Abstract
BACKGROUND
Pulse oximetry has become a cornerstone technology in healthcare, providing non-invasive monitoring of oxygen saturation levels and pulse rate. Despite its widespread use, the technology has inherent limitations and challenges that must be addressed to ensure accurate and reliable patient care.
AIM
To comprehensively evaluate the advantages, limitations, and challenges of pulse oximetry in clinical practice, as well as to propose recommendations for optimizing its use.
METHODS
A systematic literature review was conducted to identify studies related to pulse oximetry and its applications in various clinical settings. Relevant articles were selected based on predefined inclusion and exclusion criteria, and data were synthesized to provide a comprehensive overview of the topic.
RESULTS
Pulse oximetry offers numerous advantages, including non-invasiveness, real-time feedback, portability, and cost-effectiveness. However, several limitations and challenges were identified, including motion artifacts, poor peripheral perfusion, ambient light interference, and patient-specific factors such as skin pigmentation and hemoglobin variants. Recommendations for optimizing pulse oximetry use include technological advancements, education and training initiatives, quality assurance protocols, and interdisciplinary collaboration.
CONCLUSION
Pulse oximetry is crucial in modern healthcare, offering invaluable insights into patients’ oxygenation status. Despite its limitations, pulse oximetry remains an indispensable tool for monitoring patients in diverse clinical settings. By implementing the recommendations outlined in this review, healthcare providers can enhance the effectiveness, accessibility, and safety of pulse oximetry monitoring, ultimately improving patient outcomes and quality of care.
Keywords: Pulse oximetry, Oxygen saturation, Monitoring, Advantages, Limitations, Challenges, Recommendations, Clinical practice, Non-invasive, Children
Core Tip: For clinicians utilizing pulse oximetry in practice, it’s essential to remember several key considerations. Firstly, ensure proper sensor placement on well-perfused areas. Minimize motion artifacts by securing the sensor snugly but not too tightly. Establish baseline oxygen saturation levels and consider patient-specific factors like age and medical conditions. Continuous monitoring is crucial in high-risk patients. Regularly maintain and calibrate equipment, replacing sensors as needed. Educate caregivers on the importance of pulse oximetry and proper usage. Lastly, readings should be accurately documented in patient records. By adhering to these core tips, healthcare providers can optimize the effectiveness of pulse oximetry monitoring and enhance patient care.
INTRODUCTION
Pulse oximetry, a cornerstone in modern pediatric healthcare, swiftly and noninvasively offers clinicians invaluable insights into a patient’s oxygenation status and is considered the “fifth vital sign”[1]. In the pediatric setting, where oxygen saturation (SpO2) levels hold profound implications for respiratory function and overall well-being, pulse oximetry is a vital tool in the armamentarium of healthcare providers. It plays a crucial role in assessing respiratory function and oxygenation status in children of all ages, from neonates to adolescents[2]. This technology has revolutionized the management of pediatric patients by providing real-time data on oxygen levels, enabling timely intervention and improved patient outcomes. It is used in many pediatric conditions, such as monitoring respiratory status, assessment of oxygenation during procedures, screening for congenital heart defects, monitoring sleep-disordered breathing, and home monitoring for conditions that need more than usual care[3]. Pulse oximetry operates on the spectrophotometry principle, which measures light absorption by oxygenated and deoxygenated hemoglobin molecules in the blood. The pulse oximeter emits two wavelengths of light, typically red and infrared, through a translucent part of the patient’s body, such as a finger, toe, or earlobe[4]. Oxygenated hemoglobin absorbs more infrared light, while deoxygenated hemoglobin absorbs more red light. By analyzing the ratio of absorbed light at these wavelengths, the pulse oximeter calculates the SpO2 level, expressed as a percentage (%SpO2)[5].
However, as with any diagnostic modality, understanding its advantages and limitations is paramount to its effective utilization and interpretation. Understanding the technology and its limitations is vital to avoid unnecessary testing due to erroneous readings[6]. This review article is not just a theoretical exploration of pulse oximetry in pediatric care. It is a practical guide that aims to equip clinicians with the knowledge to utilize and interpret pulse oximetry in their daily practice effectively. This article provides a nuanced understanding of its utility in pediatric practice by comprehensively exploring pulse oximetry principles, clinical applications across various pediatric conditions, and inherent advantages in enhancing patient care. Moreover, this systematic review critically examines the limitations and challenges associated with pulse oximetry use in pediatrics, including factors that may impact the accuracy and reliability of readings. By addressing these considerations, clinicians can confidently navigate pulse oximetry interpretation complexities, ensuring optimal patient management and clinical decision-making. Ultimately, this systematic review underscores the pivotal role of pulse oximetry in pediatric healthcare while emphasizing the importance of maintaining a discerning approach that acknowledges its strengths and limitations. By striking this delicate balance, clinicians can harness the full potential of pulse oximetry to improve outcomes and enhance the quality of care for pediatric patients worldwide.
MATERIALS AND METHODS
Literature searching
In conducting this systematic review, a thorough search of electronic databases, including PubMed/MEDLINE, Embase, Scopus, and Google Scholar, was conducted from inception to May 11, 2024, employing a combination of Medical Subject Headings terms and keywords relevant to pulse oximetry monitoring in pediatric patients. Additionally, reference lists of pertinent articles and reviews were manually scrutinized to identify any additional relevant studies. Studies meeting the following criteria were included: (1) Focus on pulse oximetry monitoring in pediatric populations; (2) Address the advantages, limitations, challenges, or guidelines for effective use of pulse oximetry; (3) Published in English; and (4) Available as full-text articles. Two independent reviewers screened the titles and abstracts of identified articles, with subsequent full-text reviews for potentially eligible studies.
Data resources
Data from included studies were extracted using a standardized form, encompassing study characteristics, participant demographics, intervention or exposure details, outcomes assessed, and key findings. The narrative synthesis approach was employed to summarize findings, identify themes, and organize data descriptively. Quality assessment of included studies was conducted using appropriate tools based on study design, with any limitations or biases discussed within the review.
RESULTS
The systematic review identified 231 relevant studies meeting the inclusion criteria. Figure 1 shows the flow chart of the article (106 research articles, 86 review articles, 8 systematic reviews and meta-analyses, 10 case reports, 9 letters to the editors, 6 guidelines, 3 editorials, and 3 books). These studies encompassed a range of topics related to pulse oximetry monitoring in pediatric patients, including its advantages, limitations, challenges, and guidelines for effective use. The results were categorized into several key themes to facilitate comprehensive analysis and synthesis.
Figure 1.
Flow chart of the study.
Advantages of pulse oximetry
The literature highlighted pulse oximetry’s advantages, including its noninvasive nature, real-time feedback on SpO2 levels and pulse rate, early detection of hypoxemia, suitability for various medical procedures, portability and versatility, user-friendliness, continuous monitoring capabilities, integration into telemedicine platforms, and cost-effectiveness.
limitations and challenges
Conversely, limitations and challenges identified encompassed motion artifacts, poor peripheral perfusion, ambient light interference, nail polish or acrylic nails, skin pigmentation, intravascular dyes, hemoglobin variants, carbon monoxide (CO) poisoning, changes in altitude and barometric pressure, delayed measurement compared to arterial blood gas analysis, false alarms, and alarm fatigue syndrome.
Guidelines for effective use
Guidelines for effective pulse oximetry use in pediatric patients emphasized proper sensor placement, establishment of baseline SpO2 levels, continuous monitoring, consideration of patient factors, regular equipment maintenance, alternative sensor sites for patients with poor perfusion, minimizing patient movement, staff education, and accurate documentation. Overall, the results underscored the importance of pulse oximetry monitoring in pediatric care while highlighting the need to address its limitations and challenges to optimize its effectiveness.
DISCUSSION
Historical perspective
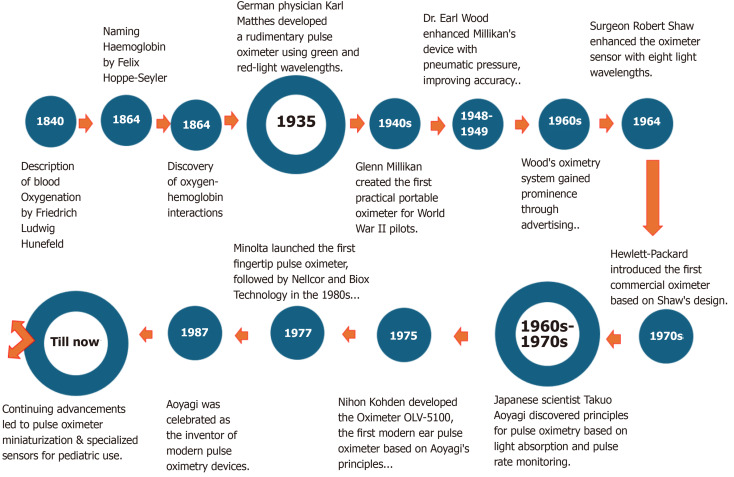
The invention of pulse oximetry reflects a success story of the continuous and successive collaboration of scientists spanning over a century and a half. In the mid-19th century, German scientists like Friedrich Ludwig Hunefeld (1840) from Germany and Felix Hoppe-Seyler (1864) began unraveling the mysteries of blood oxygenation[7]. Their discoveries laid the groundwork for the Irish-English mathematician and physicist George Gabriel Stokes in 1864 to investigate how oxygen interacts with hemoglobin in the bloodstream. Through experiments, Stokes and Hoppe-Seyler determined that hemoglobin binds to oxygen, leading to changes in blood color[8]. Despite this breakthrough, measuring blood oxygen levels remained a challenge until the development of the modern pulse oximeter, which revolutionized medical monitoring.
In 1935, German physician Karl Matthes developed a rudimentary pulse oximetry device to measure blood oxygenation through light. This device, attached to a patient’s earlobe, detects the presence of oxygenated and deoxygenated hemoglobin, which easily shines through a patient’s blood without taking a blood sample. The device detected oxygenated and deoxygenated hemoglobin using green and red-light wavelengths. Matthes later found red infrared light to be more effective. Despite its innovation, Matthes’s device was challenging to calibrate and provided saturation trends rather than precise values[9]. In the 1940s, American inventor and physiologist Glenn Millikan created the first practical and portable oximeter, primarily for World War II pilots flying at high altitudes. This device used a technique called “ear oximetry”, which involved placing a sensor on the earlobe to measure SpO2 in arterial blood. However, Millikan’s device could only measure static oxygen levels without advanced technology, offering a limited understanding of the pilot’s condition. Millikan’s original oximeter design lacked the crucial element of ensuring sufficient blood volume in the ear[7]. Recognizing this oversight, Dr Earl Wood of the Mayo Clinic substantially enhanced the device between 1948 and 1949. He ingeniously devised a method using pneumatic pressure to increase blood flow into the ear, improving accuracy and reliability for real-time readings. Wood’s innovative earpiece was a key component of his advanced oximetry system, which gained prominence through advertising in the 1960s[10].
In 1964, surgeon Robert Shaw from San Francisco enhanced the oximeter sensor by incorporating additional 8 wave lengths of light, surpassing the two utilized by Matthes’s original design. Shaw’s innovation expanded the device’s capability with eight light wave lengths, enabling more comprehensive data collection for calculating oxygenated blood levels[11]. This advancement marked the creation of the first ear oximeter, which provided absolute readings. In 1970, Hewlett Packard introduced the first commercial oximeter based on Shaw’s design. Despite being expensive and cumbersome, Shaw’s oximeter demonstrated the viability of pulse oximetry principles for commercialization. Hewlett Packard’s commercialization of the eight wave length ear oximeter paved the way for the availability of pulse oximetry devices in medical settings[12].
In the 1960s, Japanese scientist Takuo Aoyagi discovered that the ratio of red to infrared light absorption could be used to estimate arterial blood SpO2. Between 1972 and 1974, while investigating methods to enhance arterial blood flow measurement devices, he uncovered a breakthrough relevant to pulse oximetry. He realized that arterial blood oxygenation levels could be determined by monitoring the heart’s pulse rate. Aoyagi’s principle led to the development of the Oximeter OLV-5100 by Nihon Kohden in 1975, recognized as the world’s first modern ear pulse oximeter utilizing pulse oximetry based on his discovery[13]. Despite initial commercial setbacks, Aoyagi’s insight eventually gained recognition. Minolta launched the first fingertip pulse oximeter, OXIMET Met 1471, in 1977, followed by pulse oximeters from Nellcor and Biox Technology in the 1980s. By 1987, Aoyagi was celebrated as the inventor of modern pulse oximetry devices, advocating for non-invasive continuous monitoring technology in patient care. This principle has been embraced by modern pulse oximetry devices, which are now efficient and painless for patients, reflecting Aoyagi’s visionary approach to healthcare technology[14].
Over the years, pulse oximetry technology has continued to evolve. Early pulse oximeters were large, bulky, and primarily used in operating rooms and critical care settings. Technology advancements led to the miniaturization of pulse oximeters, making them more portable and suitable for use outside hospital settings. This allowed for greater flexibility in monitoring pediatric patients, both in hospitals and at home[15]. One of the key advancements in pediatric pulse oximetry was the development of sensors specifically designed to be used for infants and children. These sensors are smaller and often include adhesive attachments to secure them to a child’s finger, toe, or other appropriate site. Continuous improvement in sensor technology, sophisticated algorithms, and signal processing techniques have increased pulse oximeters’ accuracy and reliability, even in challenging pediatric populations with low perfusion or motion artifacts[16]. As a standard tool for assessing oxygenation and respiratory function across diverse medical conditions, pulse oximeters seamlessly integrate into patient monitoring systems, facilitating continuous tracking alongside vital signs like heart and respiratory rates. This integration heightens patient safety and enables early detection of respiratory issues in patients with a wide range of medical conditions. With the rise of telemedicine and remote patient monitoring technologies, pulse oximeters have assumed greater significance in pediatric care. Parents and caregivers can now monitor children’s SpO2 levels at home, guided by healthcare providers, facilitating timely interventions as needed[17]. Figure 2 shows the timeline for the discovery and invention of pulse oximetry.
Figure 2.
The Timeline for the discovery and invention of pulse oximetry.
Principles of pulse oximetry
Pulse oximetry is a non-invasive method for monitoring blood SpO2 by measuring the ratio between oxygenated and deoxygenated hemoglobin. It operates on a sophisticated understanding of hemoglobin’s behavior in response to oxygen and light. Hemoglobin is a specific blood protein responsible for carrying oxygen; every gram of hemoglobin can carry 1.34 mL of oxygen[18]. Hemoglobin exhibits positive cooperativity. When one oxygen molecule binds to one of hemoglobin’s four binding sites, it increases the affinity for oxygen in the remaining sites. This property leads to a sigmoidal oxygen dissociation curve, facilitating rapid lung oxygen loading and efficient offloading in oxygen-deficient tissues. Hemoglobin exists in two forms: Taut (T), the deoxygenated form with low oxygen affinity, and relaxed (R), the oxygenated form with high oxygen affinity[19]. These configurations result in different electromagnetic absorption properties, influencing how hemoglobin interacts with light. Pulse oximeters capitalize on these differences in light absorption between the T and R configurations[20].
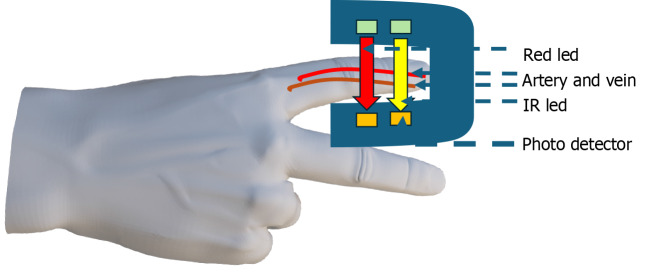
The technique involves shining light through a translucent part of the body, typically a fingertip or earlobe, and measuring the amount of light absorbed by oxygenated and deoxygenated hemoglobin (Figure 3). Pulse oximeters utilize electronic processors and light-emitting diodes (LEDs) that emit light at specific wavelengths, typically red (with a wave length of 660 nm) and infrared (with a wavelength of 940 nm)[21]. These wavelengths are chosen because they correspond to the absorption peaks of oxygenated and deoxygenated hemoglobin. Oxygenated hemoglobin absorbs more infrared light and allows more red light to pass through, while deoxygenated hemoglobin absorbs more red light and allows more infrared light to pass through. The LEDs turn on and off quickly, switching between red and infrared light about thirty times every second[20]. The oximeter measures how much light goes through (doesn’t get absorbed). These measurements change over time because there’s more blood in the arteries when the heart beats. The oximeter shows how much of the light is from the arteries by comparing the lowest and highest light readings. This helps it measure just the arterial blood. The processor then calculates the red-to-infrared light ratio, which shows how much oxygen is in the blood[22]. Following the Beer-Lambert law, the processor uses this ratio and a special table to determine the SpO2 level based on how much light gets absorbed. This law describes the relationship between the concentration of a substance in a medium and the amount of light absorbed by that substance[23]. In pulse oximetry, the absorption of light by hemoglobin is proportional to its concentration in the blood. By measuring the absorption of light at specific wavelengths, the pulse oximeter can determine the ratio of oxygenated hemoglobin to total hemoglobin and calculate the SpO2 level[24]. Pulse oximeters come in two main types: Transmittance devices and reflectance devices. Transmittance pulse oximeters, the more common type, operate by transmitting light through a body part like a fingertip or ear. The amount of oxygen in the blood affects how much light is absorbed by the tissue. A light detector on the opposite side of the probe measures the unabsorbed light, and a microprocessor computes the blood SpO2[25]. Reflectance pulse oximeters, on the other hand, are placed on the skin surface and measure the light reflected off the tissues rather than passing through them. This reflected light’s absorption is then used to determine SpO2 levels. It’s worth noting that designing reflectance devices to perform effectively is inherently more challenging[26].
Figure 3.
The pulse oximeter idea. The oximeter has a light-emitting diode on one end, and there is a phototransistor on the opposite end of the oximeter. Light-emitting diodes alternately emits red and infrared light, and part of the light will be absorbed after it passes through red blood cells in the blood vessels of the finger.
The pulsatile nature of arterial blood flow allows for the differentiation between arterial and venous blood. During each cardiac cycle, arterial blood volume increases, leading to a temporary increase in the amount of light absorbed by the pulsatile arterial blood[27]. By detecting these fluctuations in light absorption, pulse oximeters can isolate arterial blood and provide accurate SpO2 readings. The raw light absorption data collected by the pulse oximeter undergoes sophisticated signal processing algorithms to filter out noise and motion artifacts[28]. These algorithms analyze the amplitude and frequency of the pulsatile signal to extract SpO2 information accurately. These algorithms are formulated based on SaO2 measurements in healthy volunteering individuals who inhale various oxygen concentration blends, and typically, each manufacturer develops their specific algorithms. Signal processing techniques are crucial for obtaining reliable measurements, particularly in challenging conditions such as low perfusion or motion interference[29]. The SpO2 value shown reflects the average of readings taken over the preceding 3 to 6 seconds, while the information is refreshed at intervals of 0.5 to 1.0 seconds. However, pulse oximeters require periodic calibration to ensure accuracy. Calibration involves comparing the oximeter’s readings to a reference standard and making adjustments as necessary[30]. Factors such as ambient light, skin pigmentation, and device performance can affect the accuracy of pulse oximetry measurements, highlighting the importance of regular calibration procedures[31]. Therefore, pulse oximetry operates based on three main principles: Difference in light absorption by oxygenated and deoxygenated hemoglobin, pulse modulation of arterial blood flow, and sophisticated signal processing techniques. Using these three principles, pulse oximeters provide clinicians with real-time information about a patient’s oxygenation status, facilitating timely interventions and improving patient care[1]. Clinicians should realize that when there is decreased hemoglobin concentration, such as in anemia, there is a decrease in total O2 content of the blood but no change in the O2 saturation; hence, oximetry is not an effective test to evaluate oxygen status in the presence of anemia or polycythemia[32].
Special considerations of pulse oximetry in pediatric age
Several considerations are specific to pulse oximetry in pediatric patients. Children have smaller fingers and earlobes than adults. Therefore, specialized sensors designed for pediatric use are necessary to ensure proper fit and accurate readings. These sensors are often smaller in size and may include adhesive attachments to secure them to the child’s finger, toe, or other appropriate site[33]. Children, especially infants and toddlers, may be more prone to movement during monitoring, which can introduce motion artifacts and affect the accuracy of pulse oximetry readings[34]. Careful positioning of the sensor and minimizing patient movement can help mitigate this issue. Skin pigmentation can vary widely among children, which may affect the accuracy of pulse oximetry readings. Darker skin tones can absorb more light, potentially leading to lower readings. Healthcare providers should be aware of this factor and consider appropriate adjustments or alternative monitoring sites if necessary[35].
Pediatric patients, particularly neonates and infants, may have lower peripheral perfusion compared to adults, making it challenging to obtain reliable pulse oximetry readings. In such cases, choosing a monitoring site with better perfusion or using pulse oximetry in conjunction with clinical assessment can help ensure accurate monitoring[36]. Pediatric pulse oximeters may require specific calibration settings tailored to the age and size of the patient population they are intended for. Healthcare providers should ensure that pulse oximeters used in pediatric settings are appropriately calibrated to obtain accurate readings[37]. When interpreting pulse oximetry readings in pediatric patients, healthcare providers should consider factors such as the child’s age, clinical condition, and baseline SpO2 levels[38]. Contextualizing the SpO2 readings within the overall clinical picture is essential for accurate assessment and appropriate intervention. Although pulse oximetry is a valuable tool for assessing oxygenation, it has many considerations in pediatric patients[39]. Therefore, healthcare providers must be mindful of the unique considerations associated with monitoring children of varying ages and sizes. Attention to sensor size and placement, motion artifacts, skin pigmentation, perfusion, calibration, and clinical interpretation is essential for obtaining accurate and reliable pulse oximetry readings in pediatric settings. Table 1 shows some of the challenges that may face using pulse oximetry in children and how to overcome them.
Table 1.
The challenges facing using pulse oximetry in children and how to overcome them
|
Problem
|
Suggested solution
|
| Children have smaller fingers and earlobes | Use specialized sensors designed for pediatric use. These are smaller in size and may include adhesive attachments to secure them properly on the child’s finger, toe, or other appropriate site |
| Increased risk of movement during monitoring | Ensure careful positioning of the sensor and minimize patient movement during measurement to prevent motion artifacts that could affect the accuracy of pulse oximetry readings |
| Variation in skin pigmentation | Be aware that darker skin tones can absorb more light, potentially leading to lower readings. If necessary, consider appropriate adjustments or alternative monitoring sites to account for skin pigmentation differences |
| Lower peripheral perfusion in pediatric patients | Choose a monitoring site with better perfusion or use pulse oximetry in conjunction with clinical assessment to ensure accurate monitoring, especially in neonates and infants |
| Specific calibration settings for pediatric oximeters | Ensure that pulse oximeters used in pediatric settings are appropriately calibrated to obtain accurate readings, with calibration settings tailored to the age and size of the patient population |
| Consideration of clinical factors in interpretation | Contextualize SpO2 readings within the overall clinical picture, considering the child’s age, clinical condition, and baseline oxygen saturation levels for accurate assessment and appropriate intervention |
SpO2: Oxygen saturation.
Preferred placement sites for pulse oximetry probe in pediatric age
For accurate pulse oximetry readings in children, choosing the right probe placement site is crucial. In neonates, the preferable placement sites are palm of the hand followed by the sole of the foot if a reading on the palm is difficult. Some studies suggest alternative sites like the wrist or ankle in neonates, but the palm and sole are generally preferred due to better accuracy. In infants and children, the finger (index finger is the preferable one, followed by the thumb, middle finger, and the great toe), nose, earlobe, and forehead are preferable sites for pulse oximetry probe placement[40,41]. These areas have a higher vascular density than other regions, such as the chest wall, making them suitable for accurate SpO2 measurements. The choice of probe placement site depends on the clinical circumstances, and some trial and error may be necessary to find the optimal site for placement foreach patient[1]. For example, in patients with hypotension orvasoconstriction, the ear and forehead may be more reliable as these areas are less likely to have vasoconstriction than the fingers in response to catecholamines. In hypothermia, where secondary vasoconstriction occurs, the forehead probe is more reliable than the finger probe[42,43].
There are two main types of pulse oximeter probes used in pediatric patients: Reusable clip probes and single-patient adhesive probes[1]. Reusable clip probes are advantageous for their rapid deployment, ease of sampling different body sites, and cost-effectiveness, especially in outpatient settings where multiple patients are monitored sequentially with one probe[44]. On the other hand, single-patient adhesive probes offer advantages such as potentially lower transmission of infections, secure placement in patients with excessive movement, and the ability to monitor sites other than the fingers, nose, ears, and forehead. These adhesive probes are particularly useful for continuous monitoring of SpO2[45].
It is important to ensure that the probe is placed correctly on a well-perfused site. The two parts of the probe (the light emitter and the light sensor) need to be opposite each other to get an accurate reading. The probe should fit snugly but comfortably on the chosen site, avoiding excessive pressure that could restrict blood flow. In addition, the probe should not be placed on a constricted area, such as a finger with a band age or an infant’s mitten[46]. If the signal quality is poor, try shining a light on the fingertip to improve signal strength. Minimize movement during measurement to prevent interference with the readings. When using the foot for neonates or infants, secure the probe to prevent dislodging[47]. Commercially available wraps or securing methods designed for pulse oximetry on the foot can be helpful. Even after selecting the appropriate probe type, false SpO2 readings may still occur due to various factors and medical conditions[48].
For all age groups, it’s important to follow general tips to ensure accurate pulse oximetry readings. When placing the probe, ensure it fits snugly but comfortably on the chosen site, avoiding excessive pressure that could restrict blood flow. If the signal quality is poor, try shining a light on the fingertip to improve signal strength[49]. Minimize movement during measurement to prevent interference with the readings. These tips and careful consideration of clinical circumstances help optimize pulse oximetry monitoring in pediatric patients and individuals of all ages (Table 2).
Table 2.
The preferred placement sites for pulse oximeter probes in neonates, infants, and children
|
Age group
|
Preferred placement sites
|
| Neonates | The palm of the hand (preferred) |
| Sole of the foot (if palm reading is difficult) | |
| Wrist | |
| Ankle | |
| Infants | Finger (index finger preferred, followed by thumb, middle finger, and great toe), nose, earlobe, forehead |
| Children | Finger (index finger preferred, followed by thumb, middle finger, and great toe), nose, earlobe, forehead |
Clinical application and utilization of pulse oximetry in pediatric diagnosis and monitoring
Pulse oximetry is crucial in pediatric diagnosis and monitoring, offering a non-invasive and real-time assessment of SpO2 levels in children. This technology has become indispensable in various clinical settings, from neonatal intensive care units (NICUs) to general pediatric wards, emergency departments, and outpatient clinics (Table 3).
Table 3.
Clinical indications of pulse oximetry in neonates, infants, and children
|
Clinical application
|
Description
|
| Neonatal indications | |
| Fetal and neonatal care | Pulse oximetry can continuously monitor oxygen saturation during delivery and in neonates with respiratory distress syndrome, congenital heart defects, or other respiratory conditions |
| Newborn screening for CCHD | Pulse oximetry screening detects CCHD in newborns by comparing SpO2 readings between the upper and lower extremities, indicating the presence of heart defects |
| RDS | Pulse oximetry assesses oxygenation and monitors respiratory status in preterm infants with RDS, guiding oxygen therapy and evaluating response to treatment |
| BPD | Pulse oximetry monitors oxygenation and respiratory status in infants with BPD, guiding oxygen therapy, detecting complications, and assessing response to interventions |
| Apnea of prematurity | Pulse oximetry detects oxygen desaturation events associated with apnea in premature infants, allowing for prompt intervention and monitoring of respiratory status |
| PPHN | Pulse oximetry assesses oxygenation and monitors response to treatment in infants with PPHN, guiding oxygen therapy and evaluating the effectiveness of interventions |
| Neonatal methemoglobinemia | Pulse oximetry may underestimate O2 saturation in neonatal methemoglobinemia, prompting further investigation and monitoring of response to treatment |
| Postoperative care | Pulse oximetry monitors O2 saturation levels in neonates after surgery, facilitating early detection of respiratory compromise & guiding interventions for optimal recovery |
| Infancy and childhood indications | |
| Children with respiratory illnesses | Pulse oximetry is essential for managing respiratory illnesses in children. It aids in assessing oxygen saturation levels and the severity of the condition, monitoring oxygen therapy effectiveness, tracking treatment response, and guiding clinical decisions. It provides valuable insights into conditions like pneumonia, bronchiolitis, and asthma exacerbations |
| Assessment of circulatory status | Pulse oximetry is significant in evaluating circulatory status in children. It allows for the early detection of circulatory compromise and guides interventions to restore perfusion and prevent organ dysfunction. It also provides real-time feedback on treatment effectiveness, particularly in cases of shock or hypovolemia |
| Monitoring during anesthesia and sedation | Pulse oximetry is crucial for monitoring children during anesthesia and sedation. It enables continuous assessment of SpO2 levels and pulse rate. It aids in the early detection of respiratory depression, airway obstruction, and hypoxemia, ensuring patient safety during procedures requiring anesthesia or sedation |
| Management of sleep disorders | Pulse oximetry is instrumental in managing childhood sleep disorders such as OSA or central sleep apnea. It facilitates screening, assesses severity, monitors treatment effectiveness, and detects complications. It also enables home monitoring, leading to early treatment failure or disease progression detection |
| Evaluation of trauma and critical care | Pulse oximetry assists in the rapid assessment of oxygenation status in children with trauma or critical illness, aiding in the early detection of hypoxemia and respiratory compromise. It provides continuous monitoring during critical care interventions and facilitates timely escalation of care |
| Home monitoring | Pulse oximetry is valuable for monitoring various childhood disorders at home, including respiratory conditions, congenital heart diseases, neurological disorders, and neonatal complications. It enables early detection of abnormalities, prompts medical attention, and enhances accessibility to healthcare services when integrated with telemedicine technologies |
BPD: Bronchopulmonary dysplasia; CCHD: Critical congenital heart disease; OSA: Obstructive sleep apnea; PPHN: Persistent pulmonary hypertension of the newborn; RDS: Respiratory distress syndrome; SpO2: Oxygen saturation.
Fetal and neonatal care
In neonatal care, pulse oximetry can continuously monitor SpO2 during delivery in preterm infants, term neonates, and those with respiratory distress syndrome (RDS), congenital heart defects, or other respiratory conditions. Early detection of hypoxemia allows for prompt intervention, such as supplemental oxygen therapy or adjustments in ventilatory support, to optimize oxygenation and prevent hypoxic injury[50].
Fetal pulse oximetry: Fetal pulse oximetry, a technique healthcare providers use to monitor SpO2 levels in a fetus’s blood during labor and delivery, is crucial in assessing the fetus’s well-being. It provides real-time information about fetal oxygenation status, allowing for prompt intervention if necessary[51]. A specialized sensor is placed on the fetus’s scalp or another appropriate location within the birth canal to measure oxygen levels in the fetus’s blood. During labor, the fetus depends on a constant supply of oxygen from the mother’s blood through the placenta[52]. Any complications during labor, such as umbilical cord compression, placental insufficiency, or prolonged labor, can lead to fetal distress or hypoxia, which can compromise oxygen delivery to the fetus. Fetal pulse oximetry, with its ability to detect changes in fetal oxygenation, enables healthcare providers to monitor oxygen levels continuously and take immediate action[53].
Fetal pulse oximetry is a valuable tool that can be used in conjunction with other fetal monitoring methods, such as electronic fetal heart rate monitoring, to assess the overall condition of the fetus during labor. This combined approach allows healthcare providers to make timely decisions regarding interventions, such as administering oxygen to the mother, changing her position, or proceeding with a cesarean delivery if fetal distress is detected[54]. However, it is important to note that fetal pulse oximetry has limitations. It may not be suitable for all pregnancies or labor situations due to factors such as the position of the fetus, the presence of meconium, and certain maternal conditions, which may affect the accuracy and reliability of the readings. Additionally, fetal pulse oximetry is not routinely used in all labor and delivery settings[55]. It may be reserved for cases where fetal well-being is questioned or other inconclusive monitoring methods. However, the clinical effectiveness of fetal pulse oximetry is still a subject of debate[56]. For example, Bloom et al[57] discovered that monitoring fetal SpO2 does not correlate with decreased cesarean delivery rates or improved neonatal outcomes. On the other hand, East et al[58] found that employing fetal pulse oximetry to assess fetal well-being during labor led to a statistically significant decrease in operative interventions for non-reassuring fetal status compared to solely utilizing conventional cardiotocograph monitoring. This decrease occurred without any significant variance in neonatal outcomes. However, Peek et al[59] claimed that Bloom et al[57] failure to interpret the results of fetal pulse oximetry may be the cause of their findings. He also expected a higher rate of cesarean sections when using fetal pulse oximetry. He suggested having clear guidelines for intrapartum use of fetal pulse oximetry. Despite these limitations, fetal pulse oximetry remains a valuable tool in the array of fetal monitoring techniques, aiding in the comprehensive assessment of fetal health during labor and delivery[52].
Newborn screening for critical congenital heart disease: Pulse oximetry screening is recommended and integrated into routine newborn screening protocols to detect critical congenital heart disease (CCHD) in many healthcare facilities worldwide. Newborns undergo pulse oximetry screening at 24 to 48 hours of life or before discharge from the birthing facility to detect potentially life-threatening heart defects early[60]. The screening involves comparing the SpO2 readings between the upper and lower extremities to identify significant differences that may indicate underlying heart defects. Specific cutoff values for SpO2 are used to interpret the screening results. Pulse oximetry readings in neonates can indicate the presence of CCHD through several key indicators[61]. By measuring SpO2 levels simultaneously in the right hand and foot, pulse oximetry can reveal significant differences in saturation between the upper and lower extremities, suggesting impaired oxygenation or cardiac shunting characteristic of CCHD[62]. Additionally, low SpO2 levels, reflected by SpO2 readings below the normal range, may signify hypoxemia associated with certain types of CCHD. Clinical cyanosis, coupled with low SpO2 readings, further supports the diagnosis of severe hypoxemia in neonates with critical heart defects[63].
A hyperoxia-hyperventilation test can differentiate the cardiac cause of central cyanosis from the pulmonary cause in a sick newborn. This test typically involves a series of steps. Initially, an arterial blood gas sample is obtained while the neonate is breathing room air to establish baseline oxygenation levels. Subsequently, the patient is administered 100% oxygen (FiO2) for a duration of 10 minutes[64]. A repeat arterial blood gas is then performed to evaluate the response to oxygen therapy, specifically looking for an increase in the partial pressure of oxygen (PaO2) to greater than 150 mmHg. If the hypoxia is attributed to a respiratory cause, such as RDS, the PaO2 is expected to rise above the threshold of 150 mmHg with supplemental oxygen. However, in cases where hypoxia is secondary to a congenital cardiac lesion, such as a right-to-left cardiac shunt, the PaO2 may not significantly increase despite high levels of supplemental oxygen[65]. Alternatively, many physicians use pulse oximetry to monitor SpO2 levels before and after administering 10 minutes of 100% FiO2. If, after this period, the SpO2 remains below a certain threshold (typically 95%, although some references suggest 85%), it suggests that central cyanosis is likely due to an intracardiac shunt. This sequential evaluation aids in distinguishing between respiratory and cardiac causes of neonatal hypoxia, facilitating appropriate management and intervention[66].
Pulse oximetry can help the clinician expect the presence of ductal-dependent lesions in the neonates. Pulse oximetry measures SpO2 at two different sites: Pre-ductal (typically the right hand) and post-ductal (either foot). In normal circulation, there is a minor difference in SpO2 between these two sites due to the mixing of oxygenated and deoxygenated blood in the systemic circulation[67]. However, in ductal-dependent CCHD, this difference can be significant. In ductal-dependent lesions, such as hypoplastic left heart syndrome or critical aortic stenosis, oxygenated blood from the placenta preferentially flows through the patent ductus arteriosus to reach the descending aorta[68]. As a result, SpO2 is higher in the pre-ductal site (right hand) compared to the post-ductal site (lower extremities)[69]. Conversely, the lower extremities receive deoxygenated blood, leading to lower SpO2 levels post-ductal. This discrepancy in SpO2 between the pre-ductal and post-ductal sites, known as “differential cyanosis”, is highly suggestive of ductal-dependent CCHD[70]. Infants with ductal-dependent lesions may present with cyanosis and hypoxemia due to inadequate oxygen delivery to the body. Pulse oximetry readings may reveal lower SpO2 levels in the post-ductal site, reflecting impaired systemic perfusion and oxygenation[71].
In the transposition of the great arteries, the aorta and pulmonary artery are switched, causing oxygen-rich blood to be pumped back to the lungs instead of the body. This results in cyanosis and hypoxemia shortly after birth. Pulse oximetry screening in newborns can detect lower SpO2 levels, often showing a significant difference in saturation between the upper and lower extremities. This discrepancy alerts healthcare providers to the possibility of transposition of the great arteries or other forms of CCHD, prompting further diagnostic evaluation and timely intervention[72]. Tetralogy of Fallot (TOF) is a congenital heart defect characterized by four abnormalities in the heart’s structure, including a ventricular septal defect, pulmonary stenosis, overriding aorta, and right ventricular hypertrophy. In infants with TOF, SpO2 levels may be lower than normal due to decreased pulmonary blood flow and the mixing of oxygenated and deoxygenated blood[73]. Pulse oximetry screening can detect hypoxemia and cyanosis, indicating inadequate blood oxygenation. Additionally, pulse oximetry readings may reveal SpO2 discrepancies between the upper and lower extremities, reflecting the presence of a significant shunt associated with TOF[74].
Monitoring the response to interventions, such as oxygen therapy or prostaglandin infusion, allows for dynamic assessment of cardiac function and pulmonary circulation in neonates suspected of having CCHD. Continuous monitoring facilitates the identification of trends in SpO2, enabling early recognition of deteriorating cardiac status and prompt intervention[75]. Pulse oximetry is a valuable tool for detecting CCHD in neonates by assessing SpO2 discrepancies, hypoxemia, and response to interventions, leading to timely diagnosis and management of these critical conditions[76].
Neonates with abnormal pulse oximetry screening results undergo additional diagnostic tests, such as echocardiography, to confirm or rule out the presence of CCHD. Early detection of CCHD allows prompt referral to pediatric cardiology services for a comprehensive evaluation and timely intervention if necessary[77]. Pulse oximetry screening for CCHD has led to the early detection of heart defects in newborns, enabling timely interventions that can improve outcomes and reduce morbidity and mortality associated with undiagnosed CCHD. Identifying infants with CCHD before they become symptomatic allows for proactive management and appropriate planning for medical and surgical interventions[78]. While pulse oximetry screening has proven effective, it is not foolproof, and false-positive and false-negative results can occur. Factors such as low birthweight, prematurity, transient physiological changes, and technical issues with the screening process can affect the accuracy of the results[79]. Healthcare providers should be aware of these limitations and use clinical judgment to interpret screening results in the context of the individual patient’s clinical presentation.
RDS: Preterm infants are at risk of developing RDS due to immature lung development and surfactant deficiency. RDS in newborns, also known as hyaline membrane disease, is a condition characterized by inadequate lung development and surfactant deficiency, leading to respiratory distress shortly after birth[80]. While pulse oximetry alone can not definitively diagnose RDS, it plays a vital role in assessing oxygenation and monitoring respiratory status in affected infants. Infants with RDS often present with hypoxemia due to impaired gas exchange in the lungs[81]. Pulse oximetry allows for continuous non-invasive monitoring of SpO2 levels in these infants. Lower-than-normal SpO2 readings indicate inadequate oxygenation and may prompt further evaluation for RDS. Infants with RDS frequently require supplemental oxygen to maintain adequate SpO2 levels[82]. Pulse oximetry is used to titrate oxygen therapy, ensuring that SpO2 levels are within the target range while avoiding hyperoxia or hypoxia. Monitoring SpO2 trends helps healthcare providers adjust oxygen therapy as needed based on the infant’s respiratory status[83]. Pulse oximetry is valuable for assessing the response to treatment interventions in infants with RDS. Initiating interventions such as supplemental oxygen, nasal continuous positive airway pressure, or mechanical ventilation aims to improve oxygenation and respiratory function[84]. Monitoring SpO2 levels before and after treatment can gauge the effectiveness of interventions and guide further management decisions. Infants with RDS are at risk of developing complications such as respiratory failure, pneumothorax, or bronchopulmonary dysplasia (BPD)[85]. Pulse oximetry facilitates early detection of worsening respiratory status or the onset of complications by monitoring changes in SpO2 levels and providing real-time feedback to healthcare providers. Serial measurements of SpO2 over time allow for longitudinal monitoring of respiratory status and oxygenation trends in infants with RDS[86]. Continuous pulse oximetry monitoring provides valuable information on the infant’s response to treatment, disease progression, and readiness for weaning off supplemental oxygen support[87].
BPD: BPD is a chronic lung disease that primarily affects premature infants who require mechanical ventilation and oxygen therapy for an extended period. While pulse oximetry alone cannot definitively diagnose BPD, it is a valuable tool in assessing oxygenation and monitoring respiratory status in affected infants[88]. Infants with BPD often have ongoing respiratory issues and may become oxygen-dependent and require supplemental oxygen to maintain adequate SpO2 levels. Continuous pulse oximetry monitoring is used to titrate oxygen therapy, ensuring that SpO2 levels are within the target range while minimizing the risk of hyperoxia or hypoxia. Pulse oximetry allows for continuous monitoring of SpO2 trends over time[89]. In infants with BPD, fluctuations in SpO2 levels may indicate changes in respiratory status, disease progression, or response to treatment. Healthcare providers use these trends to adjust oxygen therapy and assess the effectiveness of interventions. Infants with BPD are at increased risk of respiratory distress episodes, such as apnea, desaturation, or bradycardia[90]. Pulse oximetry provides real-time monitoring of SpO2 levels during these episodes, allowing for prompt intervention and management to stabilize the infant’s respiratory status. Infants with BPD may exhibit instability in oxygenation, with fluctuations in SpO2 levels during periods of activity, feeding, or respiratory distress[91]. Pulse oximetry helps identify episodes of oxygen desaturation or instability, guiding healthcare providers in optimizing respiratory support and monitoring the infant’s response to interventions. Serial measurements of SpO2 over time enable longitudinal monitoring of respiratory status and oxygenation trends in infants with BPD[92]. Continuous pulse oximetry monitoring provides valuable information on the infant’s respiratory stability, response to treatment, and readiness for weaning off supplemental oxygen support as the disease resolves[93].
Apnea of prematurity: Apnea of prematurity (AOP) is a common condition characterized by episodes of breathing pauses in premature infants. While pulse oximetry alone cannot definitively diagnose AOP, it plays acritical role in monitoring respiratory status and detecting apnea-related oxygen desaturation events[94]. Infants with AOP may experience oxygen desaturation during apneic episodes due to decreased respiratory effort or airflow obstruction. Pulse oximetry continuously monitors SpO2 levels in these infants, allowing healthcare providers to detect and quantify episodes of oxygen desaturation associated with apnea. While pulse oximetry primarily measures SpO2, it can indirectly signal the presence of apnea by detecting associated oxygen desaturation events[95]. A sudden decrease in SpO2 levels below the normal range may indicate the onset of an apneic episode, prompting further evaluation and intervention. Apnea episodes in premature infants often coincide with bradycardia and oxygen desaturation. Pulse oximetry, in conjunction with heart rate monitoring, helps identify apnea-bradycardia events by detecting simultaneous decreases in SpO2 and heart rate[96]. These events are suggestive of apnea and warrant clinical intervention. Continuous pulse oximetry monitoring provides real-time feedback on SpO2 levels, allowing healthcare providers to assess respiratory status and response to interventions[97]. Monitoring SpO2 trends over time helps identify apnea and oxygen desaturation patterns, guiding treatment decisions and adjustments in respiratory support. Infants with AOP may require supplemental oxygen to maintain adequate oxygenation during apneic episodes. Pulse oximetry enables titration of oxygen therapy to target SpO2 levels, ensuring optimal oxygenation while minimizing the risk of hyperoxia or hypoxia during apnea events[98].
Persistent pulmonary hypertension of the newborn: Persistent pulmonary hypertension of the newborn (PPHN) is a life-threatening condition characterized by elevated pulmonary vascular resistance and right-to-left shunting of blood, resulting in hypoxemia. While pulse oximetry alone cannot definitively diagnose PPHN, it plays a crucial role in assessing oxygenation and monitoring response to treatment[70]. Infants with PPHN typically present with severe hypoxemia due to impaired oxygenation secondary to pulmonary hypertension. Pulse oximetry continuously monitors SpO2 levels, allowing healthcare providers to promptly detect and quantify the degree of hypoxemia[99]. Persistent low SpO2 readings despite oxygen therapy may raise suspicion for PPHN. Oxygen therapy isa cornerstone of management for PPHN, aimed at improving oxygenation and alleviating hypoxemia. Pulse oximetry provides real-time feedback on SpO2 levels, enabling healthcare providers to assess the effectiveness of oxygen therapy and titrate supplemental oxygen to target SpO2 levels[100]. Serial measurements of SpO2 over time help monitor SpO2 trends in infants with PPHN. Pulse oximetry allows healthcare providers to assess the stability of oxygenation, detect fluctuations in SpO2 levels, and evaluate the response to interventions such as oxygen therapy, vasodilator therapy, or mechanical ventilation[101]. Pulse oximetry helps differentiate hypoxemia associated with PPHN from other causes, such as RDS, pneumonia, or congenital heart defects. The characteristic pattern of hypoxemia in PPHN, often refractory to oxygen the rapyalone, may raise suspicion for the condition. Infants with PPHN may require intensive care management, including mechanical ventilation, inhaled nitric oxide therapy, and hemodynamic support[102]. Pulse oximetry allows for continuous monitoring of SpO2 levels during treatment, guiding adjustments in therapy and assessing the infant’s response to interventions[103].
Neonatal methemoglobinemia: Neonatal methemoglobinemia is a condition characterized by elevated levels of methemoglobin, a form of hemoglobin that cannot bind oxygen effectively. While pulse oximetry is generally reliable for detecting SpO2 levels in the presence of normal hemoglobin, it may underestimate SpO2 in the presence of methemoglobinemia[104]. Methemoglobin absorbs light differently than oxygenated or deoxygenated hemoglobin, leading to altered light absorption patterns[105]. Pulse oximeters may inaccurately measure SpO2 in the presence of methemoglobinemia, resulting in lower-than-actual readings. Despite oxygen therapy, persistent low SpO2 readings may prompt further investigation for methemoglobinemia[106]. Some advanced pulse oximeters off era feature known as methemoglobin pulse oximetry, which specifically measures the percentage of methemoglobin in the blood. Methemoglobin pulse oximetry readings can provide additional information about the presence and severity of methemoglobinemia in neonates[107]. In neonates with unexplained cyanosis or persistent hypoxemia despite adequate oxygen therapy, clinicians may suspect methemoglobinemia as a possible underlying cause. Pulse oximetry findings and clinical assessment and history can raise suspicion for methemoglobinemia and prompt further diagnostic evaluation, such as blood gas analysis or co-oximetry[108]. Treatment of neonatal methemoglobinemia typically involves the administration of methylene blue or exchange transfusion to reduce methemoglobin levels and improve oxygen-carrying capacity. Pulse oximetry can monitor the response to treatment, with increasing SpO2 levels indicating successful reversal of methemoglobinemia[109].
Postoperative care: Neonates undergoing surgical procedures, such as congenital heart or abdominal surgeries, require close monitoring of SpO2 levels during the postoperative period[110]. Pulse oximetry is used to assess respiratory status, detect hypoxemia, and monitor for complications such as atelectasis, pneumothorax, or airway obstruction. Continuous SpO2 monitoring facilitates early detection of postoperative respiratory compromise and guides interventions to optimize oxygenation and ventilation[4].
Case study: Management of a neonate with RDS.
Patient background: A full-term neonate is admitted to the NICU with respiratory distress shortly after birth. The infant was delivered via emergency cesarean section due to fetal distress during labor.
Clinical presentation: Upon admission, the neonate exhibits tachypnea (respiratory rate of 70 breaths per minute), nasal flaring, and intercostal retractions. Initial assessment reveals cyanosis of the extremities. The infant’s SpO2 on room air is 82%.
Pulse oximetry monitoring and management: Continuous pulse oximetry monitoring is initiated using a neonatal-specific pulse oximeter with appropriate sensor placement on the infant’s right hand. The pulse oximeter displays fluctuating SpO2 readings between 80% and 88%, indicating intermittent hypoxemia despite supplemental oxygen therapy via nasal cannula at 2 liters per minute.
Clinical decision-making: Based on pulse oximetry readings and clinical assessment, the NICU team adjusts the oxygen therapy to maintain SpO2 levels between 88% and 92%, aiming to balance oxygenation while avoiding hyperoxia. Frequent bedside assessments, including periodic blood gas analysis, confirm the effectiveness of therapy adjustments in improving the infant’s oxygenation status.
Outcome: Over the next 24 hours, the neonate’s respiratory distress gradually improves. Pulse oximetry continues to guide oxygen therapy adjustments, ensuring optimal SpO2 levels without compromising respiratory function. The infant is weaned off supplemental oxygen successfully by the third day of admission, and pulse oximetry monitoring is continued intermittently to monitor respiratory status during feedings and sleep.
Conclusion: This case study highlights the critical role of pulse oximetry in managing RDS in neonates. By providing continuous, non-invasive monitoring of SpO2 levels, pulse oximetry guided timely interventions and optimized oxygen therapy, contributing to improved clinical outcomes and ensuring the safe transition from NICU to regular nursery care. This case study exemplifies how pulse oximetry is used in clinical practice to monitor and manage oxygenation in neonates with respiratory distress, demonstrating its practical application and impact on patient care.
Infants and children care
Children with respiratory illnesses: Pulse oximetry plays a vital role in managing respiratory illnesses in children, providing valuable insights into their SpO2 levels and respiratory status. This non-invasive monitoring technique allows healthcare providers to promptly assess oxygen levels, particularly in conditions like pneumonia, bronchiolitis, and asthma exacerbations, where hypoxemia can occur. Detecting hypoxemia early enables timely interventions to improve oxygenation and prevent complications[111]. In pediatric respiratory illnesses, pulse oximetry serves several key purposes. Firstly, it helps gauge the severity of the condition by indicating the extent of hypoxemia. Low SpO2 levels may signal respiratory distress or failure, guiding decisions regarding the need for hospitalization, intensive care, or advanced respiratory support like non-invasive ventilation[112]. Additionally, pulse oximetry is crucial for monitoring the effectiveness of oxygen therapy. Healthcare providers can adjust oxygen flow rates by regularly assessing SpO2 levels to optimize delivery while avoiding oxygen toxicity[113]. Moreover, pulse oximetry aids in tracking the response to treatment. Changes in SpO2 levels over time can indicate the efficacy of interventions such as bronchodilators, corticosteroids, or antibiotics, helping clinicians tailor management strategies accordingly[114]. In emergency departments or hospital settings, pulse oximetry readings help make decisions regarding discharge or continued hospitalization. Stable SpO2 levels may suggest readiness for discharge, while persistent hypoxemia may necessitate further observation or treatment[115].
SpO2 is a sensitive marker for assessing disease severity in conditions characterized by ventilation/perfusion mismatch, such as asthma exacerbations, acute bronchiolitis, chronic lung disease of prematurity, and pneumonia[116]. Conversely, in cases of proximal airway obstruction (e.g., acute laryngotracheitis, vocal cord dysfunction, or foreign-body aspiration), SpO2 may not reliably reflect disease severity. Hypoxemia in such instances primarily arises from hypoventilation, leading to elevated PaCO2 levels[117]. Consequently, patients may not exhibit significantly low SpO2 readings, as a SpO2 below 90% typically corresponds to a PaCO2 level of over 80 mmHg, according to the alveolar gas equation[18]. It is important to recognize that concurrent diffuse peripheral airway obstruction (e.g., laryngotracheobronchitis) may contribute to ventilation/perfusion mismatch and decrease SpO2 levels. However, in this scenario, hemoglobin desaturation signifies a secondary physiological phenomenon rather than the disorder’s primary mechanism[118].
Pulse oximetry facilitates home monitoring for children with chronic respiratory conditions like asthma or cystic fibrosis. Portable pulse oximeters allow parents or caregivers to assess a child’s SpO2 levels regularly, especially during respiratory symptom episodes[3]. Abnormal readings prompt timely medical evaluation and intervention, enhancing the management of chronic respiratory conditions. While pulse oximetry is widely used for continuous SpO2 monitoring in infants and children, its routine use in acute respiratory illness is not universally recommended[86]. The American Academy of Family Physicians advises against continuous pulse oximetry unless the child requires supplemental oxygen. Continuous monitoring has been associated with increased admission rates and longer hospital stays, highlighting the need for judicious use based on clinical assessment[119]. However, pulse oximetry is still an invaluable tool in managing respiratory illnesses in children. It provides real-time information on oxygenation status and guides clinical decision-making. By incorporating pulse oximetry into comprehensive care protocols, healthcare providers can optimize outcomes for pediatric patients with respiratory conditions[120].
Assessment of circulatory status: Pulse oximetry, a precise and accurate tool, plays a significant role in assessing circulatory status in children. It provides valuable information about tissue perfusion and oxygen delivery. By measuring SpO2 levels in the blood, pulse oximetry enables healthcare providers to confidently evaluate the adequacy of circulatory function in pediatric patients[121]. In children with circulatory compromise, such as shock or hypovolemia, pulse oximetry can detect changes in tissue oxygenation early, allowing for prompt intervention to restore perfusion and prevent organ dysfunction[122]. Additionally, pulse oximetry can help monitor the response to interventions to improve circulatory status, such as fluid resuscitation or vasopressor therapy. By continuously monitoring SpO2 levels, pulse oximetry provides real-time feedback on the effectiveness of treatment strategies, guiding further management decisions[123]. Overall, pulse oximetry is a valuable tool in assessing circulatory status in children, aiding in the early detection of circulatory compromise and facilitating timely interventions to optimize patient outcomes[124].
Monitoring during anesthesia and sedation: Pulse oximetry is crucial in monitoring children during anesthesia and sedation, providing a continuous and non-invasive assessment of SpO2 levels and pulse rate. For instance, in a recent case, a child undergoing anesthesia experienced a sudden drop in SpO2 levels, immediately detected by the pulse oximeter. This allowed the healthcare team to intervene promptly and prevent a potential crisis[125,126]. This monitoring technique is essential for ensuring the safety and well-being of pediatric patients undergoing anesthesia or sedation procedures. During anesthesia or sedation, there is a risk of respiratory depression, airway obstruction, and hypoxemia, particularly in children, due to their unique physiological characteristics and vulnerability[127]. Pulse oximetry allows healthcare providers to detect changes in SpO2 levels early, enabling prompt intervention to prevent hypoxemia-related complications such as tissue hypoxia, organ dysfunction, or cardiac arrest[128]. The continuous monitoring of SpO2, a precise assessment tool, helps anesthesia providers assess the adequacy of ventilation and oxygenation throughout the procedure. Any decline in SpO2 can indicate airway compromise, hypoventilation, or respiratory depression, prompting immediate corrective measures such as airway repositioning, oxygen supplementation, or assisted ventilation[129].
Furthermore, pulse oximetry aids in titrating the delivery of supplemental oxygen during anesthesia or sedation. However, it’s important to note that pulse oximetry may not always accurately reflect the patient’s true oxygenation status, especially in certain clinical conditions[66]. By closely monitoring SpO2 levels, healthcare providers can adjust oxygen flow rates to maintain optimal oxygenation while minimizing the risk of hyperoxia-associated adverse effects, such as absorption atelectasis or oxygen toxicity[130]. In addition to oxygenation monitoring, pulse oximetry provides valuable information about the child’s cardiovascular status through continuous pulse rate monitoring. Changes in pulse rate can indicate hemodynamic instability, cardiovascular depression, or adverse drug reactions, prompting further assessment and intervention as needed[131]. Generally, pulse oximetry is not only an indispensable tool for ensuring the safety and effective management of children undergoing anesthesia or sedation, but it also proves to be a cost-effective investment[132]. By facilitating real-time monitoring of SpO2 and pulse rate, pulse oximetry enhances the detection of respiratory and cardiovascular compromise, allowing for timely intervention and optimization of patient outcomes, thereby reducing the need for more expensive interventions or treatments[133].
Management of sleep disorders: Pulse oximetry plays a significant role in managing childhood sleep disorders, particularly in conditions such as obstructive sleep apnea (OSA) or central sleep apnea[134]. It is a valuable screening tool for sleep-disordered breathing. It allows healthcare providers to assess SpO2 levels during sleep and detect abnormalities indicative of sleep apnea, such as recurrent oxygen desaturation events orthose associated with apnea or hypopnea episodes[135]. Additionally, pulse oximetry provides essential information about the severity of sleep-disordered breathing in children by quantifying the frequency and magnitude of oxygen desaturation events and guiding treatment decisions and interventions[136]. Moreover, it is instrumental in monitoring the effectiveness of treatments such as continuous positive airway pressure therapy, adenotonsillectomy, or weight management by assessing the response to treatment and adjusting management strategies accordingly[137]. Pulse oximetry also helps identify complications associated with childhood sleep disorders, such as nocturnal hypoxemia, hypercapnia, or cardiac arrhythmias, prompting further evaluation and management[138]. Furthermore, portable pulse oximeters enable home monitoring of SpO2 levels, allowing parents or caregivers to track overnight SpO2 trends and detect abnormalities suggestive of sleep-related breathing disturbances. This home monitoring facilitates early detection of treatment failure or disease progression, leading to timely medical intervention and improved outcomes for children with sleep-related breathing disturbances[139].
Evaluation of trauma and critical care: Pulse oximetry, a crucial tool, is instrumental in the evaluation of children with trauma and those in need of critical care[140]. In trauma cases, it aids in rapidly assessing oxygenation status, providing immediate insight into the child’s respiratory function. By measuring SpO2 levels, pulse oximetry helps identify hypoxemia, a condition that may result from respiratory compromise due to traumatic injuries such as chest trauma, pneumothorax, or airway obstruction. The early detection of hypoxemia is vital, as it allows for initiating prompt interventions to optimize oxygenation and prevent secondary complications[141]. Additionally, pulse oximetry assists healthcare providers in monitoring respiratory status during critical care interventions, such as intubation, mechanical ventilation, or oxygen therapy[142]. Continuous monitoring of SpO2 levels enables real-time assessment of treatment efficacy and early detection of respiratory deterioration or airway compromise, prompting timely adjustments to the management plan[143]. Moreover, pulse oximetry aids in the detection of potential complications associated with trauma or critical illness, including respiratory failure, acute RDS, or sepsis. By providing continuous SpO2 monitoring, pulse oximetry facilitates the timely identification of deteriorating respiratory function or oxygenation status, allowing for prompt escalation of care and initiation of lifesaving interventions[144]. Therefore, pulse oximetry enables healthcare providers to be proactive and vigilant, making timely assessments of oxygenation status and facilitating appropriate interventions to optimize patient outcomes.
Home monitoring: Pulse oximetry is an important tool for monitoring various childhood disorders at home[38]. It provides valuable information about SpO2 levels non-invasively, especially in children with chronic respiratory disorders like asthma, cystic fibrosis, or BPD. By regularly monitoring SpO2 levels at home, parents or caregivers can assess the child’s respiratory status, particularly during episodes of coughing, wheezing, or shortness of breath[3]. Abnormal readings can alert them to seek medical attention promptly. Pulse oximetry is also useful for children with sleep-related breathing disorders like OSA[145]. Continuous overnight oximetry monitoring helps identify episodes of oxygen desaturation during sleep, which is a characteristic of OSA. Parents or caregivers can record and report these findings to healthcare providers for further evaluation and management[146].
Children with congenital heart diseases, such as cyanotic heart defects, may need ongoing monitoring of their SpO2 levels at home. Pulse oximetry enables parents or caregivers to detect any fluctuations in SpO2, which could indicate worsening heart function or complications. Early abnormalities detection allows prompt medical attention and intervention[147]. Some neurological disorders, such as seizures or neuromuscular diseases, can affect respiratory function and oxygenation. Pulse oximetry monitoring at home helps assess the child’s respiratory status during periods of increased risk, such as seizures or respiratory muscle weakness[148]. Abnormal SpO2 levels may indicate the need for immediate intervention or adjustment of treatment. Premature infants or those with a history of neonatal complications may also require home monitoring of their SpO2 levels to detect episodes of desaturation or apnea[149]. Pulse oximetry allows parents or caregivers to closely monitor the infant’s respiratory status, particularly during sleep or periods of illness. Any deviations from normal SpO2 levels can prompt medical evaluation and intervention. Regular pulse oximetry monitoring at home allows for early detection of abnormalities, facilitating timely intervention and improved management of the child’s condition[150].
Pulse oximetry, integrated with telemedicine technologies, offers an innovative approach to home monitoring of childhood disorders. Caregivers can remotely share real-time pulse oximetry data with healthcare providers through telemedicine platforms, allowing for continuous monitoring and timely intervention[151]. This integration enhances the accessibility of healthcare services, especially for families living in remote areas or with limited access to specialized medical facilities. Additionally, telemedicine consultations enable healthcare providers to interpret pulse oximetry readings remotely, guiding treatment adjustments or further diagnostic steps as needed[152]. The combination of pulse oximetry with telemedicine enhances the effectiveness of home monitoring, improving the management of various childhood disorders and promoting better health outcomes[153].
Case study: Management of an infant with bronchiolitis
Patient background: A 6-month-old male infant is brought to the pediatric emergency department by his parents due to worsening cough, wheezing, and increased respiratory effort over the past two days. The infant was born full-term without complications and had no significant medical history.
Clinical presentation: Upon assessment, the infant appears anxious and breathes rapidly with nasal flaring and chest retractions. Auscultation reveals bilateral wheezing and diminished breath sounds in the lower lung fields. The infant’s initial SpO2 on room air is 88%.
Pulse oximetry monitoring and management: Continuous pulse oximetry monitoring is initiated using a pediatric pulse oximeter with a sensor placed on the infant’s right index finger. The pulse oximeter displays SpO2 readings fluctuating between 86% and 92% during periods of rest and drops to 82% during coughing episodes.
Clinical decision-making: Based on pulse oximetry readings and clinical assessment, the pediatric team administered supplemental oxygen via nasal cannula at two liters per minute to maintain SpO2 levels above 92%. Serial pulse oximetry measurements guide the titration of oxygen therapy to ensure adequate oxygenation without over-oxygenating the infant.
Outcome: Over the next 24 hours, the infant’s respiratory distress improves with oxygen therapy. Pulse oximetry monitoring continues to guide clinical decisions, including adjusting oxygen flow rate during periods of activity and sleep. The infant successfully transitions to room air after demonstrating stable SpO2 levels above 94% for several hours.
Conclusion: This case study illustrates the use of pulse oximetry in managing respiratory distress in infants with bronchiolitis. By providing real-time feedback on SpO2 levels, pulse oximetry facilitated the timely initiation and adjustment of oxygen therapy, optimizing respiratory support and contributing to the infant’s clinical improvement. This example demonstrates how pulse oximetry is integral in managing respiratory conditions in infants, ensuring appropriate oxygenation and guiding the rapeutic interventions based on continuous monitoring of SpO2 levels.
Diseases and conditions that need specific consideration when using pulse oximetry
Anaemia: Pulse oximetry plays a crucial role in monitoring patients with anemia. While pulse oximetry provides valuable information about SpO2, it’s essential to understand its limitations in the context of anemia. In patients with anemia, the reduced hemoglobin levels can affect the accuracy of SpO2 readings[1]. Since hemoglobin is responsible for carrying oxygen, lower hemoglobin concentrations mean less oxygen is available to bind to. As a result, pulse oximeters may report lower SpO2 values even if the oxygen-carrying capacity of the available hemoglobin is normal[24]. Additionally, in severe cases of anemia where tissue oxygen delivery is compromised, pulse oximetry may underestimate the severity of hypoxemia. This discrepancy occurs because pulse oximeters measure the SpO2 of hemoglobin within the blood vessels but may not accurately reflect tissue oxygenation[154].
Clinicians should interpret pulse oximetry readings in patients with anemia cautiously and consider other clinical indicators such as respiratory rate, heart rate, and clinical symptoms to accurately assess the overall oxygen status[155]. In cases of uncertainty or severe anemia, arterial blood gas analysis may be necessary to determine the PaO2 and assess tissue oxygenation more directly[156]. Despite its limitations, pulse oximetry remains a valuable tool for monitoring patients with anemia, especially in conjunction with other clinical assessments. By providing real-time information about SpO2, pulse oximetry helps guide clinical decision-making and ensures timely interventions to optimize patient care[157].
Polycythemia: Polycythemia is a condition characterized by excess red blood cells in the bloodstream. Using pulse oximetry in patients with polycythemia presents certain challenges and considerations. Polycythemia can affect the accuracy of pulse oximetry readings due to changes in blood viscosity and oxygen-carrying capacity[158]. In individuals with polycythemia, the increased number of red blood cells can result in higher hemoglobin levels, potentially leading to falsely elevated SpO2 readings on pulse oximetry and decreased oxygen affinity to hemoglobin[159]. This is because pulse oximeters measure the percentage of oxygenated hemoglobin in the blood, and the higher hemoglobin levels in polycythemia can skew these readings[154]. Furthermore, the increased viscosity of the blood in polycythemia may affect peripheral perfusion, which can also influence pulse oximetry measurements. Reduced peripheral perfusion can result in slower capillary refill times. It may lead to inaccurately low SpO2 readings on pulse oximetry, particularly in extremities where the probe is typically placed[160]. Healthcare providers must be aware of these potential limitations when interpreting pulse oximetry readings in individuals with polycythemia. Clinicians may need to consider alternative methods of assessing oxygenation in these patients, such as arterial blood gas analysis, particularly if there are concerns about the accuracy of pulse oximetry readings[161]. Pulse oximetry can differentiate polycythemia vera from secondary erythrocytosis, as normal SpO2 is one of the major criteria of polycythemia vera[162]. However, patients with polycythemia vera could have low SpO2 due to other causes of hypoxia[163].
Metabolic derangement: Pulse oximetry is invaluable in assessing patients with acid-base disorders, providing essential information about SpO2 levels. However, it’s important to understand how acid-base disturbances can influence pulse oximetry readings and interpretation. Acidosis, whether respiratory or metabolic, can significantly impact pulse oximetry readings. One notable effect is the left shift of the oxygen is sociation curve, where hemoglobin’s increased affinity for oxygen may yield higher saturation levels despite tissue hypoxia[164]. Additionally, acidosis can induce peripheral vasoconstriction, reducing blood flow to extremities and potentially affecting the accuracy of SpO2 measurements[165]. Changes in hemoglobin affinity due to acidosis may further complicate readings, with severe cases impairing oxygen binding, potentially resulting in lower saturation levels[166]. Respiratory compensation mechanisms to correct acid-base imbalances may also influence pulse oximetry readings through alterations in respiratory rate and depth[167]. Moreover, acidosis-induced changes in tissue perfusion can lead to tissue hypoxia, which may not always be reflected in SpO2 measurements due to factors like peripheral vasoconstriction[168]. Consequently, clinicians should interpret pulse oximetry readings cautiously in the context of acidosis, considering other clinical parameters for a comprehensive assessment of oxygenation status.
On the other hand, respiratory or metabolic alkalosis can also impact pulse oximetry readings, albeit indifferent ways than acidosis. In alkalosis, the oxygen dissociation curve shifts to the right, causing hemoglobin to have a decreased affinity for oxygen[169]. This shift can result in lower saturation levels detected by pulse oximetry, even in adequate tissue oxygenation. Additionally, alkalosis may induce peripheral vasodilation, increasing blood flow to extremities and potentially affecting SpO2 measurements[170]. Changes in respiratory rate and depth associated with respiratory compensation for alkalosis may also influence pulse oximetry readings, like acidosis[171]. Furthermore, alterations in tissue perfusion due to alkalosis-induced vasodilation may impact SpO2 readings, with increased blood flow potentially leading to higher saturation levels being detected[172]. Overall, clinicians should consider these effects of alkalosis when interpreting pulse oximetry readings, ensuring a comprehensive assessment of oxygenation status alongside other clinical parameters.
Cardiac arrhythmia: Arrhythmia can affect pulse oximetry readings by causing irregular blood flow in the arteries, leading to fluctuations in the pulsatile signal detected by the oximeter[173]. This irregularity can result in inaccurate SpO2 measurements, as the oximeter may have difficulty distinguishing between arterial and venous blood pulsations[174]. Moreover, arrhythmias like atrial fibrillation can cause rapid and irregular heartbeats, further complicating the interpretation of pulse oximetry readings[175]. In such cases, healthcare providers may need to rely on alternative methods for assessing oxygenation status, such as arterial blood gas analysis, to obtain more accurate measurements.
Certain modifications or considerations may be necessary to ensure accurate readings when using pulse oximetry in patients with arrhythmias. One common approach is to use pulse oximeters with advanced signal processing algorithms designed to filter out noise and motion artifacts more effectively[176]. These algorithms can help mitigate the effects of irregular pulsations caused by arrhythmias and improve the accuracy of SpO2 measurements. Additionally, some pulse oximeters may offer specific settings or modes tailored for use in patients with arrhythmias. These settings may adjust the device’s sensitivity or filtering parameters to accommodate irregular heart rhythms better and optimize signal detection[177].
Healthcare providers should also monitor the quality of the pulse oximetry wave form displayed on the device. Irregular or inconsistent waveforms may indicate poor signal quality, which could lead to unreliable SpO2 readings, particularly in patients with arrhythmias[174]. While pulse oximetry remains a valuable tool for monitoring oxygenation in patients with arrhythmias, healthcare providers should be aware of its limitations and consider supplementary assessments, such as clinical evaluation and arterial blood gas analysis, when necessary to ensure comprehensive patient care[178].
Hypothermia: Hypothermia poses challenges and can significantly affect accurate pulse oximetry readings due to its impact on peripheral perfusion and tissue oxygenation[42]. When the body temperature drops below normal levels, peripheral vasoconstriction occurs as a physiological response to conserve heat, reducing blood flow to the extremities. This decrease in peripheral perfusion can result in weaker pulsatile signals detected by the pulse oximeter, potentially leading to inaccurate readings of SpO2[179].
Furthermore, hypothermia can also affect the oxygen dissociation curve, altering hemoglobin’s affinity for oxygen[180]. As body temperature decreases, hemoglobin’s affinity for oxygen increases, making it more difficult to release oxygen to the tissues. This shift in the oxygen dissociation curve can lead to falsely elevated SpO2 readings on pulse oximetry, as hemoglobin may hold onto oxygen more tightly than usual, even in tissues experiencing oxygen deprivation[181].
In clinical practice, healthcare providers should be cautious when interpreting pulse oximetry readings in hypothermic patients, considering both false highs and false lows. To mitigate the effects of hypothermia on peripheral perfusion, local rubbing or heating of cold extremities before applying the probe may temporarily improve perfusion in infants. However, for hypothermic patients, monitoring with a forehead probe is an alternative option[182]. Furthermore, new-generation pulse oximeters are equipped with signal extraction algorithms designed to perform better in low-perfusion states, enhancing their accuracy in hypothermic patients[183]. Occasionally, supplemental assessments, such as arterial blood gas analysis, may be necessary to assess oxygenation status in these patients accurately. These advancements help healthcare providers obtain more reliable SpO2 measurements in challenging conditions such as hypothermia[184].
Jaundice: Jaundice typically does not significantly affect pulse oximetry readings, as bilirubin absorbs light at a different spectrum (around 450 nm) than that used by pulse oximeters[185]. This characteristic enables the reliable use of pulse oximetry for monitoring jaundiced patients, including neonates. However, in cases of severe hemolytic jaundice, patients may also exhibit elevated levels of carboxyhemoglobin, which can potentially cause inaccurate pulse oximetry readings[1]. Additionally, falsely low SpO2 values have been reported in rare instances of bronze baby syndrome, although this phenomenon is uncommon. Low SpO2 is due to excessive bilirubin accumulation in the skin, which can interfere with accurately detecting arterial blood SpO2 by the pulse oximeter[186]. Overall, pulse oximetry remains a valuable tool for monitoring patients with jaundice, with minimal interference from bilirubin levels in most cases.
Exposure to electromagnetic fields: Exposure to electromagnetic fields, particularly from sources such as electrosurgical cauterization units and cellular phones, can potentially interfere with the accuracy of pulse oximetry readings. This interference may result in erroneous SpO2 readings[187]. Additionally, during magnetic resonance imaging, standard pulse oximetry sensors may not be suitable due to the risk of interference with image quality and the potential for thermal injury caused by the heating of the sensor[188]. Therefore, special pulse oximetry devices equipped with fiberoptic technology are recommended during magnetic resonance imaging procedures to mitigate these risks and ensure accurate SpO2 monitoring without compromising patient safety[189]. Table 4 summarizes the diseases or conditions, the problems they pose to pulse oximetry, and potential solutions to address these challenges.
Table 4.
The diseases or conditions, the problems they pose to pulse oximetry, and potential solutions to address these challenges
|
Disease/condition
|
Problem with pulse oximetry
|
Solution
|
| Anaemia | Reduced accuracy of SpO2 readings due to lower hemoglobin levels affecting oxygen saturation | Interpret readings cautiously, consider other clinical indicators, and perform arterial blood gas analysis for severe cases |
| Polycythaemia | Falsely elevated SpO2 readings due to increased hemoglobin levels and altered blood viscosity | Be aware of potential inaccuracies and consider alternative assessment methods, such as arterial blood gas analysis |
| Metabolic derangement | Shifts in the oxygen dissociation curve and peripheral vasoconstriction can affect SpO2 readings | Interpret readings cautiously, consider other clinical parameters, and be aware of acidosis-induced left shifts or alkalosis-induced right shifts |
| Cardiac arrhythmia | Irregular blood flow causes fluctuations in pulsatile signal, leading to inaccurate readings | Use pulse oximeters with advanced signal processing algorithms, monitor waveform quality, and consider alternative assessments such as arterial blood gas analysis |
| Hypothermia | Reduced peripheral perfusion and altered oxygen dissociation curve affecting SpO2 accuracy | Apply local heating to improve perfusion, use pulse oximeters with enhanced low-perfusion algorithms, and consider supplemental assessments |
| Jaundice | Minimal interference from bilirubin with pulse oximetry readings, though COHb may cause inaccuracies | Monitor for COHb levels in severe cases; rely on pulse oximetry for most jaundiced patients |
| Electromagnetic field | Interference with pulse oximetry readings from sources such as electrosurgical units and cellular phones | Use fiberoptic pulse oximetry during MRI procedures to minimize exposure to electromagnetic fields |
COHb: Carboxyhemoglobin; MRI: Magnetic resonance imaging; SpO2: Oxygen saturation.
Advantages of pulse oximetry: Pulse oximetry, a non-invasive medical technology, offers numerous advantages across various clinical settings due to its ability to measure SpO2 levels in the blood and pulse rate accurately and continuously[190]. One of the primary advantages of pulse oximetry is its non-invasive nature. Unlike arterial blood gas sampling, which requires invasive procedures and carries some risks, pulse oximetry involves placing a sensor on a patient’s skin, typically on a finger, toe, or earlobe[191]. This makes it well-tolerated by patients, including infants and children, and reduces the risk of complications associated with invasive monitoring techniques. In addition, pulse oximetry provides real-time feedback on a patient’s SpO2 levels and pulse rate[192]. This allows healthcare providers to continuously monitor changes in oxygenation status, enabling prompt intervention if SpO2 levels drop below the normal range. Real-time monitoring is particularly crucial in critical care settings, where rapid detection of hypoxemia or changes in respiratory status can be lifesaving[193].
Pulse oximetry facilitates the early detection of hypoxemia, a condition characterized by low oxygen levels in the blood. By continuously monitoring SpO2 levels, healthcare providers can identify hypoxemia before clinical symptoms become apparent, allowing timely intervention to improve oxygenation and prevent complications associated with inadequate tissue oxygen delivery[194]. Pulse oximetry is valuable during various medical procedures, including anesthesia, sedation, and surgery. It helps anesthesia providers assess a patient’s oxygenation status throughout the procedure, detect changes in SpO2 levels early, and optimize ventilation and oxygenation to prevent hypoxemia-related complications. Similarly, pulse oximetry aids in monitoring patients undergoing sedation or procedural sedation, ensuring their safety and well-being during the procedure[126,195].
Modern pulse oximeters are portable and versatile, allowing their use in various clinical settings, including hospitals, ambulatory care facilities, and even home healthcare settings. Portable pulse oximeters are battery-operated and lightweight, making them convenient in emergency medical services settings, outpatient clinics, and remote locations where access to traditional monitoring equipment may be limited[196]. Pulse oximeters are user-friendly devices that require minimal training for healthcare providers to operate effectively. They typically feature simple interfaces and intuitive displays that provide clear and easy-to-interpret readings of SpO2 levels and pulse rate. This ease of use enhances their accessibility and utility in diverse healthcare settings[197].
Pulse oximetry enables continuous monitoring of SpO2 and pulse rates, allowing healthcare providers to track changes in a patient’s respiratory status over time. Continuous monitoring is particularly beneficial in critical care settings, where patients may require close observation for extended periods, such as during mechanical ventilation or recovery from surgery[198]. With advancements in telemedicine and remote patient monitoring technologies, pulse oximetry can now be integrated into telehealth platforms, allowing for remote monitoring of patients’ SpO2 levels and pulse rate from a distance. Remote monitoring enables healthcare providers to remotely assess patients’ respiratory status, provide timely interventions, and optimize patient care without needing in-person visits[199]. Pulse oximetry is a cost-effective monitoring tool that provides valuable clinical information at a relatively low cost compared to other monitoring techniques, such as arterial blood gas analysis. Its affordability and widespread availability make it accessible to healthcare providers in diverse healthcare settings, including resource-limited settings where access to advanced monitoring equipment may be limited[1].
Causes of limited wide-use of pulse oximetry, especially in the developing world
Despite the significant potential benefits of pulse oximetry in low- and middle-income countries (LMICs), several challenges hinder its widespread use. One major barrier is the limited availability of supplemental oxygen, which is necessary for treating hypoxemia, as identified through pulse oximetry screening and as a potential lifesaver[200]. In many low-resource settings, healthcare facilities struggle to maintain a reliable oxygen supply due to logistical and infrastructural constraints. This shortage of oxygen not only undermines the effectiveness of pulse oximetry as a diagnostic tool but also compromises the ability to provide life-saving treatment to hypoxemic patients[201].
Moreover, the initial cost of pulse oximeters and ongoing maintenance and support requirements pose significant financial challenges for healthcare systems in LMICs[197]. The high upfront expenses for procuring pulse oximeters and the need for periodic replacement of components such as probes and batteries can strain already limited budgets, often leaving healthcare providers with difficult choices. Additionally, technical barriers, including faulty equipment and inadequate training of healthcare personnel, further impede the effective use of pulse oximetry in resource-limited settings[202,203]. Provider misconceptions and lack of awareness about the importance of pulse oximetry also contribute to its underutilization. Many healthcare providers in LMICs may not fully appreciate the value of pulse oximetry in diagnosing hypoxemia and guiding treatment decisions[111]. This lack of understanding can lead to a reluctance to incorporate pulse oximetry into clinical practice and may result in missed opportunities for early intervention[204].
Addressing these challenges requires a comprehensive approach that includes urgent policy changes and substantial investment in healthcare infrastructure. Governments and international organizations must prioritize the integration of pulse oximetry into national healthcare policies and guidelines, ensuring access to both the devices and the necessary oxygen therapy[205]. Training programs should be implemented to educate healthcare providers about the benefits of pulse oximetry and how to utilize the technology in clinical practice effectively[206]. Furthermore, research efforts should focus on developing cost-effective solutions tailored to the unique needs of LMICs[207]. This may involve the development of more robust and affordable pulse oximeter models and innovations in oxygen delivery systems to improve accessibility and reliability[15]. Collaborative initiatives between public health agencies, academic institutions, and private sector partners can help drive progress in this area and ultimately improve healthcare outcomes for children in LMICs[208].
Limitations and challenges of pulse oximetry
Pulse oximetry has revolutionized the monitoring of SpO2 levels and pulse rate in healthcare settings, offering a non-invasive and convenient method for assessing respiratory status[153]. However, like any medical technology, pulse oximetry has its limitations and challenges that healthcare providers must be aware of to interpret its readings accurately. Understanding these limitations is essential for ensuring the appropriate use of pulse oximetry and avoiding misinterpretation of data. Below, we discuss some key limitations and challenges associated with pulse oximetry in clinical practice[209]. Motion artifacts can affect the accuracy of pulse oximetry readings, particularly in patients who are restless, agitated, or experiencing involuntary movements. Pediatric patients, especially infants and toddlers, are often restless and may move frequently[179]. Motion artifacts can interfere with the pulse oximeter’s ability to detect pulsatile blood flow accurately, leading to erroneous readings. Proper sensor placement and minimizing patient movement can help mitigate this challenge[210]. Inpatients with poor peripheral perfusion, such as those with hypotension, hypothermia, or vasoconstriction, pulse oximetry may not accurately reflect their true SpO2 levels. Pediatric patients, particularly those with circulatory compromise or peripheral vascular disease, may have poor peripheral perfusion[211]. Inadequate perfusion can affect the pulsatile component of the signal detected by the pulse oximeter, resulting in inaccurate readings and delaying appropriate interventions. Careful assessment of perfusion status and consideration of alternative monitoring methods may be necessary in these cases[212].
Ambient light, including sunlight or artificial light sources, can interfere with pulse oximetry readings by affecting the accuracy of the sensor’s photodetectors. Ambient light interference can result in erroneous readings or signal loss, compromising there liability of pulse oximetry measurements, particularly in brightly lit environments[22]. Nail polish, acrylic nails, or other nail enhancements can interfere with light transmission through the nail bed, affecting the accuracy of pulse oximetry readings. These substances may block or scatter light, leading to inaccurate measurements of SpO2 levels and pulse rate[213]. Skin pigmentation, particularly dark skin pigmentation, can attenuate the transmission of light through the skin, potentially affecting the accuracy of pulse oximetry readings. The absorption of light by melanin can interfere with the sensor’s ability to detect SpO2 accurately. In patients with dark skin tones, pulse oximeters may underestimate SpO2 levels, leading to misinterpretation of the patient’s respiratory status[214,215]. Certain medical conditions or interventions may involve the administration of intravascular dyes, such as methylene blue or indocyanine green. These dyes can interfere with the absorption of light by hemoglobin, affecting the accuracy of pulse oximetry readings. Healthcare providers should consider the presence of intravascular dyes when interpreting oximetry readings in pediatric patients[216].
Pediatric patients may have hemoglobin variants, such as fetal hemoglobin, which can affect the accuracy of pulse oximetry readings. Pulse oximeters are calibrated based on the absorption spectra of adult hemoglobin, and variations in hemoglobin types can lead to discrepancies in SpO2 measurements[217,218]. Pulse oximetry may produce falsely elevated SpO2 readings in patients with CO poisoning. This is because pulse oximeters cannot distinguish between oxygen and CO bound to hemoglobin, leading to overestimating SpO2 levels and masking the presence of CO toxicity[219]. Altitude and barometric pressure changes can affect the accuracy of pulse oximetry readings, particularly in high-altitude environments or during air travel. Reduced atmospheric pressure at higher altitudes may result in lower SpO2 readings, even without true hypoxemia, leading to misinterpretation of the patient’s respiratory status[220].
Pulse oximetry provides a continuous but delayed measurement of SpO2 levels compared to arterial blood gas analysis. This delay may result in a lag time between changes in a patient’s respiratory status and the detection of these changes by pulse oximetry, potentially delaying the initiation of appropriate interventions[184]. Pulse oximetry may occasionally trigger false alarms, particularly during periods of patient movement, inadequate sensor placement, or technical issues with the device. False alarms can disrupt clinical workflow, lead to unnecessary interventions or investigations, and decrease healthcare provider confidence in pulse oximetry readings[221]. Overall, while pulse oximetry is a valuable monitoring tool in healthcare, healthcare providers need to recognize its limitations and challenges and interpret its readings in conjunction with clinical assessment and other diagnostic modalities for optimal patient care[114].
Alarm fatigue syndrome
Alarm fatigue syndrome is a condition when healthcare providers become desensitized to alarm signals because of frequent exposure to false or non-actionable alarms. This can cause delayed responses or missed critical events[222]. In pulse oximetry, alarm fatigue can occur when the device generates excessive alarms, whether due to technical issues, patient conditions, or environmental factors[221]. False alarms may result from motion artifacts, poor signal quality, improper sensor placement, or fluctuations in patient physiology, such as arrhythmias or hypoperfusion. These false alarms can lead to healthcare providers ignoring or disabling alarms, which could compromise patient safety[179,223].
Several strategies can be implemented to address alarm fatigue syndrome in pulse oximetry. First, healthcare facilities should establish alarm management protocols to prioritize clinically relevant alarms and reduce unnecessary alarms. This may involve adjusting alarm thresholds based on patient characteristics and clinical context and implementing alarm delay features to minimize transient alarms[224]. Additionally, regular maintenance and calibration of pulse oximetry equipment can help prevent technical issues contributing to false alarms. Healthcare providers should also receive education and training on properly using and interpreting pulse oximetry alarms to ensure appropriate responses[34].
Furthermore, advancements in technology, such as the development of smart alarm systems and signal processing algorithms, aim to improve the specificity of alarms and reduce alarm fatigue[225]. These systems utilize artificial intelligence and machine learning to analyze physiological data and distinguish between true clinical events and artifacts, reducing the frequency of false alarms[226]. Addressing alarm fatigue syndrome in pulse oximetry requires a multifaceted approach involving technology, education, and protocol development to enhance patient safety and optimize alarm management practices[227].
Guidelines for effective pulse oximetry
Table 5 summarizes the guidelines for effective use of pulse oximetry. Effective monitoring of pulse oximetry in pediatric patients is crucial to assess their oxygenation status accurately and guide clinical management. The pulse oximeter sensor should be placed on a well-perfused area such as the finger, toe, or earlobe, depending on the child’s age and size[86]. The sensor should be secured snugly but not too tightly to prevent motion artifacts and ensure optimal signal quality. Establishing a baseline SpO2 level for each patient is essential to facilitate the interpretation of subsequent readings[192]. Age, baseline respiratory status, and underlying medical conditions should also be considered while determining the expected SpO2 range[3].
Table 5.
General guidelines for effective use of pulse oximetry
|
Guideline
|
Details
|
| Sensor placement | Place on well-perfused areas (finger, toe, earlobe) based on the child’s age and size |
| Sensor securement | Secure snugly but not too tightly to prevent motion artifacts and ensure optimal signal quality |
| Establish baseline | Establish a baseline oxygen saturation level for each patient to interpret subsequent readings accurately |
| Considerations | The expected oxygen saturation range should be determined based on age, baseline respiratory status, and underlying medical conditions |
| Continuous monitoring | Continuous monitoring should be used in critically ill or high-risk patients to promptly detect changes in oxygen saturation |
| Additional parameters | Monitor respiratory rate, heart rate, level of consciousness, and skin color alongside oxygen saturation levels |
| Minimizing artifacts | Minimize patient movement, ensure proper sensor placement, and use immobilization techniques or sedation as needed to reduce motion artifacts |
| Equipment maintenance | Regularly monitor and address technical issues and calibrate equipment according to manufacturer’s guidelines |
| Sensor replacement | Replace sensors as needed to maintain accuracy and reliability |
| Alternative sites | Alternative sensor placement sites (forehead or palm) should be used for patients with poor peripheral perfusion or compromised circulation |
| Trend monitoring | Monitor trends in oxygen saturation over time rather than relying solely on individual readings |
| Education | Educate parents, caregivers, and healthcare staff about the importance of pulse oximetry and proper sensor placement |
| Documentation | Document pulse oximetry readings, relevant clinical information, and interventions in the patient’s medical record |
| Optimization | Every effort should be made to optimize pulse oximetry monitoring effectiveness, improving patient outcomes and care quality |
Continuous pulse oximetry monitoring can promptly detect changes in SpO2, especially in critically ill or high-risk pediatric patients. It provides real-time feedback on oxygenation status and enables timely intervention if necessary[133]. SpO2 levels, respiratory rate, heart rate, level of consciousness, and skin color are crucial factors while monitoring a patient’s condition. Minimizing patient movement, ensuring proper sensor placement, and using immobilization techniques or sedation as appropriate, particularly during procedures or in agitated pediatric patients, can reduce motion artifacts and enhance signal quality[228].
Regular monitoring of pulse oximetry equipment is necessary to ensure its proper functioning. Any technical issues should be addressed promptly, and the equipment should be calibrated according to the manufacturer’s guidelines[229]. Sensors should be replaced as needed to maintain accuracy and reliability. For pediatric patients with poor peripheral perfusion or compromised circulation, alternative sites for sensor placement, such as the forehead or palm, can improve signal quality and accuracy[33]. Monitoring the trends in SpO2 over time is more important than relying solely on individual readings. Tracking changes in SpO2 levels during interventions such as oxygen therapy or respiratory treatments can help evaluate treatment efficacy and response[230].
Parents, caregivers, and healthcare staff should be educated about the importance of pulse oximetry monitoring and proper sensor placement. They should be trained to interpret oximetry readings and recognize signs of respiratory distress or hypoxemia[197]. Lastly, documenting pulse oximetry readings, relevant clinical information, and interventions in the patient’s medical record is crucial. Accurate records help track changes in oxygenation status, monitor treatment response, and facilitate communication among healthcare providers[231]. By following these practical guidelines, healthcare providers can optimize the effectiveness of pulse oximetry monitoring in pediatric patients, leading to improved patient outcomes and better quality of care.
Limitations of the study
While this review on pulse oximetry provides valuable insights into its advantages, challenges, and guidelines for effective use, several limitations must be considered. Firstly, the scope of the review may not encompass all aspects of pulse oximetry due to the complexity and vastness of the topic, potentially overlooking certain limitations or emerging issues. Additionally, publication bias could skew the findings, favoring studies with positive results and neglecting others. The review’s date range may also limit its inclusiveness of recent pulse oximetry technology and research advancements. Furthermore, the generalizability of the review’s conclusions may be limited by variations in healthcare settings, patient populations, and clinical practices. The quality of included studies, bias in selection criteria, and language bias could further impact the reliability and validity of the review’s findings. Additionally, heterogeneity among included studies and potential conflicts of interest among authors may introduce interpretation uncertainties. Lastly, incomplete accounting for confounding factors and limitations of secondary data analysis could affect the robustness of the review’s conclusions. Despite these limitations, acknowledging and addressing these potential biases and uncertainties is essential for interpreting the review’s findings accurately and guiding future research efforts in pulse oximetry.
Recommendations
Based on the insights gleaned from this review study on pulse oximetry, several specific recommendations emerge to optimize its utility and mitigate challenges in clinical application.
Advancing technological innovation: Further research is urgently needed to explore cutting-edge technologies and methodologies in pulse oximetry. This research should aim to overcome current limitations, such as motion artifacts and inaccuracies, in patients with compromised peripheral perfusion. Investing in studies assessing the efficacy and reliability of pulse oximetry across diverse patient demographics and clinical scenarios can yield evidence-based guidelines for optimal utilization.
Education and training initiatives: It is paramount to prioritize education and training on pulse oximetry for healthcare professionals, caregivers, and patients. Specific training programs should emphasize correct sensor placement, interpretation of oximetry data, and appropriate responses to alarm signals. These initiatives are crucial for fostering proficiency and awareness, which is essential for improved patient outcomes.
Healthcare infrastructure and resource allocation: Healthcare systems and policymakers should allocate resources to enhancing pulse oximetry infrastructure. This includes ensuring access to supplemental oxygen, procuring reliable pulse oximetry equipment, and implementing maintenance protocols. Overcoming logistical and financial barriers to pulse oximetry adoption, particularly in resource-constrained settings, is crucial for expanding its reach and efficacy in clinical practice.
Promoting interdisciplinary collaboration: Fostering interdisciplinary collaboration among researchers, clinicians, engineers, and industry stakeholders is imperative for driving innovation in pulse oximetry technology. Collaborative endeavors can lead to the development of novel sensor designs, advanced signal processing algorithms, and telemonitoring solutions. These innovations are essential for addressing existing limitations and effectively meeting evolving healthcare needs.
Continuous surveillance and quality assurance: Continuous surveillance and evaluation of pulse oximetry practices are vital for ensuring accuracy, reliability, and safety in clinical settings. Implementing stringent quality assurance protocols, including regular audits, performance assessments, and feedback mechanisms, can identify areas for improvement and support ongoing quality enhancement efforts.
By embracing these specific recommendations, healthcare stakeholders can enhance the effectiveness, accessibility, and reliability of pulse oximetry monitoring. This proactive approach ultimately advances patient care outcomes and ensures the optimal use of pulse oximetry in clinical practice.
CONCLUSION
This comprehensive review underscores the pivotal role of pulse oximetry in modern healthcare while illuminating its limitations, challenges, and potential solutions. Pulse oximetry is a cornerstone technology for non-invasive SpO2 and pulse rate monitoring, offering invaluable real-time feedback crucial for timely clinical interventions. However, the review elucidates various factors that can compromise the accuracy and reliability of pulse oximetry readings, including motion artifacts, poor peripheral perfusion, ambient light interference, and patient-specific factors such as skin pigmentation and hemoglobin variants. Despite these challenges, the study provides actionable recommendations to optimize pulse oximetry utilization, encompassing technological advancements, education, healthcare infrastructure, interdisciplinary collaboration, and quality assurance practices. By embracing these recommendations, healthcare stakeholders can harness the full potential of pulse oximetry, ensuring its efficacy, accessibility, and safety across diverse clinical settings. Ultimately, this review serves as a call to action for continuous innovation, education, and quality improvement efforts to enhance pulse oximetry monitoring and advance patient care worldwide.
ACKNOWLEDGEMENTS
We thank the anonymous referees and editors for their valuable suggestions.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country of origin: Egypt
Peer-review report’s classification
Scientific Quality: Grade A, Grade B, Grade C, Grade D
Novelty: Grade A, Grade B, Grade B, Grade C
Creativity or Innovation: Grade A, Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade B, Grade B, Grade C
P-Reviewer: Cui M; Pan Y; Zheng L S-Editor: Wang JJ L-Editor: A P-Editor: Wang WB
Contributor Information
Mohammed Al-Beltagi, Department of Pediatric, Faculty of Medicine, Tanta University, Tanta 31511, Alghrabia, Egypt; Department of Pediatrics, University Medical Center, King Abdulla Medical City, Arabian Gulf University, Manama 26671, Manama, Bahrain. mbelrem@hotmail.com.
Nermin Kamal Saeed, Medical Microbiology Section, Department of Pathology, Salmaniya Medical Complex, Ministry of Health, Kingdom of Bahrain, Manama 26671, Manama, Bahrain; Medical Microbiology Section, Department of Pathology, Irish Royal College of Surgeon in Bahrain, Busaiteen 15503, Muharraq, Bahrain.
Adel Salah Bediwy, Department of Pulmonology, Faculty of Medicine, Tanta University, Tanta 31527, Alghrabia, Egypt; Department of Pulmonology, University Medical Center, King Abdulla Medical City, Arabian Gulf University, Manama 26671, Manama, Bahrain.
Reem Elbeltagi, Department of Medicine, The Royal College of Surgeons in Ireland-Bahrain, Busiateen 15503, Muharraq, Bahrain.
References
- 1.Chan ED, Chan MM, Chan MM. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir Med. 2013;107:789–799. doi: 10.1016/j.rmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Popovich DM, Richiuso N, Danek G. Pediatric health care providers' knowledge of pulse oximetry. Pediatr Nurs. 2004;30:14–20. [PubMed] [Google Scholar]
- 3.Hayes D Jr, Wilson KC, Krivchenia K, Hawkins SMM, Balfour-Lynn IM, Gozal D, Panitch HB, Splaingard ML, Rhein LM, Kurland G, Abman SH, Hoffman TM, Carroll CL, Cataletto ME, Tumin D, Oren E, Martin RJ, Baker J, Porta GR, Kaley D, Gettys A, Deterding RR. Home Oxygen Therapy for Children. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2019;199:e5–e23. doi: 10.1164/rccm.201812-2276ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jubran A. Pulse oximetry. Crit Care. 2015;19:272. doi: 10.1186/s13054-015-0984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim NH, Yu SG, Kim SE, Lee EC. Non-Contact Oxygen Saturation Measurement Using YCgCr Color Space with an RGB Camera. Sensors (Basel) 2021;21 doi: 10.3390/s21186120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoneham MD, Saville GM, Wilson IH. Knowledge about pulse oximetry among medical and nursing staff. Lancet. 1994;344:1339–1342. doi: 10.1016/s0140-6736(94)90697-1. [DOI] [PubMed] [Google Scholar]
- 7.Severinghaus JW, Astrup PB. History of blood gas analysis. VI. Oximetry. J Clin Monit. 1986;2:270–288. doi: 10.1007/BF02851177. [DOI] [PubMed] [Google Scholar]
- 8.Ceredig R. George Gabriel Stokes as a biologist. Philos Trans A Math Phys Eng Sci. 2020;378:20200105. doi: 10.1098/rsta.2020.0105. [DOI] [PubMed] [Google Scholar]
- 9.Hay WW. History of Pulse Oximetry in Neonatal Medicine. NeoReviews. 2005;6:e533–e538. [Google Scholar]
- 10.Ritman EL. Earl Wood--a research career noted for development of novel instruments driven by the power of the indicator dilution concept. J Appl Physiol (1985) 2014;117:945–956. doi: 10.1152/japplphysiol.00491.2014. [DOI] [PubMed] [Google Scholar]
- 11.Blanos P, Hu S, Yan L, Alharbi S. A multiplexed electronic architecture for opto-electronic patch sensor to effectively monitor heart rate and oxygen saturation. Proceedings of the Optical Diagnostics and Sensing XVIII: Toward Point-of-Care Diagnostics; 2018 Feb 20; San Francisco, United States. United States: SPIE, 2018: 1050115. [Google Scholar]
- 12.Westhorpe RN, Ball C. The pulse oximeter. Anaesth Intensive Care. 2008;36:767. doi: 10.1177/0310057X0803600602. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya K. Takuo Aoyagi-a Tribute to the Brain Behind Pulse Oximetry. Indian J Surg. 2020;82:1332–1333. doi: 10.1007/s12262-020-02365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyasaka K, Shelley K, Takahashi S, Kubota H, Ito K, Yoshiya I, Yamanishi A, Cooper JB, Steward DJ, Nishida H, Kiani J, Ogino H, Sata Y, Kopotic RJ, Jenkin K, Hannenberg A, Gawande A. Tribute to Dr. Takuo Aoyagi, inventor of pulse oximetry. J Anesth. 2021;35:671–709. doi: 10.1007/s00540-021-02967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemomssa HD, Raj H. Development of Low-Cost and Portable Pulse Oximeter Device with Improved Accuracy and Accessibility. Med Devices (Auckl) 2022;15:121–129. doi: 10.2147/MDER.S366053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitzan M, Romem A, Koppel R. Pulse oximetry: fundamentals and technology update. Med Devices (Auckl) 2014;7:231–239. doi: 10.2147/MDER.S47319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uddin R, Koo I. Real-Time Remote Patient Monitoring: A Review of Biosensors Integrated with Multi-Hop IoT Systems via Cloud Connectivity. Appl Sci. 2024;14:1876. [Google Scholar]
- 18.O'Driscoll BR, Howard LS, Davison AG British Thoracic Society. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63 Suppl 6:vi1–v68. doi: 10.1136/thx.2008.102947. [DOI] [PubMed] [Google Scholar]
- 19.Jendroszek A, Malte H, Overgaard CB, Beedholm K, Natarajan C, Weber RE, Storz JF, Fago A. Allosteric mechanisms underlying the adaptive increase in hemoglobin-oxygen affinity of the bar-headed goose. J Exp Biol. 2018;221 doi: 10.1242/jeb.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitzan M, Nitzan I, Arieli Y. The Various Oximetric Techniques Used for the Evaluation of Blood Oxygenation. Sensors (Basel) 2020;20 doi: 10.3390/s20174844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yossef Hay O, Cohen M, Nitzan I, Kasirer Y, Shahroor-Karni S, Yitzhaky Y, Engelberg S, Nitzan M. Pulse Oximetry with Two Infrared Wavelengths without Calibration in Extracted Arterial Blood. Sensors (Basel) 2018;18 doi: 10.3390/s18103457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fluck RR Jr, Schroeder C, Frani G, Kropf B, Engbretson B. Does ambient light affect the accuracy of pulse oximetry? Respir Care. 2003;48:677–680. [PubMed] [Google Scholar]
- 23.Mannheimer PD. The light-tissue interaction of pulse oximetry. Anesth Analg. 2007;105:S10–S17. doi: 10.1213/01.ane.0000269522.84942.54. [DOI] [PubMed] [Google Scholar]
- 24.Lujan HL, DiCarlo SE. "Seeing red" reflects hemoglobin's saturation state: a discovery-based activity for understanding the science of pulse oximetry. Adv Physiol Educ. 2022;46:461–467. doi: 10.1152/advan.00093.2022. [DOI] [PubMed] [Google Scholar]
- 25.Leppänen T, Kainulainen S, Korkalainen H, Sillanmäki S, Kulkas A, Töyräs J, Nikkonen S. Pulse Oximetry: The Working Principle, Signal Formation, and Applications. Adv Exp Med Biol. 2022;1384:205–218. doi: 10.1007/978-3-031-06413-5_12. [DOI] [PubMed] [Google Scholar]
- 26.Lee H, Ko H, Lee J. Reflectance pulse oximetry: Practical issues and limitations. ICT Express. 2016;2:195–198. [Google Scholar]
- 27.Mandoki JJ, Casa-Tirao B, Molina-Guarneros JA, Jiménez-Orozco FA, García-Mondragón MJ, Maldonado-Espinoza A. Pulsatile diastolic increase and systolic decrease in arterial blood pressure: their mechanism of production and physiological role. Prog Biophys Mol Biol. 2013;112:55–57. doi: 10.1016/j.pbiomolbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Dormishian A, Schott A, Aguilar AC, Bancalari E, Claure N. Pulse oximetry reliability for detection of hypoxemia under motion in extremely premature infants. Pediatr Res. 2023;93:118–124. doi: 10.1038/s41390-022-02258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, McKnight JC, Bønnelycke ES, Bosco G, Giacon TA, Kainerstorfer JM. Self-calibrated pulse oximetry algorithm based on photon pathlength change and the application in human freedivers. J Biomed Opt. 2023;28:115002. doi: 10.1117/1.JBO.28.11.115002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basaranoglu G, Bakan M, Umutoglu T, Zengin SU, Idin K, Salihoglu Z. Comparison of SpO2 values from different fingers of the hands. Springerplus. 2015;4:561. doi: 10.1186/s40064-015-1360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi C, Goodall M, Dumville J, Hill J, Norman G, Hamer O, Clegg A, Watkins CL, Georgiou G, Hodkinson A, Lightbody CE, Dark P, Cullum N. The accuracy of pulse oximetry in measuring oxygen saturation by levels of skin pigmentation: a systematic review and meta-analysis. BMC Med. 2022;20:267. doi: 10.1186/s12916-022-02452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shobhavat L, D'Costa A, Shroff K. Anemia That Presented with Desaturation: A Focus on Core Concepts. Case Rep Pediatr. 2021;2021:5583840. doi: 10.1155/2021/5583840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedaghat-Yazdi F, Torres A Jr, Fortuna R, Geiss DM. Pulse oximeter accuracy and precision affected by sensor location in cyanotic children. Pediatr Crit Care Med. 2008;9:393–397. doi: 10.1097/PCC.0b013e3181727967. [DOI] [PubMed] [Google Scholar]
- 34.Salyer JW. Neonatal and pediatric pulse oximetry. Respir Care. 2003;48:386–96; discussion 397. [PubMed] [Google Scholar]
- 35.Al-Halawani R, Charlton PH, Qassem M, Kyriacou PA. A review of the effect of skin pigmentation on pulse oximeter accuracy. Physiol Meas. 2023;44 doi: 10.1088/1361-6579/acd51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villanueva R, Bell C, Kain ZN, Colingo KA. Effect of peripheral perfusion on accuracy of pulse oximetry in children. J Clin Anesth. 1999;11:317–322. doi: 10.1016/s0952-8180(99)00054-9. [DOI] [PubMed] [Google Scholar]
- 37.McCollum ED, King C, Colbourn T, Graham H, Bernstein M, Wilson IH, Checkley W. Pulse oximetry in paediatric primary care in low-income and middle-income countries. Lancet Respir Med. 2019;7:1001–1002. doi: 10.1016/S2213-2600(19)30358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everitt L, Roberts P, Evans HJ. Use of pulse oximetry as an investigative test for paediatric respiratory sleep disorders. Arch Dis Child Educ Pract Ed. 2023;108:429–438. doi: 10.1136/archdischild-2022-324846. [DOI] [PubMed] [Google Scholar]
- 39.Silverston P, Ferrari M, Quaresima V. Pulse oximetry in primary care: factors affecting accuracy and interpretation. Br J Gen Pract. 2022;72:132–133. doi: 10.3399/bjgp22X718769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safar H, El-dash H. Pulse Oximetry: Could Wrist and Ankle Be Alternative Placement Sites? Clin Pediatr (Phila) 2015;54:1375–1379. doi: 10.1177/0009922815584217. [DOI] [PubMed] [Google Scholar]
- 41.Rüegger C, Bucher HU, Mieth RA. Pulse oximetry in the newborn: is the left hand pre- or post-ductal? BMC Pediatr. 2010;10:35. doi: 10.1186/1471-2431-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Budidha K, Kyriacou PA. In vivo investigation of ear canal pulse oximetry during hypothermia. J Clin Monit Comput. 2018;32:97–107. doi: 10.1007/s10877-017-9975-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Branson RD, Mannheimer PD. Forehead oximetry in critically ill patients: the case for a new monitoring site. Respir Care Clin N Am. 2004;10:359–367, vi. doi: 10.1016/j.rcc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Boyd N, King C, Walker IA, Zadutsa B, Bernstein M, Ahmed S, Roy A, Hanif AAM, Saha SC, Majumder K, Nambiar B, Colbourn T, Makwenda C, Baqui AH, Wilson I, McCollum ED. Usability Testing of a Reusable Pulse Oximeter Probe Developed for Health-Care Workers Caring for Children < 5 Years Old in Low-Resource Settings. Am J Trop Med Hyg. 2018;99:1096–1104. doi: 10.4269/ajtmh.18-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clayton DG, Webb RK, Ralston AC, Duthie D, Runciman WB. Pulse oximeter probes. A comparison between finger, nose, ear and forehead probes under conditions of poor perfusion. Anaesthesia. 1991;46:260–265. doi: 10.1111/j.1365-2044.1991.tb11492.x. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal P, Pursnani N, Gautam A, Singh AP, Garg R, Pandey A, Agarwal A. Assessing the SpO(2) in a random population - Looking for the best among fingers. J Family Med Prim Care. 2022;11:5506–5509. doi: 10.4103/jfmpc.jfmpc_2596_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hlavin D, Varty M. Improving Patient Safety by Increasing Staff Knowledge of Evidence-Based Pulse Oximetry Practices. Crit Care Nurse. 2022;42:e1–e6. doi: 10.4037/ccn2022998. [DOI] [PubMed] [Google Scholar]
- 48.Satici C, Demirkol MA, Alkan M, Esatoglu SN. Falsely low values of oxygen saturation measured by pulse oximetry in patients with coronavirus disease 2019. Lung India. 2020;37:553–554. doi: 10.4103/lungindia.lungindia_392_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C, Correia R, Ballaji HK, Korposh S, Hayes-Gill BR, Morgan SP. Optical Fibre-Based Pulse Oximetry Sensor with Contact Force Detection. Sensors (Basel) 2018;18 doi: 10.3390/s18113632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaonkar PM, Mutha SR, Sanghani IM. Enhancing Neonatal Care: The Vital Role of Pulse Oximetry in the Early Screening of Critical Congenital Heart Diseases and Respiratory Diseases in Rural Areas. Cureus. 2024;16:e58398. doi: 10.7759/cureus.58398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikolov A, Dimitrov A. [Fetal pulse oximetry: a clinical methodological study] Akush Ginekol (Sofiia) 2000;39:47–50. [PubMed] [Google Scholar]
- 52.East CE, Begg L, Colditz PB, Lau R. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database Syst Rev. 2014;2014:CD004075. doi: 10.1002/14651858.CD004075.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luttkus AK, Dudenhausen JW. Fetal pulse oximetry. Baillieres Clin Obstet Gynaecol. 1996;10:295–306. doi: 10.1016/s0950-3552(96)80039-3. [DOI] [PubMed] [Google Scholar]
- 54.Garite TJ, Dildy GA, McNamara H, Nageotte MP, Boehm FH, Dellinger EH, Knuppel RA, Porreco RP, Miller HS, Sunderji S, Varner MW, Swedlow DB. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049–1058. doi: 10.1067/mob.2000.110632. [DOI] [PubMed] [Google Scholar]
- 55.Nijland R, Jongsma HW, Nijhuis JG, Oeseburg B. Accuracy of fetal pulse oximetry and pitfalls in measurements. Eur J Obstet Gynecol Reprod Biol. 1997;72 Suppl:S21–S27. doi: 10.1016/s0301-2115(97)02714-0. [DOI] [PubMed] [Google Scholar]
- 56.Valverde M, Puertas AM, Lopez-Gallego MF, Carrillo MP, Aguilar MT, Montoya F. Effectiveness of pulse oximetry versus fetal electrocardiography for the intrapartum evaluation of nonreassuring fetal heart rate. Eur J Obstet Gynecol Reprod Biol. 2011;159:333–337. doi: 10.1016/j.ejogrb.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 57.Bloom SL, Spong CY, Thom E, Varner MW, Rouse DJ, Weininger S, Ramin SM, Caritis SN, Peaceman A, Sorokin Y, Sciscione A, Carpenter M, Mercer B, Thorp J, Malone F, Harper M, Iams J, Anderson G National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Fetal pulse oximetry and cesarean delivery. N Engl J Med. 2006;355:2195–2202. doi: 10.1056/NEJMoa061170. [DOI] [PubMed] [Google Scholar]
- 58.East CE, Brennecke SP, King JF, Chan FY, Colditz PB FOREMOST Study Group. The effect of intrapartum fetal pulse oximetry, in the presence of a nonreassuring fetal heart rate pattern, on operative delivery rates: a multicenter, randomized, controlled trial (the FOREMOST trial) Am J Obstet Gynecol. 2006;194:606.e1–606.16. doi: 10.1016/j.ajog.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 59.Peek MJ, Condous GS, Nanan RK. Fetal pulse oximetry and cesarean delivery. N Engl J Med. 2007;356:1377; author reply 1377–1377; author reply 1378. doi: 10.1056/NEJMc063582. [DOI] [PubMed] [Google Scholar]
- 60.Narvey M, Wong KK, Fournier A. Pulse oximetry screening in newborns to enhance detection of critical congenital heart disease. Paediatr Child Health. 2017;22:494–503. doi: 10.1093/pch/pxx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engel MS, Kochilas LK. Pulse oximetry screening: a review of diagnosing critical congenital heart disease in newborns. Med Devices (Auckl) 2016;9:199–203. doi: 10.2147/MDER.S102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murni IK, Wibowo T, Arafuri N, Oktaria V, Dinarti LK, Panditatwa D, Patmasari L, Noormanto N, Nugroho S. Feasibility of screening for critical congenital heart disease using pulse oximetry in Indonesia. BMC Pediatr. 2022;22:369. doi: 10.1186/s12887-022-03404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Movahedian AH, Mosayebi Z, Sagheb S. Evaluation of Pulse Oximetry in the Early Detection of Cyanotic Congenital Heart Disease in Newborns. J Tehran Heart Cent. 2016;11:73–78. [PMC free article] [PubMed] [Google Scholar]
- 64.Katona M, Kertész E, Bartyik K, Gábor K. [Hyperoxia-hyperventilation test in the diagnosis and therapy of persistent neonatal pulmonary hypertension] Orv Hetil. 1986;127:1003–1008. [PubMed] [Google Scholar]
- 65.Bouferrache B, Filtchev S, Leke A, Marbaix-Li Q, Freville M, Gaultier C. The hyperoxic test in infants reinvestigated. Am J Respir Crit Care Med. 2000;161:160–165. doi: 10.1164/ajrccm.161.1.9904012. [DOI] [PubMed] [Google Scholar]
- 66.Jubran A, Tobin MJ. Reliability of pulse oximetry in titrating supplemental oxygen therapy in ventilator-dependent patients. Chest. 1990;97:1420–1425. doi: 10.1378/chest.97.6.1420. [DOI] [PubMed] [Google Scholar]
- 67.de-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganäs L, Eriksson M, Segerdahl N, Agren A, Ekman-Joelsson BM, Sunnegårdh J, Verdicchio M, Ostman-Smith I. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037. doi: 10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fricke K, Ryd D, Weismann CG, Hanséus K, Hedström E, Liuba P. Fetal cardiac magnetic resonance imaging of the descending aorta in suspected left-sided cardiac obstructions. Front Cardiovasc Med. 2023;10:1285391. doi: 10.3389/fcvm.2023.1285391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Said Habib H. Oxygen saturation trends in the first hour of life in healthy full-term neonates born at moderate altitude. Pak J Med Sci. 2013;29:903–906. doi: 10.12669/pjms.294.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma V, Berkelhamer S, Lakshminrusimha S. Persistent pulmonary hypertension of the newborn. Matern Health Neonatol Perinatol. 2015;1:14. doi: 10.1186/s40748-015-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mawson IE, Babu PL, Simpson JM, Fox GF. Pulse oximetry findings in newborns with antenatally diagnosed congenital heart disease. Eur J Pediatr. 2018;177:683–689. doi: 10.1007/s00431-018-3093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Mukhaini KS, Mohamed AM. Transposition of the Great Arteries and Coarctation of the Aorta in an Infant Presenting with Bronchiolitis: An incidental finding. Sultan Qaboos Univ Med J. 2017;17:e348–e351. doi: 10.18295/squmj.2017.17.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karl TR, Stocker C. Tetralogy of Fallot and Its Variants. Pediatr Crit Care Med. 2016;17:S330–S336. doi: 10.1097/PCC.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 74.Mathur NB, Gupta A, Kurien S. Pulse Oximetry Screening to Detect Cyanotic Congenital Heart Disease in Sick Neonates in a Neonatal Intensive Care Unit. Indian Pediatr. 2015;52:769–772. doi: 10.1007/s13312-015-0714-y. [DOI] [PubMed] [Google Scholar]
- 75.Ko WJ, Chang CI, Chiu IS, Chu SH. Continuous monitoring of venous oxygen saturation in critically-ill infants. J Formos Med Assoc. 1996;95:258–262. [PubMed] [Google Scholar]
- 76.Jain D, Jain M, Lamture Y. Pulse Oximetry Screening for Detecting Critical Congenital Heart Disease in Neonates. Cureus. 2022;14:e32852. doi: 10.7759/cureus.32852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plana MN, Zamora J, Suresh G, Fernandez-Pineda L, Thangaratinam S, Ewer AK. Pulse oximetry screening for critical congenital heart defects. Cochrane Database Syst Rev. 2018;3:CD011912. doi: 10.1002/14651858.CD011912.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar P. Universal Pulse Oximetry Screening for Early Detection of Critical Congenital Heart Disease. Clin Med Insights Pediatr. 2016;10:35–41. doi: 10.4137/CMPed.S33086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine--results from a prospective multicenter study. Eur J Pediatr. 2010;169:975–981. doi: 10.1007/s00431-010-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma CC, Ma S. The role of surfactant in respiratory distress syndrome. Open Respir Med J. 2012;6:44–53. doi: 10.2174/1874306401206010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niknafs P, Norouzi E, Bahman Bijari B, Baneshi MR. Can we Replace Arterial Blood Gas Analysis by Pulse Oximetry in Neonates with Respiratory Distress Syndrome, who are Treated According to INSURE Protocol? Iran J Med Sci. 2015;40:264–267. [PMC free article] [PubMed] [Google Scholar]
- 82.Mascoll-Robertson KK, Viscardi RM, Woo HC. The Objective Use of Pulse Oximetry to Predict Respiratory Support Transition in Preterm Infants: An Observational Pilot Study. Respir Care. 2016;61:416–422. doi: 10.4187/respcare.04102. [DOI] [PubMed] [Google Scholar]
- 83.Tarnow-Mordi W, Kirby A. Current Recommendations and Practice of Oxygen Therapy in Preterm Infants. Clin Perinatol. 2019;46:621–636. doi: 10.1016/j.clp.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 84.Tana M, Tirone C, Aurilia C, Lio A, Paladini A, Fattore S, Esposito A, De Tomaso D, Vento G. Respiratory Management of the Preterm Infant: Supporting Evidence-Based Practice at the Bedside. Children (Basel) 2023;10 doi: 10.3390/children10030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramaswamy VV, Bandyopadhyay T, Abiramalatha T, Pullattayil S AK, Szczapa T, Wright CJ, Roehr CC. Clinical decision thresholds for surfactant administration in preterm infants: a systematic review and network meta-analysis. EClinicalMedicine. 2023;62:102097. doi: 10.1016/j.eclinm.2023.102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wick KD, Matthay MA, Ware LB. Pulse oximetry for the diagnosis and management of acute respiratory distress syndrome. Lancet Respir Med. 2022;10:1086–1098. doi: 10.1016/S2213-2600(22)00058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahant S, Wahi G, Giglia L, Pound C, Kanani R, Bayliss A, Roy M, Sakran M, Kozlowski N, Breen-Reid K, Lavigne M, Premji L, Moretti ME, Willan AR, Schuh S, Parkin PC. Intermittent versus continuous oxygen saturation monitoring for infants hospitalised with bronchiolitis: study protocol for a pragmatic randomised controlled trial. BMJ Open. 2018;8:e022707. doi: 10.1136/bmjopen-2018-022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davidson LM, Berkelhamer SK. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J Clin Med. 2017;6 doi: 10.3390/jcm6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kapur N, Nixon G, Robinson P, Massie J, Prentice B, Wilson A, Schilling S, Twiss J, Fitzgerald DA. Respiratory management of infants with chronic neonatal lung disease beyond the NICU: A position statement from the Thoracic Society of Australia and New Zealand. Respirology. 2020;25:880–888. doi: 10.1111/resp.13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balink S, Onland W, Vrijlandt EJLE, Andrinopoulou ER, Bos AF, Dijk PH, Goossens L, Hulsmann AR, Nuytemans DH, Reiss IKM, Sprij AJ, Kroon AA, van Kaam AH, Pijnenburg M SOS BPD study group. Supplemental oxygen strategies in infants with bronchopulmonary dysplasia after the neonatal intensive care unit period: study protocol for a randomised controlled trial (SOS BPD study) BMJ Open. 2022;12:e060986. doi: 10.1136/bmjopen-2022-060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singer L, Martin RJ, Hawkins SW, Benson-Szekely LJ, Yamashita TS, Carlo WA. Oxygen desaturation complicates feeding in infants with bronchopulmonary dysplasia after discharge. Pediatrics. 1992;90:380–384. [PMC free article] [PubMed] [Google Scholar]
- 92.Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, Kleinman R, Klijanowicz A, Martinez F, Ozdemir A, Panitch HB, Nickerson B, Stein MT, Tomezsko J, Van Der Anker J American Thoracic Society. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168:356–396. doi: 10.1164/rccm.168.3.356. [DOI] [PubMed] [Google Scholar]
- 93.Bonafide CP, Xiao R, Brady PW, Landrigan CP, Brent C, Wolk CB, Bettencourt AP, McLeod L, Barg F, Beidas RS, Schondelmeyer A Pediatric Research in Inpatient Settings (PRIS) Network. Prevalence of Continuous Pulse Oximetry Monitoring in Hospitalized Children With Bronchiolitis Not Requiring Supplemental Oxygen. JAMA. 2020;323:1467–1477. doi: 10.1001/jama.2020.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin RJ. Review of diagnosis and treatment of neonatal cardiorespiratory events. Pediatr Med. 2021;4:20. [Google Scholar]
- 95.Martin RJ, Wang K, Köroğlu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology. 2011;100:303–310. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Williamson M, Poorun R, Hartley C. Apnoea of Prematurity and Neurodevelopmental Outcomes: Current Understanding and Future Prospects for Research. Front Pediatr. 2021;9:755677. doi: 10.3389/fped.2021.755677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warburton A, Monga R, Sampath V, Kumar N. Continuous pulse oximetry and respiratory rate trends predict short-term respiratory and growth outcomes in premature infants. Pediatr Res. 2019;85:494–501. doi: 10.1038/s41390-018-0269-4. [DOI] [PubMed] [Google Scholar]
- 98.van Zanten HA, Tan RN, van den Hoogen A, Lopriore E, te Pas AB. Compliance in oxygen saturation targeting in preterm infants: a systematic review. Eur J Pediatr. 2015;174:1561–1572. doi: 10.1007/s00431-015-2643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mathew B, Lakshminrusimha S. Persistent Pulmonary Hypertension in the Newborn. Children (Basel) 2017;4 doi: 10.3390/children4080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chandrasekharan P, Rawat M, Lakshminrusimha S. How Do We Monitor Oxygenation during the Management of PPHN? Children (Basel) 2020;7 doi: 10.3390/children7100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiao SY, Ou CN. Validation of oxygen saturation monitoring in neonates. Am J Crit Care. 2007;16:168–178. [PMC free article] [PubMed] [Google Scholar]
- 102.Lakshminrusimha S, Keszler M. Persistent Pulmonary Hypertension of the Newborn. Neoreviews. 2015;16:e680–e692. doi: 10.1542/neo.16-12-e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vali P, Underwood M, Lakshminrusimha S. Hemoglobin oxygen saturation targets in the neonatal intensive care unit: Is there a light at the end of the tunnel? (1) Can J Physiol Pharmacol. 2019;97:174–182. doi: 10.1139/cjpp-2018-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth. 2014;28:1043–1047. doi: 10.1053/j.jvca.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 105.Kuo WC, Wu TC, Wang JS. Design and Application of a Flexible Blood Oxygen Sensing Array for Wearable Devices. Micromachines (Basel) 2022;13 doi: 10.3390/mi13101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Watcha MF, Connor MT, Hing AV. Pulse oximetry in methemoglobinemia. Am J Dis Child. 1989;143:845–847. doi: 10.1001/archpedi.1989.02150190095030. [DOI] [PubMed] [Google Scholar]
- 107.Ward J, Motwani J, Baker N, Nash M, Ewer AK, Toldi G. Congenital Methemoglobinemia Identified by Pulse Oximetry Screening. Pediatrics. 2019;143 doi: 10.1542/peds.2018-2814. [DOI] [PubMed] [Google Scholar]
- 108.Lyle ANJ, Spurr R, Kirkey D, Albert CM, Billimoria Z, Perez J, Puia-Dumitrescu M. Case report of congenital methemoglobinemia: an uncommon cause of neonatal cyanosis. Matern Health Neonatol Perinatol. 2022;8:7. doi: 10.1186/s40748-022-00142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Viršilas E, Timukienė L, Liubšys A. Congenital methemoglobinemia: Rare presentation of cyanosis in newborns. Clin Pract. 2019;9:1188. doi: 10.4081/cp.2019.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nasr VG, Markham LW, Clay M, DiNardo JA, Faraoni D, Gottlieb-Sen D, Miller-Hance WC, Pike NA, Rotman C American Heart Association Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Council on Cardiovascular Radiology and Intervention. Perioperative Considerations for Pediatric Patients With Congenital Heart Disease Presenting for Noncardiac Procedures: A Scientific Statement From the American Heart Association. Circ Cardiovasc Qual Outcomes. 2023;16:e000113. doi: 10.1161/HCQ.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 111.Sheikh M, Ahmad H, Ibrahim R, Nisar I, Jehan F. Pulse oximetry: why oxygen saturation is still not a part of standard pediatric guidelines in low-and-middle-income countries (LMICs) Pneumonia (Nathan) 2023;15:3. doi: 10.1186/s41479-023-00108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hendaus MA, Jomha FA, Alhammadi AH. Pulse oximetry in bronchiolitis: is it needed? Ther Clin Risk Manag. 2015;11:1573–1578. doi: 10.2147/TCRM.S93176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walsh BK, Smallwood CD. Pediatric Oxygen Therapy: A Review and Update. Respir Care. 2017;62:645–661. doi: 10.4187/respcare.05245. [DOI] [PubMed] [Google Scholar]
- 114.Sylvies F, Nyirenda L, Blair A, Baltzell K. The impact of pulse oximetry and Integrated Management of Childhood Illness (IMCI) training on antibiotic prescribing practices in rural Malawi: A mixed-methods study. PLoS One. 2020;15:e0242440. doi: 10.1371/journal.pone.0242440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lancet EA, Gonzalez D, Alexandrou NA, Zabar B, Lai PH, Hall CB, Braun J, Zeig-Owens R, Isaacs D, Ben-Eli D, Reisman N, Kaufman B, Asaeda G, Weiden MD, Nolan A, Teo H, Wei E, Natsui S, Philippou C, Prezant DJ. Prehospital hypoxemia, measured by pulse oximetry, predicts hospital outcomes during the New York City COVID-19 pandemic. J Am Coll Emerg Physicians Open. 2021;2:e12407. doi: 10.1002/emp2.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Solé D, Komatsu MK, Carvalho KV, Naspitz CK. Pulse oximetry in the evaluation of the severity of acute asthma and/or wheezing in children. J Asthma. 1999;36:327–333. doi: 10.3109/02770909909068225. [DOI] [PubMed] [Google Scholar]
- 117.Wheeler DS. Life-Threatening Diseases of the Upper Respiratory Tract. Pediatr Crit Care Me. 2014:19–39. [Google Scholar]
- 118.Sarkar M, Niranjan N, Banyal PK. Mechanisms of hypoxemia. Lung India. 2017;34:47–60. doi: 10.4103/0970-2113.197116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530. doi: 10.1001/archpedi.158.6.527. [DOI] [PubMed] [Google Scholar]
- 120.Hedible GB, Louart S, Neboua D, Catala L, Anago G, Sawadogo AG, Kargougou GD, Meda B, Kolié JS, Hema A, Keita S, Niome M, Savadogo AS, Peters-Bokol L, Agbeci H, Zair Z, Lenaud S, Vignon M, Ouedraogo Yugbare S, Abarry H, Diakite AA, Diallo IS, Lamontagne F, Briand V, Dahourou DL, Cousien A, Ridde V, Leroy V AIRE Research Study Group. Evaluation of the routine implementation of pulse oximeters into integrated management of childhood illness (IMCI) guidelines at primary health care level in West Africa: the AIRE mixed-methods research protocol. BMC Health Serv Res. 2022;22:1579. doi: 10.1186/s12913-022-08982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fouzas S, Priftis KN, Anthracopoulos MB. Pulse oximetry in pediatric practice. Pediatrics. 2011;128:740–752. doi: 10.1542/peds.2011-0271. [DOI] [PubMed] [Google Scholar]
- 122.Perkins GD, Graesner JT, Semeraro F, Olasveengen T, Soar J, Lott C, Van de Voorde P, Madar J, Zideman D, Mentzelopoulos S, Bossaert L, Greif R, Monsieurs K, Svavarsdóttir H, Nolan JP European Resuscitation Council Guideline Collaborators. European Resuscitation Council Guidelines 2021: Executive summary. Resuscitation. 2021;161:1–60. doi: 10.1016/j.resuscitation.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 123.Pinsky MR. Functional hemodynamic monitoring. Crit Care Clin. 2015;31:89–111. doi: 10.1016/j.ccc.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amsbaugh S, Scott SD, Foss K. Pulse Oximetry Screening for Critical Congenital Heart Disease: Bringing Evidence Into Practice. J Pediatr Nurs. 2015;30:591–597. doi: 10.1016/j.pedn.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 125.Anderson JA, Vann WF Jr. Respiratory monitoring during pediatric sedation: pulse oximetry and capnography. Pediatr Dent. 1988;10:94–101. [PubMed] [Google Scholar]
- 126.Divatia J. Pulse oximetry: Mandatory for sedation during regional/local Anaesthesia (but watch for hypoventilation!) Indian J Anaesth. 2011;55:217–219. doi: 10.4103/0019-5049.82649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saraswat V. Effects of anaesthesia techniques and drugs on pulmonary function. Indian J Anaesth. 2015;59:557–564. doi: 10.4103/0019-5049.165850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maity A, Saha D, Swaika S, Maulik SG, Choudhury B, Sutradhar M. Detection of hypoxia in the early postoperative period. Anesth Essays Res. 2012;6:34–37. doi: 10.4103/0259-1162.103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karcz M, Papadakos PJ. Respiratory complications in the postanesthesia care unit: A review of pathophysiological mechanisms. Can J Respir Ther. 2013;49:21–29. [PMC free article] [PubMed] [Google Scholar]
- 130.Abdelbaky AM, Elmasry WG, Awad AH. Lower Versus Higher Oxygenation Targets for Critically Ill Patients: A Systematic Review. Cureus. 2023;15:e41330. doi: 10.7759/cureus.41330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schondelmeyer AC, Dewan ML, Brady PW, Timmons KM, Cable R, Britto MT, Bonafide CP. Cardiorespiratory and Pulse Oximetry Monitoring in Hospitalized Children: A Delphi Process. Pediatrics. 2020;146 doi: 10.1542/peds.2019-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burn SL, Chilton PJ, Gawande AA, Lilford RJ. Peri-operative pulse oximetry in low-income countries: a cost-effectiveness analysis. Bull World Health Organ. 2014;92:858–867. doi: 10.2471/BLT.14.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hardy MS, Dallaire C, Bouchlaghem MA, Hajji I. The impact of the use of continuous pulse oximetry monitoring to monitor patients at high risk of respiratory depression on nursing practice. Nurs Open. 2023;10:6136–6142. doi: 10.1002/nop2.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bitners AC, Arens R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung. 2020;198:257–270. doi: 10.1007/s00408-020-00342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Álvarez D, Alonso-Álvarez ML, Gutiérrez-Tobal GC, Crespo A, Kheirandish-Gozal L, Hornero R, Gozal D, Terán-Santos J, Del Campo F. Automated Screening of Children With Obstructive Sleep Apnea Using Nocturnal Oximetry: An Alternative to Respiratory Polygraphy in Unattended Settings. J Clin Sleep Med. 2017;13:693–702. doi: 10.5664/jcsm.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xiao L, Barrowman N, Momoli F, Murto K, Bromwich M, Proulx F, Katz SL. Polysomnography parameters as predictors of respiratory adverse events following adenotonsillectomy in children. J Clin Sleep Med. 2021;17:2215–2223. doi: 10.5664/jcsm.9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nigro CA, Borsini EE, Dibur E, Larrateguy LD, Cazaux A, Elias C, de-la-Vega M, Berrozpe C, Maggi S, Grandval S, Cambursano H, Visentini D, Criniti J, Nogueira F. CPAP indication based on clinical data and oximetry for patients with suspicion of obstructive sleep apnea: A multicenter trial. Sleep Sci. 2019;12:249–256. doi: 10.5935/1984-0063.20190089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burke RM, Maxwell B, Hunter C, Graham D, O'Donoghue D, Shields MD. Night-to-night variation of pulse oximetry in children with sleep-disordered breathing. Arch Dis Child. 2016;101:1095–1099. doi: 10.1136/archdischild-2015-308981. [DOI] [PubMed] [Google Scholar]
- 139.Garde A, Dehkordi P, Karlen W, Wensley D, Ansermino JM, Dumont GA. Development of a screening tool for sleep disordered breathing in children using the phone Oximeter™. PLoS One. 2014;9:e112959. doi: 10.1371/journal.pone.0112959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Athale J, Suffredini AF. Pulse Oximetry: A Necessary Imperfect Tool in Critical Care. Crit Care Med. 2023;51:1246–1248. doi: 10.1097/CCM.0000000000005891. [DOI] [PubMed] [Google Scholar]
- 141.Helm M, Lampl L, Forstner K, Maier B, Tisch M, Bock KH. [Respiratory disorders in trauma patients. Pulse oximetry as an extension of prehospital diagnostic and therapeutic possibilities] Unfallchirurg. 1991;94:281–286. [PubMed] [Google Scholar]
- 142.Rackley CR. Monitoring During Mechanical Ventilation. Respir Care. 2020;65:832–846. doi: 10.4187/respcare.07812. [DOI] [PubMed] [Google Scholar]
- 143.Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, Te Pas A, Plavka R, Roehr CC, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GHA, Halliday HL. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023;120:3–23. doi: 10.1159/000528914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ergan B, Nasiłowski J, Winck JC. How should we monitor patients with acute respiratory failure treated with noninvasive ventilation? Eur Respir Rev. 2018;27 doi: 10.1183/16000617.0101-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garde A, Hoppenbrouwer X, Dehkordi P, Zhou G, Rollinson AU, Wensley D, Dumont GA, Ansermino JM. Pediatric pulse oximetry-based OSA screening at different thresholds of the apnea-hypopnea index with an expression of uncertainty for inconclusive classifications. Sleep Med. 2019;60:45–52. doi: 10.1016/j.sleep.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 146.Coverstone AM, Bird M, Sicard M, Tao Y, Grange DK, Cleveland C, Molter D, Kemp JS. Overnight pulse oximetry for evaluation of sleep apnea among children with trisomy 21. J Clin Sleep Med. 2014;10:1309–1315. doi: 10.5664/jcsm.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schmitt HJ, Schuetz WH, Proeschel PA, Jaklin C. Accuracy of pulse oximetry in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 1993;7:61–65. doi: 10.1016/1053-0770(93)90120-a. [DOI] [PubMed] [Google Scholar]
- 148.Racca F, Vianello A, Mongini T, Ruggeri P, Versaci A, Vita GL, Vita G. Practical approach to respiratory emergencies in neurological diseases. Neurol Sci. 2020;41:497–508. doi: 10.1007/s10072-019-04163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seddon P, Sobowiec-Kouman S, Wertheim D. Infant home respiratory monitoring using pulse oximetry. Arch Dis Child. 2018;103:603–605. doi: 10.1136/archdischild-2016-310712. [DOI] [PubMed] [Google Scholar]
- 150.Fierro J, Herrick H, Fregene N, Khan A, Ferro DF, Nelson MN, Brent CR, Bonafide CP, DeMauro SB. Home pulse oximetry after discharge from a quaternary-care children's hospital: Prescriber patterns and perspectives. Pediatr Pulmonol. 2022;57:209–216. doi: 10.1002/ppul.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rizzo L, Barbetta E, Ruberti F, Petz M, Tornesello M, Deolmi M, Fainardi V, Esposito S. The Role of Telemedicine in Children with Obstructive Sleep Apnea Syndrome (OSAS): A Review of the Literature. J Clin Med. 2024;13 doi: 10.3390/jcm13072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anawade PA, Sharma D, Gahane S. A Comprehensive Review on Exploring the Impact of Telemedicine on Healthcare Accessibility. Cureus. 2024;16:e55996. doi: 10.7759/cureus.55996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alboksmaty A, Beaney T, Elkin S, Clarke JM, Darzi A, Aylin P, Neves AL. Effectiveness and safety of pulse oximetry in remote patient monitoring of patients with COVID-19: a systematic review. Lancet Digit Health. 2022;4:e279–e289. doi: 10.1016/S2589-7500(21)00276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gylys R, Feiner J, Pologe J, Delianides T, Sutter S, Bickler P, Lipnick MS. Quantifying pulse oximeter accuracy during hypoxemia and severe anemia using an in vitro circulation system. J Clin Monit Comput. 2023;37:1441–1449. doi: 10.1007/s10877-023-01031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jay GD, Hughes L, Renzi FP. Pulse oximetry is accurate in acute anemia from hemorrhage. Ann Emerg Med. 1994;24:32–35. doi: 10.1016/s0196-0644(94)70158-x. [DOI] [PubMed] [Google Scholar]
- 156.Gadrey SM, Lau CE, Clay R, Rhodes GT, Lake DE, Moore CC, Voss JD, Moorman JR. Imputation of partial pressures of arterial oxygen using oximetry and its impact on sepsis diagnosis. Physiol Meas. 2019;40:115008. doi: 10.1088/1361-6579/ab5154. [DOI] [PubMed] [Google Scholar]
- 157.Torres-Robles A, Allison K, Poon SK, Shaw M, Hutchings O, Britton WJ, Wilson A, Baysari M. Patient and Clinician Perceptions of the Pulse Oximeter in a Remote Monitoring Setting for COVID-19: Qualitative Study. J Med Internet Res. 2023;25:e44540. doi: 10.2196/44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen G, Zhao L, Liu Y, Liao F, Han D, Zhou H. Regulation of blood viscosity in disease prevention and treatment. Kexve Tongbao. 2012;57:1946–1952. [Google Scholar]
- 159.Johansson P, Brudin G, Nilsson B, Andréasson B. Oxygen affinity is decreased in patients with polycythaemia vera and essential thrombocythaemia. Scand J Clin Lab Invest. 2004;64:71–75. doi: 10.1080/00365510310004128. [DOI] [PubMed] [Google Scholar]
- 160.Lenz C, Rebel A, Waschke KF, Koehler RC, Frietsch T. Blood viscosity modulates tissue perfusion: sometimes and somewhere. Transfus Altern Transfus Med. 2008;9:265–272. doi: 10.1111/j.1778-428X.2007.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wong AI, Charpignon M, Kim H, Josef C, de Hond AAH, Fojas JJ, Tabaie A, Liu X, Mireles-Cabodevila E, Carvalho L, Kamaleswaran R, Madushani RWMA, Adhikari L, Holder AL, Steyerberg EW, Buchman TG, Lough ME, Celi LA. Analysis of Discrepancies Between Pulse Oximetry and Arterial Oxygen Saturation Measurements by Race and Ethnicity and Association With Organ Dysfunction and Mortality. JAMA Netw Open. 2021;4:e2131674. doi: 10.1001/jamanetworkopen.2021.31674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stuart BJ, Viera AJ. Polycythemia vera. Am Fam Physician. 2004;69:2139–2144. [PubMed] [Google Scholar]
- 163.Tefferi A. Polycythemia vera: a comprehensive review and clinical recommendations. Mayo Clin Proc. 2003;78:174–194. doi: 10.4065/78.2.174. [DOI] [PubMed] [Google Scholar]
- 164.Opdahl H, Strømme TA, Jørgensen L, Bajelan L, Heier HE. The acidosis-induced right shift of the HbO₂ dissociation curve is maintained during erythrocyte storage. Scand J Clin Lab Invest. 2011;71:314–321. doi: 10.3109/00365513.2011.565366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lima A, van Genderen ME, Klijn E, Bakker J, van Bommel J. Peripheral vasoconstriction influences thenar oxygen saturation as measured by near-infrared spectroscopy. Intensive Care Med. 2012;38:606–611. doi: 10.1007/s00134-012-2486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nechipurenko YD, Semyonov DA, Lavrinenko IA, Lagutkin DA, Generalov EA, Zaitceva AY, Matveeva OV, Yegorov YE. The Role of Acidosis in the Pathogenesis of Severe Forms of COVID-19. Biology (Basel) 2021;10 doi: 10.3390/biology10090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohammed HM, Abdelatief DA. Easy blood gas analysis: Implications for nursing. Egypt J Chest Dis Tuberc. 2016;65:369–376. [Google Scholar]
- 168.Galley HF, Webster NR. Acidosis and tissue hypoxia in the critically ill: how to measure it and what does it mean. Crit Rev Clin Lab Sci. 1999;36:35–60. doi: 10.1080/10408369991239178. [DOI] [PubMed] [Google Scholar]
- 169.Langley R, Cunningham S. How Should Oxygen Supplementation Be Guided by Pulse Oximetry in Children: Do We Know the Level? Front Pediatr. 2016;4:138. doi: 10.3389/fped.2016.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Celotto AC, Capellini VK, Baldo CF, Dalio MB, Rodrigues AJ, Evora PR. Effects of acid-base imbalance on vascular reactivity. Braz J Med Biol Res. 2008;41:439–445. doi: 10.1590/s0100-879x2008005000026. [DOI] [PubMed] [Google Scholar]
- 171.Yee J, Frinak S, Mohiuddin N, Uduman J. Fundamentals of Arterial Blood Gas Interpretation. Kidney360. 2022;3:1458–1466. doi: 10.34067/KID.0008102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thijssen M, Janssen L, le Noble J, Foudraine N. Facing SpO(2) and SaO(2) discrepancies in ICU patients: is the perfusion index helpful? J Clin Monit Comput. 2020;34:693–698. doi: 10.1007/s10877-019-00371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pereira T, Tran N, Gadhoumi K, Pelter MM, Do DH, Lee RJ, Colorado R, Meisel K, Hu X. Photoplethysmography based atrial fibrillation detection: a review. NPJ Digit Med. 2020;3:3. doi: 10.1038/s41746-019-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Webb RK, Ralston AC, Runciman WB. Potential errors in pulse oximetry. II. Effects of changes in saturation and signal quality. Anaesthesia. 1991;46:207–212. doi: 10.1111/j.1365-2044.1991.tb09411.x. [DOI] [PubMed] [Google Scholar]
- 175.McMANUS DD, Chong JW, Soni A, Saczynski JS, Esa N, Napolitano C, Darling CE, Boyer E, Rosen RK, Floyd KC, Chon KH. PULSE-SMART: Pulse-Based Arrhythmia Discrimination Using a Novel Smartphone Application. J Cardiovasc Electrophysiol. 2016;27:51–57. doi: 10.1111/jce.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee J, Jung W, Kang I, Kim Y, Lee G. Design of filter to reject motion artifact of pulse oximetry. Comput Stand Inter. 2004;26:241–249. [Google Scholar]
- 177.Fridman V, Saponieri C, Sherif NE, Turitto G. Cardiac Rhythm Device Threshold Testing Via Pulse Oxymetry. J Atr Fibrillation. 2016;8:1389. doi: 10.4022/jafib.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.da Costa JC, Faustino P, Lima R, Ladeira I, Guimarães M. Research: Comparison of the Accuracy of a Pocket versus Standard Pulse Oximeter. Biomed Instrum Technol. 2016;50:190–193. doi: 10.2345/0899-8205-50.3.190. [DOI] [PubMed] [Google Scholar]
- 179.Petterson MT, Begnoche VL, Graybeal JM. The effect of motion on pulse oximetry and its clinical significance. Anesth Analg. 2007;105:S78–S84. doi: 10.1213/01.ane.0000278134.47777.a5. [DOI] [PubMed] [Google Scholar]
- 180.Woyke S, Brugger H, Ströhle M, Haller T, Gatterer H, Dal Cappello T, Strapazzon G. Effects of Carbon Dioxide and Temperature on the Oxygen-Hemoglobin Dissociation Curve of Human Blood: Implications for Avalanche Victims. Front Med (Lausanne) 2021;8:808025. doi: 10.3389/fmed.2021.808025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kaufman DP, Kandle PF, Murray IV, Dhamoon AS. Physiology, Oxyhemoglobin Dissociation Curve. 2023 Jul 31. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan- [PubMed] [Google Scholar]
- 182.Wintermark P, Hansen A, Gregas MC, Soul J, Labrecque M, Robertson RL, Warfield SK. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am J Neuroradiol. 2011;32:2023–2029. doi: 10.3174/ajnr.A2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shah N, Ragaswamy HB, Govindugari K, Estanol L. Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers. J Clin Anesth. 2012;24:385–391. doi: 10.1016/j.jclinane.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 184.Abraham EA, Verma G, Arafat Y, Acharya S, Kumar S, Pantbalekundri N. Comparative Analysis of Oxygen Saturation by Pulse Oximetry and Arterial Blood Gas in Hypoxemic Patients in a Tertiary Care Hospital. Cureus. 2023;15:e42447. doi: 10.7759/cureus.42447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lampert R, Brandt L. [The effect of hyperbilirubinemia on the measurement of oxygenated hemoglobin (O2Hb), carboxyhemoglobin (COHb) and methemoglobin (MetHb) using multiwavelength oximeters in mixed venous blood] Anaesthesist. 1993;42:702–709. [PubMed] [Google Scholar]
- 186.Hussain SA. Pulse oximetry interference in bronze baby syndrome. J Perinatol. 2009;29:828–829. doi: 10.1038/jp.2009.35. [DOI] [PubMed] [Google Scholar]
- 187.Bensghir M, Houba A, El Hila J, Ahtil R, Azendour H, Kamili ND. Henna dye: A cause of erroneous pulse oximetry readings. Saudi J Anaesth. 2013;7:474–475. doi: 10.4103/1658-354X.121052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sung SJ, Park YS, Cho JY. Full Thickness Burn on the Finger due to Pulse Oximetry during Magnetic Resonance Imaging in a Conscious Patient. Arch Plast Surg. 2016;43:612–613. doi: 10.5999/aps.2016.43.6.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dziuda Ł. Fiber-optic sensors for monitoring patient physiological parameters: a review of applicable technologies and relevance to use during magnetic resonance imaging procedures. J Biomed Opt. 2015;20:010901. doi: 10.1117/1.JBO.20.1.010901. [DOI] [PubMed] [Google Scholar]
- 190.Addison PS, Watson JN, Mestek ML, Ochs JP, Uribe AA, Bergese SD. Pulse oximetry-derived respiratory rate in general care floor patients. J Clin Monit Comput. 2015;29:113–120. doi: 10.1007/s10877-014-9575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Spiss CK, Mauritz W, Zadrobilek E, Draxler V. [Non-invasive pulse oximetry for the measurement of oxygen saturation in intensive care patients] Anaesthesist. 1985;34:405–408. [PubMed] [Google Scholar]
- 192.Hassan AT, Ahmed SM, AbdelHaffeez AS, Mohamed SAA. Accuracy and precision of pulse oximeter at different sensor locations in patients with heart failure. Multidiscip Respir Med. 2021;16:742. doi: 10.4081/mrm.2021.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brochard L, Martin GS, Blanch L, Pelosi P, Belda FJ, Jubran A, Gattinoni L, Mancebo J, Ranieri VM, Richard JC, Gommers D, Vieillard-Baron A, Pesenti A, Jaber S, Stenqvist O, Vincent JL. Clinical review: Respiratory monitoring in the ICU - a consensus of 16. Crit Care. 2012;16:219. doi: 10.1186/cc11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Comesaña-Campos A, Casal-Guisande M, Cerqueiro-Pequeño J, Bouza-Rodríguez JB. A Methodology Based on Expert Systems for the Early Detection and Prevention of Hypoxemic Clinical Cases. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17228644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gupta K, Chopra R, Kulkarni P. Use of pulse oximetry during nitrous oxide- oxygen inhalation sedation: mandatory or recommended? Eur Arch Paediatr Dent. 2022;23:647–652. doi: 10.1007/s40368-022-00717-7. [DOI] [PubMed] [Google Scholar]
- 196.Schrading WA, McCafferty B, Grove J, Page DB. Portable, consumer-grade pulse oximeters are accurate for home and medical use: Implications for use in the COVID-19 pandemic and other resource-limited environments. J Am Coll Emerg Physicians Open. 2020;1:1450–1458. doi: 10.1002/emp2.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Peterson ME, Docter S, Ruiz-Betancourt DR, Alawa J, Arimino S, Weiser TG. Pulse oximetry training landscape for healthcare workers in low- and middle-income countries: A scoping review. J Glob Health. 2023;13:04074. doi: 10.7189/jogh.13.04074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Davoudi A, Shickel B, Tighe PJ, Bihorac A, Rashidi P. Potentials and Challenges of Pervasive Sensing in the Intensive Care Unit. Front Digit Health. 2022;4:773387. doi: 10.3389/fdgth.2022.773387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vegesna A, Tran M, Angelaccio M, Arcona S. Remote Patient Monitoring via Non-Invasive Digital Technologies: A Systematic Review. Telemed J E Health. 2017;23:3–17. doi: 10.1089/tmj.2016.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chew R, Zhang M, Chandna A, Lubell Y. The impact of pulse oximetry on diagnosis, management and outcomes of acute febrile illness in low-income and middle-income countries: a systematic review. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-007282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Graham HR, Bagayana SM, Bakare AA, Olayo BO, Peterson SS, Duke T, Falade AG. Improving Hospital Oxygen Systems for COVID-19 in Low-Resource Settings: Lessons From the Field. Glob Health Sci Pract. 2020;8:858–862. doi: 10.9745/GHSP-D-20-00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chew R, Greer RC, Tasak N, Day NPJ, Lubell Y. Modelling the cost-effectiveness of pulse oximetry in primary care management of acute respiratory infection in rural northern Thailand. Trop Med Int Health. 2022;27:881–890. doi: 10.1111/tmi.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dempsey K, Lindsay M, Tcheng JE, Ian Wong AK. The High Price of Equity in Pulse Oximetry: A cost evaluation and need for interim solutions. medRxiv. 2023 [Google Scholar]
- 204.Wolk CB, Schondelmeyer AC, Barg FK, Beidas R, Bettencourt A, Brady PW, Brent C, Eriksen W, Kinkler G, Landrigan CP, Neergaard R, Bonafide CP Pediatric Research in Inpatient Settings (PRIS) Network. Barriers and Facilitators to Guideline-Adherent Pulse Oximetry Use in Bronchiolitis. J Hosp Med. 2021;16:23–30. doi: 10.12788/jhm.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Duke T, Graham SM, Cherian MN, Ginsburg AS, English M, Howie S, Peel D, Enarson PM, Wilson IH, Were W Union Oxygen Systems Working Group. Oxygen is an essential medicine: a call for international action. Int J Tuberc Lung Dis. 2010;14:1362–1368. [PMC free article] [PubMed] [Google Scholar]
- 206.Walters TP. Pulse oximetry knowledge and its effects on clinical practice. Br J Nurs. 2007;16:1332–1340. doi: 10.12968/bjon.2007.16.21.27721. [DOI] [PubMed] [Google Scholar]
- 207.van Zyl C, Badenhorst M, Hanekom S, Heine M. Unravelling 'low-resource settings': a systematic scoping review with qualitative content analysis. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Clarence C, Shiras T, Zhu J, Boggs MK, Faltas N, Wadsworth A, Bradley SE, Sadruddin S, Wazny K, Goodman C, Awor P, Bhutta ZA, Källander K, Hamer DH. Setting global research priorities for private sector child health service delivery: Results from a CHNRI exercise. J Glob Health. 2020;10:021201. doi: 10.7189/jogh.10.021201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Oyewole D, Boon R. Uses and limitations of pulse oximetry. Br J Hosp Med. 1996;55:222. [PubMed] [Google Scholar]
- 210.Yan YS, Zhang YT. An efficient motion-resistant method for wearable pulse oximeter. IEEE Trans Inf Technol Biomed. 2008;12:399–405. doi: 10.1109/titb.2007.902173. [DOI] [PubMed] [Google Scholar]
- 211.MacLeod DB, Cortinez LI, Keifer JC, Cameron D, Wright DR, White WD, Moretti EW, Radulescu LR, Somma J. The desaturation response time of finger pulse oximeters during mild hypothermia. Anaesthesia. 2005;60:65–71. doi: 10.1111/j.1365-2044.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 212.Gudelunas MK, Lipnick M, Hendrickson C, Vanderburg S, Okunlola B, Auchus I, Feiner JR, Bickler PE. Low Perfusion and Missed Diagnosis of Hypoxemia by Pulse Oximetry in Darkly Pigmented Skin: A Prospective Study. Anesth Analg. 2024;138:552–561. doi: 10.1213/ANE.0000000000006755. [DOI] [PubMed] [Google Scholar]
- 213.Coté CJ, Goldstein EA, Fuchsman WH, Hoaglin DC. The effect of nail polish on pulse oximetry. Anesth Analg. 1988;67:683–686. [PubMed] [Google Scholar]
- 214.Setchfield K, Gorman A, Simpson AHRW, Somekh MG, Wright AJ. Effect of skin color on optical properties and the implications for medical optical technologies: a review. J Biomed Opt. 2024;29:010901. doi: 10.1117/1.JBO.29.1.010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cabanas AM, Martín-Escudero P, Shelley KH. Improving pulse oximetry accuracy in dark-skinned patients: technical aspects and current regulations. Br J Anaesth. 2023;131:640–644. doi: 10.1016/j.bja.2023.07.005. [DOI] [PubMed] [Google Scholar]
- 216.Baek HY, Lee HJ, Kim JM, Cho SY, Jeong S, Yoo KY. Effects of intravenously administered indocyanine green on near-infrared cerebral oximetry and pulse oximetry readings. Korean J Anesthesiol. 2015;68:122–127. doi: 10.4097/kjae.2015.68.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rajadurai VS, Walker AM, Yu VY, Oates A. Effect of fetal haemoglobin on the accuracy of pulse oximetry in preterm infants. J Paediatr Child Health. 1992;28:43–46. doi: 10.1111/j.1440-1754.1992.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 218.Pritišanac E, Urlesberger B, Schwaberger B, Pichler G. Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin-A Systematic Review. Children (Basel) 2021;8 doi: 10.3390/children8050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hampson NB. Pulse oximetry in severe carbon monoxide poisoning. Chest. 1998;114:1036–1041. doi: 10.1378/chest.114.4.1036. [DOI] [PubMed] [Google Scholar]
- 220.Dohmen LME, Spigt M, Melbye H. The effect of atmospheric pressure on oxygen saturation and dyspnea: the Tromsø study. Int J Biometeorol. 2020;64:1103–1110. doi: 10.1007/s00484-020-01883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nguyen H, Jang S, Ivanov R, Bonafide CP, Weimer J, Lee I. Reducing Pulse Oximetry False Alarms Without Missing Life-Threatening Events. Smart Health (Amst) 2018;9-10:287–296. doi: 10.1016/j.smhl.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sendelbach S, Funk M. Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24:378–86; quiz 387. doi: 10.1097/NCI.0b013e3182a903f9. [DOI] [PubMed] [Google Scholar]
- 223.Sukor JA, Redmond SJ, Lovell NH. Signal quality measures for pulse oximetry through waveform morphology analysis. Physiol Meas. 2011;32:369–384. doi: 10.1088/0967-3334/32/3/008. [DOI] [PubMed] [Google Scholar]
- 224.Berg KJ, Johnson DP, Nyberg G, Claeys C, Ausmus A, Wilkinson E, Clark NA. Reducing the Frequency of Pulse Oximetry Alarms at a Children's Hospital. Pediatrics. 2023;151 doi: 10.1542/peds.2022-057465. [DOI] [PubMed] [Google Scholar]
- 225.Imhoff M, Kuhls S, Gather U, Fried R. Smart alarms from medical devices in the OR and ICU. Best Pract Res Clin Anaesthesiol. 2009;23:39–50. doi: 10.1016/j.bpa.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 226.Au-Yeung WM, Sahani AK, Isselbacher EM, Armoundas AA. Reduction of false alarms in the intensive care unit using an optimized machine learning based approach. NPJ Digit Med. 2019;2:86. doi: 10.1038/s41746-019-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shaoru C, Hui Z, Su W, Ruxin J, Huiyi Z, Hongmei Z, Hongyan Z. Determinants of Medical Equipment Alarm Fatigue in Practicing Nurses: A Systematic Review. SAGE Open Nurs. 2023;9:23779608231207227. doi: 10.1177/23779608231207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tobin RM, Pologe JA, Batchelder PB. A characterization of motion affecting pulse oximetry in 350 patients. Anesth Analg. 2002;94:S54–S61. [PubMed] [Google Scholar]
- 229.Batchelder PB, Raley DM. Maximizing the laboratory setting for testing devices and understanding statistical output in pulse oximetry. Anesth Analg. 2007;105:S85–S94. doi: 10.1213/01.ane.0000268495.35207.ab. [DOI] [PubMed] [Google Scholar]
- 230.Brekke IJ, Puntervoll LH, Pedersen PB, Kellett J, Brabrand M. The value of vital sign trends in predicting and monitoring clinical deterioration: A systematic review. PLoS One. 2019;14:e0210875. doi: 10.1371/journal.pone.0210875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Paul M, Maglaras L, Ferrag MA, Almomani I. Digitization of healthcare sector: A study on privacy and security concerns. ICT Express. 2023;9:571–588. [Google Scholar]