Abstract
A complex interaction has evolved between the host's peripheral nervous system (PNS) and herpes simplex virus type 1 (HSV-1). Sensory neurons are permissive for viral replication, yet the virus can also enter a latent state in these cells. The interplay of viral and neuronal signals that regulate the switch between the viral lytic and latent states is not understood. The latency-associated transcript (LAT) regulates the establishment of the latent state and is required for >65% of the latent infections established by HSV-1 (R. L. Thompson and N. M. Sawtell, J. Virol. 71:5432–5440, 1997). To further investigate how LAT functions, a 1.9-kb deletion that includes the entire LAT promoter and 827 bp of the 5′ end of the primary LAT mRNA was introduced into strain 17syn+. The wild-type parent, three independently derived deletion mutants, and two independently derived genomically rescued variants of the mutants were analyzed in a mouse ocular model. The number of latent sites established in trigeminal ganglion (TG) neurons was determined using a single-cell quantitative PCR assay for the viral genome on purified TG neurons. It was found that the LAT null mutants established ∼75% fewer latent infections than the number established by the parental strain or rescued variant. The reduced establishment phenotype of LAT null mutants was due at least in part to a dramatic increase in the loss of TG neurons in animals infected with the LAT mutants. Over half of the neurons in the TG were destroyed following infection with the LAT mutants, and this was significantly more than were lost following infection with wild type. This is the first demonstration that the HSV LAT locus prevents the destruction of sensory neurons. The death of these neurons did not appear to be the result of increased apoptosis as measured by a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay. Animals latently infected with the LAT null mutants reactivated less frequently in vivo and this was consistent with the reduction in the number of neurons in which latency was established. Thus, one function of the LAT gene is to protect sensory neurons and enhance the establishment of latency in the PNS.
A complex interaction between the virus and the host determines the fate of cells infected by herpes simplex virus type 1 (HSV-1). At the body surface, about 77 known viral lytic-phase genes are actively transcribed during productive infection, which is invariably lethal for the cell. Latent infections are characterized by the down regulation of these lytic-phase genes in innervating sensory neurons of the trigeminal ganglia (TG), and these cells survive infection. The continued expression of the latency-associated transcript (LAT) is detectable by in situ hybridization in about 100 to 200 sensory neurons per mouse TG (for review, see references 1 and 87). The discovery of the LAT RNAs over a decade ago led to speculation that that they must play a central role in viral latency (70), but the function(s) of the LAT gene is not yet understood. The LAT gene is not required for the reactivation of HSV-1 from latently infected mouse dorsal root ganglia cultured in vitro (32, 62). However, other reports have suggested that LAT mutants were reactivated from fewer neurons (35) or with reduced kinetics (5, 6, 69) from cultured TG. This apparent discrepancy is due at least in part to a difference in the behavior of LAT mutants in dorsal root ganglia versus TG (59).
When assayed in vivo, mutations within the LAT locus result in a reduced frequency of induced (29) or spontaneous (48) virus shedding in the tear film of infected rabbits (for review, see reference 87). LAT null mutants reactivate at a reduced frequency in mouse TG in vivo (59, 79). These findings have been interpreted as strong support for a direct role of the LAT locus in virus reactivation from latency (reviewed in reference 87). However, to date no empiric evidence of a direct role of LAT in reactivation has been reported. The frequency of reactivation measured as an endpoint can be influenced by a variety of factors that affect the establishment of latency. Factors that contribute to the number of latent infections established can be relatively obvious, such as the general ability of a virus to replicate (25, 33, 63) or the inoculation titer employed (56). Even when equivalent numbers of latent infections are established, more subtle virally regulated parameters, such as the number of viral genomes harbored within a latently infected neuron, also apparently influence reactivation frequency (58). Thus, it may be that the LAT gene exerts an influence long before the host encounters a reactivation stimulus.
Results obtained with noncongenic LAT promoter–β-galactosidase reporter viruses first suggested that the LATs might be required for the efficient establishment of latency (59). These early studies were later confirmed with several different paired congenic LAT null mutants and genomically restored isolates (79). These latter studies obviated the need for detection of a reporter molecule as a marker for latency (i.e., β-galactosidase or LAT RNA) through the use of a single-neuron PCR assay for the presence of the viral genome. This assay, termed contextual analysis of DNA (CXA-D), offered compelling evidence that the LAT gene functions to increase the number of latent infections established (79). A promoter-reporter strategy was recently employed in the rabbit model to quantify latency. Unfortunately, the reactivation phenotypes of the mutants could not be determined since none of the viruses reactivated. However, a reduced number of promoter-positive neurons was observed with the LAT null mutant (51), consistent with the earlier studies in the mouse model (59, 79).
The LAT-dependent establishment function we identified in the mouse was mapped to a 2.3-kb fragment that encodes the stable LAT locus introns known as the LATs (79). How this genomic region enhances the establishment of latency is not yet known. It has been reported that apoptosis is widespread in ganglia infected with LAT null mutants and that at least some of the cells undergoing apoptosis are neurons (49). While no quantitative analysis of the loss of neurons was performed, the authors speculated that the LAT gene might prevent apoptosis in neurons. However, the antibody employed to immunohistochemically label apoptotic rabbit neurons does not work on rabbit cells (81), and the validity of the assays employed to detect apoptosis and the results obtained in that study have therefore been questioned (81). The LAT locus may serve to down regulate viral protein expression in neurons. We previously demonstrated that the disruption of the LAT locus resulted in more viral protein expression in neurons that express the LAT promoter during the acute stage of infection (59). Further, stressing mice infected with LAT null mutants during the acute stage of infection suppressed that LAT-reactivation phenotype and also reduced the expression of viral protein in LAT promoter-positive neurons to wild-type levels (79). With the caveat that it must be assumed that an equivalent number of neurons were infected by mutant and wild-type isolates, a subsequent study suggested that LAT might function at the level of mRNA expression, serving to down regulate viral mRNA production in neurons (23). In support of this hypothesis, the overexpression of the LAT locus in transformed tissue culture cells resulted in an inhibition of virus production and immediate-early gene expression at the level of mRNA production (41). In addition, there may be more than one function located within the LAT locus. Thomas et al. have suggested that the LAT locus encodes a protein that, when dysregulated, actually enhances the ability of the virus to replicate in certain cell lines of neuronal and nonneuronal origin (75). Conversely. Lock and colleagues reported evidence that the open reading frame identified by Thomas et al. may not be expressed in the context of the virus (38). Obviously, the role(s) of the LAT gene in latency and its possible mechanisms of action are not yet understood. However, taken together, these diverse findings, on the main, offer support for the hypothesis that the LAT gene is required for the efficient establishment of latency.
Our initial investigations of the LAT-dependent establishment function were performed with HSV-1 strain KOS/M (59, 79), which reactivates less well in vivo than some other strains (25, 59, 60, 71). We therefore sought to confirm and extend our previous results with HSV-1 strain 17syn+, which reactivates with high efficiency in the murine hyperthermic stress (HS) model (56, 58–60). Three independently generated LAT null mutants in strain 17syn+ and corresponding genomically restored isolates were produced and analyzed. Importantly, over 50% of the TG neurons were destroyed following infection with the mutants and this was significantly more than that lost following infection with wild-type or genomically restored isolates. These findings offer compelling support for the hypothesis that the LAT gene is important to inhibit viral replication in neurons and that this down-regulatory function of LAT serves to prevent the death of sensory neurons and increase the number of latent sites established (59).
MATERIALS AND METHODS
Cells and viruses.
The wild-type HSV-1 strain 17syn+ was originally obtained from J. Subak-Sharpe of the Medical Research Council Virology Unit in Glasgow, Scotland, and plaque purified as described elsewhere (82, 84). Virus stocks were generated by routine propagation in rabbit skin cell (RSC) monolayers as previously described (82, 84).
Construction of viral mutants.
All viral DNA sequences were derived from the isolate of strain 17syn+ described above. The construction of the 1,964-bp deletion that encompasses the LAT promoter and 828 bp of the 5′ end of the primary LAT is shown schematically in Fig. 1. In brief, a deletion spanning bp 117674 to 119638 was recombined into the HSV-1 genome as previously described in detail (77–79, 84). The progeny of single plaques were screened for the presence of the deletion in both copies of the long terminal repeat sequences and subsequently plaque purified to homogeneity to generate the isolate named 17-AH. The mutated fragments corresponding to the BamHI fragments B and E of strain 17syn+ were cloned from the LAT null mutant 17-AH, and the region surrounding the deletion was sequenced to confirm the genomic structure. Both strands of both copies of the deleted genomic region were sequenced (Fig. 1 and data not shown). The fragment corresponding to the HpaI fragment R (bp 117009 to 120300 with bases 117674 to 119638 deleted) was subcloned from the BamHI fragment B of 17-AH and employed to generate two additional mutant isolates, designated 17-FA91 and 17-FA94. To ensure that any changes in phenotypic properties were due to the deletion, the mutated genomes were restored to wild type by recombination with the 17syn+ HpaI fragment R (bp 117009 to 120300) to yield 17-AHRI (produced from 17-AH) and 17-FA94R (from 17-FA94) as described above. Both copies of the deleted sequences were restored (Fig. 1 and data not shown). All restriction enzyme sites and base-pair numbering are referred to as the corresponding positions in the published HSV-1 sequence of strain 17syn+ (45, 53) as presented in GenBank (g1944536).
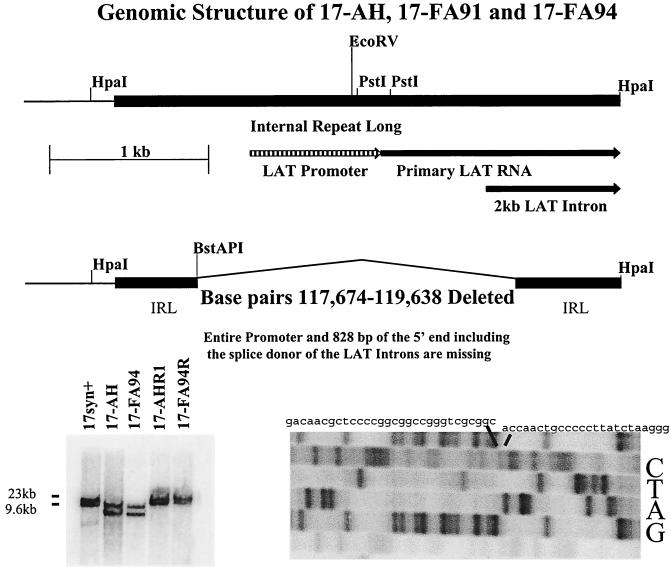
FIG. 1.
(Top) Schematic representation of the 17syn+ HpaI fragment R (bp 117009 to 120300). The internal long repeat (IRL) is indicated by a heavy black bar and the LAT promoter, start site of the primary LAT RNA, and 5′ end of the 2-kb LAT intron are shown. (Middle) Schematic representation of the same region from the LAT null mutants 17-AH, 17-FA91, and 17-FA94. The deletion removes the entire LAT promoter, start site of the primary LAT RNA, as well as the splice donor for the stable LAT introns. (Bottom, left) Viral DNA was cleaved with BamHI, electrophoresed on 0.8% agarose gels, Southern blotted, and probed with a 32P-labeled probe (bp 120300 to 120468). The presence of the deletion in both the BamHI fragment B (bp 113322 to 123461) and BamHI fragment E (bp 2905 to 11818) confirms that both copies of the LAT locus were mutated in isolates 17-AH and 17-FA94. Similarly, both copies of the LAT locus were genomically restored to wild type in isolates 17-AHRI and 17-AHR2, as evidenced by the wild-type migration of both hybridizing fragments. (Bottom, right) Fragments equivalent to the HpaI fragment R (from BamHI fragment B) and S (from BamHI fragment E) of 17syn+ were cloned from the genome of 17-AH and sequenced. Both strands of both fragments were sequenced. Shown is the region of the gel that confirms the presence of the deletion.
Inoculation of mice.
Male, outbred, Swiss Webster mice (Charles River Breeding Laboratories, Kingston, N.Y., or Harlan Laboratories) were used throughout these studies. Animals were housed in American Association for Laboratory Animal Care-approved quarters with unlimited access to food and water. Mice were anesthetized by intraperitoneal injection of sodium pentobarbital (Nembutal) at a dosage of 50 mg/kg of body weight. Both corneas were scarified and a total inoculum of 2 × 105 PFU of 17syn+ or the mutant isolates was applied to the cornea as described previously (59, 60, 79). Mice that displayed signs of severe central nervous system infection were euthanatized. Under these conditions, 10 to 20% of the animals were lost, and this did not vary between groups.
In vitro and in vivo acute replication kinetics.
Single- and multistep replication kinetic analyses were performed on slightly subconfluent RSC monolayers following infection at a high multiplicity of infection (MOI) (10 PFU/cell) or low MOI (0.01 PFU/cell). Cells and media were harvested at 4, 8, 12, 18, and 24 h postinfection (p.i.) (high MOI) or 4, 24, 48, and 72 h p.i. (low MOI) and subjected to three cycles of freeze and thaw. Virus titers were determined on RSC monolayers as described previously (82, 83).
Groups of 45 mice were inoculated with each of the five viruses analyzed (225 mice total) as described above. At the indicated times p.i., the animals (four per time point) were sacrificed and the appropriate tissues were removed, snap-frozen, and stored at −80°C. These tissues were homogenized in 1 ml of minimal essential medium (MEM), clarified at 5,000 × g for 5 min, and assayed for infectious virus titer on RSC monolayers (82, 83). The remaining mice were maintained for at least 30 days p.i. and employed in latency studies as described below.
Reactivation of latent HSV-1 by explant cultivation in vitro and by HS in vivo.
Mice inoculated as above were maintained for at least 30 days p.i. and TG were explanted into MEM supplemented with 10% fetal bovine serum. The explanted cultures were maintained for 5 days and the tissues with medium were harvested, homogenized, and assayed for infectious virus on RSC monolayers.
To determine the reactivation potential of the mutants in vivo, infected mice were subjected to the transient hyperthermia induction procedure as described elsewhere (60). Briefly, mice were inoculated as detailed above and maintained for at least 30 days. Animals were then subjected to 10 min of hyperthermia at 43°C and at 22 h posttreatment TG pairs were removed from sacrificed animals, homogenized, and plated on RSC monolayers to detect infectious virus as described previously (60). DNA was purified from positive cultures, and the genomic structure of the reactivating virus was confirmed by Southern blot restriction fragment length polymorphism analysis as above. In all cases, the reactivated virus had a genomic structure indistinguishable from that of the infecting strain or mutant (data not shown).
Analysis of mRNA expression.
RSC monolayers were infected with an MOI of 10 PFU per cell. At 4 or 10 h p.i. RNA was isolated using Ultraspec RNA (Biotecx Laboratories) according to the manufacturer's directions. Ten micrograms of RNA was glyoxylated, electrophoresed, transferred to nylon membranes (GeneScreen), and probed as described below (79).
Mice were inoculated as above and at various times p.i. the TG from three animals were removed and snap-frozen in liquid nitrogen. The tissues were homogenized in Ultraspec RNA and total RNA was obtained as recommended by the manufacturer (Biotecx Laboratories). Ten micrograms of RNA was glyoxylated, electrophoresed, transferred to nylon membranes (GeneScreen), and probed as described previously (79). The blots were incubated for 2 h at 50°C in prehybridization mix and a 32P-labeled probe specific either for the second exon of ICP0 (bp 122709 to 123030) or the stable LAT introns (bp 120468 to 120904) was then added and incubated at 50°C for an additional 24 h as previously described (79). The blots were washed in 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 1% sodium dodecyl sulfate at 65°C and exposed to a storage phosphor screen (Molecular Dynamics). Plates were analyzed using the STORM 860 phosphorimaging system and ImageQuant software.
Quantification of HSV DNA in latently infected ganglia by PCR.
A quantitative PCR (QPCR) for the viral genome was carried out on DNA isolated from latently infected ganglia essentially as originally described by Katz et al. (33) with minor modifications as described previously (55, 59, 79, 80). It was found that the maximum reproducibility required care in the dissections in order to obtain equivalent amounts of tissue from each animal as well as precise quantification and control of the amount of total DNA in each sample. Mice were infected as above and after at least 30 days p.i. the ganglia were harvested, lysed in 10 mM Tris–10 mM EDTA–0.2% sodium dodecyl sulfate–RNase A (10 μg/ml), and digested at 50°C with proteinase K (Bethesda Research Laboratories; 10 μg/ml). Following purification with phenol-chloroform-isoamyl alcohol and ethanol precipitation, the DNA was quantified with a GeneQuant spectrophotometer, and 100 ng of total DNA was amplified using primers specific for the single-copy mouse adipsin gene as described previously (33). One-tenth of the product was electrophoresed on a 10% acrylamide gel, transferred to GeneScreen plus membrane, and probed with a 32P-end-labeled oligo internal to the amplification primers. The resulting blots were scanned in a Molecular Dynamics PhosphorImager for quantification using ImageQuant software and were compared to standards derived from amplification of twofold dilutions of normal mouse DNA spanning 12.5 to 400 ng and that were performed with each PCR run. Where necessary, the original samples were readjusted to contain 100 ng of DNA/5 μl, as determined by the adipsin signals obtained, and amplified using primers specific for the thymidine kinase and/or adipsin to obtain the final values.
Contextual analysis of latency.
The single-neuron QPCR determination of the percentage of latently infected neurons (CXA-D) was performed as previously detailed (55, 56, 58, 79, 80). Mice were infected as above and maintained for at least 30 days p.i. Animals were anesthetized with sodium pentobarbital and perfused with Streck's tissue fixative. Fixed TG were dissociated into single-cell suspensions with collagenase, and neurons were purified on Percoll (Pharmacia) gradients. Neurons were stained with Ponceau-S and aliquoted into 200-μl PCR tubes. The tube contents were visualized microscopically and only tubes containing a single neuron were employed. Following treatment with immobilized DNase (Mobitec), intracellular DNA was liberated with proteinase K and analyzed for the presence of the HSV-1 genome by CXA-D (55) using a QPCR assay (33). Products were electrophoresed, probed with an internal 32P-labeled oligonucleotide, and quantified on a STORM 860 Phosphorimager (Molecular Dynamics) using ImageQuant software.
Contextual analysis of neuronal survival.
CXA provided a new method to quantify neuronal death following infection. The procedure was adapted to determine the number of neurons present in TG as described previously (56). Additional mice infected with 17-AH or 17-AHRI and maintained for at least 30 days p.i. served as a source of tissues. Individual dissociated right and left ganglia from each of four animals per group were resuspended in a 500-μl volume and two sets of triplicate 1-μl aliquots of each was affixed to glass slides. The number of neurons in each sample was counted and the total number in the ganglia was calculated. Neurons were identified on the basis of size, morphology, and in some instances positive immunohistochemical reaction with antineurofilament antibodies (56).
TUNEL and immunohistochemical staining.
RSCs were grown on glass slides to confluence, infected at an MOI of 0.0001 with 17-AH or 17-AHRI, and overlaid with culture medium supplemented with 0.3% human immunoglobulin G (Gammastan). The resulting plaques were allowed to grow for 3.5 days p.i., and the slides were processed as described below. Mice were infected as described above. On days 4 and 7 p.i. with 17syn+, 17-AHRI, or 17-AH, four mice per group were perfusion fixed with 4% paraformaldehyde and TG were removed, postfixed, dehydrated, and embedded in paraffin. Eight-micrometer sections were cut on a rotary microtome and placed on glass slides. The DeadEnd colorimetric apoptosis detection system (Promega) was utilized according to the manufacturer's instructions. Positive terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was indicated by a brown precipitate. In a second experiment, mice were inoculated as above with 2 × 105 PFU of 17syn+, 17-AHRI, 17-AH, 17-FA94, or 17-FA94R, groups of four mice were processed as above at days 6, 10, or 15 p.i., and 8-μm sections were analyzed for TUNEL-positive cells. To facilitate interpretation of the results, the following additional slides were analyzed: (i) RSCs acutely infected with HSV, (ii) RSCs pretreated with DNase, or (iii) TG from uninfected mice induced to undergo apoptosis by explantation into culture medium containing 0.15 μM staurosporine (Sigma). In order to link any apoptotic neurons that were observed with HSV infection, slides from the day 4 and 7 samples were subsequently stained immunohistochemically for HSV lytic proteins using rabbit anti-HSV-1 (Accurate). Selected slides from these and other time points were stained immunohistochemically to identify neurons or T cells with antibodies specific for neurofilament (Sigma) or CD3-ɛ (M-20; Santa Cruz Biotechnology). The primary antibody was followed by an appropriate biotinylated secondary antibody and either an avidin-alkaline phosphatase conjugate or an avidin-horseradish peroxidase conjugate (Vector) as previously detailed (59, 60). Fast Red (Sigma) and naphthol-AS-MX-phosphate (Sigma) were used to visualize sites of complex deposition (a red precipitate) with alkaline phosphatase, whereas diaminobenzidine was employed with the peroxidase conjugate (brown precipitate).
RESULTS
Virus construction and replication properties.
A schematic representation of the mutants employed in this study is shown in Fig. 1. HSV-1 strain 17syn+ is wild type, and 17-AH, 17-FA91, and 17-FA94 are independently derived isolates that carry the 1.964-kb deletion in both copies of the LAT gene. This deletion eliminates the entire LAT promoter, including the upstream enhancer regions (39) and the long-term expression sequences (15), which also demonstrate some promoter activity (9). In addition, 828 bp of the 5′ end of the primary LAT, including the splice donor for the stable LAT introns (21), was removed. This deletion does not disrupt the ICP0 mRNA. As described above in Materials and Methods, the mutated portion of 17-AH was cloned from the virus and sequenced to confirm the nature of the deletion (Fig. 1 and data not shown). Two independently derived rescued isolates of the mutants were generated and designated 17-AHRI (derived from 17-AH) and 17-FA94R (derived from 17-FA94). The genomic structures of these isolates were confirmed by restriction fragment length polymorphism analysis employing at least five different restriction endonucleases and a variety of probes from the LAT region as well as probes from other genomic regions (Fig. 1 and data not shown). As seen in the middle panel of Fig. 1, the faster migration of both the BamHI B and E fragments of 17-AH and 17-FA94 demonstrated that both copies of the LAT gene were disrupted. Likewise, both copies of the LAT gene have been genomically restored to a wild-type migration pattern in the isolates 17-AHRI and 17-FA94R.
Viral gene transcription in the region of the deletion.
We next analyzed the transcription of the viral genome near the region that was deleted in the LAT null mutants. The 1.9-kb deletion in the LAT null mutants should not adversely affect the expression of any known HSV-1 genes other than LAT. Several transcripts thought to be the result of readthrough of genes located in the unique long portion of the genome have been localized to the region deleted (64). An additional 0.7-kb transcript that is contained entirely within the region deleted was recently reported (88). The significance of this transcript is unclear since it was found in only two of four laboratory strains examined and was not produced by any of eight clinical isolates tested. The only known gene that maps near the deleted region is ICP0 (Fig. 1). The deletion is over 1 kb 3′ of the polyadenylation signal for ICP0 and is therefore not likely to affect its expression. To ensure that this was the case, total RNA was extracted from infected RSCs at 4 or 10 h p.i. and employed in Northern blot analysis as described above. Representative blots are shown in Fig. 2. ICP0 mRNA was readily detected in RSC monolayers at 4 h p.i. regardless of infecting virus strain. Shown are the results obtained with 17syn+, 17-AH, and 17-AHRI (Fig. 2, bottom left). Similar results were obtained with 17-FA91, 17-FA94, and 17-FA94R (data not shown). Thus, as predicted by the genomic structure, the engineered deletion did not grossly affect the expression of ICP0 in the mutants.
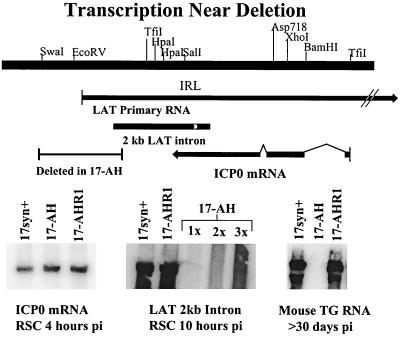
FIG. 2.
(Top) Schematic representation of the region near the deletion present in the LAT null mutants. (Middle) Known transcripts within this region are indicated, including the primary LAT RNA, the 2-kb LAT intron, and the spliced ICP0 mRNA. (Bottom) Representative Northern blots. Left, total RNA was isolated from infected RSCs, electrophoresed, blotted, and probed with 32P-labeled sequences specific for the second exon of ICP0 (bp 122709 to 123030) as described in Materials and Methods. Middle, a similar blot probed with sequences specific for the stable LAT introns (bp 120300 to 120468). No specific signal was detected in the 17-AH lanes, even when up to three times the amount of RNA was loaded and the blot was overexposed. Right, similar blotting performed on RNA extracted from latently infected mouse TG.
The engineered deletion was predicted to completely eliminate the 2.0- and 1.5-kb stable LAT RNAs. As shown in Fig. 2, expression of the 2.0-kb form of the stable LAT introns was only detected in RSCs infected with either 17syn+ or 17-AHRI. The 2-kb transcript was not detected in 17-AH-infected cells even when a threefold greater amount of RNA was loaded and the blots were deliberately overexposed (Fig. 2, bottom center). Similar experiments showed that neither 17-FA91 nor 17-FA94 expressed detectable levels of the 2-kb intron (data not shown). Likewise, the LAT null mutants did not express detectable levels of either the 2.0- or 1.5-kb LAT introns in mouse TG during the latent phase of infection (Fig. 2, bottom right). Both the 1.5- and 2.0-kb forms of the LAT RNA were readily detected in mouse TG latently infected with either 17syn+ or 17-AHRI. Similarly, mutants 17-FA91 and 17-FA94 did not produce any detectable LAT transcripts, whereas the rescued isolate 17-FA94R produced both the 2.0- and 1.5-kb RNAs in latently infected mouse TG (data not shown).
Viral replication in vitro and in vivo.
Replication-deficient or -incompetent viral mutants can establish at least some latent infections (10, 11, 16, 18–20, 22, 31, 33, 36, 47, 52, 68). However, viral replication at the body surface and, importantly, replication efficiency within the sensory ganglia are critical parameters affecting the number of neurons in which latent infections are established and the number of viral genome copies contained within them (80). Therefore, replication kinetic experiments were performed to ensure that all viruses employed in this study replicated equivalently to the parental strain, 17syn+.
Initial experiments were performed in RSCs under both high and low MOI (5 and 0.01 PFU/cell, respectively). These experiments were repeated at least three times for each virus isolate. The results of some of the experiments are shown graphically in Fig. 3 (top). There was no detectable difference in the ability of the deletion mutants and genomically wild-type isolates to replicate in RSCs under conditions of either low or high MOI. Thus, no generalized replication defect was detected in any of the virus isolates under study.
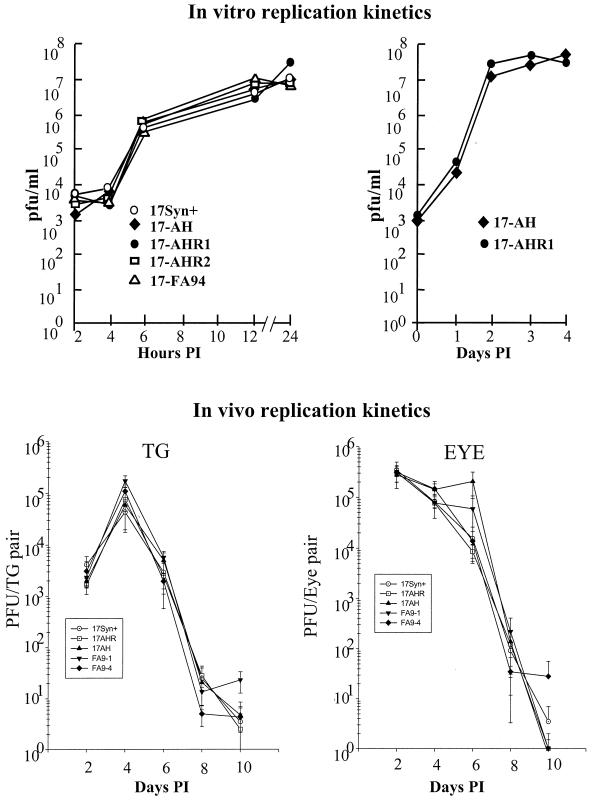
FIG. 3.
(Top) Replication kinetics curves generated in vitro in RSC monolayers. Left, monolayers were infected at an MOI of 5 PFU/cell. At the indicated times postinfection, triplicate cultures were harvested, pooled, and assayed for virus content. Right, similar monolayers were infected at a low MOI of 0.01 PFU/cell. At the indicated times postinfection, triplicate cultures were harvested and assayed for virus content. (Bottom) Replication kinetics curves generated in vivo. Groups of 20 mice (100 mice total) were inoculated on both scarified corneas with 2 × 105 PFU of each of the viruses tested as described in the text. At the indicated days postinfection, tissues were harvested from four animals from each group, homogenized in 1 ml of MEM, and assayed for virus content. Each point represents the geometric mean ± the standard error.
To examine viral replicative capacity in vivo, groups of 45 mice were infected via both scarified corneas with each of the viruses (225 mice total). At the indicated times p.i., four animals per group were individually examined for virus titer in eyes and TG as described in Materials and Methods. As shown in Fig. 3 (bottom), no differences in the ability of the mutants or wild-type strains were detected in vivo in the eye or TG. Statistical comparisons of mean peak titers and areas under the curves demonstrated that there were no significant differences in replication efficiencies between any of the mutant or genomically wild-type isolates. This experiment was repeated at least twice for each mutant or genomically rescued isolate, with similar results. There were no significant differences between the groups or between results obtained in the replicate experiments, compared to the data shown in Fig. 3, demonstrating that the inoculation procedures were consistent within and between experiments (data not shown). Therefore, any latency phenotypes exhibited by the engineered strains could not be attributed to differences in general replication or to their ability to replicate at the body surface, invade the nervous system, and replicate within the TG during the acute stage of infection.
In vitro reactivation frequency.
One commonly employed assay to quantify reactivation is explant cultivation of latently infected ganglia. It has been suggested that the frequency at which virus is isolated from explant cultures, or the amount of time it takes for the cultures to become positive (5, 35, 43, 60, 67, 85), reflects the ability of virus isolates to reactivate. We employed this assay to investigate the capacity of isolates to reactivate by this measure and to perform a direct comparison to the results obtained by reactivation in vivo, described in the next section. Additional animals from the replication kinetic experiments described above were maintained for at least 30 days p.i. The ganglia from individual mice (five animals per virus tested) were removed and placed into culture as previously described (58, 80). Five days postexplant, the entire contents of each organ culture were homogenized and assayed for infectious virus on RSC monolayers as previously described. Regardless of the infecting strain, all cultures tested were positive at 5 days postexplant. Thus, by this criterion the LAT null mutants did not display a reduced reactivation phenotype.
In vivo reactivation phenotype.
The HS induction model was employed to examine the ability of the mutants to reactivate in vivo. Additional animals were subjected to the HS reactivation protocol. This procedure, which consists of raising the core body temperature of the mouse to 43°C over a period of 10 min, results in the induced in vivo reactivation of HSV-1 in a significant percentage of mice within 14 to 24 h post-HS (60). As expected, about 75 to 80% of the animals infected with either the wild-type parent 17syn+ or the genomically restored isolate 17-AHRI reactivated (8 of 10 and 9 of 12 mice positive per number tested, respectively). The ability of 17-AH to reactivate following HS in vivo was reduced to 40% (12 of 30 mice positive per number tested). Likewise, the frequencies of reactivation of two additional isolates with identical deletions at the LAT locus were also reduced compared to that of the genomically restored isolate (17-FA91, 8 of 21, 38%; and 17-FA94, 6 of 17, 35%), whereas 17-FA94R was similar to wild type (11 of 17, 65%). Viruses in which the LAT gene was intact reactivated in a significantly higher percentage of the animals than did those containing the deletion (P = 0.014; Student's t-test). Clearly, when assayed in vivo the LAT null mutants had a reduced reactivation phenotype.
Analysis of the amount of HSV DNA present in latently infected TG.
The reduced reactivation phenotype observed with the mutants was not the result of reduced efficiency of replication at the body surface or a reduced ability to enter into or replicate in the peripheral nervous system. Therefore, either the ability to reactivate had been impaired directly, or the mutants were less efficient at establishing latency. The QPCR method of Katz et al. (33) was employed to determine the number of latent viral genomes present in whole ganglia as a measure of the ability of the viruses to establish latency (Fig. 4). TG were removed from additional mice from the groups infected for the reactivation studies above. Total DNA was isolated from each pair of TG and subjected to QPCR for the viral thymidine kinase gene as well as for the mouse adipsin gene as previously described (33).
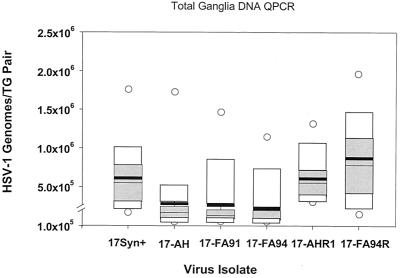
FIG. 4.
Box diagram of the total number of latent HSV-1 genomes present within each pair of mouse TG. Additional mice from the experiment shown in Fig. 3 were maintained for at least 30 days p.i. Total DNA was isolated from the TG and processed for QPCR detection of the viral genome as described previously (34). Each box diagram represents the compiled results from 14 animals (28 TG). The mean value obtained is shown as a heavy horizontal black bar and the 25th and 75th percentile values are indicated as gray boxes. White boxes indicate the 95th percentile values, and open circles indicate the maximum and minimum values.
The amount of viral DNA present in each pair of latently infected TG was variable, which is consistent with previous reports employing similar methods (8, 33, 34, 58, 59, 79). A comparison of the mean genome copy number per animal and a one-way analysis of variance revealed a statistically significant difference between the genomically wild-type strains 17syn+, 17-AHRI, and 17-FA94R (mean, 6.1 × 105, 6.2 × 105, and 1.1 × 106, respectively) and the deletion mutant isolates 17-AH, 17-FA91, and 17-FA94 (mean, 2.8 × 105, 2.6 × 105, and 2.2 × 105, respectively; analysis of variance, P = 0.027). The total amount of HSV-1 DNA present in the ganglia of the mutants was reduced to about one-third of that found in the genomically wild-type strains. Some previous studies have failed to show a statistically significant difference between LAT null and wild-type isolates in terms of the amount of DNA present in mouse TG measured by similar methods (6, 8, 14, 28, 48). It should be noted that in some prior studies a two- or threefold difference between groups was seen (as is reported here) but was not considered significant (14). It is possible that technical differences, such as variability in the inoculation procedures, may have broadened the range of values obtained, masking the effect of LAT. In this report, group sizes of 14 (28 ganglia) were required to show a statistically significant difference. With this group size and under the infection conditions described in Materials and Methods, all pairwise comparisons between genomically wild-type and mutant isolates demonstrated statistically significant differences at the 95% confidence interval with the exception of 17-FA91 versus 17-AHRI, which was significant at the 94.5% confidence interval (P = 0.055).
Percentage of neurons in which latency is established correlates with ability to reactivate.
The 17syn+ LAT mutants were deficient for the establishment of latency as measured by the amount of viral DNA present in latently infected ganglia. The reduced amount of latent DNA present in mouse TG latently infected with the mutants might have been due to the establishment of fewer latent infections or the establishment of latent infections that contained fewer HSV genomes per latently infected neuron, or both. CXA was employed to quantify the percentage of neurons that harbored the viral genome in latently infected trigeminal ganglionic neurons to distinguish between these two possibilities. Groups of mice were inoculated on scarified corneas with a total of 2 × 105 PFU of either 17-AH, 17-AHRI, or 17syn+. At >30 days p.i., three mice from each group were perfusion fixed, the ganglia were dissociated, and enriched neuronal cell populations were harvested and utilized to determine the percentage of neurons which were latently infected, as previously described in detail (55–58, 79, 80). Of the 138 single neuron samples analyzed from the 17-AH-infected mice, 17 (12%) contained HSV DNA. In the 17-AHRI-infected group, 31% of the neurons were positive (33 out of 106 single neurons tested) and 28% (21 of 75) were positive with 17syn+, representing a ∼2.5-fold difference (P = 0.0028). These results recapitulate those observed with KOS-based LAT mutants as we previously reported (79), and they demonstrate that the establishment function provided by LAT is not unique to strain KOS. As detailed above, the ability of 17-AH to reactivate following HS in vivo was reduced to a frequency of 40% of the latently infected animals, compared to an ∼75 to 80% reactivation frequency in mice infected with the wild-type strains. A central question is whether the reduction in the number of latent sites established by this mutant can fully account for the reduced reactivation frequency seen in vivo. In a previous study, mice were infected with strain 17syn+ with the titer that resulted in latent infections established in 11.5% of the TG neurons. In these animals, 30% (6 of 20) of mice reactivated following HS (56). Thus, the reactivation frequency seen in the mice latently infected with 17-AH was consistent with the reduced number of latent infections detected in these animals.
HSV-1 LAT gene functions to promote neuronal survival.
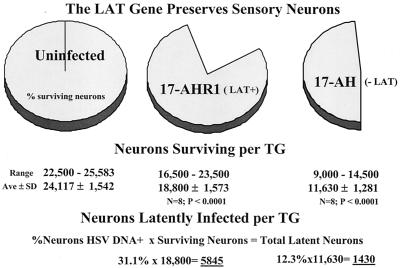
The mechanism whereby the LAT gene mediates an increase in the number of latent sites established is not yet known. Our working hypothesis is that this gene functions to down regulate viral gene expression in neurons (59, 79, 80). If this were the case, one would predict that more neurons would survive infection with wild-type strains than would survive following infection with the LAT null mutants. CXA provided a new method to determine if this were the case. The CXA procedure was modified, as described above and elsewhere (56), to enable an accurate determination of the number of neurons present in individual TG to be determined. The results are summarized in Fig. 5. As shown, in uninfected mice the number of neurons in a TG ranged from 22,500 to 25,583 with a mean (± standard deviation) of 24,117 ± 1,542 neurons (56). This method was used to determine the number of neurons in the ganglia following acute infection with 17-AH compared to 17-AHRI. Individual dissociated right and left ganglia from each of four animals per group were resuspended in a 500-μl volume and two sets of triplicate 1-μl aliquots of each were affixed to glass slides. The number of neurons in each sample was counted and the total number in the ganglia was calculated. At 30 days p.i., far fewer neurons were present in the TG of mice infected with 17-AH. Indeed, more than half of the TG neurons were lost following infection with 17-AH (Fig. 5). In contrast, the genomically rescued isolate 17-AHRI resulted in the death of far fewer neurons per TG. This difference in the loss of neurons was statistically significant (P < 0.001; t test).
FIG. 5.
(Top) Pie charts demonstrating the percentage of sensory neurons remaining in the TG at 30 days p.i. (Middle) The range, mean (Ave), and standard deviation (SD) of the number of neurons per ganglia for 17-AHRI compared to uninfected and 17-AH compared to 17-AHRI (t test P values are shown). (Bottom) Total number of latently infected neurons per ganglion was calculated by multiplying the percentage of neurons positive for the viral genome by CXA-D (see text) by the number of neurons present in the ganglia at 30 days p.i.
The increase in neuronal loss that occurred in the TG infected with the LAT null mutant also impacts the interpretation of the CXA-D analysis for latently infected neurons. The single-neuron PCR assay employed determined the percentage of neurons that harbor one or more latent viral genomes. Because far fewer neurons survived in mice infected with the LAT null mutant, there were actually considerably fewer latently infected neurons present in these animals than indicated by the percent that were positive for the viral genome. Normalization of the values with the number of surviving neurons revealed a >400% increase in the number of latent sites present in mice infected with the LAT+ viruses (Fig. 5). This increase in the number of neurons harboring the latent genome in the TG of mice infected with wild-type virus correlated with the increased amount of viral DNA detected in these ganglia by QPCR (Fig. 4).
Increase in neuronal dropout mediated by LAT null mutants does not appear to be due to an increase in TUNEL-positive neurons.
It has been reported that expression of the bovine herpesvirus 1 LAT (61) or portions of the HSV-1 LAT gene (30, 49) inhibit chemically induced apoptosis in certain cell lines. One report concluded that infection of rabbits with a LAT null mutant resulted in widespread apoptosis in TG, and at least some of this was thought to be in neurons (49). An increase in the apoptosis of neurons in animals infected with the LAT null mutants employed in this study would explain the observed increased loss of neurons described above. Therefore, the potential role of the LAT gene in ameliorating apoptosis was examined. If, as suggested by Perng et al., the LAT gene prevents widespread apoptosis during the peak of viral replication in the TG (49), we would expect to see this effect in mouse TG on or about day 4 p.i. In this case, one would predict 20 to 50% of the neurons examined would show signs of apoptosis at some time during days 4 to 7 p.i. In this case, power statistics predict that examining 50 neurons per group would reveal a statistically significant difference in the groups at the 95% confidence level. Alternatively, LAT might exert some long-term effect on neuronal survival (49). Since about 50% of the neurons in TG infected with LAT null mutants were lost during the first 30 days p.i. (versus ∼20% for wild type), one would anticipate about 1.6% of these neurons would be lost per day if the effect of LAT was mediated equally throughout the 30-day period. This simplistic model predicts that 1 out of ∼66 neurons examined should be undergoing apoptosis on any given day. In this case, examining ∼1,200 neurons from each group of infected TG would reveal a statistically significant increase in apoptosis of neurons infected with LAT null mutants.
Mice were infected on scarified corneas with 2 × 105 PFU of either 17syn+, 17-AH, or 17-AHRI. On days 4, 6, 7, 10, and 15 p.i., the TG from four mice per group were sectioned and examined for the presence of TUNEL-positive neurons. To facilitate the interpretation of the results, the following additional slides were analyzed: (i) RSCs acutely infected with HSV; (ii) RSCs pretreated with DNase; and (iii) mouse TG incubated in the presence of staurosporine to induce apoptosis. In order to link any apoptotic neurons that may be observed with HSV infection, slides from the day 4 and 7 p.i. samples were subsequently stained for HSV lytic proteins. The findings are summarized in Table 1.
TABLE 1.
Frequency of TUNEL-positive neurons in TG
| Day p.i. | No. of neurons TUNEL positive/no. examined for strain
|
||||
|---|---|---|---|---|---|
| 17-AH | 17-AHRI | 17-FA94 | 17-FA94R | 17syn+ | |
| 4 | 0/1,875 | 1/1,900 | NDa | ND | 0/1,900 |
| 6 | 0/1,900 | 0/1,920 | 0/1,800 | 2/1,940 | 2/1,970 |
| 7 | 0/1,900 | 0/1,900 | ND | ND | 0/1,900 |
| 10 | 1/1,600 | 2/1,920 | 2/1,900 | 2/2,000 | 2/1,780 |
| 15 | 1/1,620 | 2/1,800 | 1/1,900 | 1/1,800 | 1/1,940 |
ND, not determined.
At least 1,600 neurons were examined for signs of possible apoptosis (positive TUNEL) from each time point for each virus. The criteria employed for distinguishing neurons from other cell types in the ganglia are discussed and illustrated further below. Of the 33,265 neurons examined in all of the samples, only 20 were positive by the TUNEL assay. This is consistent with a recent report that found that cells undergoing apoptosis in ganglia infected with wild-type strain 17syn+ were restricted primarily to nonneuronal cells (86). This low frequency of potential apoptosis of neurons (0.06%) was not significantly different among any of the groups. Further, this frequency of TUNEL-positive neurons was much too low to account for the loss of neurons that was observed in either the wild-type- or the LAT null mutant-infected TG.
Viral replication per se does not result in a positive TUNEL signal.
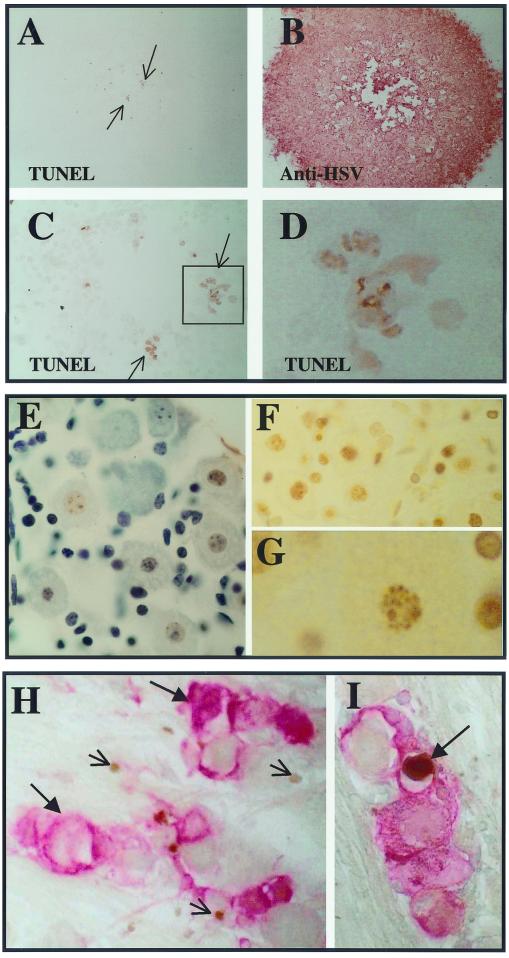
RSCs grown on slides were infected at a low MOI with 17syn+ or 17-AH and plaques were allowed to develop for 3.5 days in the presence of anti-HSV antibody to prevent spread through the medium. The slides were processed for the TUNEL assay and subsequently immunohistochemically stained for HSV proteins. The vast majority (>99%) of acutely infected RSCs were negative for TUNEL, demonstrating that the mere presence of replicating viral DNA did not register as a positive result under the conditions employed (Fig. 6A and B). A plaque of 17syn+ on RSCs is shown at the same magnification, stained for TUNEL (Fig. 6A), and subsequently stained immunohistochemically for HSV proteins (Fig. 6B). As expected, the DNase-treated RSC positive control showed strong nuclear staining in virtually all of the cells (results not shown). A few TUNEL-positive RSCs can be seen at the center of the plaque in panel A, and this region is enlarged in panel C. Two TUNEL-positive cells that demonstrate nuclear blebbing typical of cells undergoing apoptosis are evident in panel D. Most of the cells positive by TUNEL were localized to the center of the 3.5-day-old plaque. Since viral DNA replication occurs between 6 and 14 h p.i., replication of viral DNA per se was not detected as positive TUNEL.
FIG. 6.
(A to D) Results of TUNEL assay on infected RSCs. Panels A and B are at the same magnification. (A) Positive TUNEL staining is indicated by a brown precipitate (arrows) (B) The same plaque was subsequently stained immunohistochemically for HSV proteins (red precipitate). (C) The TUNEL-positive region is enlarged and positive cells are boxed. The boxed region is further enlarged in panel D. (E to G) Micrographs of an uninfected TG that was cultured in the presence of staurosporine to induce apoptosis. The DeadEnd (Promega) colorimetric apoptosis detection system was utilized according to the manufacturer's instructions. (E) A section stained for TUNEL (brown) and subsequently counterstained (blue). Many TUNEL-positive cells, including neurons, are evident. (F) A similar section to that in panel E, prior to counterstaining. Many TUNEL-positive nuclei (brown) are evident. (G) A greater magnification of one of the TUNEL-positive neuronal nuclei of panel F is shown. (H and I) Mice were infected with 17-AH (panel H) or 17-AHRI (panel I) as described in Materials and Methods. Ganglia were processed for TUNEL staining 4 days p.i. The sections were subsequently processed for the immunohistochemical detection of viral proteins as described in Materials and Methods. HSV proteins are stained red (large arrows) and TUNEL staining is brown (small arrows). A positive putative neuron is indicated by an arrow.
Apoptosis induced by staurosporine in cultured TG is readily detected by TUNEL.
As an additional positive control, mouse TG were removed and incubated with staurosporine to induce apoptosis. This treatment resulted in widespread apoptosis in many cell types in the TG, including most neurons. In Fig. 6E, a section with many TUNEL-positive cells was lightly counterstained to show the cell bodies and nuclei. The counterstain masked some of the positive TUNEL signal. A similar section prior to counterstaining is shown in panel F and many neuronal nuclei positive for TUNEL staining are evident. A photomicrograph at high magnification revealed nuclear changes characteristic of apoptosis in a TUNEL-positive neuron (Fig. 6G). Clearly, positive TUNEL signal was readily detectable in neurons in which apoptosis was induced with staurosporine.
Viral protein expression in neurons does not correlate with positive TUNEL signal.
At the peak of acute infection in TG, immunohistochemical analysis revealed that many neurons were positive for HSV proteins. However, these HSV protein-positive neurons were not TUNEL positive, regardless of whether infected with LAT+ or LAT null mutants (Fig. 6H to I). Evidence for possible apoptosis (a positive TUNEL signal) was apparent in a population of small round cells, which were apparently infiltrating inflammatory cells. The frequency and distribution of these cells did not appear different between the groups. Of the thousands of neurons examined in the day 4 p.i. samples, only one was positive by TUNEL and this was found in the 17-AHRI (LAT+)-infected group (Fig. 6I).
TUNEL-positive neurons were exceedingly rare regardless of the infecting viral genotype.
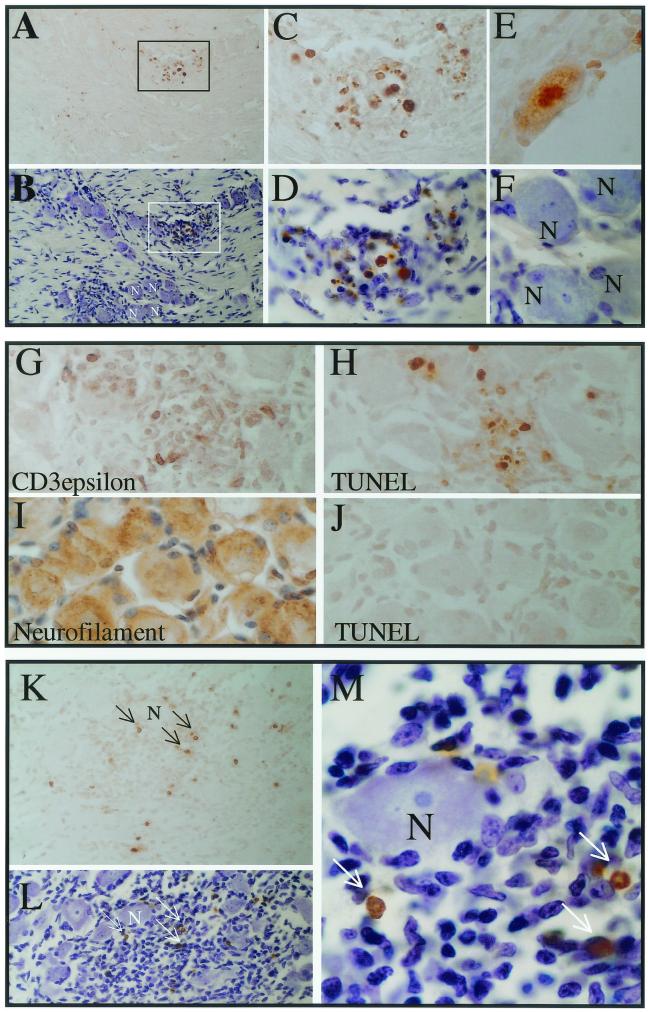
By day 6 p.i., immunohistochemical staining demonstrated that very few cells within the ganglia were positive for HSV proteins. As shown in Fig. 7, small, round TUNEL-positive cells were still readily detected along with very rare TUNEL-positive neurons. Figure 7A through F shows photomicrographs of TG sections from 17-AH-infected mice on day 6 p.i. Panel A shows a low-magnification field of a section of a TG infected with 17-AH on day 6 p.i. Many TUNEL-positive cells can be seen. This same section was subsequently counterstained to reveal the ganglion cellular architecture. Numerous neurons were present on the section (Fig. 7B). The positive TUNEL signal was in small round cells that appeared to be infiltrating immune cells (Fig. 7C and D). The only identified TUNEL-positive neuron from the day 10 p.i. 17-AH ganglia is shown in Fig. 7E. For comparative purposes, the neurons labeled “N” in panel B are shown in panel F at the same magnification. This set of data is representative of the thousands of neuronal profiles examined for each virus and time point tested, and it demonstrates that it is possible to distinguish TUNEL-positive neurons from other TUNEL-positive cell types based on their size and morphology.
FIG. 7.
Detection of TUNEL-positive cells in infected TG. Mice were infected and processed for TUNEL staining as described in Materials and Methods. (A to F) Sections from 17-AH-infected ganglia at day 6 p.i. Panels A and B are at equal magnification; a region of TUNEL staining (boxed) is shown before (A) and after (B) counterstaining. Many neurons can be seen in panel B, some of which are labeled “N.” Panels C, D, E, and F are all at the same magnification. The boxed region is shown in panels C and D. Panel F shows the labeled neurons from panel B. Of the 1,875 neuronal profiles examined on day 6 p.i., none were TUNEL positive (Table 1). For comparative purposes, panel E shows a positive neuron from a ganglion infected with 17-AH at 10 days p.i. (G to J) Photomicrographs of sections from ganglia infected with 17-AH on day 6 p.i.; all are shown at the same magnification. Panel G was stained with antibody to CD3-ɛ, which labels T cells (positive cells are brown). A similar region of cellular infiltrate was stained for TUNEL in panel H (positive cells are brown). Panel I is a section stained with an antineurofilament antibody (positive cells are brown), and panel J is a similar region stained for TUNEL (no positive cells were seen). (K to M) Ganglia infected 10 days prior with 17syn+. Panels K and L are the same region at the same magnification following staining for TUNEL (brown, arrows) and subsequent counterstaining (blue). Many TUNEL-positive cells are evident (arrows) and many neurons can be seen in panel L (one is labeled “N”). This same region is shown at a greater magnification in panel N, with the same TUNEL-positive cells (brown) indicated by arrows.
To confirm the identity of the neurons and to analyze the nature of the presumed immune cell infiltrates, additional sections were stained immunohistochemically for neurofilament (specific for neurons) and CD3-ɛ (a general marker for T cells). Shown in Fig. 7G through J are additional sections from the day 6 p.i. 17-AH-infected TG samples. These panels are all shown at the same magnification. As seen in panel G, many cells within the regions of presumed immune cell infiltrate were positive for CD3-ɛ, demonstrating the presence of T cells. In a similar region in panel H, many TUNEL-positive cells are evident. This does not mean that all TUNEL-positive cells were T cells, but it does demonstrate that the TUNEL-positive cells reside within regions of infiltrating immune cells. Panels H, I, and J illustrate the difference in size and shape between TG neurons (panel I) and the TUNEL-positive cells (panel H). Again TUNEL-positive neurons were very rare, and none were present in this field.
Small round TUNEL-positive cells were still readily detectable in ganglia at days 10 and 15 p.i. There were no differences apparent between ganglia infected with the LAT null mutants or those infected with genomically restored isolates. As was true for earlier time points, very rare TUNEL-positive neurons were detectable in these ganglia (Table 1). An example of TUNEL-positive cells in a ganglion infected with 17syn+ at 10 days p.i. is shown in Fig. 7K through M. Numerous small round TUNEL-positive cells were seen (Fig. 7K). This same section was subsequently counterstained, which reveals many neurons on the section (panel L) as well as an infiltrate of small cells (small darkly staining nuclei). The TUNEL-positive cells were seen localized within the cellular infiltrate, while the neuron present in this field was devoid of TUNEL signal.
DISCUSSION
Acceptance that LAT functions to increase the establishment of latency has been less than universal. Indeed, it is most commonly stated that LAT plays no role in establishment but is important for efficient reactivation, as is reviewed in reference 87. This misperception results primarily from the fact that most studies examining LAT null mutants have either failed to assess the establishment of latency or used insufficiently quantitative measures to do so. In addition, the results obtained with viral mutants have been inconsistent. Much of the controversy about the role of LAT in the pathobiology of HSV and discrepancies about where the function maps on the viral genome may actually be the result of unmeasured differences in the replication efficiency of the viral mutants employed in published studies. Efficient viral replication at the body surface as well as within TG is required for the efficient establishment of latent infections (80). As an example, it has been reported that a 348-bp region that maps downstream of the start site of the primary LAT that is deleted in mutant 17Δ348 is required for efficient epinephrine-induced ocular virus shedding in the rabbit model (6). However, it was recently reported that mutant 17Δ348 has a significant replication defect that is evident even in cultured cells (D. Garber and D. M. Knipe, 24th International Herpes Virus Workshop, abstr. 1.012, 1999). A consensus has formed that mutation of the LAT locus does not adversely affect viral replication (87), and therefore the replication defect must be due to an unsuspected secondary mutation. Such a secondary mutation would have a serious negative impact on the induced reactivation frequency seen in rabbits.
A similar 371-bp deletion (which includes 230 of those base pairs deleted in 17Δ348) in the strain McKrae background (mutant DLAT371) did not effect spontaneous or induced ocular shedding (50). Conversely, a mutant containing an identical 371-bp deletion in 17syn+ (17ΔSty) was deficient in both of these properties in the rabbit (40). The authors concluded that differences in the genetic background must account for the differences displayed by the 371-bp deletions in strains McKrae and 17syn+ in the rabbit (40). While this may be true, replication kinetics in eyes and TG were not performed, and therefore the results cannot be unambiguously interpreted. This same genomic region is not required for either the efficient establishment of latency (79) or efficient reactivation (42, 79) in the mouse. Taken together with the negative results reported for DLAT371 (50), it seems unlikely that a functional element resides within this region.
The purpose of the present study was to confirm and extend our earlier work in which LAT mutants constructed in the HSV-1 strain KOS were utilized (59, 60, 79). Viral replication efficiency might be a contributing factor for the latency phenotypes of strain KOS-based LAT mutants. KOS is less neuroinvasive than most other HSV-1 strains (76). Further, strain KOS establishes latent infections that contain fewer viral genome copies than either strain 17syn+ or McKrae (58) and also reactivates in vivo less efficiently in the rabbit (25, 72) and in the mouse following UV light (71) or HS induction (58–60, 79). It was therefore important to demonstrate that the reduced establishment of the latency phenotype of KOS-based LAT null mutants was not unique to this strain but was also evident in the genetic background of a more virulent HSV-1 strain, such as 17syn+.
Engineered LAT mutation prevented expression of the LAT locus but did not impair replication in TG.
Expression of the stable LAT 2.0- and 1.5-kb transcripts, as detected by Northern blot analysis, was completely eliminated both in infected cultured cells and in latently infected mouse TG by the deletion engineered into mutants 17-AH, 17-FA91, and 17-FA94, as was predicted (Fig. 2). Of critical importance, the deletion did not adversely affect the ability of the virus to replicate either at the body surface or in the TG (Fig. 3). It is somewhat counterintuitive that the LAT mutants caused the loss of more neurons but did not appear to replicate with an increased efficiency in TG. When viewed in the context of the overall infection process, this would be expected. We (59) and others (44) reported in 1992 that three broad classes of infected neurons could be identified during the acute stage of infection. These are neurons expressing lytic-phase proteins (the great majority of infected neurons), neurons expressing only the LAT promoter (a minor class that presumably had entered latency), and neurons expressing both lytic proteins and the LAT promoter (also a minor subset). This latter subset is presumably where LAT exerted its influence (59, 79). About 20% of the LAT promoter-positive neurons expressed viral proteins when the LAT locus was intact. However, 70% of the LAT promoter-positive neurons in TG infected with a LAT null mutant expressed viral lytic-phase proteins (59, 79). These and other findings lead us to hypothesize that LAT is important for the down regulation of viral protein production in neurons and functions to enhance the establishment of latency (59, 79). This effect of LAT would be masked by the permissive replication that occurs in most neurons infected by HSV.
The 1,964-bp region deleted in the LAT null mutants characterized in this study includes several regions that have been implicated in regulating herpetic latency. These include regions upstream of the basal LAT promoter, the basal promoter itself, and deletions in the 5′ end of the primary LAT (5–7, 27–29, 35, 40, 42, 48, 59, 79). It has been reported that a 2.6-kb fragment that includes the LAT promoter and upstream sequences as well as 811 bp of the primary LAT can partially restore a wild-type spontaneous ocular shedding frequency to that of a LAT null mutant (17). The promoter and first 828 bp of the primary LAT are deleted in the mutants employed in this study. It remains to be determined whether it is the deleted sequences or the lack of expression of the sequences downstream of the deletion that is important for the efficient establishment of latency.
HSV LAT gene preserves sensory neurons and enhances the establishment of latency.
A single-neuron PCR assay (CXA-D [55]) was employed to quantify the number of viral genome-containing neurons in mouse ganglia latently infected with LAT null or wild-type isolates of strain 17syn+. In our initial experiments, it was noted that the number of neurons isolated from mouse TG latently infected with the LAT null mutant 17-AH was consistently lower than that recovered from ganglia infected with wild-type or genomically restored isolates. This observation led to the most significant finding in this report: namely, that ganglia latently infected with the LAT null mutants contain 32% fewer neurons than those infected with LAT+ strains. This is the first reported empiric evidence that an intact LAT locus is associated with increased neuronal survival.
As was observed with strain KOS-based mutants previously (79), LAT was also found to play a critical role in the establishment of latency, increasing the number of latent sites by fourfold. A direct correlation between the number of latent sites established and the frequency with which mice reactivate following HS was previously reported for strain 17syn+ (56). Based on this earlier study, one would predict a corresponding decrease in the frequency of reactivation with the LAT null mutants. As predicted, following HS the reactivation frequency of the LAT null mutants was 35 to 40%, versus 70 to 80% for the genomically rescued variants. Importantly, the reactivation frequency observed with the mutants was consistent with what was predicted for strain 17syn+ in animals with similar numbers of latent sites per ganglion (58). This strongly suggests that the primary phenotype of LAT null mutants is an impaired establishment of latency and that the reduced reactivation observed by many investigators reflects this function of LAT rather than a direct role in reactivation.
Increased neuronal loss in LAT null mutant-infected TG was not due to an increase in apoptosis of neurons as assessed by TUNEL.
It is not yet known how the LAT gene functions to promote the establishment of latency, but the preservation of infected neurons is one likely possibility. Infection of mice via the footpad with wild-type HSV-1 (73) or HSV-2 (26) results in the death of a significant number of sensory neurons in dorsal root ganglia L4 and L5. The destruction of neurons by HSV may be dependent on the virus or mouse strain, inoculation titer, or inoculation route. Significant death of neurons was not detected in the dorsal root ganglia of mice infected via the flank model (65, 66). Under the conditions employed in this study, infection with strain 17syn+ resulted in the loss of ∼22% of the neurons in the TG. We report here that far more sensory ganglion neurons were killed following infection with LAT null mutants (52%) than were killed by LAT+ strains. We hypothesize that the increased survival of neurons mediated by the LAT locus is responsible for the increase in the number of latent sites established by LAT+ viruses.
The process whereby the LAT gene inhibits the death of sensory neurons is also not known. It has been hypothesized that LAT functions to inhibit the apoptosis of neurons (49). However, the validity of the assays employed to detect apoptosis and the results obtained in that study have been questioned (81). We found no evidence that LAT null mutants induced more extensive apoptosis in neurons in mouse TG. Indeed, apoptosis, as defined by positive TUNEL (Fig. 6 and 7) and positive immunohistochemical staining with an antibody directed against the cleaved form of caspase 3 (unpublished data), was found to be restricted mainly to small cells resembling infiltrating immune cells. Apoptosis of infiltrating immune cells is found during the resolution of infection and thought to be an immune regulatory process (4, 24, 37). The frequency and distribution of these small round TUNEL-positive cells did not appear to be different among the groups infected with wild-type or mutant viruses in this study. Very rare TUNEL-positive neurons were found in TG infected with both LAT+ and LAT null viruses on days 4 through 15 p.i., but the frequency of positive cells did not vary between the groups. In addition, the very low frequency of positive neurons detected could not account for loss of neurons following infection with either wild-type or mutant strains. The systematic approach employed to detect TUNEL-positive cells would have revealed a significant difference between the groups if either the bulk of neuronal dropout occurred during the peak of viral replication in the ganglia (days 4 to 7 p.i.), or if the loss was random throughout the first 15 days p.i. We therefore conclude that the disruption of LAT did not result in a significant increase in the apoptosis of neurons as measured by the TUNEL assay.
We hypothesize that one function of LAT is to help protect neurons from high-MOI infection.
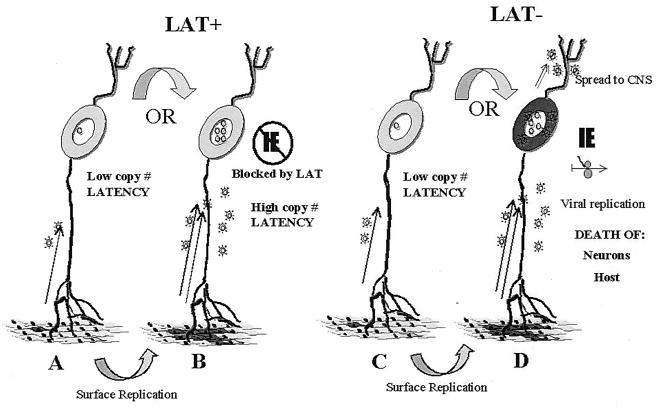
Figure 8 summarizes our working hypothesis of how the LAT locus functions directly to promote the establishment of latency and thereby indirectly to increase the probability of reactivation. This model is based on several recent observations. Latently infected neurons vary in the amount of viral genetic information they contain. The majority contain fewer than 10 HSV-1 genomes, but a significant portion contain hundreds, and rare latent sites contain more than 1,000 (55, 56, 58, 79, 80). The biological significance of high-genome-copy latent sites is not yet known, but the viral genome copy number might influence reactivation (54–56). We have shown that the average latent genome copy number is virus-strain specific, and that viral strains that produce latent sites with a high average copy number reactivate more frequently than strains that do not (58). Further, viral DNA replication in ganglia is not required to establish high-genome-copy latent infections, but efficient replication at the body surface is required (80). This latter finding suggests that numerous copies of the viral genome found in some latently infected neurons result from the transport of multiple virions from the surface into a single neuron cell body.
FIG. 8.
Schematic representation of a hypothesis for how LAT might function to decrease neuronal death and increase the establishment of latency. The gray oval objects represent neuronal cell bodies with their axons and dendrites indicated as gray lines. The body surface is indicated at the bottom. Virions are indicated by stellate objects and viral genomes are indicated by circles present within the neuronal nucleus. It is hypothesized that initial limited replication at the body surface results in a low MOI of neurons and only one or a very few virions enter any particular neuronal cell body (A or C). More extensive replication at the surface (indicated by dark regions) results in a higher MOI for neurons (B or D). In the absence of LAT, this high MOI results in entry into the lytic pathway and death of the neuron. In the presence of LAT, immediate-early gene expression is reduced or eliminated and a latent infection that contains hundreds or even thousands of HSV-1 genomes is established within the neuron.
We hypothesize that the LAT gene is a negative factor that inhibits viral replication when hundreds or even thousands of virions enter a neuron. That is, we hypothesize that the expression of LAT in neurons prevents progression to lytic infection when they are subsequently superinfected as the acute stage of infection proceeds. The regulation of the LAT gene is not completely delineated, but the promoter contains neuron-specific expression elements (2, 3, 9, 46, 89) and its strongest expression in terms of both intensity and number of neurons is during the peak days of acute virus replication in TG (44, 59). These findings suggest that neurons that initially enter the latent pathway (e.g., that are infected with one or a few virions) begin to express the LAT-dependent establishment function. The arborization of sensory neurons (12, 13, 74) suggests that virus spread at the body surface could lead to the entry of virions into a given neuron that is protracted over many days (80). As the virus spreads across the body surface, it may encounter additional axonal branches which innervate cell bodies that have already been infected (Fig. 8B). The expression of LAT in these previously infected neurons is hypothesized to further decrease their permissivity and repress viral gene expression despite the higher MOI. Latency would therefore be maintained, and high-viral-genome-copy-number latent sites would be established (Fig. 8A and C). In the absence of functional LAT, this hypothesis predicts that a high MOI would frequently result in entry into the lytic transcriptional program and ultimately the death of the neuron. This in turn would reduce the number of latent infections established (as well as their average latent virus genome copy number) and indirectly result in a reduced probability of subsequent virus reactivation.
ACKNOWLEDGMENTS
This work was supported primarily by NIH grant AI 32121. Additional support was provided by EY13168.
REFERENCES
- 1.Ahmed R, Stevens J G. Viral persistence. In: Fields B N, et al., editors. Fields virology. New York, N.Y: Raven Press; 1990. pp. 241–265. [Google Scholar]
- 2.Batchelor A H, O'Hare P. Regulation and cell-type-specific activity of a promoter located upstream of the latency-associated transcript of herpes simplex virus type 1. J Virol. 1990;64:3269–3279. doi: 10.1128/jvi.64.7.3269-3279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batchelor A H, Wilcox K W, O'Hare P. Binding and repression of the latency-associated promoter of herpes simplex virus by the immediate early 175K protein. J Gen Virol. 1994;75:753–767. doi: 10.1099/0022-1317-75-4-753. [DOI] [PubMed] [Google Scholar]
- 4.Behrens T W, Mueller D L. Bcl-x and the regulation of survival in the immune system. Immunol Res. 1997;16:149–160. doi: 10.1007/BF02786359. [DOI] [PubMed] [Google Scholar]
- 5.Block T M, Deshmane S, Masonis J, Maggioncalda J, Valyi-Nagi T, Fraser N W. An HSV LAT null mutant reactivates slowly from latent infection and makes small plaques on CV-1 monolayers. Virology. 1993;192:618–630. doi: 10.1006/viro.1993.1078. [DOI] [PubMed] [Google Scholar]
- 6.Bloom D C, Hill J M, Devi-Rao G, Wagner E K, Feldman L T, Stevens J G. A 348-base-pair region in the latency-associated transcript facilitates herpes simplex virus type 1 reactivation. J Virol. 1996;70:2449–2459. doi: 10.1128/jvi.70.4.2449-2459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom D C, Stevens J G, Hill J M, Tran R K. Mutagenesis of a cAMP response element within the latency-associated transcript promoter of HSV-1 reduces adrenergic reactivation. Virology. 1997;236:202–207. doi: 10.1006/viro.1997.8723. [DOI] [PubMed] [Google Scholar]
- 8.Chen S H, Kramer M F, Schaffer P A, Coen D M. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1997;71:5878–5884. doi: 10.1128/jvi.71.8.5878-5884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Schmidt M C, Goins W F, Glorioso J C. Two herpes simplex virus type 1 latency-active promoters differ in their contributions to latency-associated transcript expression during lytic and latent infections. J Virol. 1995;69:7899–7908. doi: 10.1128/jvi.69.12.7899-7908.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements G B, Stow N D. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J Gen Virol. 1989;70:2501–2506. doi: 10.1099/0022-1317-70-9-2501. [DOI] [PubMed] [Google Scholar]
- 11.Coen D M, Kosz-Vnenchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coggeshall R E, Carlton S M. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol. 1998;391:78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Cruz M C, Jeanmonod D, Meier K, Van der Loos H. A silver and gold technique for axons and axon-bundles in formalin-fixed central and peripheral nervous tissue. J Neurosci Methods. 1984;10:1–8. doi: 10.1016/0165-0270(84)90073-6. [DOI] [PubMed] [Google Scholar]
- 14.Devi-Rao G B, Bloom D C, Stevens J G, Wagner E K. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J Virol. 1994;68:1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobson A T, Margolis T P, Gomes W A, Feldman L T. In vivo deletion analysis of the herpes simplex virus type 1 latency-associated transcript promoter. J Virol. 1995;69:2264–2270. doi: 10.1128/jvi.69.4.2264-2270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson A T, Margolis T P, Sedarati F, Stevens J G, Feldman L T. A latent, nonpathogenic HSV-1-derived vector stably expresses beta-galactosidase in mouse neurons. Neuron. 1990;5:353–360. doi: 10.1016/0896-6273(90)90171-b. [DOI] [PubMed] [Google Scholar]
- 17.Drolet B S, Perng G C, Villosis R J, Slanina S M, Nesburn A B, Wechsler S L. Expression of the first 811 nucleotides of the herpes simplex virus type 1 latency-associated transcript (LAT) partially restores wild-type spontaneous reactivation to a LAT-null mutant. Virology. 1999;253:96–106. doi: 10.1006/viro.1998.9492. [DOI] [PubMed] [Google Scholar]
- 18.Ecob-Prince M S, Preston C M, Rixon F J, Hassan K, Kennedy P G. Neurons containing latency-associated transcripts are numerous and widespread in dorsal root ganglia following footpad inoculation of mice with herpes simplex virus type 1 mutant in1814. J Gen Virol. 1993;74:985–994. doi: 10.1099/0022-1317-74-6-985. [DOI] [PubMed] [Google Scholar]
- 19.Ecob-Prince M S, Rixon F J, Preston C M, Hassan K, Kennedy P G. Reactivation in vivo and in vitro of herpes simplex virus from mouse dorsal root ganglia which contain different levels of latency-associated transcripts. J Gen Virol. 1993;74:995–1002. doi: 10.1099/0022-1317-74-6-995. [DOI] [PubMed] [Google Scholar]
- 20.Efstathiou S, Kemp S, Darby G, Minson A C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989;70:869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- 21.Farrell M J, Dobson A T, Feldman L T. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci USA. 1991;88:790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedrich A, Kleim J P, Schneweis K E. Detection of latent thymidine kinase-deficient herpes simplex virus in trigeminal ganglia of mice using the polymerase chain reaction. Arch Virol. 1990;113:107–113. doi: 10.1007/BF01318359. [DOI] [PubMed] [Google Scholar]
- 23.Garber D A, Schaffer P A, Knipe D M. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J Virol. 1997;71:5885–5893. doi: 10.1128/jvi.71.8.5885-5893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold R, Archelos J J, Hartung H P. Mechanisms of immune regulation in the peripheral nervous system. Brain Pathol. 1999;9:343–360. doi: 10.1111/j.1750-3639.1999.tb00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon Y J, Romanowski E G, Araullo-Cruz T, Kinchington P R. The proportion of trigeminal ganglionic neurons expressing herpes simplex virus type 1 latency-associated transcripts correlates to reactivation in the New Zealand rabbit ocular model. Graefes Arch Clin Exp Ophthalmol. 1995;233:649–654. doi: 10.1007/BF00185286. [DOI] [PubMed] [Google Scholar]
- 26.Henken D B, Goldstein M E, Martin J R. Herpes simplex virus type-2 infection by a footpad route results in neuronal death in mouse spinal ganglia. J Neurol Sci. 1993;115:177–183. doi: 10.1016/0022-510x(93)90222-k. [DOI] [PubMed] [Google Scholar]
- 27.Hill J M, Garza H H, Jr, Su Y H, Meegalla R, Hanna L A, Loutsch J M, Thompson H W, Varnell E D, Bloom D C, Block T M. A 437-base-pair deletion at the beginning of the latency-associated transcript promoter significantly reduced adrenergically induced herpes simplex virus type 1 ocular reactivation in latently infected rabbits. J Virol. 1997;71:6555–6559. doi: 10.1128/jvi.71.9.6555-6559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill J M, Maggioncalda J B, Garza H H, Jr, Su Y H, Fraser N W, Block T M. In vivo epinephrine reactivation of ocular herpes simplex virus type 1 in the rabbit is correlated to a 370-base-pair region located between the promoter and the 5′ end of the 2.0-kilobase latency-associated transcript. J Virol. 1996;70:7270–7274. doi: 10.1128/jvi.70.10.7270-7274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill J M, Sedarati F, Javier R T, Wagner E K, Stevens J G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990;174:117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- 30.Inman M, Perng G C, Henderson G, Ghiasi H, Nesburn A B, Wechsler S L, Jones C. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J Virol. 2001;75:3636–3646. doi: 10.1128/JVI.75.8.3636-3646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson J G, Leib D A, Goldstein D J, Bogard C L, Schaffer P A, Weller S K, Coen D M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989;173:276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- 32.Javier R T, Stevens J G, Dissette V B, Wagner E K. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology. 1988;166:254–257. doi: 10.1016/0042-6822(88)90169-9. [DOI] [PubMed] [Google Scholar]
- 33.Katz J P, Bodin E T, Coen D M. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer M F, Coen D M. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leib D A, Bogard C L, Kosz-Vnenchak M, Hicks K A, Coen D M, Knipe D M, Schaffer P A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenardo M J. The molecular regulation of lymphocyte apoptosis. Semin Immunol. 1997;9:1–5. doi: 10.1006/smim.1996.0050. [DOI] [PubMed] [Google Scholar]
- 38.Lock M, Miller C, Fraser N W. Analysis of protein expression from within the region encoding the 2.0-kilobase latency-associated transcript of herpes simplex virus type 1. J Virol. 2001;75:3413–3426. doi: 10.1128/JVI.75.7.3413-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lokensgard J R, Berthomme H, Feldman L T. The latency-associated promoter of herpes simplex virus type 1 requires a region downstream of the transcription start site for long-term expression during latency. J Virol. 1997;71:6714–6719. doi: 10.1128/jvi.71.9.6714-6719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loutsch J M, Perng G C, Hill J M, Zheng X, Marquart M E, Block T M, Ghiasi H, Nesburn A B, Wechsler S L. Identical 371-base-pair deletion mutations in the LAT genes of herpes simplex virus type 1 McKrae and 17syn+ result in different in vivo reactivation phenotypes. J Virol. 1999;73:767–771. doi: 10.1128/jvi.73.1.767-771.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mador N, Goldenberg D, Cohen O, Panet A, Steiner I. Herpes simplex virus type 1 latency-associated transcripts suppress viral replication and reduce immediate-early gene mRNA levels in a neuronal cell line. J Virol. 1998;72:5067–5075. doi: 10.1128/jvi.72.6.5067-5075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maggioncalda J, Mehta A, Fraser N W, Block T M. Analysis of a herpes simplex virus type 1 LAT mutant with a deletion between the putative promoter and the 5′ end of the 2.0-kilobase transcript. J Virol. 1994;68:7816–7824. doi: 10.1128/jvi.68.12.7816-7824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maggioncalda J, Mehta A, Su Y H, Fraser N W, Block T M. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology. 1996;225:72–81. doi: 10.1006/viro.1996.0576. [DOI] [PubMed] [Google Scholar]
- 44.Margolis T P, Sedarati F, Dobson A T, Feldman L T, Stevens J G. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology. 1992;189:150–160. doi: 10.1016/0042-6822(92)90690-q. [DOI] [PubMed] [Google Scholar]
- 45.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 46.Morrow J A, Rixon F J. Analysis of sequences important for herpes simplex virus type 1 latency-associated transcript promoter activity during lytic infection of tissue culture cells. J Gen Virol. 1994;75:309–316. doi: 10.1099/0022-1317-75-2-309. [DOI] [PubMed] [Google Scholar]
- 47.Perng G C, Chokephaibulkit K, Thompson R L, Sawtell N M, Slanina S M, Ghiasi H, Nesburn A B, Wechsler S L. The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J Virol. 1996;70:282–291. doi: 10.1128/jvi.70.1.282-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perng G C, Dunkel E C, Geary P A, Slanina S M, Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol. 1994;68:8045–8055. doi: 10.1128/jvi.68.12.8045-8055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perng G C, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, Slanina S M, Hofman F M, Ghiasi H, Nesburn A B, Wechsler S L. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 50.Perng G C, Slanina S M, Ghiasi H, Nesburn A B, Wechsler S L. A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J Virol. 1996;70:2014–2018. doi: 10.1128/jvi.70.3.2014-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perng G C, Slanina S M, Yukht A, Ghiasi H, Nesburn A B, Wechsler S L. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J Virol. 2000;74:1885–1891. doi: 10.1128/jvi.74.4.1885-1891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perng G C, Thompson R L, Sawtell N M, Taylor W E, Slanina S M, Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J Virol. 1995;69:3033–3041. doi: 10.1128/jvi.69.5.3033-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry L J, McGeoch D J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- 54.Roizman B, Sears A. Herpes simplex viruses and their replication. In: Fields B N, et al., editors. Fields virology. Philadelphia, Pa: Raven Press; 1996. pp. 2231–2295. [Google Scholar]
- 55.Sawtell N M. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997;71:5423–5431. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawtell N M. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol. 1998;72:6888–6892. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawtell N M, Bernstein D I, Stanberry L R. A temporal analysis of acyclovir inhibition of induced herpes simplex virus type 1 in vivo reactivation in the mouse trigeminal ganglia. J Infect Dis. 1999;180:821–823. doi: 10.1086/314958. [DOI] [PubMed] [Google Scholar]
- 58.Sawtell N M, Poon D K, Tansky C S, Thompson R L. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol. 1998;72:5343–5350. doi: 10.1128/jvi.72.7.5343-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawtell N M, Thompson R L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawtell N M, Thompson R L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schang L M, Hossain A, Jones C. The latency-related gene of bovine herpesvirus 1 encodes a product which inhibits cell cycle progression. J Virol. 1996;70:3807–3814. doi: 10.1128/jvi.70.6.3807-3814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedarati F, Izumi K M, Wagner E K, Stevens J G. Herpes simplex virus type 1 latency-associated transcription plays no role in establishment or maintenance of a latent infection in murine sensory neurons. J Virol. 1989;63:4455–4458. doi: 10.1128/jvi.63.10.4455-4458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sedarati F, Margolis T P, Stevens J G. Latent infection can be established with drastically restricted transcription and replication of the HSV-1 genome. Virology. 1993;192:687–691. doi: 10.1006/viro.1993.1089. [DOI] [PubMed] [Google Scholar]
- 64.Singh J, Wagner E K. Transcriptional analysis of the herpes simplex virus type 1 region containing the TRL/UL junction. Virology. 1993;196:220–231. doi: 10.1006/viro.1993.1470. [DOI] [PubMed] [Google Scholar]
- 65.Speck P G, Simmons A. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J Virol. 1991;65:4001–4005. doi: 10.1128/jvi.65.8.4001-4005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Speck P G, Simmons A. Synchronous appearance of antigen-positive and latently infected neurons in spinal ganglia of mice infected with a virulent strain of herpes simplex virus. J Gen Virol. 1992;73:1281–1285. doi: 10.1099/0022-1317-73-5-1281. [DOI] [PubMed] [Google Scholar]
- 67.Spivack J G, Fraser N W. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J Virol. 1988;62:1479–1485. doi: 10.1128/jvi.62.5.1479-1485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steiner I, Spivack J G, Deshmane S L, Ace C I, Preston C M, Fraser N W. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol. 1990;64:1630–1638. doi: 10.1128/jvi.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steiner I, Spivack J G, Lirette R P, Brown S M, MacLean A R, Subak-Sharpe J H, Fraser N W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989;8:505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevens J G, Wagner E K, Devi-Rao G B, Cook M L, Feldman L T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 71.Strelow L I, Laycock K A, Jun P Y, Rader K A, Brady R H, Miller J K, Pepose J S, Leib D A. A structural and functional comparison of the latency-associated transcript promoters of herpes simplex virus type 1 strains KOS and McKrae. J Gen Virol. 1994;75:2475–2480. doi: 10.1099/0022-1317-75-9-2475. [DOI] [PubMed] [Google Scholar]
- 72.Stroop W G, Banks M C. Herpes simplex virus type 1 strain KOS-63 does not cause acute or recurrent ocular disease and does not reactivate ganglionic latency in vivo. Acta Neuropathol. 1994;87:14–22. doi: 10.1007/BF00386250. [DOI] [PubMed] [Google Scholar]
- 73.Tenser R B, Edris W A, Hay K A. Neuronal control of herpes simplex virus latency. Virology. 1993;195:337–347. doi: 10.1006/viro.1993.1384. [DOI] [PubMed] [Google Scholar]
- 74.Tervo T, Joo F, Huikuri K T, Toth I, Palkama A. Fine structure of sensory nerves in the rat cornea: an experimental nerve degeneration study. Pain. 1979;6:57–70. doi: 10.1016/0304-3959(79)90140-4. [DOI] [PubMed] [Google Scholar]
- 75.Thomas S K, Gough G, Latchman D S, Coffin R S. Herpes simplex virus latency-associated transcript encodes a protein which greatly enhances virus growth, can compensate for deficiencies in immediate-early gene expression, and is likely to function during reactivation from virus latency. J Virol. 1999;73:6618–6625. doi: 10.1128/jvi.73.8.6618-6625.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson R L, Cook M L, Devi-Rao G B, Wagner E K, Stevens J G. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986;58:203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson R L, Devi-Rao G V, Stevens J G, Wagner E K. Rescue of a herpes simplex virus type 1 neurovirulence function with a cloned DNA fragment. J Virol. 1985;55:504–508. doi: 10.1128/jvi.55.2.504-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson R L, Rogers S K, Zerhusen M A. Herpes simplex virus neurovirulence and productive infection of neural cells is associated with a function which maps between 0.82 and 0.832 map units on the HSV genome. Virology. 1989;172:435–450. doi: 10.1016/0042-6822(89)90186-4. [DOI] [PubMed] [Google Scholar]
- 79.Thompson R L, Sawtell N M. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompson R L, Sawtell N M. Replication of herpes simplex virus type 1 within trigeminal ganglia is required for high frequency but not high viral genome copy number latency. J Virol. 2000;74:965–974. doi: 10.1128/jvi.74.2.965-974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson R L, Sawtell N M, Wechsler S L, Perng G C, Ghiasi H, Nesburn A B, Jones C. HSV latency-associated transcript and neuronal apoptosis. Science. 2000;289:1651. [PubMed] [Google Scholar]
- 82.Thompson R L, Stevens J G. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983;131:171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- 83.Thompson R L, Stevens J G. Replication at body temperature selects a neurovirulent herpes simplex virus type 2. Infect Immun. 1983;41:855–857. doi: 10.1128/iai.41.2.855-857.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson R L, Wagner E K, Stevens J G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983;131:180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- 85.Trousdale M D, Steiner I, Spivack J G, Deshmane S L, Brown S M, MacLean A R, Subak-Sharpe J H, Fraser N W. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J Virol. 1991;65:6989–6993. doi: 10.1128/jvi.65.12.6989-6993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valyi-Nagy T, Olson S J, Valyi-Nagy K, Montine T J, Dermody T S. Herpes simplex virus type 1 latency in the murine nervous system is associated with oxidative damage to neurons. Virology. 2000;278:309–321. doi: 10.1006/viro.2000.0678. [DOI] [PubMed] [Google Scholar]
- 87.Wagner E K, Bloom D C. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev. 1997;10:419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu J, Kang W, Marquart M E, Hill J M, Zheng X, Block T M, Fraser N W. Identification of a novel 0.7-kb polyadenylated transcript in the LAT promoter region of HSV-1 that is strain specific and may contribute to virulence. Virology. 1999;265:296–307. doi: 10.1006/viro.1999.0057. [DOI] [PubMed] [Google Scholar]
- 89.Zwaagstra J C, Ghiasi H, Nesburn A B, Wechsler S L. Identification of a major regulatory sequence in the latency associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1) Virology. 1991;182:287–297. doi: 10.1016/0042-6822(91)90672-x. [DOI] [PubMed] [Google Scholar]