Abstract
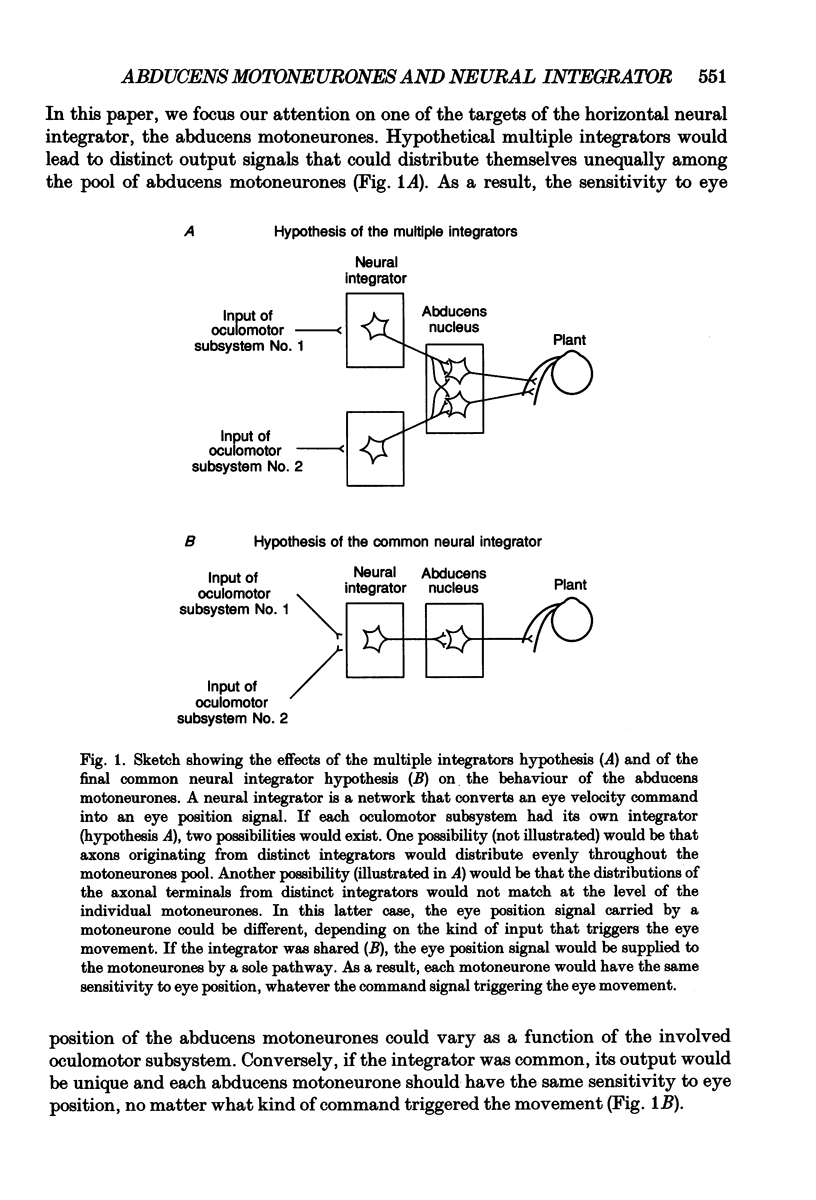
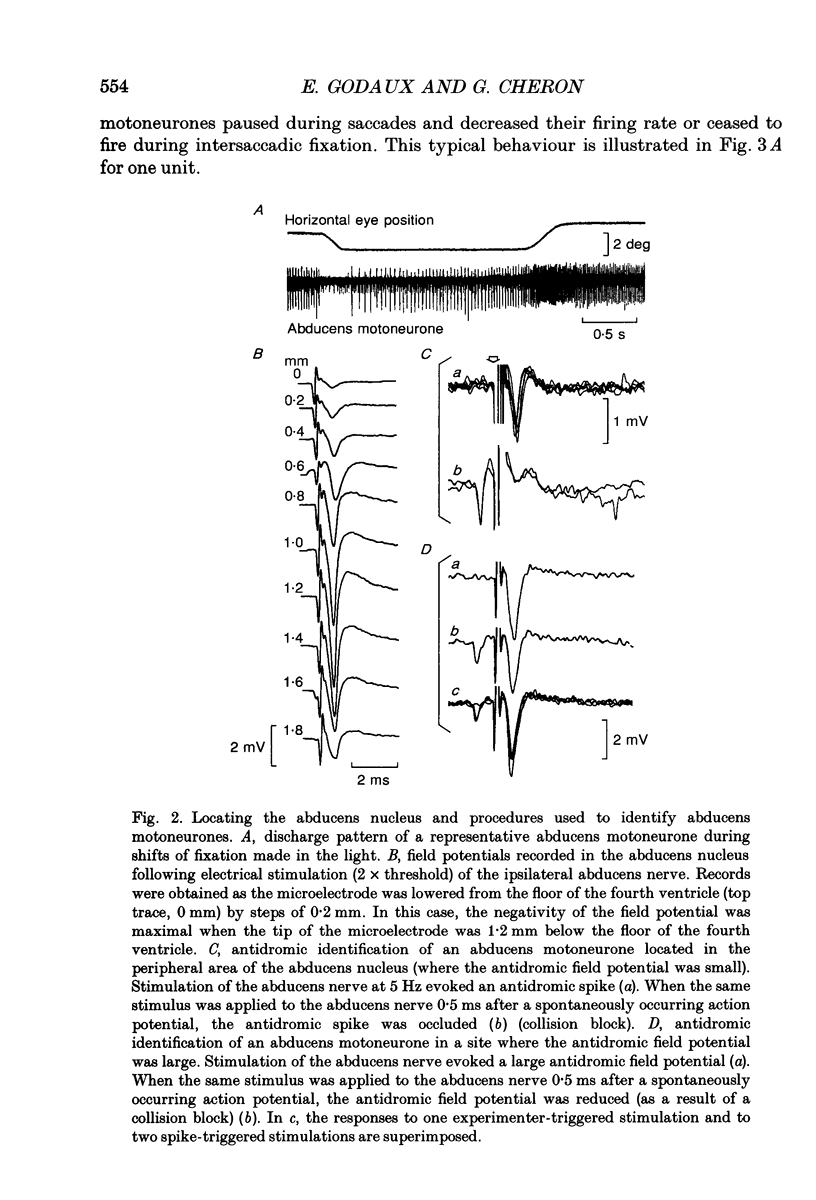
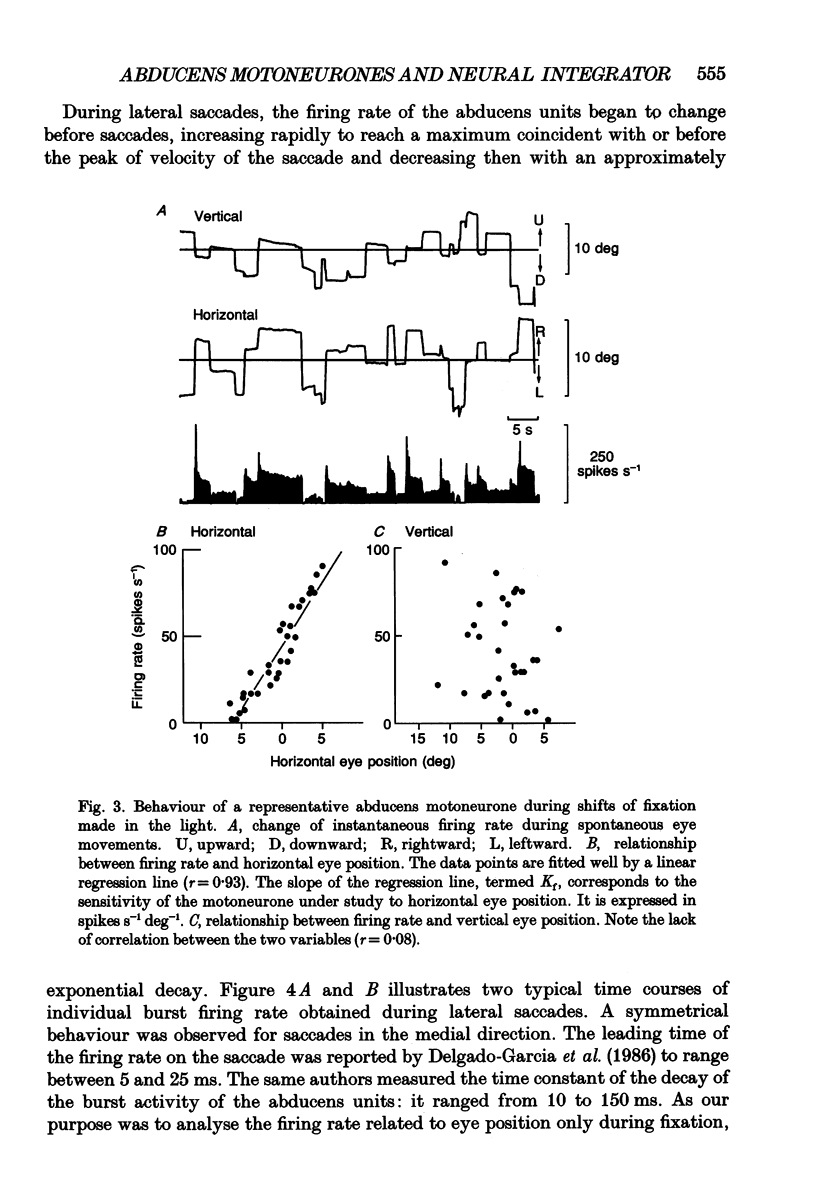
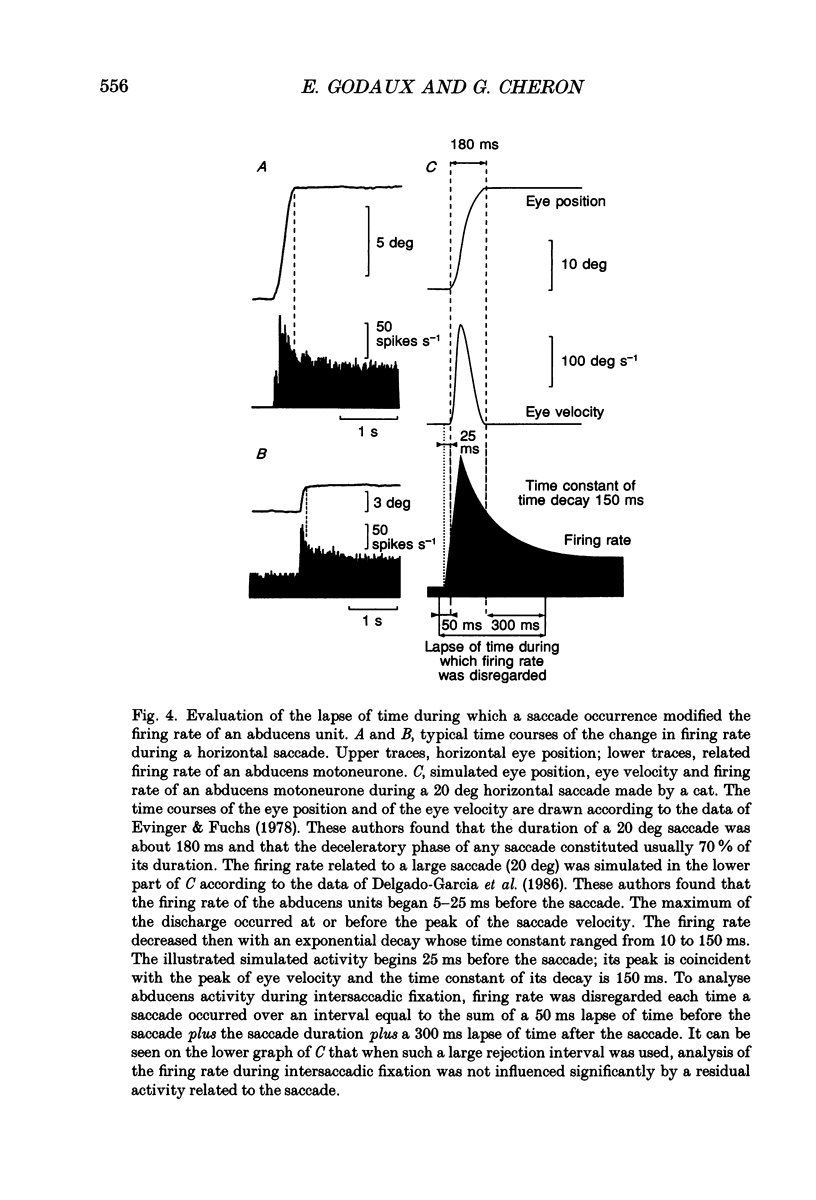
1. As far as horizontal eye movements are concerned, the well-known hypothesis of a common neural integrator states that the eye-position signal is generated by a common network, regardless of the type of versional movement. The aim of this study was to evaluate the validity of this hypothesis by analysing the behaviour of the abducens motoneurones, the system into which the horizontal neural integrator(s) project(s). If there were a common neural integrator, the different motoneurones would receive the eye position signal through the same pathway and the sensitivity to eye position would be the same regardless of the type of versional movement. If there were multiple integrators, the sensitivity to eye position in one type of versional movement might be different from the sensitivity to eye position in another type of versional movement, at least for occasional motoneurones. 2. The discharge of thirty-one antidromically identified abducens motoneurones was recorded in the alert cat during spontaneous eye movements made in the light and in response to sinusoidal rotations of the head in complete darkness. 3. All of the abducens motoneurones exhibited a burst of action potentials for lateral saccades. During fixation between saccades, they maintained a steady firing rate that increased as the cat fixated increasingly lateral eye positions. 4. For each abducens motoneurone, the sensitivity to eye position (Kf) was determined from measurements carried out during intersaccadic fixations. Kf was calculated from the slope of the firing rate-eye position linear regression line. 5. The discharge rate of the identified motoneurones was observed during four sinusoidal vestibular stimulations (+/- 10 deg, 0.10 Hz; +/- 20 deg, 0.10 Hz; +/- 30 deg, 0.10 Hz; +/- 40 deg, 0.10 Hz). The motoneurones exhibited a burst of activity during fast phases in the lateral direction and paused during fast phases in the opposite direction. During slow phases, motoneurones modulated their activity as a function of the vestibularly induced eye movements except for slow phases that occurred in position ranges below their recruitment threshold. In these cases their activity was cut off. 6. A new method was developed to measure the sensitivity to eye position of neurones during vestibular slow phases. The difficulty came from the fact that, during slow phases, eye velocity and eye position changed simultaneously and that each of those two variables could influence neuronal activity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



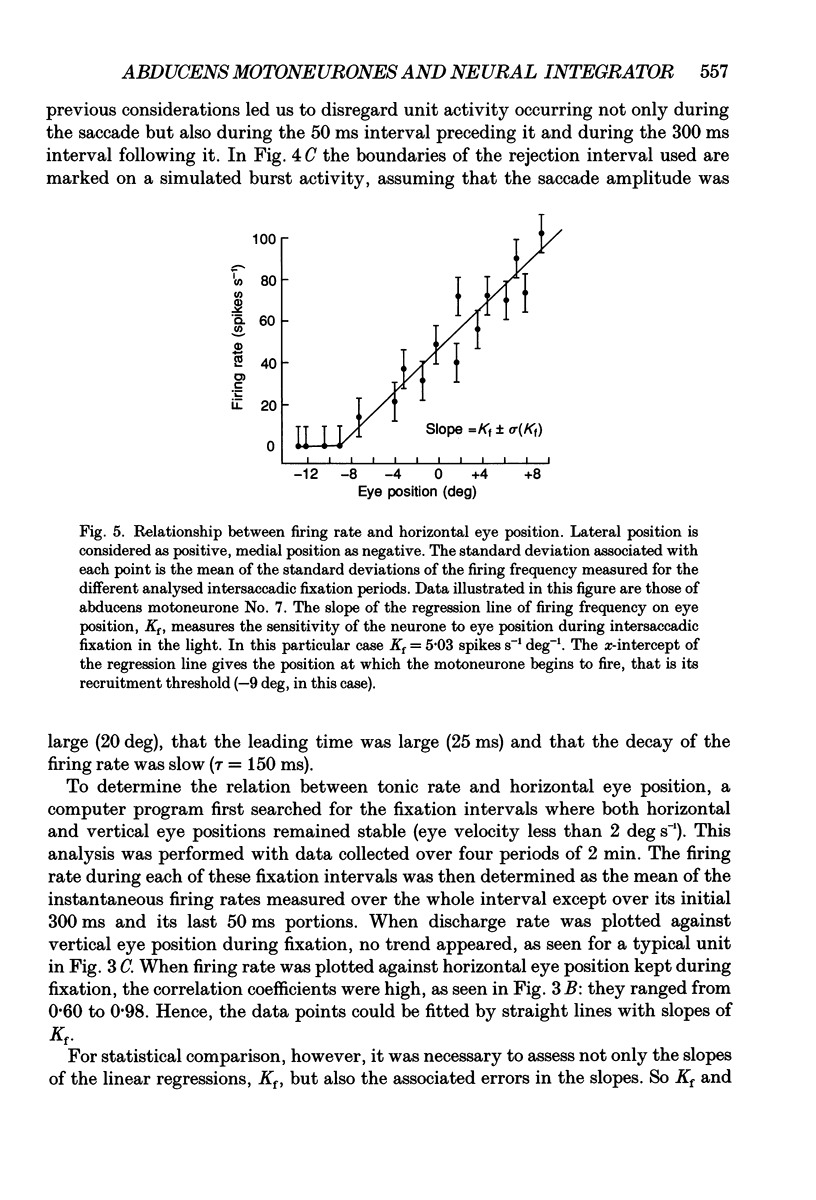
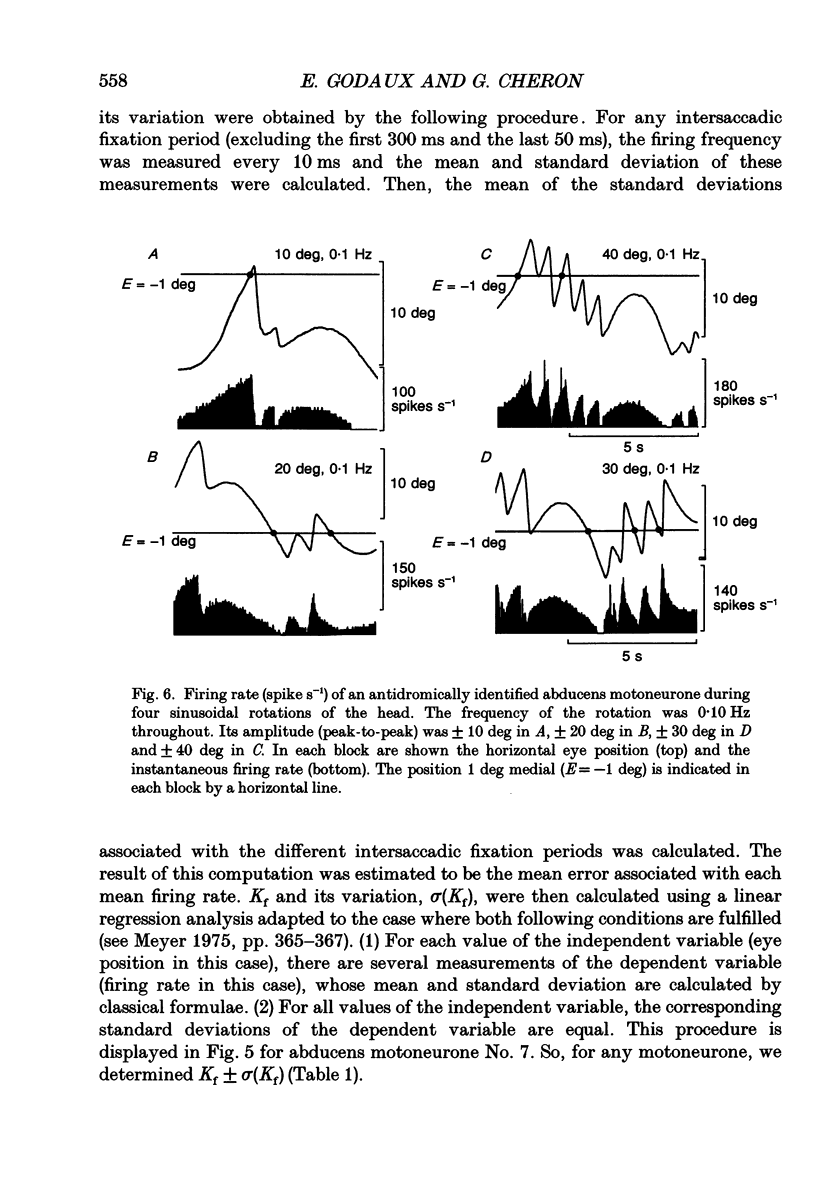
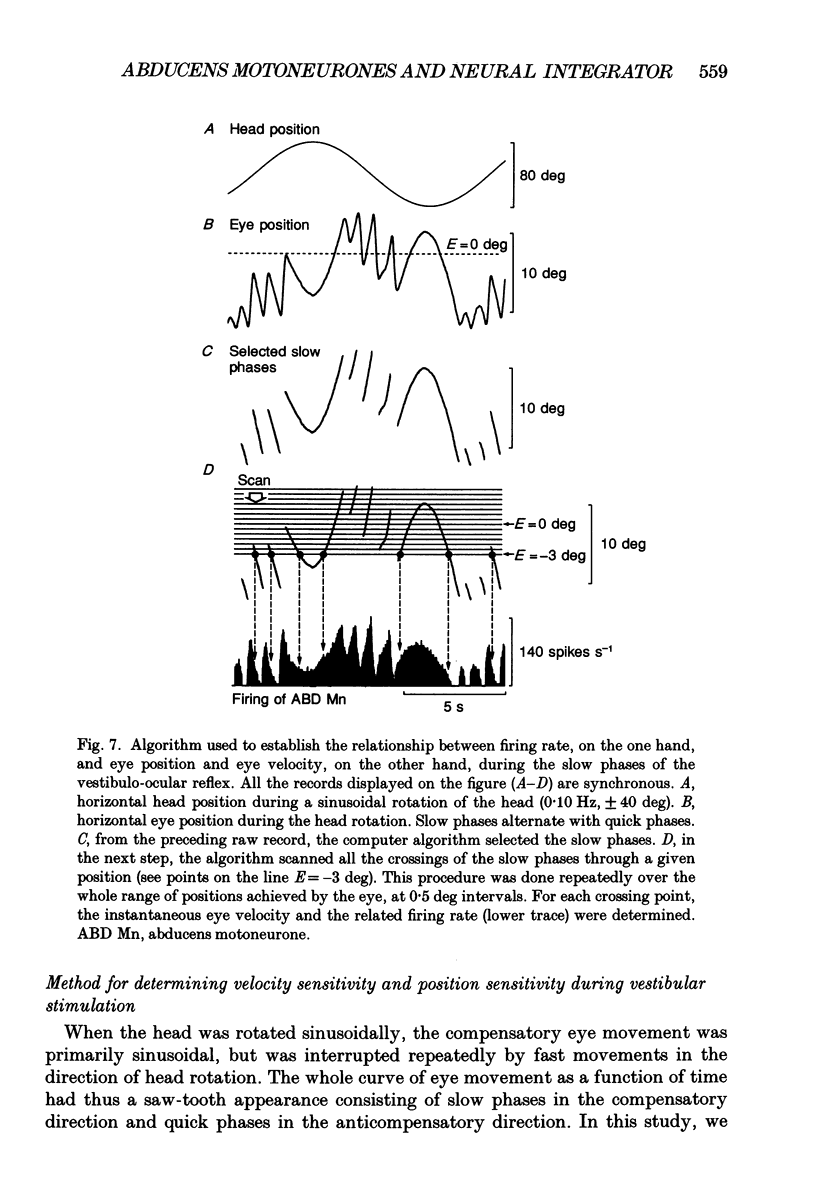
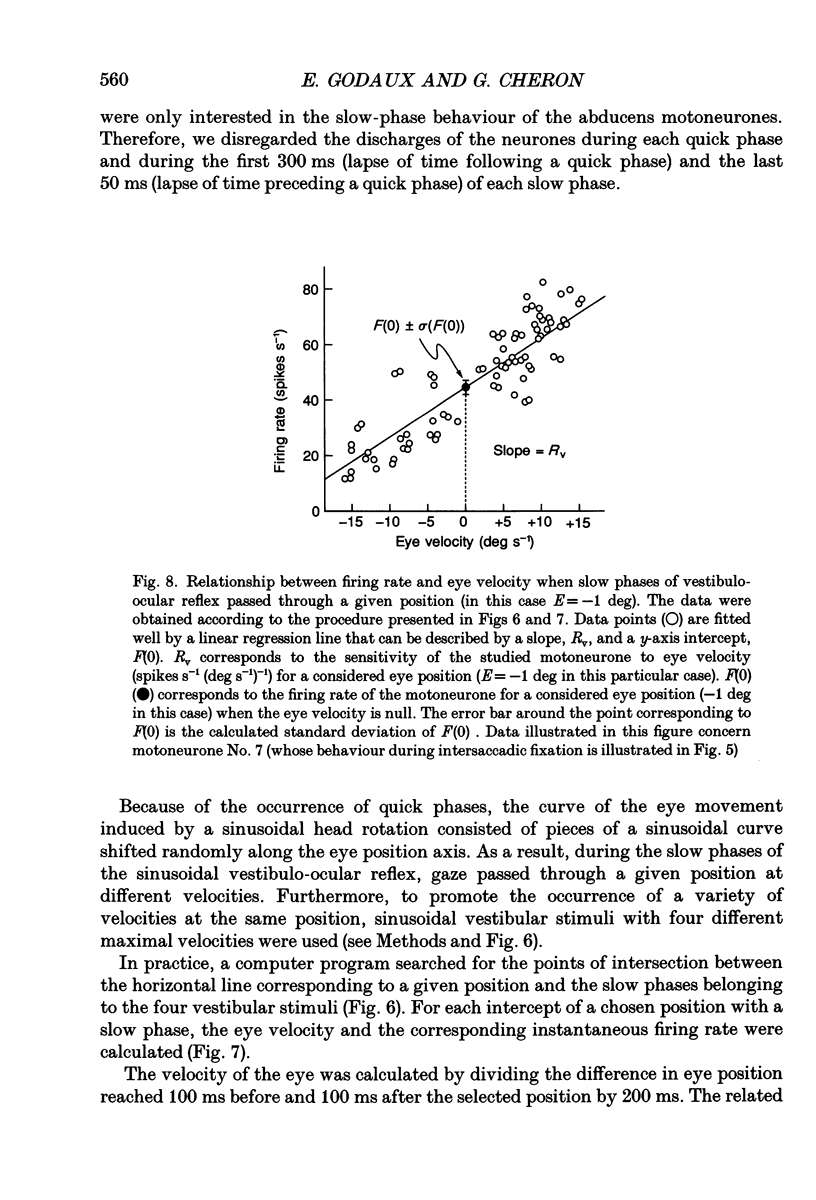
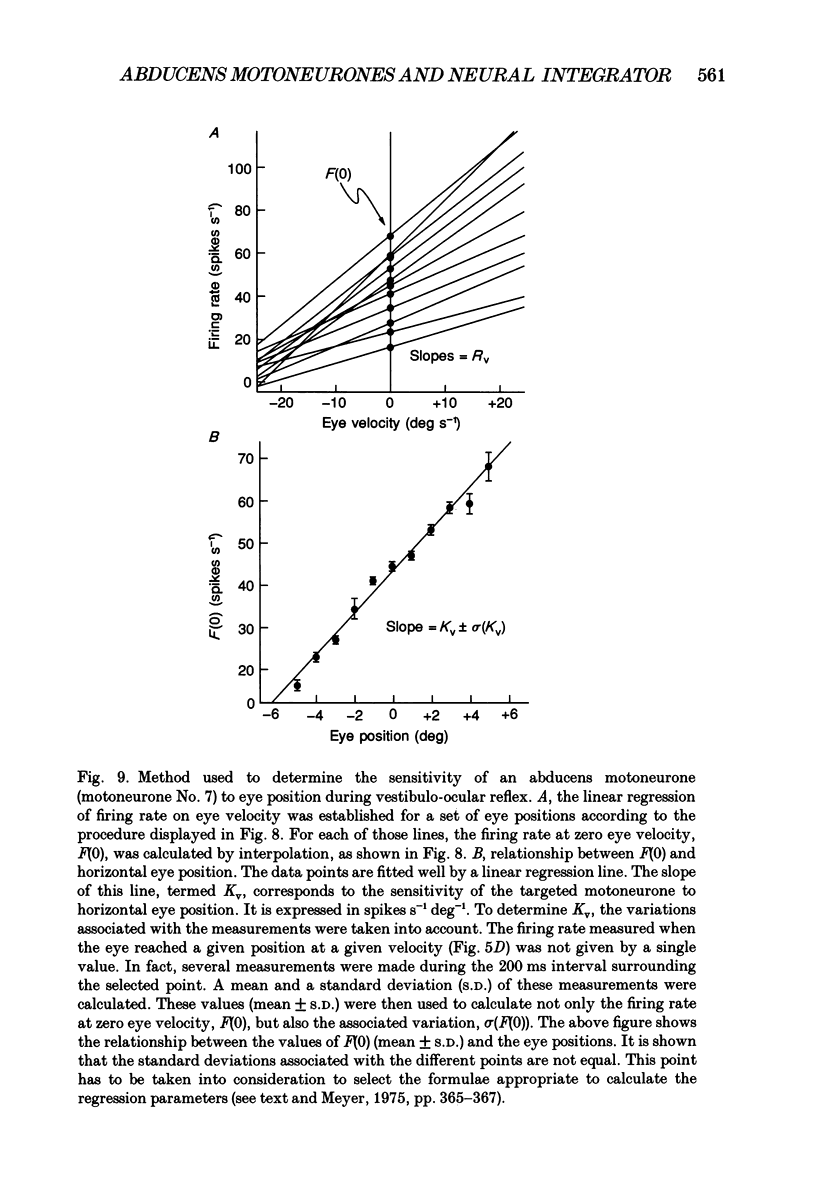
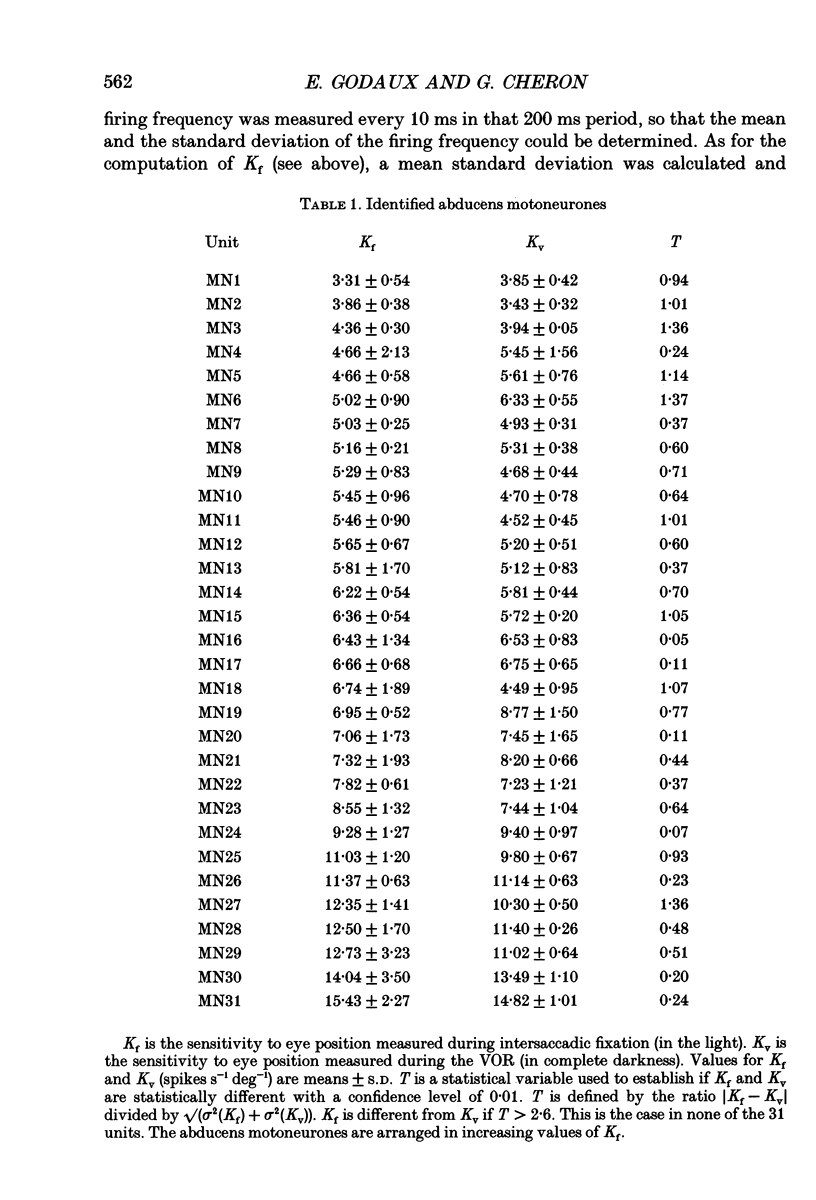
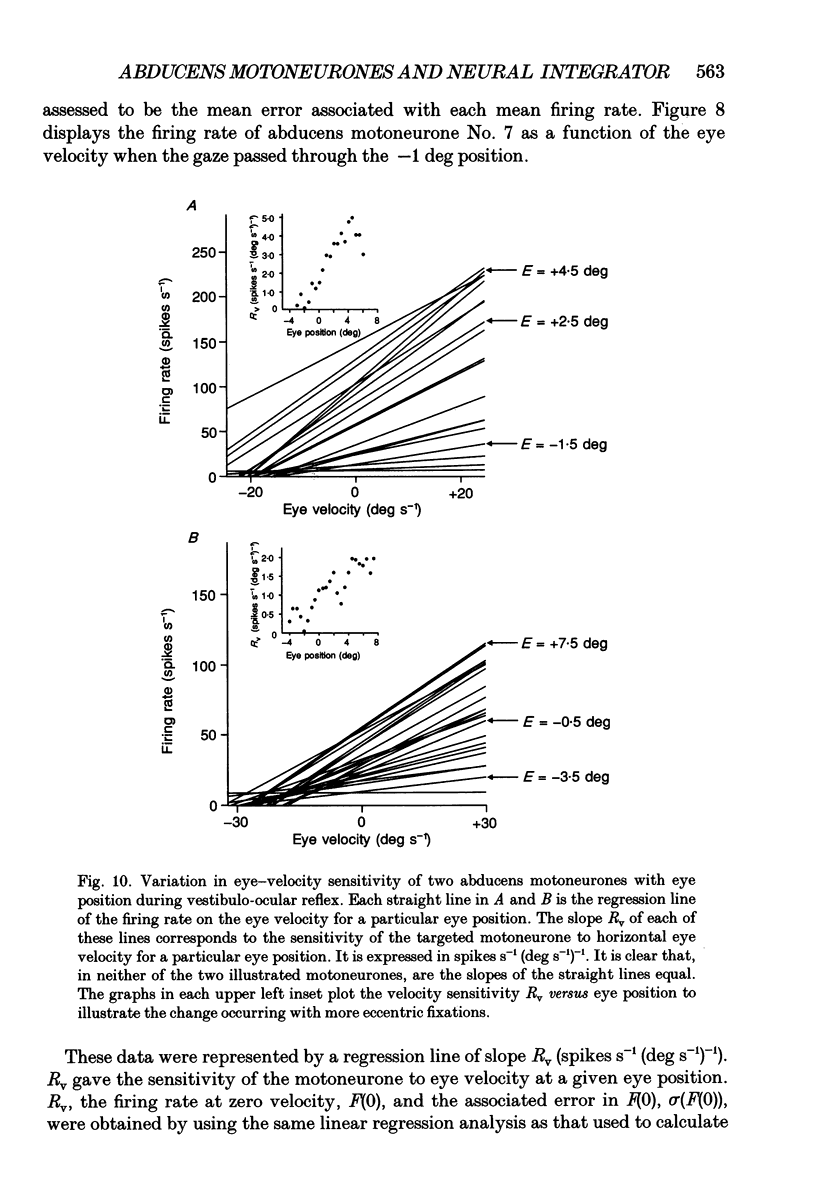
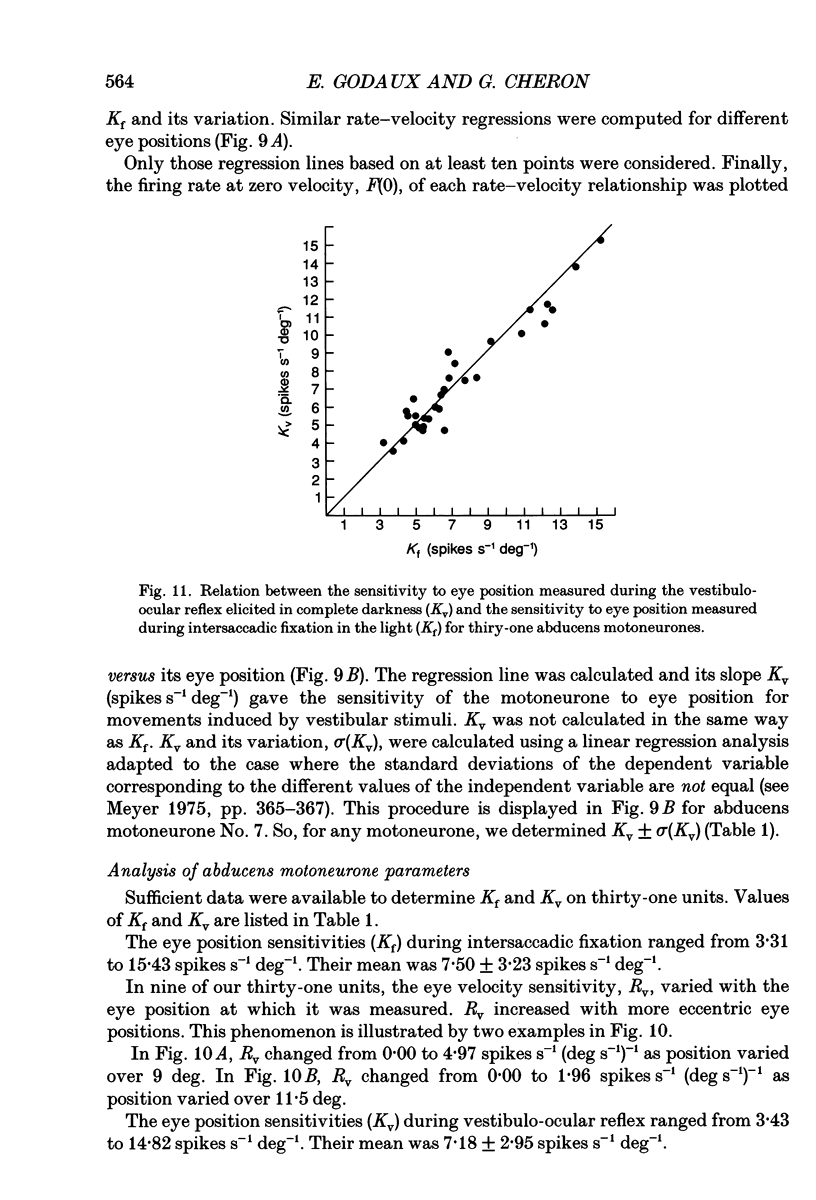






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baland J. F., Godaux E. R., Cheron G. A. Algorithms for the analysis of the nystagmic eye movements induced by sinusoidal head rotations. IEEE Trans Biomed Eng. 1987 Oct;34(10):811–816. doi: 10.1109/tbme.1987.325923. [DOI] [PubMed] [Google Scholar]
- Berthoz A., Droulez J., Vidal P. P., Yoshida K. Neural correlates of horizontal vestibulo-ocular reflex cancellation during rapid eye movements in the cat. J Physiol. 1989 Dec;419:717–751. doi: 10.1113/jphysiol.1989.sp017895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S. C., Robinson D. A. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J Neurophysiol. 1987 May;57(5):1383–1409. doi: 10.1152/jn.1987.57.5.1383. [DOI] [PubMed] [Google Scholar]
- Cheron G., Godaux E. Disabling of the oculomotor neural integrator by kainic acid injections in the prepositus-vestibular complex of the cat. J Physiol. 1987 Dec;394:267–290. doi: 10.1113/jphysiol.1987.sp016870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G., Godaux E., Laune J. M., Vanderkelen B. Lesions in the cat prepositus complex: effects on the vestibulo-ocular reflex and saccades. J Physiol. 1986 Mar;372:75–94. doi: 10.1113/jphysiol.1986.sp015998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G., Mettens P., Godaux E. Gaze holding defect induced by injections of ketamine in the cat brainstem. Neuroreport. 1992 Jan;3(1):97–100. doi: 10.1097/00001756-199201000-00026. [DOI] [PubMed] [Google Scholar]
- Cohen B., Matsuo V., Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977 Sep;270(2):321–344. doi: 10.1113/jphysiol.1977.sp011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H. Direction-selective units in the rabbit's nucleus of the optic tract. Brain Res. 1975 Dec 26;100(3):489–508. doi: 10.1016/0006-8993(75)90154-7. [DOI] [PubMed] [Google Scholar]
- Crawford J. D., Cadera W., Vilis T. Generation of torsional and vertical eye position signals by the interstitial nucleus of Cajal. Science. 1991 Jun 14;252(5012):1551–1553. doi: 10.1126/science.2047862. [DOI] [PubMed] [Google Scholar]
- Delgado-Garcia J. M., del Pozo F., Baker R. Behavior of neurons in the abducens nucleus of the alert cat--I. Motoneurons. Neuroscience. 1986 Apr;17(4):929–952. doi: 10.1016/0306-4522(86)90072-2. [DOI] [PubMed] [Google Scholar]
- Evinger C., Fuchs A. F. Saccadic, smooth pursuit, and optokinetic eye movements of the trained cat. J Physiol. 1978 Dec;285:209–229. doi: 10.1113/jphysiol.1978.sp012568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Goldberg J. M. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971 Jul;34(4):661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Luschei E. S. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol. 1970 May;33(3):382–392. doi: 10.1152/jn.1970.33.3.382. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Robinson D. A. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966 May;21(3):1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Scudder C. A., Kaneko C. R. Discharge patterns and recruitment order of identified motoneurons and internuclear neurons in the monkey abducens nucleus. J Neurophysiol. 1988 Dec;60(6):1874–1895. doi: 10.1152/jn.1988.60.6.1874. [DOI] [PubMed] [Google Scholar]
- Fukushima K., Fukushima J., Harada C., Ohashi T., Kase M. Neuronal activity related to vertical eye movement in the region of the interstitial nucleus of Cajal in alert cats. Exp Brain Res. 1990;79(1):43–64. doi: 10.1007/BF00228872. [DOI] [PubMed] [Google Scholar]
- Fukushima K. The interstitial nucleus of Cajal and its role in the control of movements of head and eyes. Prog Neurobiol. 1987;29(2):107–192. doi: 10.1016/0301-0082(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Gamlin P. D., Gnadt J. W., Mays L. E. Abducens internuclear neurons carry an inappropriate signal for ocular convergence. J Neurophysiol. 1989 Jul;62(1):70–81. doi: 10.1152/jn.1989.62.1.70. [DOI] [PubMed] [Google Scholar]
- Goldstein H. P., Robinson D. A. Hysteresis and slow drift in abducens unit activity. J Neurophysiol. 1986 May;55(5):1044–1056. doi: 10.1152/jn.1986.55.5.1044. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Schoppmann A. Retinal input to direction selective cells in the nucleus tractus opticus of the cat. Brain Res. 1975 Dec 5;99(2):359–366. doi: 10.1016/0006-8993(75)90037-2. [DOI] [PubMed] [Google Scholar]
- Judge S. J., Richmond B. J., Chu F. C. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Wurtz R. H. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988 Aug;60(2):580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Miles F. A., Optican L. M., Eighmy B. B. Optokinetic response in monkey: underlying mechanisms and their sensitivity to long-term adaptive changes in vestibuloocular reflex. J Neurophysiol. 1981 May;45(5):869–890. doi: 10.1152/jn.1981.45.5.869. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Morris E. J., Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Westbrook L. E. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985 Jun;5(6):1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays L. E., Porter J. D. Neural control of vergence eye movements: activity of abducens and oculomotor neurons. J Neurophysiol. 1984 Oct;52(4):743–761. doi: 10.1152/jn.1984.52.4.743. [DOI] [PubMed] [Google Scholar]
- Pastor A. M., Torres B., Delgado-Garcia J. M., Baker R. Discharge characteristics of medial rectus and abducens motoneurons in the goldfish. J Neurophysiol. 1991 Dec;66(6):2125–2140. doi: 10.1152/jn.1991.66.6.2125. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. Eye movement control in primates. The oculomotor system contains specialized subsystems for acquiring and tracking visual targets. Science. 1968 Sep 20;161(3847):1219–1224. doi: 10.1126/science.161.3847.1219. [DOI] [PubMed] [Google Scholar]
- Skavenski A. A., Robinson D. A. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973 Jul;36(4):724–738. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- Tomlinson R. D., Robinson D. A. Signals in vestibular nucleus mediating vertical eye movements in the monkey. J Neurophysiol. 1984 Jun;51(6):1121–1136. doi: 10.1152/jn.1984.51.6.1121. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen J. A., Robinson D. A., Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. J Neurophysiol. 1981 Mar;45(3):417–442. doi: 10.1152/jn.1981.45.3.417. [DOI] [PubMed] [Google Scholar]


