [DOI] [PubMed] [Google Scholar]
- Schwieterman W., Sorrentino D., Potter B. J., Rand J., Kiang C. L., Stump D., Berk P. D. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A. 1988 Jan;85(2):359–363. doi: 10.1073/pnas.85.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Inoue K., Tani Y., Yamada H. Enzymatic microdetermination of serum free fatty acids. Anal Biochem. 1979 Oct 1;98(2):341–345. doi: 10.1016/0003-2697(79)90151-9. [DOI] [PubMed] [Google Scholar]
- Sorrentino D., Stump D., Potter B. J., Robinson R. B., White R., Kiang C. L., Berk P. D. Oleate uptake by cardiac myocytes is carrier mediated and involves a 40-kD plasma membrane fatty acid binding protein similar to that in liver, adipose tissue, and gut. J Clin Invest. 1988 Sep;82(3):928–935. doi: 10.1172/JCI113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Berk P. D. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte L. P., Kiens B., Richter E. A. Saturation kinetics of palmitate uptake in perfused skeletal muscle. FEBS Lett. 1991 Feb 25;279(2):327–329. doi: 10.1016/0014-5793(91)80180-b. [DOI] [PubMed] [Google Scholar]
- Turcotte L. P., Richter E. A., Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992 Jun;262(6 Pt 1):E791–E799. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]


