Abstract
To establish normal reference ranges for fetal right ventricular modified myocardial performance index (RV Mod-MPI) using automatic synchronization of the RV inflow and outflow images (MPI+TM). Additionally, we aimed to clinically apply RV Mod-MPI to investigate its changes in fetal right congenital diaphragmatic hernia (CDH) compared to normal fetuses. This prospective study included uncomplicated singleton pregnancies between 16 and 38 weeks of gestational age. Cases with any maternal or fetal complications that developed during the enrollment period were excluded. Two experienced operators measured the RV Mod-MPI using the automated and manual methods. The intraclass correlation coefficients (ICC) were calculated for intra- and inter-operator reproducibility. The mean differences between the manual and automated measurements were also compared. The RV Mod-MPI was then compared between the right CDH fetuses and normal fetuses. Seventy normal fetuses were analyzed for the feasibility of an automated system, and 364 examinations from 272 fetuses were analyzed for developing the normal references. The automated system showed significantly higher intra- and inter-operator reproducibility of Mod-MPI than those of manual measurements (ICC = 0.962 vs. 0.913 and 0.961 vs. 0.889, respectively). The mean difference in Mod-MPI between the manual and automated method was 0.0002 ± 0.0586 with a 95% confidence interval of -0.0095–0.0099. The Mod-MPI and isovolumetric relaxation time increased throughout the gestational weeks. The isovolumetric contraction time increased until 24 weeks of gestation and then slightly decreased afterwards, and the ejection time also increased until 31 weeks of gestation and then decreased. There was no significant difference in the Mod-MPI between right CDH and normal fetuses. The automated system showed high inter- and intra-operator reproducibility. Furthermore, the normal reference values of Mod-MPI for each gestational age were established. Our results suggest that the automated system might be clinically feasible for evaluating fetal cardiac function.
Subject terms: Cardiovascular biology, Intrauterine growth
Introduction
Assessment of cardiac function as well as structural abnormalities of the heart has been recognized as an essential component of fetal echocardiography1,2. Fetal cardiac function is an early marker of the fetal adaptive response to various insults, providing helpful information for fetal surveillance in several conditions such as twin-to-twin transfusion syndrome3–5, fetal growth restriction6–10, and gestational diabetes11–13. In addition, the fetal programming hypothesis has been suggested that the changes in fetal cardiac function in the intrauterine environment are closely linked to cardiovascular disease in adulthood14,15. Therefore, fetal cardiac function can be considered an important predictor of long-term cardiovascular outcomes as well as perinatal outcomes.
The myocardial performance index (MPI) is an indicator that evaluates global cardiac function measured by using a conventional pulsed-wave Doppler. MPI was first proposed by Tei16, and later in 2005, the modified method (Mod-MPI), which is measured using valve click, was introduced17. Since then, an automated system for MPI measurement was developed and is now globally used18. Until now, most studies have been conducted on the left heart because of the convenience of the measurement. However, measurement of right cardiac function is also important since the fetal heart shows right dominancy.
Mod-MPI with synchronized images of right ventricular (RV) inflow and outflow was developed as a technique for measuring fetal right cardiac function5. With this method, isovolumetric contraction time (ICT) and isovolumetric relaxation time (IRT) can be measured independently. Recently, this technique was advanced to an automated system (MPI + ™, Samsung Medison Co., Ltd., Seoul, Korea) to improve the reproducibility of RV Mod-MPI measurement.
Using this newly developed automated system, this study aimed to evaluate the feasibility of MPI + ™ and establish normal reference values for RV Mod-MPI. Afterwards, these reference values were applied to evaluating the RV function in fetuses with right congenital diaphragmatic hernia (CDH).
Results
Feasibility and reproducibility of MPI + ™
Two experienced operators were able to successfully measure RV Mod-MPI in all 70 cases. Intra- and inter-operator reproducibility of the RV Mod-MPI measurements by manual and automated methods are summarized in Table 1. The automated measurements demonstrated higher ICCs in both intra- and inter-operator reproducibility than the manual measurements. The highest ICC was achieved in the automated measurement of ET for both intra- and inter-operator reproducibility. Also, the ICCs were noticeably improved when IRTs were measured automatically. The mean difference between automated and manual measurement of the Mod-MPI, ICT, IRT, and ET were 0.0002 ± 0.0586 (95% CI, -0.0095 to 0.0099), 0.8575 ± 8.4241 (95% CI, -0.541 to 2.2559), 1.4278 ± 4.9515 (95% CI, 0.6058 to 2.2498), and 4.5203 ± 7.4951 (95% CI, 3.276 to 5.7645), respectively. The Bland–Altman plots for the Mod-MPI and its components measured by automated and manual methods are shown in Fig. 1.
Table 1.
Intra-operator and inter-operator reproducibility of measurements of the right ventricular modified myocardial performance index and its components.
| Intra-operator reproducibility | Inter-operator reproducibility | ||||||
|---|---|---|---|---|---|---|---|
| Value | Method | ICC | 95% CI | Value | Method | ICC | 95% CI |
| Mod-MPI | Manual | 0.913 | 0.879 − 0.941 | Mod-MPI | Manual | 0.889 | 0.862 − 0.912 |
| Automated | 0.962 | 0.946 − 0.975 | Automated | 0.961 | 0.952 − 0.970 | ||
| ICT | Manual | 0.904 | 0.866 − 0.935 | ICT | Manual | 0.883 | 0.855 − 0.907 |
| Automated | 0.964 | 0.949 − 0.976 | Automated | 0.964 | 0.955 − 0.972 | ||
| IRT | Manual | 0.863 | 0.812 − 0.906 | IRT | Manual | 0.843 | 0.806 − 0.874 |
| Automated | 0.963 | 0.948 − 0.976 | Automated | 0.962 | 0.953 − 0.970 | ||
| ET | Manual | 0.94 | 0.916 − 0.960 | ET | Manual | 0.928 | 0.910 − 0.943 |
| Automated | 0.977 | 0.967 − 0.985 | Automated | 0.976 | 0.971 − 0.982 | ||
ICC, intraclass correlation coefficient; CI, confidence interval; Mod-MPI, modified myocardial performance index; ICT, isovolumetric contraction time; IRT, isovolumetric relaxation time; ET, ejection time.
Fig. 1.
Bland–Altman plots demonstrating the mean differences for automated and manual measurement of right ventricular modified myocardial performance index and its components. (a) RV Mod-MPI (b) ICT (c) IRT (d) ET. RV Mod-MPI, modified myocardial performance index; ICT, isovolumetric time; IRT, isovolumetric relaxation time; ET, ejection time.
Development of reference values for RV Mod-MPI using MPI + ™
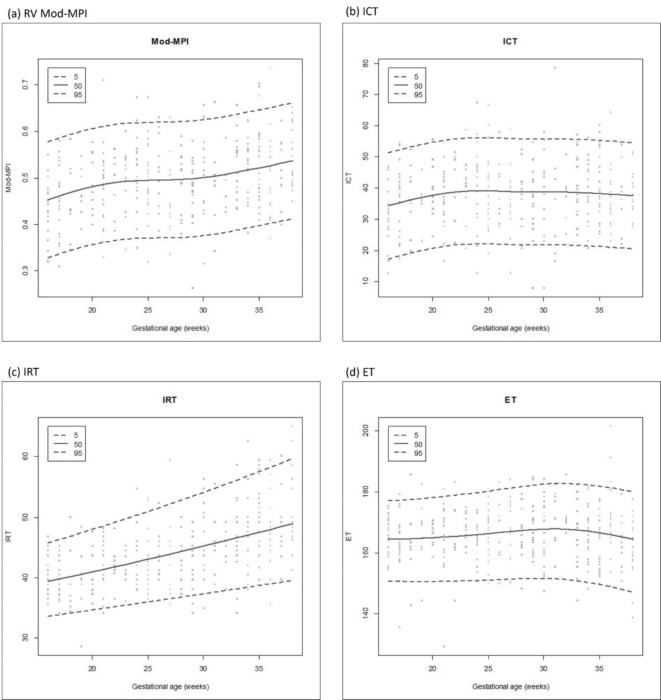
For reference values, 363 examinations were performed on 272 pregnant women. The median age of the pregnant women participating in the study was 34.0 years (range, 22–44 years), and the median BMI at the time of measurement was 24.2 (range, 14.6–35.7). All the pregnant women were Asian. The median gestational age at delivery was 38.5 weeks (range, 37.0 to 40.5 weeks), and the median birth weight was 3200 g (range, 2530 to 3770 g). The distribution of the cases by gestational age are demonstrated in Table 2. The normal reference values and scatter plots of Mod-MPI and its components corresponding to gestational age are shown in Table 3; Fig. 2. The median Mod-MPI increased from 0.45 at 16.0 weeks of gestation to 0.53 at 38.0 weeks of gestation. At the same time, IRT also increased from 39.4 ms to 48.9 ms between the same gestational week intervals. The median ICT increased from 34.3 ms to 39.2 ms until 24 weeks of gestation and then slightly decreased afterwards. The median ET also increased from 164.6 ms to 168.0 ms until 31 weeks of gestation, and it decreased to 164.5 ms at 38 weeks of gestation.
Table 2.
Distribution of the cases by gestational age.
| Gestational age (weeks) | Case (n) | Gestational age (weeks) | Case (n) | Gestational age (weeks) | Case (n) |
|---|---|---|---|---|---|
| 16 | 20 | 24 | 18 | 32 | 7 |
| 17 | 22 | 25 | 23 | 33 | 18 |
| 18 | 9 | 26 | 11 | 34 | 19 |
| 19 | 9 | 27 | 15 | 35 | 26 |
| 20 | 12 | 28 | 14 | 36 | 19 |
| 21 | 22 | 29 | 20 | 37 | 12 |
| 22 | 8 | 30 | 18 | 38 | 20 |
| 23 | 13 | 31 | 8 | Total | 363 |
Table 3.
Normal reference values for the right ventricular modified myocardial performance index and its components throughout gestation.
| GA (weeks) |
Mod-MPI | ICT (ms) | IRT (ms) | ET (ms) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th percentile | 10th percentile | 50th percentile | 90th percentile | 95th percentile | 5th percentile | 10th percentile | 50th percentile | 90th percentile | 95th percentile | 5th percentile | 10th percentile | 50th percentile | 90th percentile | 95th percentile | 5th percentile | 10th percentile | 50th percentile | 90th percentile | 95th percentile | |
| 16 | 0.34 | 0.36 | 0.45 | 0.56 | 0.59 | 17.3 | 21.4 | 34.3 | 47.2 | 51.3 | 33.6 | 34.8 | 39.4 | 44.3 | 45.8 | 150.8 | 154.2 | 164.6 | 174.3 | 177.2 |
| 17 | 0.34 | 0.37 | 0.46 | 0.56 | 0.60 | 18.3 | 22.4 | 35.3 | 48.2 | 52.3 | 33.9 | 35.1 | 39.8 | 44.8 | 46.3 | 150.7 | 154.1 | 164.6 | 174.4 | 177.4 |
| 18 | 0.35 | 0.37 | 0.46 | 0.57 | 0.60 | 19.2 | 23.3 | 36.2 | 49.1 | 53.2 | 34.1 | 35.4 | 40.1 | 45.3 | 46.9 | 150.6 | 154.1 | 164.7 | 174.6 | 177.6 |
| 19 | 0.36 | 0.38 | 0.47 | 0.58 | 0.61 | 20.0 | 24.1 | 37.0 | 49.9 | 54.0 | 34.4 | 35.7 | 40.5 | 45.8 | 47.4 | 150.6 | 154.1 | 164.8 | 174.8 | 177.9 |
| 20 | 0.36 | 0.38 | 0.48 | 0.58 | 0.61 | 20.7 | 24.8 | 37.7 | 50.6 | 54.7 | 34.6 | 36.0 | 41.0 | 46.4 | 48.0 | 150.6 | 154.2 | 165.0 | 175.1 | 178.2 |
| 21 | 0.36 | 0.39 | 0.48 | 0.59 | 0.62 | 21.3 | 25.4 | 38.3 | 51.2 | 55.3 | 34.9 | 36.3 | 41.4 | 46.9 | 48.6 | 150.7 | 154.3 | 165.2 | 175.5 | 178.6 |
| 22 | 0.37 | 0.39 | 0.49 | 0.59 | 0.62 | 21.7 | 25.8 | 38.8 | 51.7 | 55.8 | 35.2 | 36.6 | 41.8 | 47.4 | 49.1 | 150.8 | 154.4 | 165.5 | 175.9 | 179.0 |
| 23 | 0.37 | 0.39 | 0.49 | 0.59 | 0.62 | 22.0 | 26.1 | 39.0 | 52.0 | 56.1 | 35.4 | 36.9 | 42.2 | 48.0 | 49.7 | 150.9 | 154.6 | 165.8 | 176.3 | 179.4 |
| 24 | 0.37 | 0.40 | 0.49 | 0.59 | 0.62 | 22.2 | 26.3 | 39.2 | 52.1 | 56.2 | 35.7 | 37.2 | 42.6 | 48.6 | 50.3 | 151.0 | 154.8 | 166.1 | 176.7 | 179.9 |
| 25 | 0.37 | 0.40 | 0.49 | 0.59 | 0.62 | 22.1 | 26.2 | 39.1 | 52.0 | 56.1 | 36.0 | 37.5 | 43.0 | 49.1 | 51.0 | 151.2 | 154.9 | 166.4 | 177.1 | 180.4 |
| 26 | 0.37 | 0.40 | 0.50 | 0.59 | 0.62 | 22.0 | 26.1 | 39.0 | 51.9 | 56.0 | 36.2 | 37.8 | 43.5 | 49.7 | 51.6 | 151.3 | 155.1 | 166.8 | 177.6 | 180.9 |
| 27 | 0.37 | 0.40 | 0.50 | 0.59 | 0.62 | 21.8 | 25.9 | 38.8 | 51.7 | 55.8 | 36.5 | 38.1 | 43.9 | 50.3 | 52.2 | 151.5 | 155.3 | 167.1 | 178.1 | 181.4 |
| 28 | 0.37 | 0.40 | 0.50 | 0.59 | 0.62 | 21.7 | 25.8 | 38.7 | 51.6 | 55.7 | 36.8 | 38.4 | 44.3 | 50.9 | 52.8 | 151.6 | 155.5 | 167.4 | 178.5 | 181.9 |
| 29 | 0.37 | 0.40 | 0.50 | 0.59 | 0.62 | 21.7 | 25.8 | 38.7 | 51.6 | 55.7 | 37.1 | 38.7 | 44.8 | 51.5 | 53.5 | 151.7 | 155.7 | 167.7 | 178.9 | 182.3 |
| 30 | 0.38 | 0.41 | 0.50 | 0.60 | 0.62 | 21.7 | 25.8 | 38.7 | 51.6 | 55.7 | 37.3 | 39.0 | 45.2 | 52.1 | 54.1 | 151.7 | 155.7 | 167.9 | 179.2 | 182.6 |
| 31 | 0.38 | 0.41 | 0.50 | 0.60 | 0.63 | 21.7 | 25.8 | 38.7 | 51.6 | 55.7 | 37.6 | 39.3 | 45.7 | 52.7 | 54.8 | 151.6 | 155.6 | 168.0 | 179.4 | 182.9 |
| 32 | 0.39 | 0.41 | 0.51 | 0.60 | 0.63 | 21.7 | 25.8 | 38.7 | 51.6 | 55.7 | 37.9 | 39.6 | 46.1 | 53.3 | 55.5 | 151.3 | 155.4 | 167.9 | 179.4 | 182.9 |
| 33 | 0.39 | 0.42 | 0.51 | 0.61 | 0.64 | 21.6 | 25.7 | 38.6 | 51.6 | 55.7 | 38.1 | 39.9 | 46.6 | 53.9 | 56.2 | 150.9 | 155.0 | 167.6 | 179.3 | 182.7 |
| 34 | 0.40 | 0.42 | 0.52 | 0.61 | 0.64 | 21.5 | 25.6 | 38.5 | 51.4 | 55.5 | 38.4 | 40.2 | 47.0 | 54.6 | 56.8 | 150.4 | 154.5 | 167.2 | 178.9 | 182.4 |
| 35 | 0.40 | 0.43 | 0.52 | 0.62 | 0.65 | 21.3 | 25.4 | 38.3 | 51.2 | 55.3 | 38.7 | 40.5 | 47.5 | 55.2 | 57.6 | 149.7 | 153.9 | 166.7 | 178.5 | 182.0 |
| 36 | 0.41 | 0.43 | 0.52 | 0.62 | 0.65 | 21.1 | 25.2 | 38.1 | 51.0 | 55.1 | 39.0 | 40.8 | 48.0 | 55.9 | 58.3 | 149.0 | 153.2 | 166.0 | 177.9 | 181.5 |
| 37 | 0.42 | 0.44 | 0.53 | 0.63 | 0.66 | 20.8 | 24.9 | 37.8 | 50.8 | 54.8 | 39.2 | 41.2 | 48.4 | 56.5 | 59.0 | 148.1 | 152.4 | 165.3 | 177.2 | 180.8 |
| 38 | 0.42 | 0.45 | 0.53 | 0.64 | 0.67 | 20.6 | 24.7 | 37.6 | 50.5 | 54.6 | 39.5 | 41.5 | 48.9 | 57.2 | 59.7 | 147.3 | 151.6 | 164.5 | 176.5 | 180.1 |
Mod-MPI, modified myocardial performance index; ICT, isovolumetric time; IRT, isovolumetric relaxation time; ET, ejection time.
Fig. 2.
Scatter plots of the right ventricular modified myocardial performance index and its components according to gestational age. (a) RV Mod-MPI (b) ICT (c) IRT (d) ET. RV Mod-MPI, modified myocardial performance index; ICT, isovolumetric time; IRT, isovolumetric relaxation time; ET, ejection time.
Clinical application to fetuses with right CDH
A total of 31 measurements were obtained from 16 right CDH fetuses, and ten of them survived and six died. Since the RV Mod-MPI values showed a near plateau between 22 and 31 weeks of gestational age (Table 3), we compared the Mod-MPI and its components between the right CDH fetuses and normal fetuses in that gestational age (21 measurements from 13 CDH fetuses vs. 148 measurements from 134 fetuses). No significant differences were observed in all measured values between the right CDH and normal groups (Table 4). The scatter plots of Mod-MPI and its components in right CDH fetuses are demonstrated in Fig. 3. No fetuses showed a Mod-MPI above the 95th percentile among survivors, whereas three fetuses showed a Mod-MPI above the 95th percentile among non-survivors.
Table 4.
A comparison of the right ventricular modified myocardial performance index and its components between the fetuses with right congenital diaphragmatic hernia and normal fetuses.
| Right CDH (n = 13) |
Normal (n = 134) |
P value | |
|---|---|---|---|
| Mod-MPI | 0.47 (0.44–0.51) | 0.50 (0.48–0.51) | 0.276 |
| ICT (ms) | 38.3 (32.6–44.0) | 38.8 (37.0-40.7) | 0.860 |
| IRT (ms) | 41.9 (39.5–44.3) | 43.7 (42.9–44.5) | 0.167 |
| ET (ms) | 169.9 (165.4-174.4) | 166.9 (165.5-168.4) | 0.213 |
Values are given as estimate with a 95% confidence interval.
CDH, congenital diaphragmatic hernia; Mod-MPI, modified myocardial performance index; ICT, isovolumetric time; IRT, isovolumetric relaxation time; ET, ejection time.
Fig. 3.
Scatter plots of the right ventricle modified myocardial performance index and its components for survivors (blue filled triangle) and non-survivors (red filled circle) in right congenital diaphragmatic hernia. (a) RV Mod-MPI (b) ICT (c) IRT (d) ET. RV Mod-MPI, modified myocardial performance index; ICT, isovolumetric time; IRT, isovolumetric relaxation time; ET, ejection time; CDH, congenital diaphragmatic hernia.
Discussion
The current study presented (1) the feasibility of an automated RV Mod-MPI system, (2) normal reference values for RV Mod-MPI for each gestational age, and (3) the clinical applicability of RV Mod-MPI in right CDH fetuses. We found that the automated RV Mod-MPI system showed excellent intra- and inter-operator reproducibility. Mod-MPI and IRT increased throughout the gestational weeks, whereas ICT and ET increased until certain gestational weeks and thereafter decreased. We also clinically applied the automated system by comparing the cardiac function between the right CDH and normal fetuses.
Conventionally, the inflow and outflow waveforms of the right ventricle are obtained separately to measure the RV Mod-MPI17,19. Because the heart rate varies according to each cycle, previous studies investigating the RV Mod-MPI showed lower intra- and inter-operator reproducibility19. Moreover, detailed evaluation of cardiac function has been hampered by a lack of available ICT and IRT data. Although right cardiac function is more important in the fetus, RV Mod-MPI has not been widely used so far due to the aforementioned limitations. To overcome this, the synchronized method was introduced to measure the RV Mod-MPI5, and the automated system was developed.
The automated RV Mod-MPI system showed a high ICC of over 0.95 in the Mod-MPI, ICT, IRT, and ET. Among them, the ET showed the highest reproducibility, which is consistent with the previous studies5,18. Noteworthily, the reproducibility of IRT was the lowest in the manual measurements, but it noticeably improved using the automated measurements. This is probably due to the superiority of the automated system in synchronizing two optimal images based on the pulmonary valve closure clicks, which is sometimes difficult to operate accurately with the manual method. The automated system can detect the beginning point of a pulmonary valve click more accurately than the manual method, which leads to more consistent synchronization and higher reproducibility.
It is well-established that the left ventricular (LV) Mod-MPI increases with gestational age20–23, ranging from 0.39 to 0.57. The previously reported RV MPI values range widely from 0.35 to 0.53 in normal singleton fetuses24–27, and the associations between gestational age and MPI are inconsistent. A few studies reported that the RV MPI was independent of gestational age24–26. In those studies, they did not provide clear explanations on the measurement method (i.e., whether the conventional or modified method was used or where the calipers on each valve click were placed). Furthermore, they included cases with maternal complications, and MPI was not measured at each week of gestational age. These might have contributed to the discrepant findings in previous studies. Another relevant study conducted under similar study settings to ours with the conventional method demonstrated that the RV MPI increased throughout gestational age27, which is consistent with our finding.
CDH is commonly associated with lung hypoplasia, pulmonary hypertension, and cardiac dysfunction28. Especially, ventricular dysfunction is the well-known pathophysiology29. Several previous studies have attempted to detect cardiac dysfunction early in utero30–33. The results of this study showed that there was no difference in LV and RV MPI between fetuses with right CDH and normal fetuses, although right ventricular dimension and cardiac volume were reduced in fetuses with right CDH32. Even if fetuses with CDH have pulmonary hypoplasia with vascular resistance due to herniated organs, there may be little compromise of cardiac function because blood flow can be diverted to the foramen ovale and ductus arteriosus during the fetal period. However, most non-survivors in right CDH fetuses showed Mod-MPI and ICT values above the 50th percentile of normal references. We postulate that despite the redistribution of blood flow in fetal life, severe pulmonary resistance might influence the cardiac function in non-survivors. This finding is consistent with postnatal studies, which have supported the association between early right ventricular dysfunction and poor prognosis in right CDH neonates28,34,35. However, our findings were limited by a small sample size, necessitating further large-scale research.
There are a few limitations to this study. First, the automated RV Mod-MPI system is provided by a specific ultrasound device. Second, although we attempted to enroll cases evenly throughout the entire gestational period, the number of cases was uneven during some gestational ages, and consequently, less than 10 cases were available at a few gestational ages. Furthermore, this study only evaluated reproducibility between 20 and 24 weeks. To assess reproducibility accurately, clear images of the valve click in each inflow and outflow are essential. However, obtaining high-quality images can be challenging in fetuses with advanced gestational ages due to several factors, including fetal position, bone shadowing, and fetal breathing movements. Evaluation of reproducibility at later gestation might be required for a more comprehensive assessment.
In conclusion, the automated Mod-MPI system showed high inter- and intra-operator reproducibility. Furthermore, the normal reference ranges of RV Mod-MPI for each gestational age were established. Our results suggest that the automated RV Mod-MPI might be clinically feasible for evaluating cardiac function.
Materials and methods
Study design
This was a prospective study conducted with the cases of uncomplicated singleton pregnant women between 16 weeks and 38 weeks of gestational age who visited Asan Medical Center from May 2017 to July 2022. The inclusion criteria were: (1) pregnant women without any underlying diseases such as preexisting hypertension, diabetes, thyroid disease, and others; and (2) the fetuses with appropriate growth for their gestational age (estimated fetal weight between the 10th and 90th percentile). The accurate gestational ages of all fetuses were confirmed with ultrasound during the first trimester. Cases later diagnosed with maternal complications such as gestational diabetes mellitus, preeclampsia, and preterm birth, as well as fetal complications such as small or large for gestational age at birth, were further excluded. The study protocol was approved by Asan Medical Center Institutional Review Board (IRB numbers: 2017 − 0710 and 2020 − 1731) and all patients provided written informed consent. All methods were performed in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Obtaining images of inflow and outflow in right ventricle
All ultrasonographic measurements were performed using a WS80A or HERA W10 (Samsung Medison Co., Ltd., Seoul, Korea) ultrasound device with associated transabdominal probes. Doppler waveform images were obtained as previously described:5,18 (1) The RV inflow image was obtained in the apical four-chamber view by placing the cursor just beneath the tricuspid valve leaflet; (2) the RV outflow image was obtained in the RV outflow tract view by placing the cursor just above the pulmonary valve leaflet. All obtained images included the opening and closure clicks of the pulmonary valve. Only heart rate (HR) differences of ≤ 5 beats/min between RV inflow and outflow were included5. Ultrasonographic settings were as follows: 2–3 mm sample volume size, 420 Hz Doppler sweep frequency, low Doppler gain, 200–400 Hz wall motion filter, and < 15° angle of insonation.
Automated measurement of RV Mod-MPI
With clear images of both RV inflow and outflow obtained previously, the Mod-MPI was calculated using the MPI + ™ (Samsung Medison Co., Ltd., Seoul, Korea). MPI + ™ operated as follows (Fig. 4): (1) The operator classified the RV images into inflow and outflow and selected one cardiac cycle each from inflow and outflow with an HR difference of ≤ 5 beats/min; (2) If there was an HR difference between inflow and outflow, the system would automatically adjust the HR of outflow to that of inflow; (3) Then, the system automatically synchronized the inflow and outflow images based on the pulmonary valve closure clicks and placed the calipers at the beginning of each valve click; (4) And finally, the system automatically calculated the RV Mod-MPI and its components.
Fig. 4.

Measurement of the right ventricular modified myocardial performance index (RV Mod-MPI) using MPI + ™. (a) The operator classifies the RV inflow and outflow and presses the “Start” key. (b) When the operator selects one cardiac cycle from each inflow and outflow image, the system automatically calculates the heart rate (138 bpm in inflow and 137 bpm in outflow). (c) When the operator clicks the “Confirm” key, then the system automatically adjusts the outflow’s HR to that of the inflow, automatically synchronizes the inflow and outflow images based on the pulmonary valve closure clicks, automatically places the calipers at the beginning of each valve click, and automatically calculates the values of Mod-MPI and its components.
Feasibility and reproducibility of MPI + ™
Seventy uncomplicated singleton pregnancies between 20.0 and 24.4 weeks of gestation were prospectively conducted at Asan Medical Center from May 2017 to December 2020. Our study employed Intraclass Correlation Power Analysis, which determined that a sample size of 61 subjects, each observed twice, would achieve 80% power to detect a change in intraclass correlation from 0.65 to 0.80, with an alpha of 0.05. To enhance the robustness of our conclusions, we increased the sample size to 70 subjects. Two experienced operators (M.Y.L. and S.Y.K.) independently measured the RV Mod-MPI both manually and automatically twice for each method. To evaluate intra-operator reproducibility, the two measurements were performed on different dates. The measurements were blinded between the operators to evaluate inter-operator reproducibility.
Development of reference values for RV Mod-MPI using MPI + ™
Uncomplicated singleton pregnant women between 16 weeks and 38 weeks of gestational age participated in the study at Asan Medical Center between December 2020 and July 2022. To minimize inter-operator variability, all sonographic images were taken by one experienced operator (S. Y. K.) and the measurements were done automatically using MPI + ™.
Clinical application of RV Mod-MPI to the fetuses with right CDH
RV Mod-MPI and its components were automatically measured by MPI + ™ with stored images of the fetuses with right CDH between January 2018 and February 2022. The measured values of right CDH fetuses were compared to those of normal fetuses. Since the Mod-MPI and its components change with gestational age, we compared the values of the fetuses who are in certain gestational weeks where the Mod-MPI shows the least change according to reference values. We also plotted the RV Mod-MPI and its components of survivors and non-survivors in the right CDH fetuses.
Statistical analysis
Intraclass correlation coefficients (ICC) with a 95% confidence interval (CI) were calculated for intra- and inter-operator reproducibility. The automated and manual measurements were compared using mean differences and Bland-Altman plots. Reference values for the 5th, 10th, 50th, 90th, and 95th percentiles for each gestational age were evaluated with generalized additive models for location, scale, and shape (GAMLSS)36. The model selection was performed by the Generalized Akaike Information Criterion (GAIC). The model diagnostic was performed using residual plots from the final model and worm plots from the residuals of the model. We compared the values between CDH and normal fetuses after considering the clustering effect on the same patient using the covariance pattern model in a linear mixed model. For GAMLSS model fitting, the “gamlss” package of R software (version 4.1.1, http://www.r-project.org/) was used. Other statistical analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC, USA), with statistical significance defined as P < 0.05.
Author contributions
SYK and MYL drafted and revised the manuscript. SYK, MYL, JHC, HSW contributed to the data acquisition and investigation of the study. HJK and MJK analyzed the data. MYL designed and led the study. All authors commented on and approved the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crispi, F., Valenzuela-Alcaraz, B., Cruz-Lemini, M. & Gratacos, E. Ultrasound assessment of fetal cardiac function. Australas J. Ultrasound Med.16, 158–167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakata, M., Sakuma, J., Takano, M. & Nagasaki, S. Assessment of fetal cardiac function with echocardiography. J. Obstet. Gynaecol. Res.46, 31–38 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Ortiz, J. U. et al. Differential Changes in Myocardial Performance Index and its Time intervals in Donors and recipients of twin-to-twin transfusion syndrome before and after laser therapy. Fetal Diagn. Ther.44, 305–310 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Eschbach, S. J., Gijtenbeek, M., van Geloven, N., Oepkes, D. & Haak, M. C. Measurement of cardiac function by cardiac time intervals, applicability in normal pregnancy and twin-to-twin transfusion syndrome. J. Echocardiogr. 17, 129–137 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Kang, O. J. et al. Novel technique for the measurement of fetal right modified myocardial performance index using synchronized images of right ventricular inflow and outflow and clinical application to twin-to-twin transfusion syndrome. J. Ultrasound Med.40, 2467–2475 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Comas, M. et al. Usefulness of myocardial tissue Doppler vs conventional echocardiography in the evaluation of cardiac dysfunction in early-onset intrauterine growth restriction. Am. J. Obstet. Gynecol.203, 45e41–45e47 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Martinez, R., Figueras, F., Hernandez-Andrade, E., Oros, D. & Gratacos, E. Changes in myocardial performance index and aortic isthmus and ductus venosus Doppler in term, small-for-gestational age fetuses with normal umbilical artery pulsatility index. Ultrasound Obstet. Gynecol.38, 400–405 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Zhang, L. et al. Assessment of fetal modified myocardial performance index in early-onset and late-onset fetal growth restriction. Echocardiography. 36, 1159–1164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen, T. T. N. et al. Assessment of myocardial performance index in late-onset fetal growth restriction. Nagoya J. Med. Sci.83, 259–268 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevan, P., Bradshaw, C. M., Roberts, M. H. & Szabadi, E. The effect of microelectrophoretically applied mescaline on cortical neurones. Neuropharmacology. 13, 1033–1045 (1974). [DOI] [PubMed] [Google Scholar]
- 11.Alkan, F., Alanyali, M. O., Ulkumen, B. A. & Coskun, S. Evaluation of the effect of gestational diabetes mellitus on fetal cardiac functions with myocardial performance index. Minerva Obstet. Gynecol.10.23736/S2724-606X.21.04941-1 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Depla, A. L. et al. Effect of maternal diabetes on fetal heart function on echocardiography: systematic review and meta-analysis. Ultrasound Obstet. Gynecol.57, 539–550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senol, G. et al. Evaluation of right side foetal myocardial performance index in pregestational and gestational diabetes mellitus. J. Obstet. Gynaecol.42, 91–96 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Barker, D. J. In utero programming of chronic disease. Clin. Sci. (Lond). 95, 115–128 (1998). [PubMed] [Google Scholar]
- 15.Kwon, E. J. & Kim, Y. J. What is fetal programming? A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci.60, 506–519 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tei, C. et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function–a study in normals and dilated cardiomyopathy. J. Cardiol.26, 357–366 (1995). [PubMed] [Google Scholar]
- 17.Hernandez-Andrade, E. et al. A modified myocardial performance (Tei) index based on the use of valve clicks improves reproducibility of fetal left cardiac function assessment. Ultrasound Obstet. Gynecol.26, 227–232 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Lee, M. Y. et al. Feasibility of using auto Mod-MPI system, a novel technique for automated measurement of fetal modified myocardial performance index. Ultrasound Obstet. Gynecol.43, 640–645 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Maheshwari, P., Henry, A. & Welsh, A. W. The Fetal Modified Myocardial Performance Index: Is Automation the Future? Biomed Res Int 215910 (2015). (2015). [DOI] [PMC free article] [PubMed]
- 20.Lee, M. Y. et al. Fetal left modified myocardial performance index measured by the auto Mod-MPI system: development of reference values and application to recipients of twin-to-twin transfusion syndrome. Prenat Diagn.36, 424–431 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Cruz-Martinez, R. et al. Normal reference ranges from 11 to 41 weeks’ gestation of fetal left modified myocardial performance index by conventional doppler with the use of stringent criteria for delimitation of the time periods. Fetal Diagn. Ther.32, 79–86 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Meriki, N. & Welsh, A. W. Development of Australian reference ranges for the left fetal modified myocardial performance index and the influence of caliper location on time interval measurement. Fetal Diagn. Ther.32, 87–95 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Luewan, S., Tongprasert, F., Srisupundit, K., Traisrisilp, K. & Tongsong, T. Reference ranges of myocardial performance index from 12 to 40 weeks of gestation. Arch. Gynecol. Obstet.290, 859–865 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Clur, S. A., Oude Rengerink, K., Mol, B. W., Ottenkamp, J. & Bilardo, C. M. Fetal cardiac function between 11 and 35 weeks’ gestation and nuchal translucency thickness. Ultrasound Obstet. Gynecol.37, 48–56 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Ghawi, H. et al. Fetal left and right ventricle myocardial performance index: defining normal values for the second and third trimesters–single tertiary center experience. Pediatr. Cardiol.34, 1808–1815 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Parasuraman, R., Osmond, C. & Howe, D. T. Gestation-specific reference intervals for right and left ventricular ejection force from 12 to 40 weeks of gestation. J. Obstet. Gynaecol. Res.38, 160–164 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Meriki, N. et al. Development of normal gestational ranges for the right myocardial performance index in the Australian population with three alternative caliper placements. Fetal Diagn. Ther.36, 272–281 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Critser, P. J. & Levy, P. T. Risk Assessment and Monitoring of right ventricular function in congenital diaphragmatic hernia. Ann. Am. Thorac. Soc.17, 1380–1381 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel, N., Massolo, A. C. & Kipfmueller, F. Congenital diaphragmatic hernia-associated cardiac dysfunction. Semin Perinatol.44, 151168 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Van Mieghem, T. et al. Left ventricular cardiac function in fetuses with congenital diaphragmatic hernia and the effect of fetal endoscopic tracheal occlusion. Ultrasound Obstet. Gynecol.34, 424–429 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Byrne, F. A. et al. Severe left diaphragmatic hernia limits size of fetal left heart more than does right diaphragmatic hernia. Ultrasound Obstet. Gynecol.46, 688–694 (2015). [DOI] [PubMed] [Google Scholar]
- 32.DeKoninck, P. et al. Cardiac assessment in fetuses with right-sided congenital diaphragmatic hernia: case-control study. Ultrasound Obstet. Gynecol.43, 432–436 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Kaya, B., Tayyar, A., Sezer, S. & Kaya, S. The assessment of cardiac function with tissue doppler imaging in fetuses with congenital diaphragmatic hernia. J. Matern Fetal Neonatal Med.33, 1233–1238 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Patel, N. et al. Ventricular dysfunction is a critical determinant of mortality in congenital diaphragmatic hernia. Am. J. Respir Crit. Care Med.200, 1522–1530 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Avitabile, C. M. et al. Right ventricular strain, brain natriuretic peptide, and mortality in congenital diaphragmatic hernia. Ann. Am. Thorac. Soc.17, 1431–1439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigby, R. A. & Stasinopoulos, D. M. Generalized additive models for location, scale and shape. J. Roy. Stat. Soc.: Ser. C (Appl. Stat.). 54, 507–554 (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.