Abstract
1. We expressed a novel 5-hydroxytryptamine receptor (SRL) in Xenopus oocytes and monitored cytosolic Ca2+ through the endogenous Ca(2+)-dependent Cl- channel activity using the double electrode voltage-clamp technique. 2. 5-Hydroxytryptamine (5-HT; 200 nM) led to an initial rapid oscillatory current followed by a pronounced secondary one, which lasted long after 5-HT wash-out (20-40 min) and was not affected by the receptor antagonist yohimbine. 3. Both phases of the current were abolished by heparin demonstrating a key role for IP3-induced Ca2+ release. 4. Caffeine (10 mM) alone did not evoke a current but reduced both phases of the current evoked by 5-HT. Ryanodine had no effect. No evidence for Ca(2+)-induced Ca2+ release was found. 5. The secondary current activated by 5-HT was sensitive to changes in extracellular Ca2+, suggesting it was evoked by Ca2+ influx. Reducing external Na+ did not affect this current, demonstrating that it was rather specific for Ca2+. 6. The Ca2+ influx pathway was much more sensitive to Cd2+ than other divalent ions (Co2+, Mn2+, Sr2+, Ba2+). It was insensitive to verapamil. 7. Injection of D-myo-inositol 1,4,5-trisphosphate, 3-deoxy-3-fluoro (IP3-F; an analogue not metabolized to D-myo-inositol 1,3,4,5-tetrakisphosphate (IP4)), evoked either an oscillatory current or a rapid current followed by a sustained secondary one. The latter was sensitive to external Ca2+ and was blocked by Cd2+. Heparin dramatically reduced the IP3-F-evoked current. 8. Perfusion in Ca(2+)-free solution, once a secondary current had been generated, significantly decreased the amount of intracellular Ca2+ mobilized by 5-HT, indicating that the Ca2+ influx pathway plays an important role in pool refilling. 9. Block of Ca2+ influx by Cd2+ in cells that were oscillating transiently increased the amplitude and then either abolished the oscillations or made them irregular. This effect was also elicited by increasing external Ca2+. 10. These results demonstrate that 5-HT, acting via IP3, both releases Ca2+ from internal stores and evokes a pronounced Ca2+ influx. This last step is activated by pool depletion and is important for both refilling of the agonist-sensitive stores and modifying the oscillatory pattern.
Full text
PDF


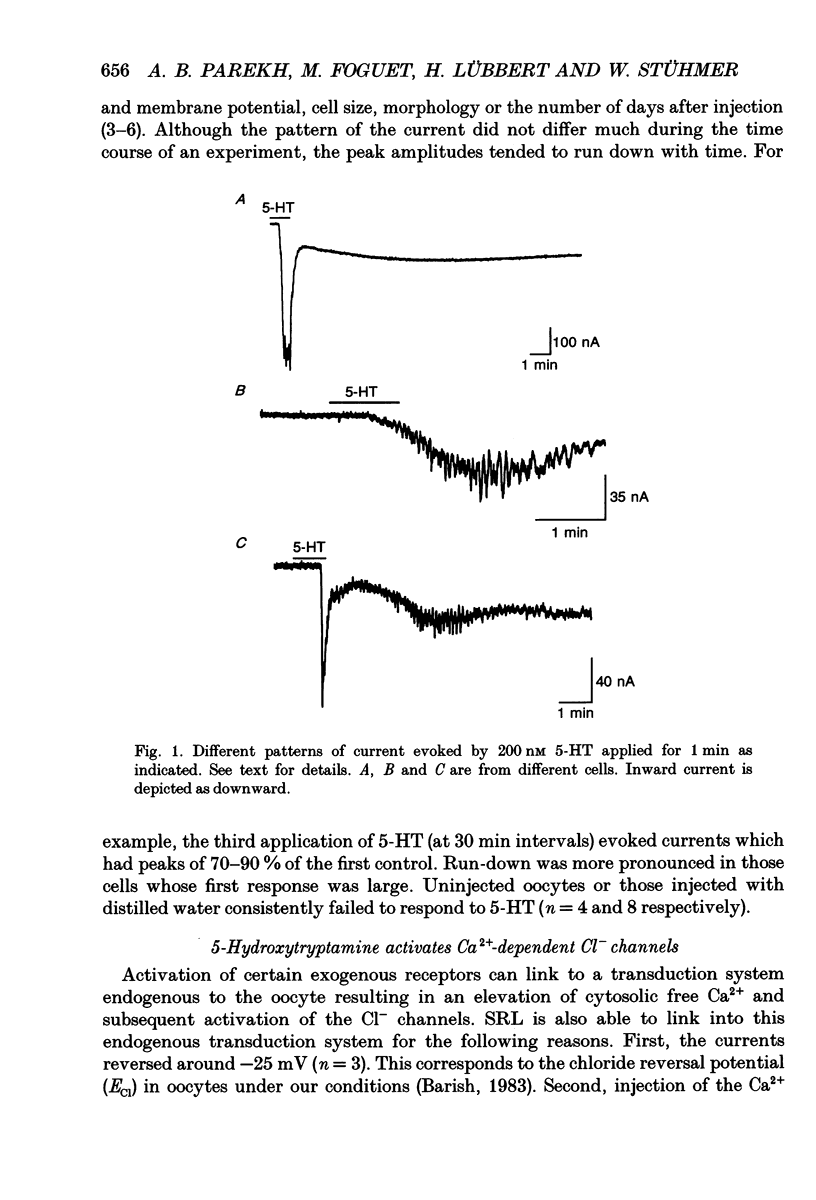
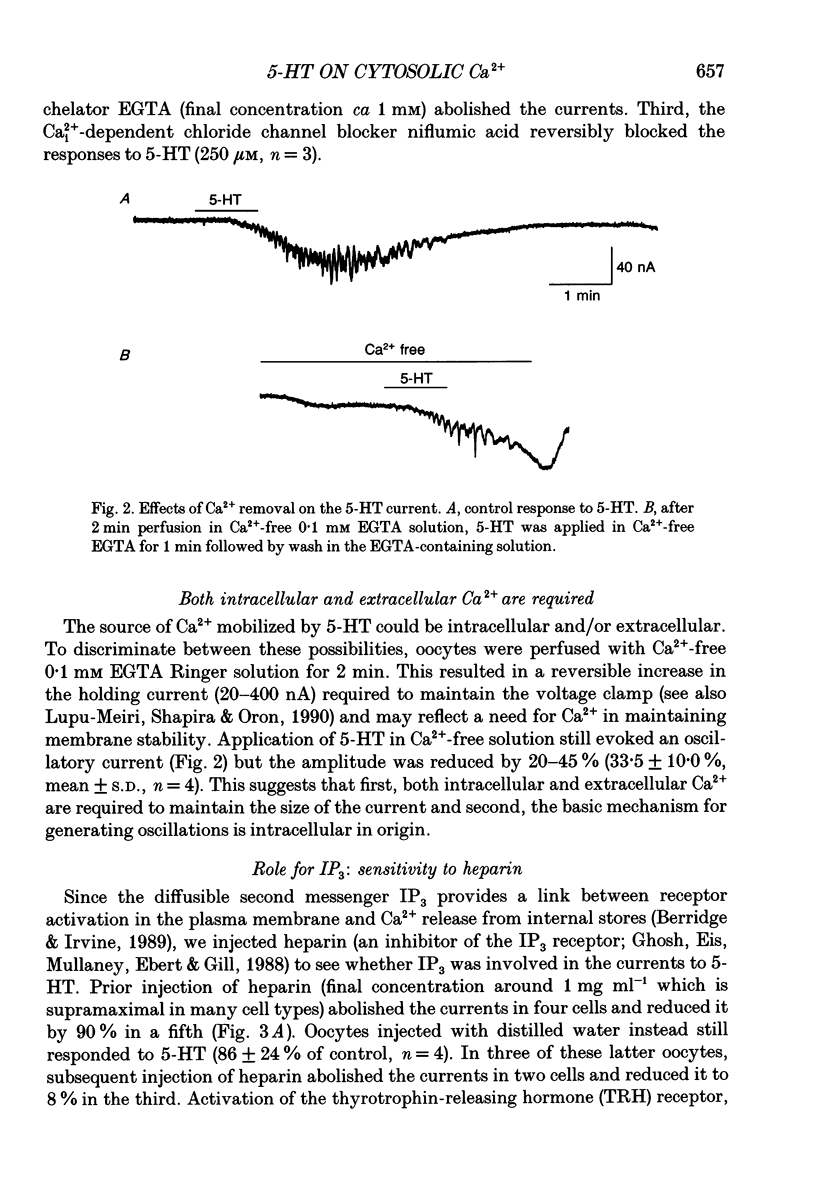
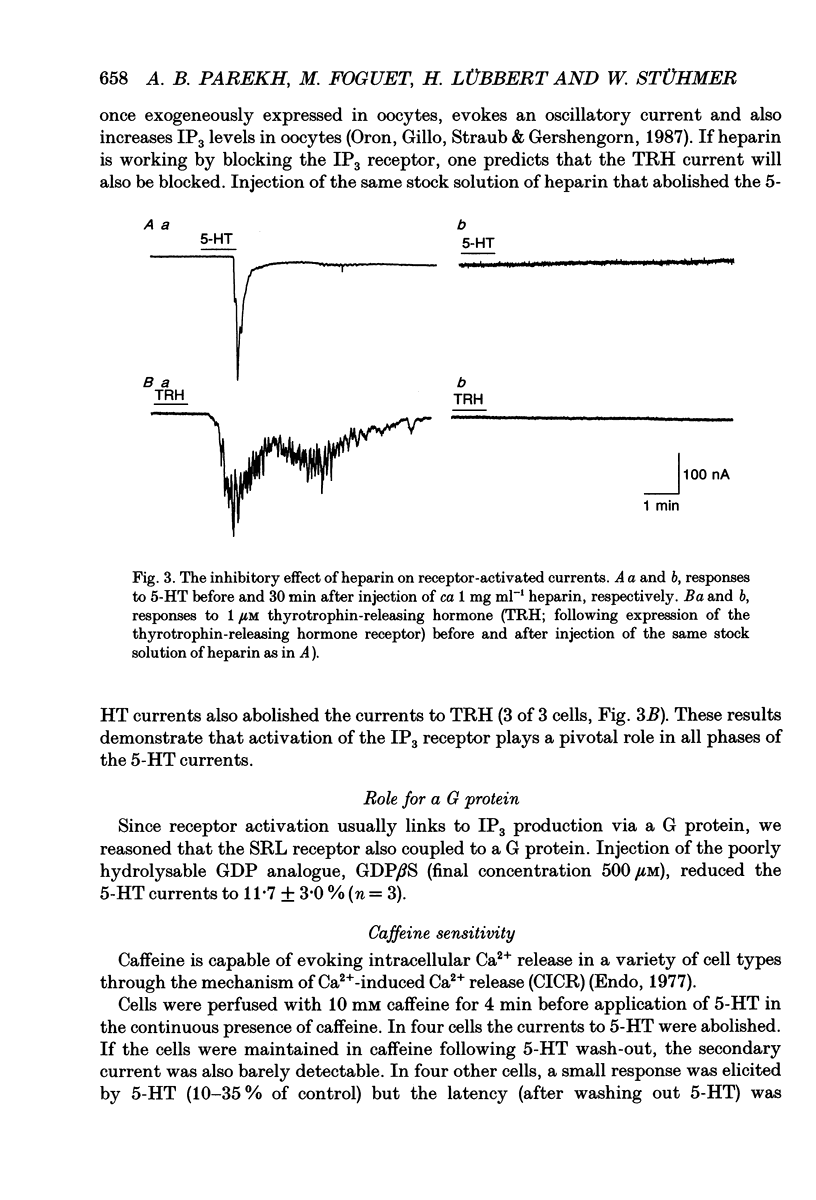
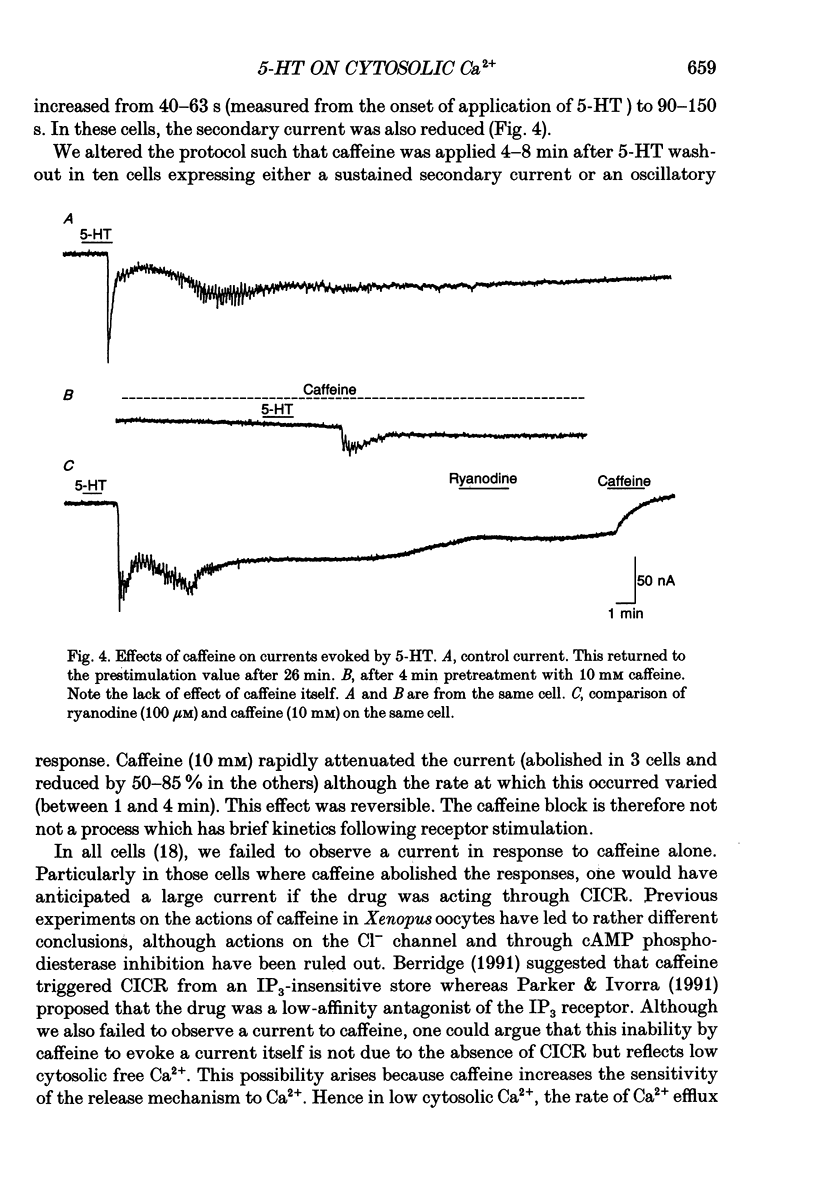
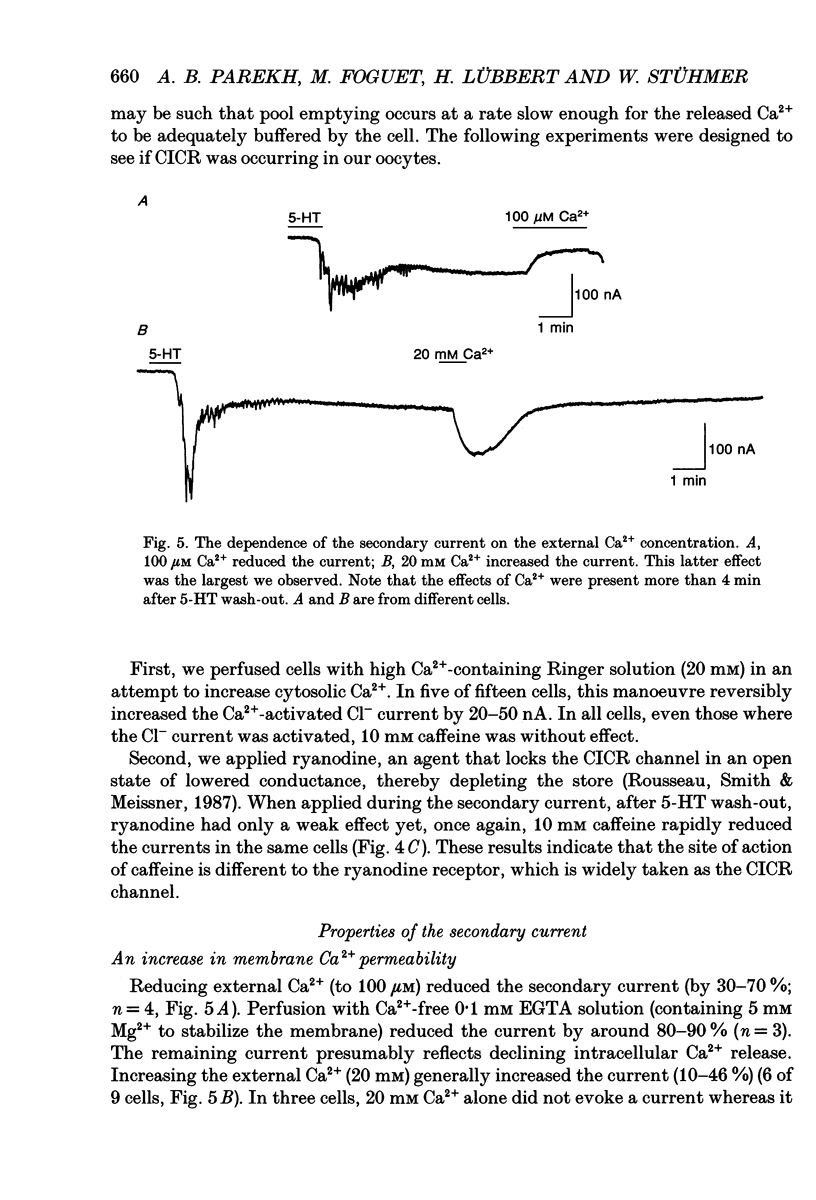
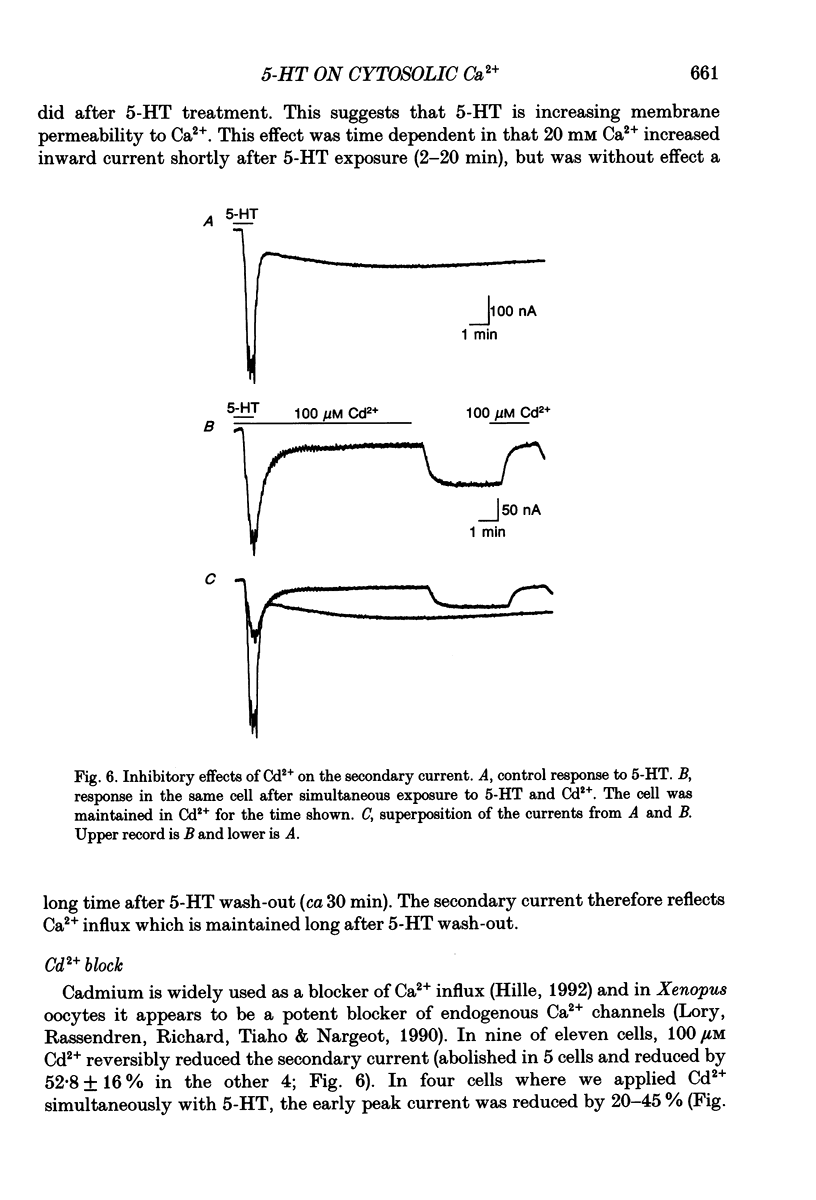

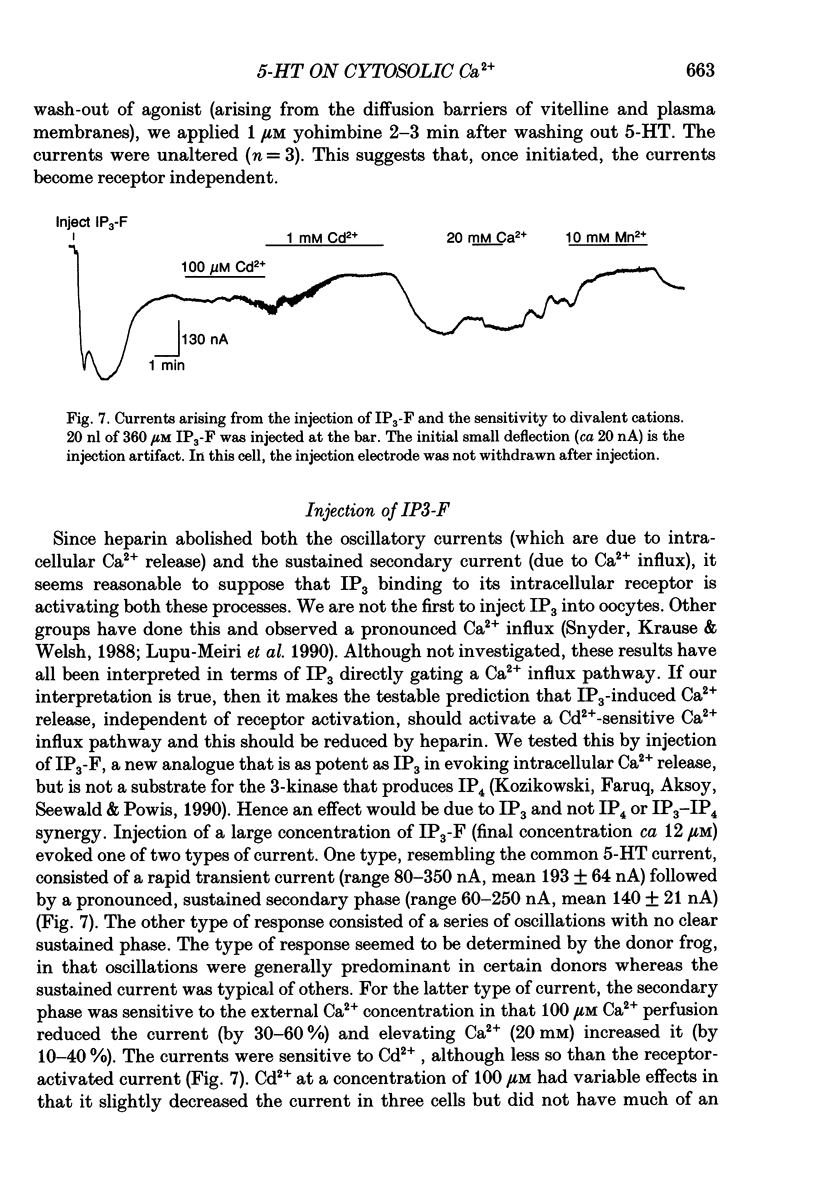
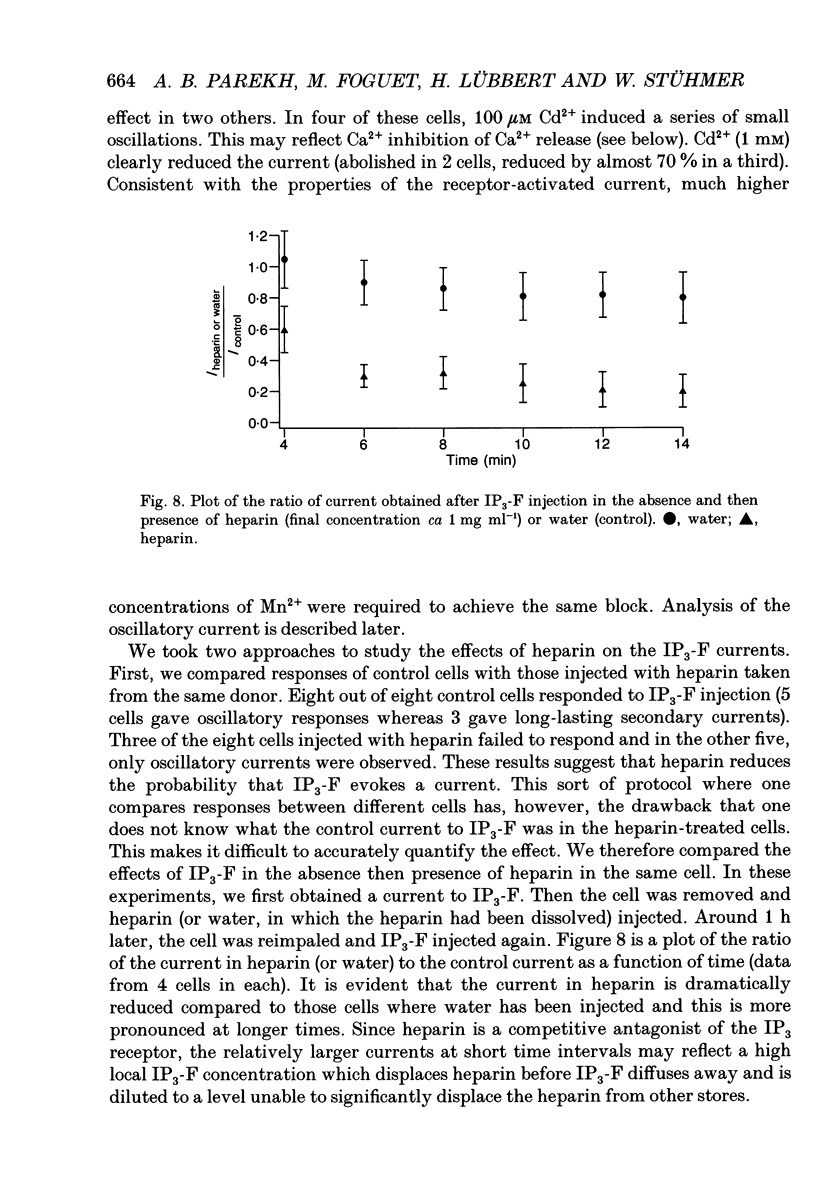







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Caffeine inhibits inositol-trisphosphate-induced membrane potential oscillations in Xenopus oocytes. Proc Biol Sci. 1991 Apr 22;244(1309):57–62. doi: 10.1098/rspb.1991.0051. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Brown G. R., Sayers L. G., Kirk C. J., Michell R. H., Michelangeli F. The opening of the inositol 1,4,5-trisphosphate-sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. Biochem J. 1992 Mar 1;282(Pt 2):309–312. doi: 10.1042/bj2820309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle S., Krause K. H., Denning G., Potter B. V., Welsh M. J. Effect of inositol trisphosphate and calcium on oscillating elevations of intracellular calcium in Xenopus oocytes. J Biol Chem. 1990 Jul 15;265(20):11726–11730. [PubMed] [Google Scholar]
- DeLisle S., Pittet D., Potter B. V., Lew P. D., Welsh M. J. InsP3 and Ins(1,3,4,5)P4 act in synergy to stimulate influx of extracellular Ca2+ in Xenopus oocytes. Am J Physiol. 1992 Jun;262(6 Pt 1):C1456–C1463. doi: 10.1152/ajpcell.1992.262.6.C1456. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fadool D. A., Ache B. W. Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron. 1992 Nov;9(5):907–918. doi: 10.1016/0896-6273(92)90243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C., Pandiella A., Meldolesi J., Pozzan T. Generation of inositol phosphates, cytosolic Ca2+, and ionic fluxes in PC12 cells treated with bradykinin. J Biol Chem. 1988 Nov 25;263(33):17350–17359. [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Foguet M., Hoyer D., Pardo L. A., Parekh A., Kluxen F. W., Kalkman H. O., Stühmer W., Lübbert H. Cloning and functional characterization of the rat stomach fundus serotonin receptor. EMBO J. 1992 Sep;11(9):3481–3487. doi: 10.1002/j.1460-2075.1992.tb05427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lory P., Rassendren F. A., Richard S., Tiaho F., Nargeot J. Characterization of voltage-dependent calcium channels expressed in Xenopus oocytes injected with mRNA from rat heart. J Physiol. 1990 Oct;429:95–112. doi: 10.1113/jphysiol.1990.sp018246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu-Meiri M., Shapira H., Oron Y. Extracellular calcium participates in responses to acetylcholine in Xenopus oocytes. FEBS Lett. 1990 Mar 26;262(2):165–169. doi: 10.1016/0014-5793(90)80180-q. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nakai J., Imagawa T., Hakamat Y., Shigekawa M., Takeshima H., Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990 Oct 1;271(1-2):169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Neher E. Cell physiology. Controls on calcium influx. Nature. 1992 Jan 23;355(6358):298–299. doi: 10.1038/355298a0. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Kaneko S., Kato K., Yamagishi S., Sugiyama H. Inositol phosphate formation and chloride current responses induced by acetylcholine and serotonin through GTP-binding proteins in Xenopus oocyte after injection of rat brain messenger RNA. Brain Res. 1987 Jul;388(2):113–123. doi: 10.1016/s0006-8993(87)80004-5. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Oron Y., Gillo B., Straub R. E., Gershengorn M. C. Mechanism of membrane electrical response to thyrotropin-releasing hormone in Xenopus oocytes injected with GH3 pituitary cell messenger ribonucleic acid. Mol Endocrinol. 1987 Dec;1(12):918–925. doi: 10.1210/mend-1-12-918. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol. 1991 Feb;433:229–240. doi: 10.1113/jphysiol.1991.sp018423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 1991 Dec 23;246(1317):269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Shuttleworth T. J. Fluoroaluminate activation of different components of the calcium signal in an exocrine cell. Biochem J. 1990 Jul 15;269(2):417–422. doi: 10.1042/bj2690417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder P. M., Krause K. H., Welsh M. J. Inositol trisphosphate isomers, but not inositol 1,3,4,5-tetrakisphosphate, induce calcium influx in Xenopus laevis oocytes. J Biol Chem. 1988 Aug 15;263(23):11048–11051. [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]


