Abstract
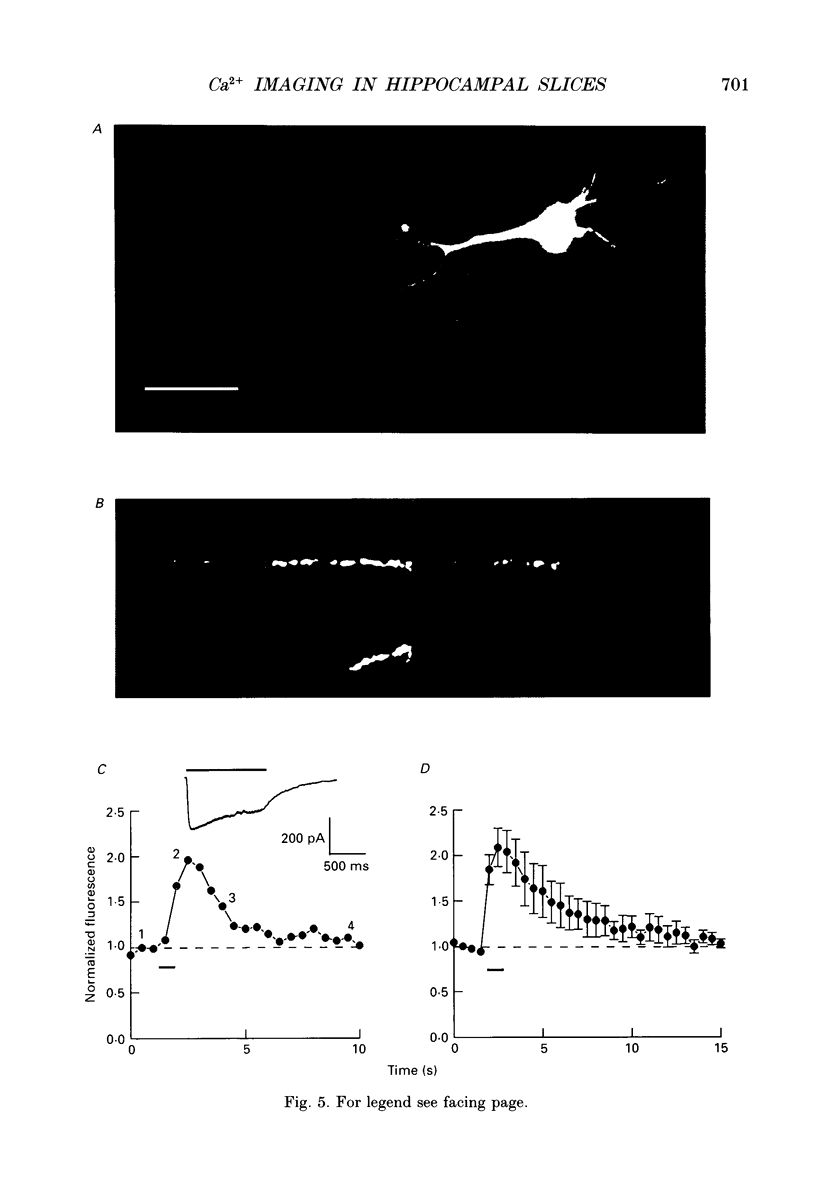
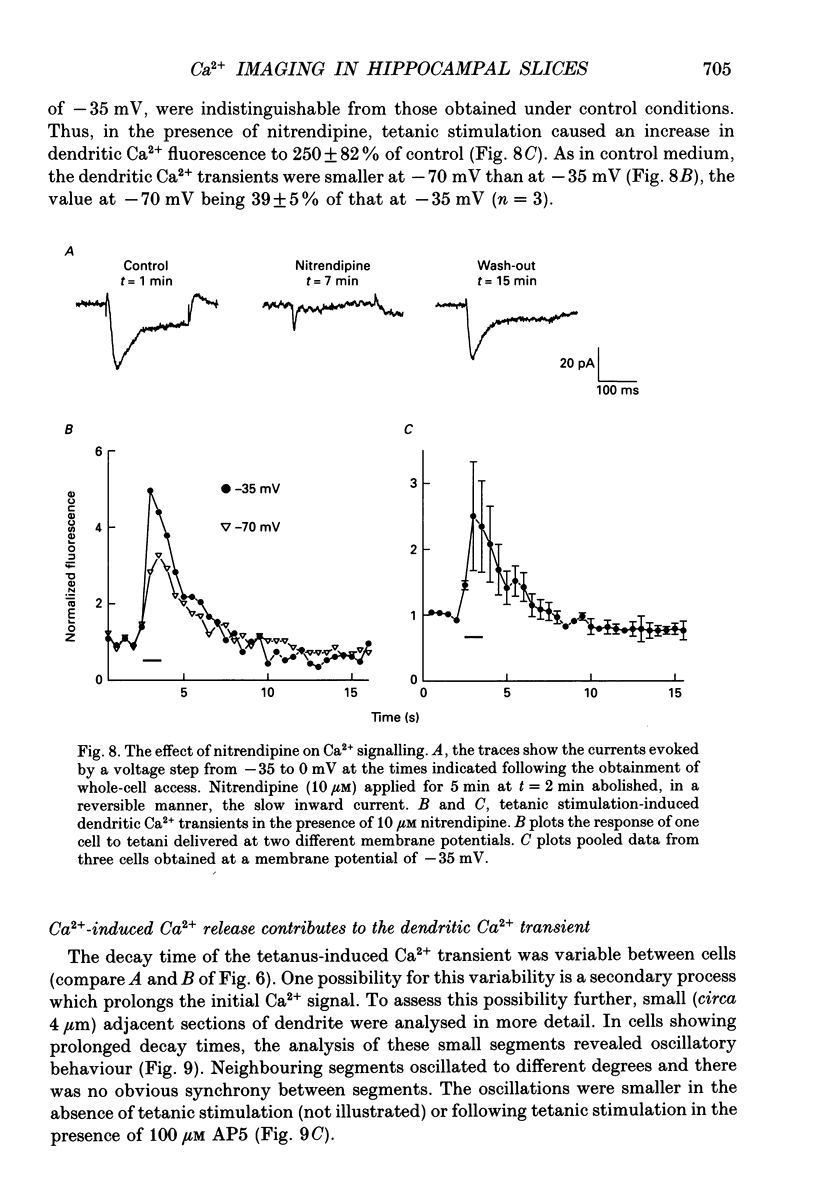
1. A combination of confocal microscopy, whole-cell patch-clamp recording, intracellular dialysis and pharmacological techniques have been employed to study Ca2+ signalling in CA1 pyramidal neurones, within rat hippocampal slices. 2. In the soma of CA1 neurones, depolarizing steps applied through the patch-pipette resulted in transient increases in the fluorescence emitted by the Ca2+ indicator fluo-3. The intensity of the fluorescence transients was proportional to the magnitude of the Ca2+ currents recorded through the pipette. Both the somatic fluorescence transients and the voltage-activated Ca2+ currents ran down in parallel over a period of between approximately 15-45 min. The fluorescence transients were considered, therefore, to be caused by increases in cytosolic free Ca2+. 3. Under current-clamp conditions, high-frequency (tetanic) stimulation (100 Hz, 1 s) of the Schaffer collateral-commissural pathway led to compound excitatory postsynaptic potentials (EPSPs) and somatic Ca2+ transients. The somatic Ca2+ transients were sensitive to the N-methyl-D-aspartate (NMDA) receptor antagonist D-2-amino-5-phosphonopentanoate (AP5; 100 microM). These transients, but not the EPSPs, disappeared with a time course similar to that of the run-down of voltage-gated Ca2+ currents. Tetanus-induced somatic Ca2+ transients could not be elicited under voltage-clamp conditions. 4. Fluorescence images were obtained from the dendrites of CA1 pyramidal neurones starting at least 30 min after obtaining whole-cell access to the neurone. Measurements were obtained only after voltage-gated Ca2+ channel activity had run down completely. 5. Tetanic stimulation of the Schaffer collateral-commissural pathway resulted in compound EPSPs and excitatory postsynaptic currents (EPSCs), under current- and voltage-clamp, respectively. In both cases, these were invariably associated with dendritic Ca2+ transients. In cells voltage-clamped at -35 mV, the fluorescent signal increased on average 2-fold during the tetanus and decayed to baseline values with a half-time (t1/2) of approximately 5 s. 6. The alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 microM) partially reduced the tetanus-induced EPSC without affecting the Ca2+ transients. In contrast, AP5, which also depressed the EPSC, substantially reduced or eliminated the Ca2+ transients. 7. In normal (i.e. 1 mM Mg(2+)-containing) medium, NMDA receptor-mediated synaptic currents displayed the typical region of negative slope conductance in the peak I-V relationship (between -90 and -35 mV). The dendritic tetanus-induced Ca2+ transients also displayed a similar anomalous voltage dependence, decreasing in size from -35 to -90 mV.(ABSTRACT TRUNCATED AT 400 WORDS)
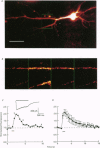
Full text
PDF


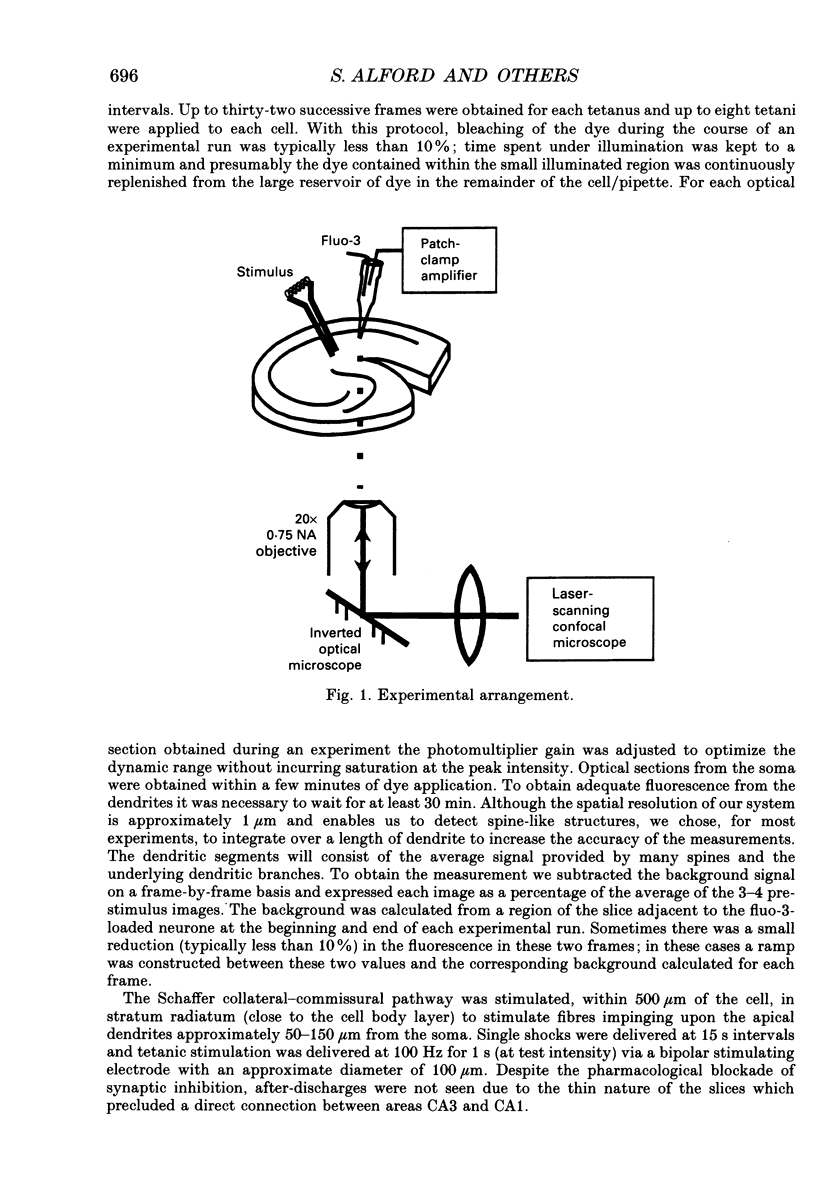

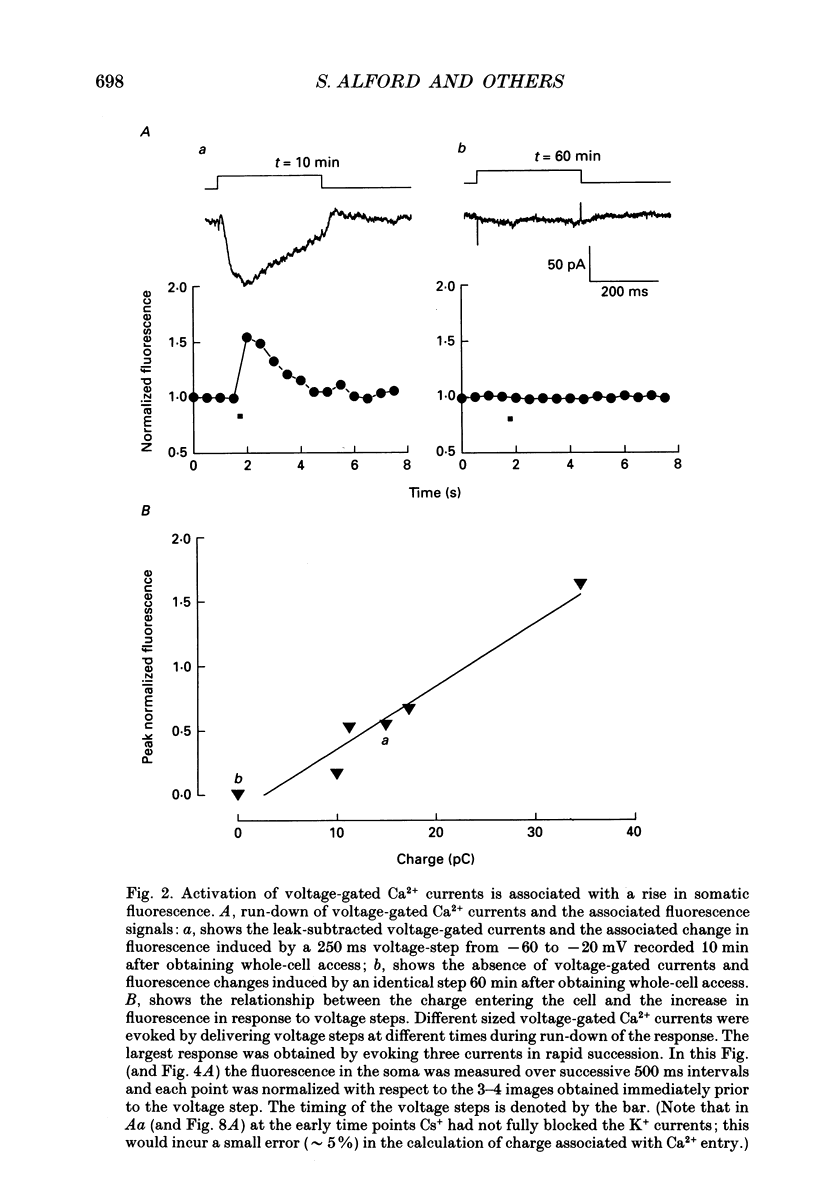
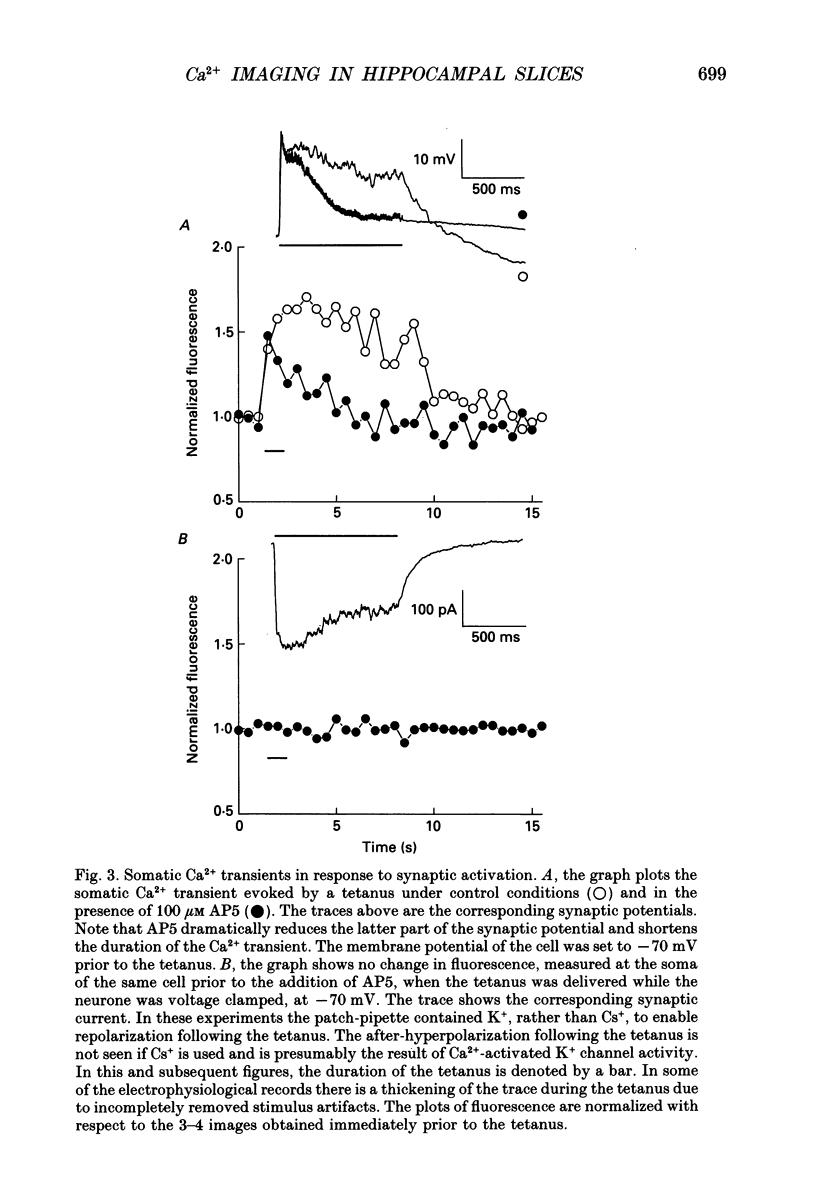
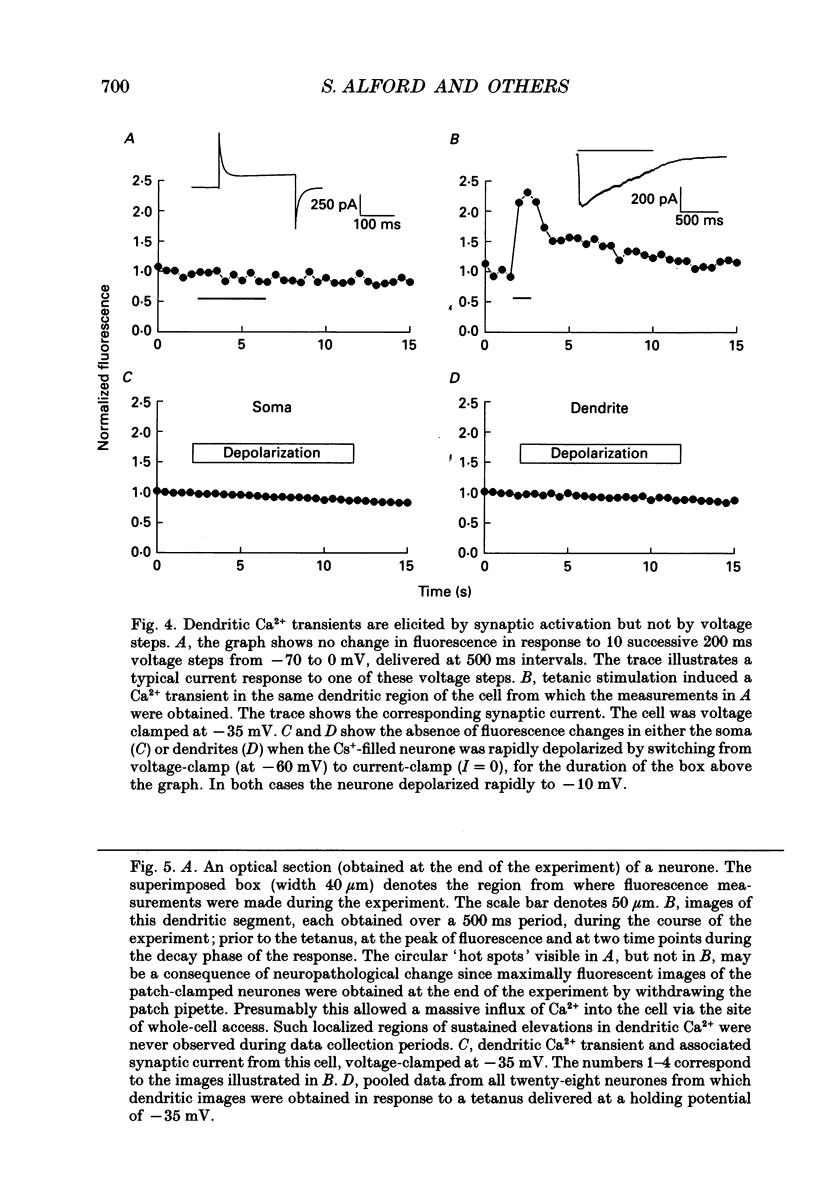

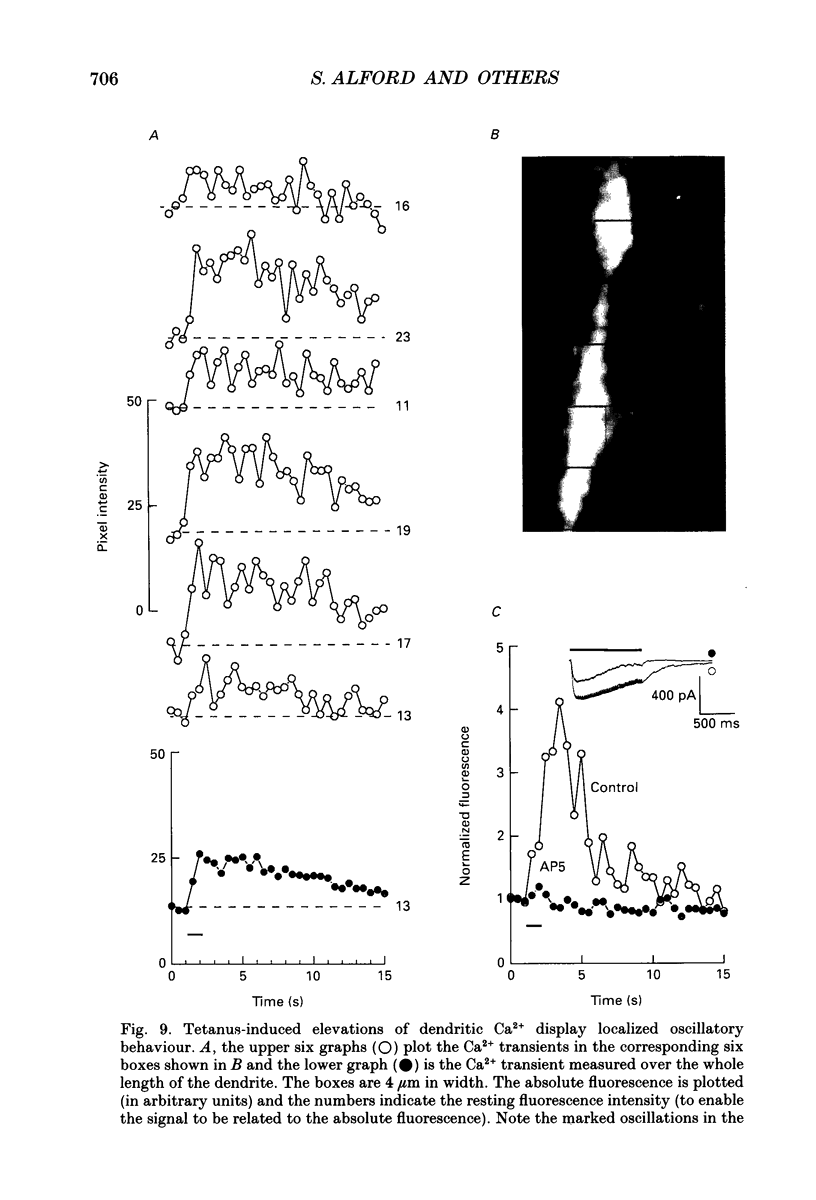
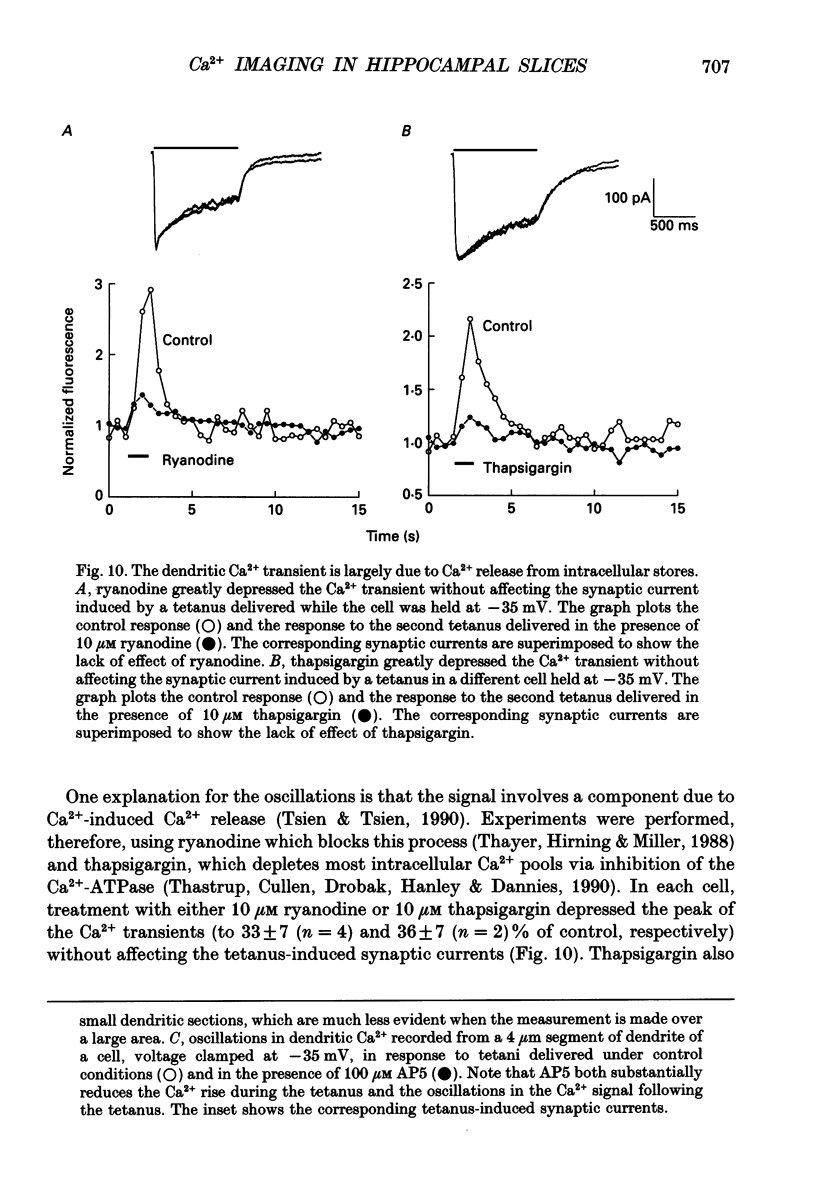
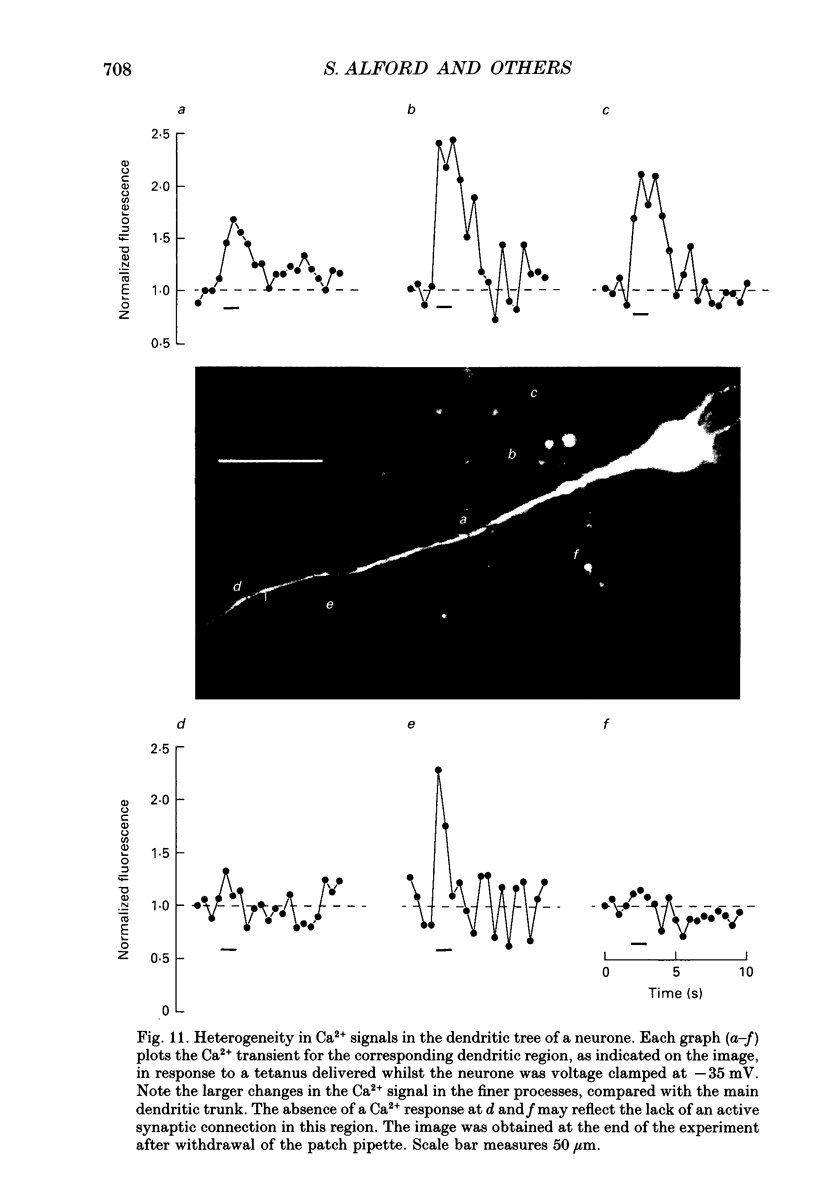
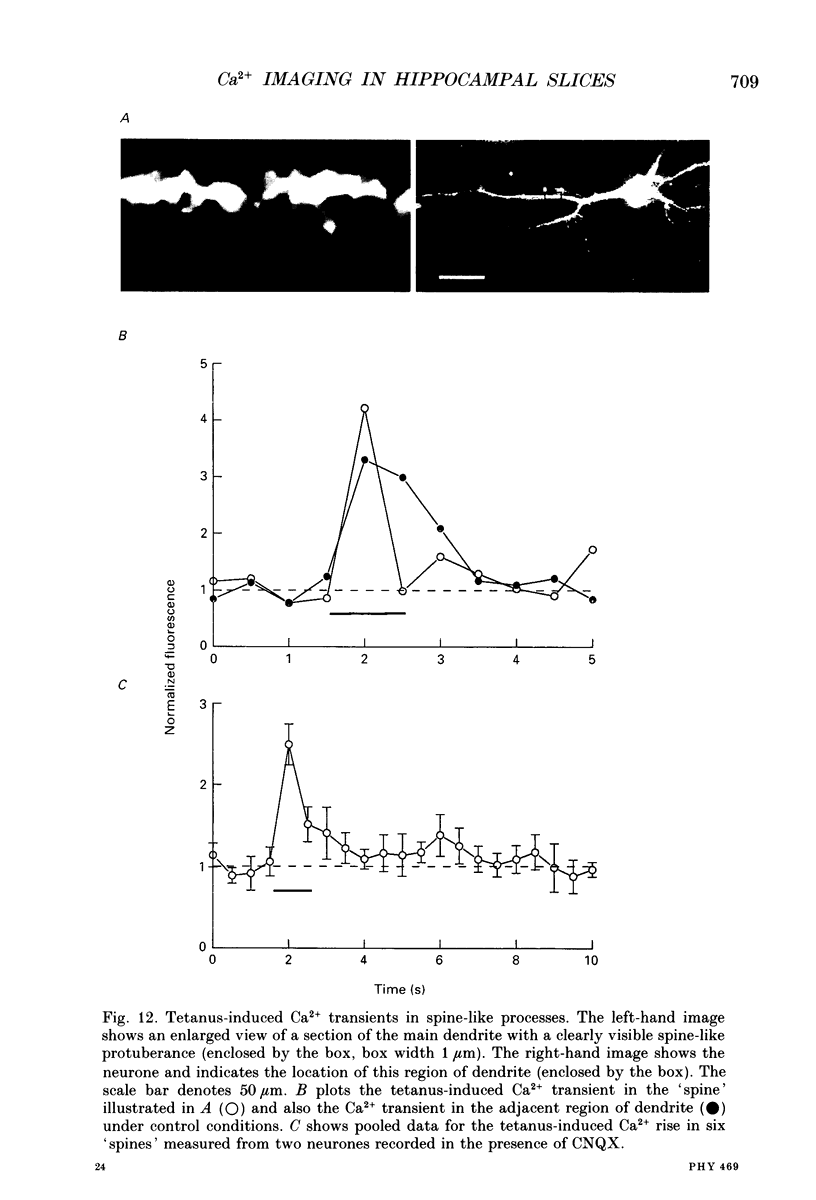







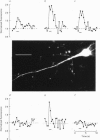
Images in this article
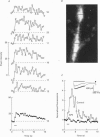
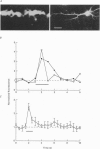
Selected References
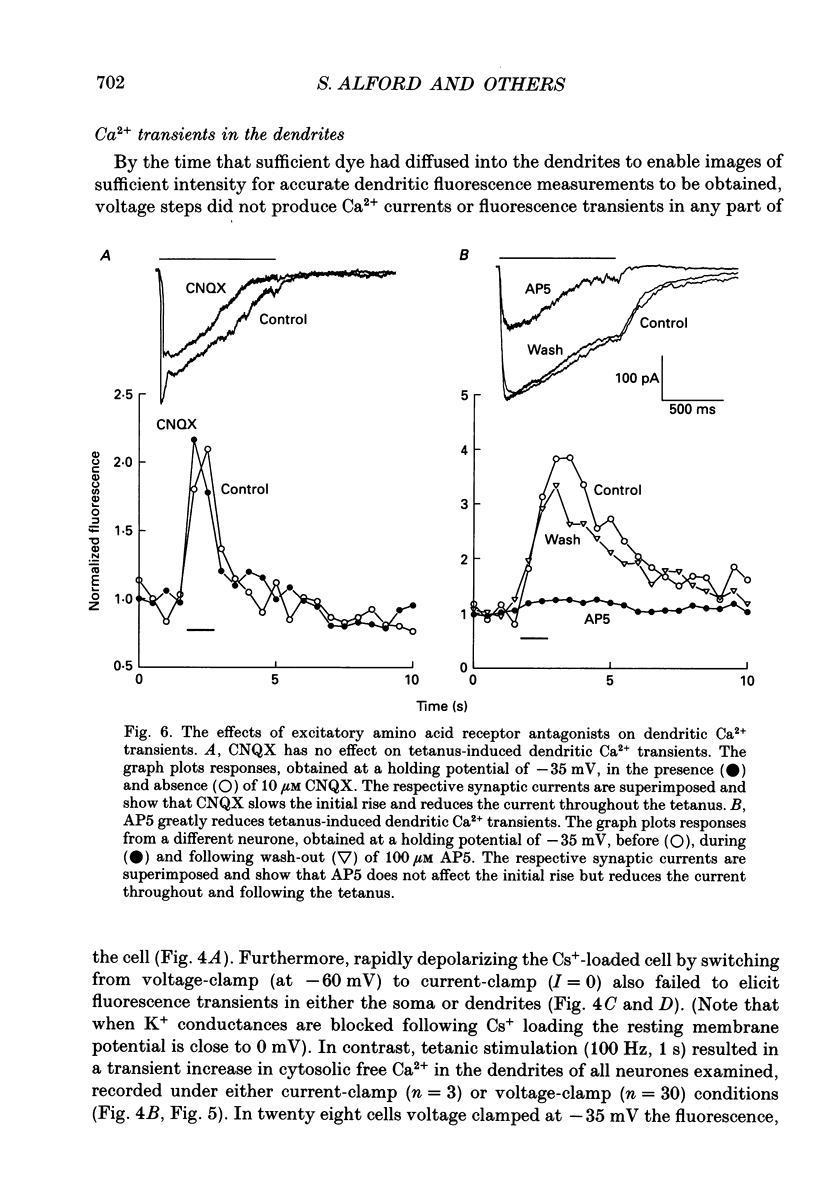
These references are in PubMed. This may not be the complete list of references from this article.
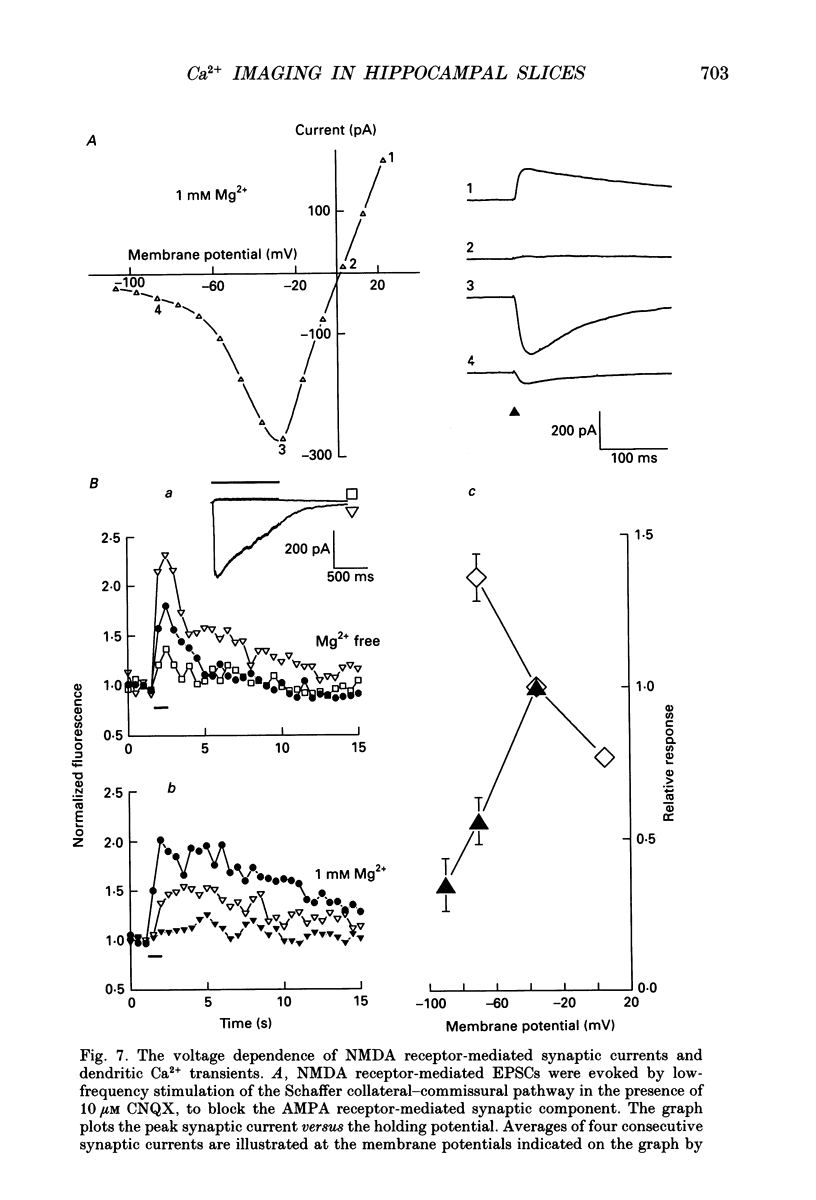
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J. F., Yates R. G., Brown M. W., Collingridge G. L. 6-Cyano-7-nitroquinoxaline-2,3-dione as an excitatory amino acid antagonist in area CA1 of rat hippocampus. Br J Pharmacol. 1989 May;97(1):71–76. doi: 10.1111/j.1476-5381.1989.tb11925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bortolotto Z. A., Collingridge G. L. Characterisation of LTP induced by the activation of glutamate metabotropic receptors in area CA1 of the hippocampus. Neuropharmacology. 1993 Jan;32(1):1–9. doi: 10.1016/0028-3908(93)90123-k. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher M. J., Collins J. F., Meldrum B. S. Anticonvulsant action of excitatory amino acid antagonists. Science. 1982 May 21;216(4548):899–901. doi: 10.1126/science.7079744. [DOI] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Collingridge G. L. Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):373–384. doi: 10.1098/rspb.1989.0028. [DOI] [PubMed] [Google Scholar]
- Fine A., Amos W. B., Durbin R. M., McNaughton P. A. Confocal microscopy: applications in neurobiology. Trends Neurosci. 1988 Aug;11(8):346–351. doi: 10.1016/0166-2236(88)90056-2. [DOI] [PubMed] [Google Scholar]
- Frey U., Krug M., Brödemann R., Reymann K., Matthies H. Long-term potentiation induced in dendrites separated from rat's CA1 pyramidal somata does not establish a late phase. Neurosci Lett. 1989 Feb 13;97(1-2):135–139. doi: 10.1016/0304-3940(89)90152-3. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc Biol Sci. 1991 Jan 22;243(1306):39–45. doi: 10.1098/rspb.1991.0007. [DOI] [PubMed] [Google Scholar]
- Harvey J., Collingridge G. L. Thapsigargin blocks the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1992 May 25;139(2):197–200. doi: 10.1016/0304-3940(92)90551-h. [DOI] [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving A. J., Collingridge G. L., Schofield J. G. Interactions between Ca2+ mobilizing mechanisms in cultured rat cerebellar granule cells. J Physiol. 1992 Oct;456:667–680. doi: 10.1113/jphysiol.1992.sp019360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Ballanyi K., Yaari Y. Voltage sensitivity of NMDA-receptor mediated postsynaptic currents. Exp Brain Res. 1990;81(1):209–212. doi: 10.1007/BF00230117. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Perkel D. J., Manabe T., Nicoll R. A. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992 Dec;9(6):1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Nicoll R. A. The impact of postsynaptic calcium on synaptic transmission--its role in long-term potentiation. Trends Neurosci. 1989 Nov;12(11):444–450. doi: 10.1016/0166-2236(89)90094-5. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Lancaster B., Zucker R. S. Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron. 1992 Jul;9(1):121–128. doi: 10.1016/0896-6273(92)90227-5. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Connor J. A. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature. 1991 Nov 7;354(6348):73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- Obenaus A., Mody I., Baimbridge K. G. Dantrolene-Na (Dantrium) blocks induction of long-term potentiation in hippocampal slices. Neurosci Lett. 1989 Mar 27;98(2):172–178. doi: 10.1016/0304-3940(89)90505-3. [DOI] [PubMed] [Google Scholar]
- Randall A. D., Schofield J. G., Collingridge G. L. Whole-cell patch-clamp recordings of an NMDA receptor-mediated synaptic current in rat hippocampal slices. Neurosci Lett. 1990 Jul 3;114(2):191–196. doi: 10.1016/0304-3940(90)90070-p. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. Calcium concentration dynamics produced by synaptic activation of CA1 hippocampal pyramidal cells. J Neurosci. 1992 Nov;12(11):4202–4223. doi: 10.1523/JNEUROSCI.12-11-04202.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. Postsynaptic NMDA receptor-mediated calcium accumulation in hippocampal CA1 pyramidal cell dendrites. Nature. 1990 Jun 28;345(6278):807–810. doi: 10.1038/345807a0. [DOI] [PubMed] [Google Scholar]
- Segal M., Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. J Physiol. 1992 Mar;448:655–676. doi: 10.1113/jphysiol.1992.sp019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Hirning L. D., Miller R. J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988 Nov;34(5):664–673. [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci. 1988 Oct;11(10):419–424. doi: 10.1016/0166-2236(88)90192-0. [DOI] [PubMed] [Google Scholar]