Abstract
Inflammatory bowel disease (IBD) is a chronic condition characterized by immune-mediated inflammation in the gastrointestinal tract, which follows a relapsing and remitting course. Apart from affecting the gastrointestinal tract, IBD also has extra-intestinal manifestations (EIMs). While the etiology of extraintestinal manifestation remains unclear, it is theorized to be based on immunological responses influenced by genetic factors. Renal involvement is one of the EIMs observed in ulcerative colitis and Crohn’s disease. The renal manifestations in IBD patients encompass a range of conditions including nephrolithiasis, amyloidosis, tubulointerstitial nephritis, glomerulonephritis (GN), obstructive pathologies, and chronic kidney disease (CKD). The incidence of CKD in IBD patients varies from 5%-15%. The decline in renal function can stem from various factors such as direct inflammatory damage to the kidneys leading to glomerular or tubular injury, or from complications like recurrent stones, amyloidosis, or GN. Additionally, nephrotoxic medications used in treating IBD, such as TNF-α inhibitors, calcineurin inhibitors, and aminosalicylates, can exacerbate the decline in renal function. Currently, there is a lack of consensus regarding these patients' screening and renal function monitoring. This review aims to assess the existing literature on the different renal complications among individuals with IBD, shedding light on their pathophysiology and management.
Keywords: Inflammatory bowel disease, Glomerulonephritis, Amyloidosis, Extra-intestinal manifestations, Nephrotoxicity, chronic kidney disease
Core Tip: Renal manifestations have been described in 4%-23% of the patients with Inflammatory bowel disease, leading to significant morbidity and mortality. Glomerulonephritis, nephrolithiasis, amyloidosis, and tubulointerstitial nephritis are common with approximately 5%-15% of the patients developing chronic kidney disease. Serum markers such as creatinine levels, especially cystatin-c levels, should be used to monitor renal function, especially in patients receiving nephrotoxic medications such as aminosalicylates and TNF-α inhibitors.
INTRODUCTION
Inflammatory bowel disease (IBD) is a debilitating chronic inflammatory disorder of the gastrointestinal tract, with a global disease burden of around 4.9 million cases[1]. Besides affecting the intestine, this disease can also present with extra-intestinal manifestations (EIMs), the prevalence of which varies from 6% to 46%[2]. The most common EIMs reported in the literature are joint (peripheral and axial arthropathies), skin (erythema nodosum, aphthous ulcers, pyoderma gangrenosum), biliary tract (primary sclerosing cholangitis, peri cholangitis), and eye (uveitis, scleritis) involvement[3,4]. In addition to these, renal manifestations have also been described in around 4%-23% of the patients with IBD[5,6].
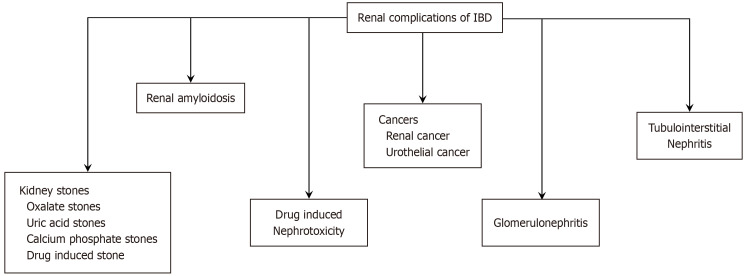
Nephrolithiasis, amyloidosis, tubulointerstitial nephritis, glomerulonephritis (GN), amyloidosis, fistulas, and urothelial cancers are common renal manifestations (Figure 1). Similar to other EIMs, the pathogenesis of renal EIMs is unclear. Immune response dysregulation, susceptible loci, and shared biological pathways are all expected to play a role[7]. Furthermore, treatment for IBD requires various immunomodulators that can be nephrotoxic and can lead to a reduction in kidney function.
Figure 1.
Common renal manifestation among patients with inflammatory bowel disease. IBD: Inflammatory bowel disease.
As a result, IBD is considered to be a risk factor for the progression of chronic kidney disease (CKD). A prospective study by Liu et al[8] reported patients with IBD to have 57% and 96% higher odds of developing CKD and acute kidney injury (AKI) compared to patients without IBD. This relationship was noted to be even stronger among young patients with the disease, who are known to have a higher rate of EIMs compared to adults[9-11]. This manuscript aims to review the current literature on renal complications of IBD, pathophysiological mechanisms, the diagnostic workup, and the treatment options for patients with these conditions.
IBD AND RISK OF CKD
Literature has documented that the presence of IBD may lead to the progression of CKD by multiple mechanisms. IBD has been shown to affect renal function by causing glomerular or renal tubular injury[12,13]. Glomerular insults and GN may lead to proteinuria and reduced glomerular filtration rate (GFR), while direct tubular injury can result in AKI or increased excretion of tubular proteins. Animal models of colitis induced with dextran sulfate sodium (DSS) have been extensively employed to investigate changes in renal function and structure. Chang et al[14], utilizing this model, observed a decrease in kidney size and weight following colitis induction. They also noted a reduction in type IV collagen, a constituent of the glomerular basement membrane's (GBM) supportive extracellular matrix, and deposition of type I and type V collagens in the renal interstitium. Damage to podocytes was also noted, particularly the loss of podocyte cytoskeletal proteins such as synaptopodin and podocalyxin. In a study by Ducasa et al[15], colitis induction by DSS resulted in increased bacterial translocation to the kidney and AKI, compared to controls.
The data regarding whether active IBD results in the progression of CKD is conflicting. Recent systematic reviews and large database studies have reported an increased risk of CKD in patients with IBD. In a retrospective study that included 10117 patients with IBD, it was noted that the hazard ratio of receiving a CKD diagnosis in patients with IBD was 1.24 (95%CI: 1.10-1.40) compared to patients without IBD[16]. Another study with a similar design notes that the hazard ratio of AKI decreases with age, with the risk being 7.88 (95%CI: 2.56–24.19) at age 16 which decreased to 1.13 (95%CI: 1.01–1.25) at age 77[17]. Other studies have also demonstrated this finding of increased CKD risk in the younger population[18]. In a retrospective study involving children diagnosed with IBD, a non-significant trend of declining GFR was observed over six years since disease onset (113 mL/min/1.73 m² vs 103 mL/min/1.73 m², one-way ANOVA, P = 0.17)[19].
Kidney function can be clinically monitored by following creatinine levels or by proteinuria. Mahmud et al[20] in 1994 were the first to demonstrate that patients with active IBD exhibited significantly higher levels of microalbuminuria compared to those in remission (206 µg/min vs 65 µg/min, P < 0.001)[20]. Wu et al[21] utilized genome-wide associate study data to investigate the relationship between IBD and renal changes. They demonstrated a causal relation between ulcerative colitis (UC) and elevated albumin levels in urine in only one of their models (Inverse variance weighted method) but did observe a causal relationship between Crohn's disease (CD) and albuminuria. Similarly, in a prospective study involving 86 patients, Poulou et al[22] failed to demonstrate a difference in albuminuria between active and inactive IBD. Overall, the clinical evidence of microalbuminuria in IBD is conflicting and is of limited utility in clinical settings.
Reduction in GFR has been noted in patients with active IBD however the evidence is conflicting[23]. A retrospective review found IBD to be associated with an increased risk of CKD development; however, they failed to demonstrate a reduction in estimated GFR (eGFR) in patients with active IBD (−0.10 mL/min/1.73 m²; 95%CI: -0.33 to 0.48)[17]. For monitoring changes in GFR, cystatin C has been noted to be more sensitive than creatinine levels, particularly in patients with severe IBD[19]. We also hypothesize that associated factors such as an increased risk of stone formation, urinary tract infections (UTIs), and drug-induced nephrotoxicity may also play a more significant role in CKD development. Thus, regular monitoring of kidney function is generally recommended.
NEPHROLITHIASIS
Nephrolithiasis is one of the most common renal manifestations in IBD patients and has been described in case reports as old as the 1970s[24]. While the prevalence varies with different studies, ranging from 9%-28%, there is little doubt that stone formation is more common in patients with IBD compared to the general population[25-27]. The incidence of asymptomatic nephrolithiasis has been reported as high as 38% of IBD patients in some studies[28]. Among patients with IBD, there is a greater risk of stone formation in adults, males, patients using non-steroidal anti-inflammatory drugs and with lower levels of physical activity, patients with CD rather than UC, and higher in patients who have undergone surgical procedures, particularly terminal ileum resection or intestinal bypass[26-30]. Cury et al[28] also demonstrated that the extent of bowel disease, and ileocolonic involvement rather than just ileal or colonic involvement was also a relevant contributor.
Recurrent nephrolithiasis and the number of procedures it necessitates are both risk factors associated with the development of CKD in patients with IBD[26]. Additionally, compared to a patient without IBD, those who develop kidney stones with concomitant IBD are more prone to UTIs, sepsis, AKI, and end-organ failure and are more likely to need hospital admission[30]. Owing to their recurrent nature and requirement for repeated interventions, kidney stones in IBD patients are associated with increased morbidity[26]. The description of various stones among patients with IBD is presented below.
Calcium oxalate
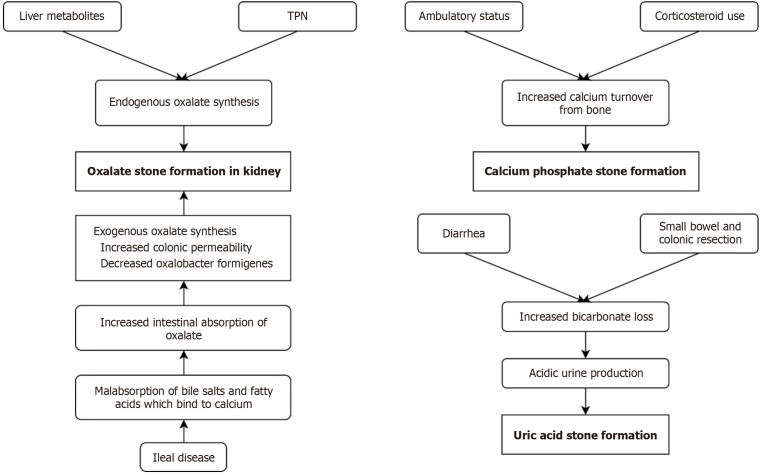
The pathogenesis of oxalate stones in IBD is multimodal. Involvement of terminal ileum results in malabsorption of bile salts and fatty acids. This causes calcium to bind to fatty acids rather than forming calcium oxalate complexes, leading to increased intestinal absorption of oxalate with consequent “enteric hyperoxaluria”, defined as urinary oxalate concentration > 45 mg/dL[31]. Additional proposed mechanisms include altered colonic mucosal permeability to oxalate and changes to the gut microbiome (Figure 2). The latter refers to the decolonization of Oxalobacter formingens, which reduces colonic oxalate catabolism[5]. The importance of this bacterium is supported by the finding that oral Oxalobacter administration is associated with reduced urinary concentrations of oxalate[32]. The role of hyperoxaluria may not be limited to oversaturation and precipitation. Studies have shown that oxalate proves toxic to the renal tubular epithelium and causes changes in gene expression, mitochondrial function, and cell death by oxidative damage, a series of events termed “oxalate nephropathy”[33]. Chronic exposure of tubular epithelium to oxalate can cause desensitization and thus impair protective countermeasures, predisposing patients to recurrent stone formation and CKD[34].
Figure 2.
Pathogenesis of stones among patients with inflammatory bowel disease. TPN: Total parenteral nutrition.
Uric acid stones
Diarrhea is common in IBD and leads to dehydration as well as loss of bicarbonate, leading to the excretion of concentrated, acidic urine which promotes the formation of uric acid stones[34]. Surgical procedures involving small bowel and colonic resection contribute[34]. This is accompanied by a reduced concentration of stone-inhibiting factors such as citrate and magnesium, all of which likely play a synergistic role in stone formation[26]. Serum or urinary levels of uric acid do not need to be increased to promote stone formation, rendering their monitoring unnecessary[35].
Calcium phosphate
Calcium phosphate stones are mainly seen in pediatric IBD patients, the pathogenesis of which is not well-understood[36]. It may represent increased calcium mobilization from the bone in the setting of fat-soluble Vitamin D malabsorption(Figure 2)[29].
Drug-induced nephrolithiasis
Treatment of IBD can also carry a risk of nephrolithiasis. There have been infrequent reports of sulfasalazine causing crystalluria and stone formation[26]. Vedolizumab, a monoclonal antibody directed against α4 β7 integrin is used in the treatment of moderate to severe IBD, especially UC[37]. A study found that vedolizumab use, as well as using two or more biologic drugs for treatment was associated with a greater risk of nephrolithiasis as compared to no biologic use. It is unclear if this is attributable to drug use or a consequence of more severe IBD in the patients who need this treatment[37]. However, it may be valid to consider stopping drugs that induce stone formation in these patients[38]. Discussion regarding the other nephrotoxic effects of drugs for IBD is presented below in a separate section.
Given the morbidity carried by nephrolithiasis in IBD, it is essential to adopt a meticulous approach to early diagnosis and treatment of the same. Diagnosis of nephrolithiasis can be challenging as the pain of nephrolithiasis can be confused with pain from an IBD flare. Low-dose computed tomography (CT) of kidneys, ureters, and bladder is mostly employed for diagnosis in the emergency setting[30]. Currently, guidelines do not advocate for routine urinary imaging[26]. However, IBD patients are still exposed to a high amount of radiation overall. Thus, current research is focusing on the possibility of using ultra-low radiation techniques for follow-up of IBD patients with nephrolithiasis[30].
Prevention and treatment are based on a mixture of dietary, medical, and surgical measures[26]. Dietary modifications include increased water intake to minimize dehydration from diarrhea and ostomies, increased citrate consumption, bile salt sequestrants, and pyridoxine supplementation[27]. Citrate therapy has been shown to prevent stone formation as well as help in stone expulsion[39]. It is also recommended that magnesium and citrate replacement should focus on correcting urinary levels of these substances instead of serum levels[30]. Consumption of oxalate and fat should be reduced especially in patients with CD and ileal resection[26]. Prevention of uric acid stones involves reducing dietary intake of purines, hydration, oral potassium citrate, and urine alkalinization[27]. Allopurinol and other xanthine oxidase inhibitors reduce uric acid levels in both serum and urine[30]. Treatment of stones ranges from conservative to extracorporeal shockwave lithotripsy and surgery, depending on the size of the stones and the presence of complications such as UTI and hydronephrosis.
RENAL AMYLOIDOSIS
CD has been reported to be the fourth leading cause of secondary amyloidosis, after chronic inflammatory arthropathy, chronic infections, and periodic fever syndromes[40,41]. Chronic immune-mediated inflammation in IBD leads to an increased risk of AA amyloidosis. Incidence is noted to be higher in patients with CD (0.3%-10.9%) compared to UC (0%-0.7%)[42-45]. This is usually attributed to the fact that there is a wider extension of inflammation in CD compared to the UC[46,47]. In one study it was noted that 54% of the cases with amyloidosis secondary to IBD had extensive ileocolonic involvement[48]. Interestingly it was also noted that the development of amyloidosis was twice as prevalent in men as compared to women diagnosed with IBD[48]. Amyloidosis typically occurs as a longstanding sequelae of IBD, however simultaneous diagnosis is not uncommon due to diagnostic delays[45,49,50].
Renal amyloidosis in IBD usually presents proteinuria in the setting of nephrotic syndrome, eventually leading to renal impairment. However, approximately 15% of the cases, have neither at presentation, and thus a high index of suspicion is required[48]. In many IBD patients, amyloidosis can also present as malabsorption which is not explained by the underlying disease activity[48]. Renal biopsy is the gold standard test for diagnosis. A high majority of cases have both mesangium and glomerular involvement. In many cases when the suspicion of renal amyloidosis is high but renal biopsy is difficult to obtain, abdominal fat pad or rectal biopsies can be done.
Since renal amyloidosis is a rare EIM, there is a lack of therapeutic studies and thus the effectiveness of treatment is not established[42,43,51]. The primary objective of treatment is to control the underlying inflammatory state, decrease the formation and deposition of circulating AA protein, and reverse the deposits already present in the affected organs[49]. Immunosuppressive drugs (methotrexate, cyclosporine, and azathioprine), colchicine, dimethylsulfoxide, and corticosteroid have all been, without established benefits. Initially, colchicine was proposed as an effective therapy, based on the favorable response seen in cases of amyloidosis secondary to periodic fever syndromes[52].
Even if started early on colchicine, proteinuria was observed to have been reduced and renal function would remain stable over a while but no benefit was observed in the reduction of amyloid depositions[53-58]. Favorable outcomes have also been noted with anti-TNF drugs such as infliximab and adalimumab[44,59-62]. Combining anti-TNF, immunosuppressants, and colchicine could improve prognosis. Many studies have also reported clinical improvement of IBD-related amyloidosis after surgical resection of the involved intestinal segment[63-65]. The suggested mechanism behind it is the decrease in the production of serum amyloid A after the surgical resection of the affected organ. Newer treatment options include drugs targeting interleukin (IL)-6 such as tocilizumab or drugs targetting serum amyloid P component such as (R)-1-[6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid and therapeutic IgG anti-SAP antibodies[66-69].
Even with the recent biochemical advancements the treatment needs to be individualized as there is a lack of understanding of the long-term efficacy and safety of biological agents in clinical settings. In addition to management of the IBD, medical management of renal injury is also imperative in these patients. Therapy for nephrotic syndrome is symptomatic with restriction of sodium intake, use of angiotensin-converting enzyme inhibitors, and statin may be needed in certain scenarios. Monitoring proteinuria and serum amyloid A protein can be useful to assess the response to treatment and prognosis. Many patients may end up with end-stage renal disease requiring dialysis and/or transplantation.
RENAL AND UROTHELIAL CANCERS IN IBD
Extraintestinal cancers are significant concerns for individuals with IBD. However, there's a lack of research on the link between IBD and urothelial cancer. In individuals with IBD, there exists a significant overrepresentation of malignancies originating in the kidneys and urinary tract with the relative risk being five times higher compared to the general population[70,71]. Although the mechanism underlying the relationship between IBD and oncogenesis is not fully understood, there is evidence suggesting that inflammation not only serves as the body's response to malignant tumors but also plays a role in triggering carcinogenesis[72]. Additionally, immunosuppressive medications frequently utilized in the treatment of IBD are believed to be associated with a heightened risk of various malignancies, including non-Hodgkin lymphoma, acute myeloid leukemias, non-melanoma skin cancers, and urinary tract cancers[73].
A recent meta-analysis of 9 cohort studies indicated a significantly elevated risk of renal cancer in individuals with IBD, particularly those diagnosed with CD. In contrast, no significant increase in risk was observed among patients with UC when compared to the general population[74]. Previous epidemiological investigations have established a robust association between IBD and an increased risk of various cancers[75]. However, conclusive evidence supporting a direct link between IBD and bladder cancer risk remains elusive. Kappelman et al[76] conducted a comprehensive study spanning over 30 years and reported a slight increase in the risk of bladder cancer among patients with CD [standardized incidence rate (SIR): 1.1; 95%CI: 0.8 to 1.6].
In patients diagnosed with CD, a significant correlation has been established between cigarette smoking and urological malignancies. However, such an association has not been consistently observed in individuals with UC. In a study conducted by Madanchi et al[77], which analyzed the incidence of malignancies in IBD centers over 7 years, a higher occurrence of bladder cancer was observed in IBD patients compared to those without IBD (21.7 per 100000). Conversely, Algaba et al[70], in a cohort study, did not identify a significant overall increase in the risk of cancer among individuals with IBD. However, they did find that the risk of bladder cancer remained elevated (RR: 5.23; 95%CI: 1.95–13.87). Both studies by Madanchi et al[77] and Algaba et al[70] are single-center cohort studies indicating a statistically significant increase in urothelial cancer among IBD patients. However, the sample sizes differ (1026 vs 590 patients), potentially introducing bias due to the smaller cohorts. Conversely, Pedersen et al[78] observed no difference in the risk of bladder cancer between patients with IBD and the general population (SIR: 0.99; 95%CI: 0.63–1.54). In a study encompassing nearly 19500 patients diagnosed with IBD, 16 individuals developed urological malignancies. Upon conducting a multivariate analysis, the use of thiopurines was found to be associated with a threefold increased risk of urinary tract cancers[79]. Further investigation through larger case-control studies is essential to comprehensively comprehend the underlying mechanisms and refine clinical approaches for renal cancer surveillance in IBD patients. Additionally, there remains a significant gap in understanding the association between IBD and urinary tract malignancies, necessitating continued research efforts to address unanswered questions in this area.
GN
GN is a relatively infrequent complication of IBD but has been extensively studied. These include IgA nephropathy (IgAN), membranoproliferative GN, membranous glomerulopathy, minimal change disease (MCD), C3 glomerulopathy, and anti-GBM GN[26,80]. Of these, IgAN is the most prevalent, and reports of the association date back to 1984[26,80]. A study of renal biopsies in IBD patients with acute or chronic renal failure demonstrated IgAN in a majority of the samples[81].
The pathogenesis of GN in IBD is poorly understood and different theories have been proposed. One theory is that IBD and IgAN share genetic predisposition. Both have shown an association with HLA-DR1[82]. Novel genetic loci have also been identified in IgAN recently. These include CARD9, VAV, and PSMB 8/9, genes involved in the activation of NF-kB, maintaining the gut mucosal barrier, and regulating local inflammation[83,84]. Additional regions include TNFSF13 and DEFA[84,85]. DEFA encodes alpha-defensins, a type of antimicrobial formed by Paneth cells in the small intestine. Reduced levels of alpha-defensins have been reported in CD patients[86]. Inflammation from cytokines could contribute to both IBD and glomerular damage. Complement activity also plays a role in both diseases. It is known to promote glomerular sclerosis and interstitial fibrosis in IgAN[87]. Activated C3b has also been seen in intestinal mucosa and resected ileocecal specimens in patients with CD[88]. Mucosal inflammation in IBD alters gut mucosal permeability leading to antigen exposure, formation of autoantibodies, and consequent deposition of antigen-antibody complexes[35]. Considering that IgA acts as a mucosal antibody, it is reasonable that gut mucosal inflammation would be associated with IgA dysregulation. IgAN patients have been known to possess antibodies to dietary antigens, which have been recovered from sera as well as immune complexes deposited in the glomeruli[89]. A similar pathogenesis was first suggested for the association of IBD with celiac disease[90] Ileostomy and colostomy have also been associated with IgAN, which may be attributable to exposure of skin flora to the gut and the ensuing immune response[91]. The gut microbiome can also produce B cells activating TNF factors and overstimulate B cells, which causes a shift from IgA2 to IgA1 generation[92,93]. Moreover, TNFSF13, identified as a susceptibility locus for IgAN encodes APRIL, a TNF ligand involved in B-cell maturation, response to mucosal antigens, and IgA production in gut-associated lymphoid tissue[91]. Studies have also shown aberrant O-linked glycosylation of IgA in CD patients[94]. Additionally, the role of T-cells has also been questioned, given the importance of costimulation in gut immunity. In animal models of IgAN, T-cells have been observed to play a critical role in controlling intestinal inflammation[95]. MCD development might also be associated with IBD treatment[35].
Another possibility is that GN is simply a concurrent EIM of IBD. This is supported by the frequent appearance of GN with IBD flares and improvement after treatment of gut disease[35]. Overall, it is unclear if IgAN occurs as a consequence of IBD flares exclusively or develops separately as individuals prone to IBD likely have a genetic makeup that predisposes to IgAN. Aside from IgAN, the pathophysiology behind other forms of GN is less clear[27].
Diagnosis of IgAN is mostly suspected based on occult blood and/or proteinuria on urinalysis and confirmed on biopsy[91]. Elevated serum IgA levels have been noted but are not a consistent finding[81]. Patients who are suspected to have nephropathy based on labs but not referred for biopsy are labeled as “suspected IgAN”[91]. Treatment of IBD-associated IgAN mainly focuses on treating the IBD, mostly with steroids[36]. In most cases, this improves renal function. Clearance of IgA deposits as well as mesangial proliferation has been observed on follow-up biopsy after treating IBD, suggesting sufficient healing[96]. A course of enteric budesonide has resulted in improvements in proteinuria and normalization of kidney function, further supporting this theory[97,98].
It is currently unclear if concomitant IBD forbodes a poor prognosis in IgAN. Some studies have reported similar rates of kidney failure in IgAN with and without IBD, but some have described a renal failure rate of up to 50% in IBD-related IgAN[99,100]. This might be explained by shorter follow-ups in some studies, which could miss the development of kidney failure[80]. A study found that CD-associated IgAN patients were more likely to experience aggressive IgAN, with glomerulosclerosis, extensive tubular atrophy, and interstitial fibrosis[84]. It thus appears reasonable to maintain a high index of suspicion for IgAN in IBD patients and follow these patients closely to prevent potentially adverse outcomes.
Other patterns of glomerulopathy are less prevalent but have been reported in IBD. An Egyptian study reported that crescentic GN is the most common pattern after IgAN[101]. It was associated with the presence of ASCA, which tends to present in more severe forms of IBD. Moreover, it showed associations with p-ANCA and c-ANCA, possibly indicating that ANCA found in IBD patients might be targeting tissues similar to those in ANCA-associated renal vasculitis[101]. A case report described a 75-year-old patient with UC who presented with symptoms of an IBD flare and worsening renal function. A renal biopsy revealed C3 glomerulopathy. The mechanism is uncertain, but complement activation and deposition are presumed to play a critical role in UC and may be responsible for glomerulopathy as well[102]. Another study from China found membrane glomerulopathy to be the most prevalent pattern after IgAN[103]. Genetic associations between IBD and glomeruli nephropathies other than IgAN have not been found yet[28]. Further research is needed to assess the molecular mechanisms responsible for these associations.
TUBULOINTERSTITIAL NEPHRITIS
In a retrospective review of renal biopsy specimens among patients with IBD and renal injury, interstitial nephritis was noted to be the second most common diagnosis after IgAN[81]. Tubulointerstitial injury has been noted to occur secondary to systemic inflammatory and immunological reactions, however, there is still no consensus in regards to the exact mechanism. In animal models, renal tubular injury has been associated with neutrophil infiltration and the expression of cytokines and chemokines, resembling the pathophysiology of colitis characterized by neutrophil infiltration in the colonic epithelium. This is proposed to be mediated via keratinocyte chemoattractant (KC) receptors[104]. Ranganathan et al[105] demonstrated that genetic deletion of CXCR2, a type of KC receptor, led to the suppression of AKI in animal models. In a similar study design, overexpression of Netrin-1, a chemotropic cue with anti-inflammatory properties, in proximal tubular epithelial cells suppressed cytokine expression and neutrophil infiltration[13].
In addition, 5-aminosalicylates (ASAs), cyclosporine A (CsA), and TNF-α inhibitor exposure are known to cause tubulointerstitial nephritis. As 5-ASA compounds have shown good efficacy in maintaining disease remission, frequent and long-term use of these compounds is common. One study noted serious renal impairment in 1 of 500 patients treated with 5-ASA derivatives[106,107]. Both direct injury secondary to inflammation and medication can independently cause tubulointerstitial damage. Larchet et al[108] were the first to describe an association of interstitial nephritis with IBD in an adolescent population. Since then, numerous studies have reported the incidence of nephritis even in the early stages of the disease.
Clinically, these changes manifest as increased urinary levels of tubular proteins such as N-acetyl-B-D-glucosaminidase (NAG), α-1-microglobulin, and beta-2-microglobulin. Fraser et al[109] noted that over 50% of patients with new diagnoses of IBD had increased levels of NAG and α-1-microglobulin. However, no correlation was found between protein levels and disease activity, and treatment did not significantly alter these levels[109,110]. Thus, apart from demonstrating kidney injury, their clinical significance for screening or indicating a decrease in disease activity has not been documented.
The association between TIN in IBD patients and drug therapy is bolstered by those reports demonstrating a strong temporal relationship to drug exposure, recovery of renal function after withdrawal of the drug, and recurrence of renal injury upon rechallenge[111-113]. In cases of TIN secondary 5-ASA, complete recovery of renal function has been reported if TIN is diagnosed within 10 months from the start of treatment. The treatment for drug-induced AIN is the discontinuation of the offending drug and steroid therapy[114,115]. If diagnosis is delayed beyond 18 months, only one-third of cases show any recovery of renal function[107]. Unfortunately, many of the cases of TIN with exposure to nephrotoxic agents and concomitant IBD end up with ESRD within 3 years of diagnosis[116]. Therefore, for patients on regular 5-ASA and mesalamine therapy, it is now recommended that renal function should be assessed before initiation of medical therapy with repeat testing every 3 months for a year and then twice every year[107,115,117].
DRUGS AND RENAL TOXICITY
Drug-induced kidney damage is a prevalent issue in clinical practice, with drug-related AKI occurring in up to 60% of cases[118,119]. This condition often necessitates extensive and costly interventions, potentially including hospitalization[120]. It is crucial to note that all parts of the kidney can be impacted, leading to the development of classic clinical renal syndromes such as AKI, tubulopathies, proteinuric renal disease, and CKD (Table 1)[121]. The conservative management of IBD typically involves a combination of medications, including ASAs, steroids, antibiotics, immunosuppressants, and biologic agents. While the nephrotoxic effects of ASAs and CsA are well recognized, recent evidence suggests a potential role of biologic agents such as infliximab and adalimumab in contributing to renal impairment[122].
Table 1.
Potential nephrotoxic effects of pharmaceuticals used in the treatment of inflammatory bowel disease
|
Pharmaceutical treatment
|
Potential nephrotoxic effects
|
| Sulfasalazine | Tubulointerstitial nephritis, gloermurlonephritis |
| CsA | Nephritis, interstitial fibrosis |
| TNFα Inhibitors | Glomerulonephritis |
| Methotrexate | None at conventional doses |
| Azathioprine | No direct evidence of a renal effect |
| Corticosteroids | None |
| Antibiotics (e.g., ciprofloxacin) | Acute kidney injury |
| Vedolizumab | Acute interstitial nephritis |
| Tofacitinib | Acute kidney injury |
| Filgotinib | Increased drug concentration in renal impairment |
CsA: Cyclosporine A.
ASAs
5-ASAs are the primary treatment for IBD. Different formulations have been developed to enhance 5-ASA absorption in the inflamed intestine. Both sulfasalazine (5-ASA bound to sulfapyridine) and coated 5-ASA (mesalazine, olsalazine) have been linked to renal toxicity[123]. Research indicates that 5-ASA administration can directly impact renal function, with renal impairment occurring in up to 1 in 100 patients, but clinically significant damage affecting only 1 in 500 patients[107,124]. Renal toxicity due to 5-ASA may present as GN, minimal-change nephropathy with nephrotic syndrome, and interstitial nephritis, which may be associated with nephrogenic diabetes insipidus. Several instances of renal toxicity have been documented with sulfasalazine, presenting as an idiosyncratic, dose-independent occurrence within a broader hypersensitivity reaction[125]. Conversely, mesalazine has been associated with a notable incidence of nephritis development in patients during its use. Mesalazine has the potential to induce acute or chronic interstitial nephritis[115]. Salicylate is filtered and actively secreted in the kidney via proximal tubules, with subsequent passive reabsorption leading to elevated cortical and medullary concentrations. Inhibition of intrarenal prostaglandin synthesis by salicylates disrupts intrarenal blood flow regulation and mitochondrial oxidative phosphorylation, potentially causing regional hypoxia. Moreover, high intrarenal salicylate levels inhibit the pentose phosphate shunt, reducing renal glutathione and making the kidney vulnerable to oxidative damage.
Cyclosporine
Low-dose CsA administration showed no significant nephrotoxicity, while high-dose CsA led to renal impairment in 6% of patients[126,127]. Overall, patients receiving CsA treatment for autoimmune diseases, including IBD, experience a 20% reduction in their GFR[128]. CsA can induce acute renal dysfunction by causing significant vasoconstriction of the afferent arterioles. This constriction leads to a decrease in renal blood flow and GFR, accompanied by elevated serum creatinine levels. Typically, renal function improves within 5 to 7 days after reducing or discontinuing CsA treatment[129]. CsA can lead to chronic renal impairment, although the precise mechanism of this nephrotoxicity is not fully understood. However, it has been suggested that the activation of the renin-angiotensin system within the kidney may play a significant role in the development of chronic CsA nephrotoxicity[130]. The recommended duration of CsA therapy should not surpass 4–6 months, after which an alternative remission maintenance drug should be considered. CsA dosages should be lowered if baseline serum creatinine levels increase by more than 30%[131]. It's important to avoid concurrent use of other nephrotoxic agents, and patients with preexisting renal dysfunction should likely not be treated with CsA[127].
TNF-α inhibitors
While TNF-α inhibitors, specifically infliximab and adalimumab, have demonstrated significant efficacy in inducing and maintaining clinical remission in IBD, they are associated with serious adverse effects. Despite earlier reports indicating the potential benefits of TNF-α inhibitors in the treatment of GN, recent studies and increased experience with these inhibitors have raised questions about their efficacy in GN and highlighted a potential role in the development of renal complications, including GN. Although data on GN in IBD patients due to TNF-α inhibitor therapy are limited, recent reports suggest a potential triggering effect of TNF-α inhibitors on renal impairment, indicating a significant adverse effect[132]. One possible mechanism for the development of renal complications during anti-TNF-α administration involves the interaction of anti-TNF-α antibodies with TNF-α present in glomerular visceral epithelial cells[133].
Nephrotoxicity induced by other therapeutic agents in IBD
Vedolizumab, a humanized monoclonal antibody targeting α4β7 integrin and primarily acting in the gut, is utilized to manage UC and CD[134]. The therapy has demonstrated efficacy in both initiating and sustaining remission in IBD. It is generally well received and is perceived to possess a favorable safety profile compared to alternative 'biologics' utilized in managing IBD[135]. Recent case studies propose that vedolizumab might contribute to the development of acute interstitial nephritis. Reported cases have emerged indicating occurrences of interstitial nephritis believed to be linked to the use of vedolizumab. This underscores the necessity for ongoing awareness regarding rare adverse drug reactions, even in treatments that are typically well-tolerated.
Tofacitinib is an orally administered medication, that acts as a partially selective inhibitor of Janus kinase (JAK). This small molecule functions intracellularly to impede JAK-dependent cytokine signaling. By blocking these enzymes, tofacitinib helps regulate immune and inflammatory reactions[136]. Fixed-dose regimens of tofacitinib achieved improved kidney function and showed comparable effectiveness to CsA in kidney transplant patients. However, this came with a higher likelihood of experiencing certain adverse events[137].
Filgotinib is a medication taken by mouth, designed as a preferential inhibitor of JAK1. By primarily targeting JAK1, it influences a specific set of proinflammatory cytokines within the JAK-signal transducer and activator of the transcription pathway. This subset differs from those affected by inhibition of JAK2 or JAK3. Pharmacokinetic investigations have revealed elevated drug concentrations in individuals with an eGFR < 60 mL/min/1.73 m². Therefore, it is recommended to consider dose reduction for such patients. Filgotinib hasn't been studied in patients with end-stage renal disease (eGFR < 15 mL/min/1.73 m2)[138]. As a result, its use is not recommended in this population. Ustekinumab, an anti-IL-23 biologic, presents an alternative treatment for individuals with moderate to severe UC and CD. However, it is important to note that its use may be linked to nephrotic syndrome arising from focal segmental glomerulosclerosis[139].
Other therapies utilized in IBD therapy, include corticosteroids, thiopurines (azathioprine and 6-mercaptopurine), methotrexate, and mycophenolate mofetil, which have shown no direct significant effects on renal function. Specifically, methotrexate, despite the potential for nephrotoxicity at high doses, does not exhibit nephrotoxic effects at conventional doses typically used in IBD treatment[140]. Concerning azathioprine, there is no evidence indicating a direct renal effect. The prolonged use of antibiotics like ciprofloxacin and metronidazole, as well as the use of total parenteral or enteral nutrition, does not seem to induce renal impairment. However, there have been a few case reports suggesting nephrotoxic effects associated with ciprofloxacin[141]. Furthermore, there is abundant data regarding the role of corticosteroids on kidney function which is beyond the scope of this article. Distinguishing extraintestinal renal dysfunction from drug-induced injury is complex and vigilant renal function monitoring is required to diagnose drug-induced nephrotoxicity as prompt cessation of toxic medications is vital to prevent further harm and potentially reverse renal injury.
FISTULAS AND HYDRONEPHROSIS
CD is characterized by sustained transmural inflammation of the bowel wall leading to fistula formation which can be noted in about one-third of the patients with CD[142]. The inflamed intestine can adhere to the bladder wall leading to the formation of an enterovesical fistula (EVF). CD is the third most common cause of EVF behind diverticular disease and malignancy[143]. The incidence of EVF is rare with a reported incidence of 2%-5% among patients with CD, with ileovesical fistula being the most common (64.9%)[144]. Males are noted to be affected by EVFs at a higher rate than women in most of the major studies, and this has been attributed to the anatomical barriers imposed by the uterus and vagina[144-146].
Clinically EVF is characterized by pneumaturia, fecaluria, recurrent or persistent UTIs, and urorrhea. Recurrent UTIs in a patient with CD justify a diagnostic workup for a urinary system fistula[147-149]. Enteric bacteria, Escherichia coli are the usual infective agents. Historically, poppy seed tests, plain abdominal X-rays, and barium enema were used, but these have been superseded by cross-sectional imaging[150,151]. Although not routinely recommended, Cystoscopy should be used in patients with EVF secondary to malignancy to rule out bladder involvement[152]. It can also be used to exclude other etiologies such as bladder stones and interstitial cystitis[153].
An abdominopelvic CT with oral or rectal contrast (but not IV contrast) is the imaging test of choice for diagnosing EVF and some authors have reported that CT can accurately detect the presence of EVF in up to 90 to 100 percent of patients even though direct visualization of the tract is limited[143]. Suggestive CT findings include intravesical contrast or air without prior instrumentation. Radiation dose is one of the major deterrents against CT scan and most of the CD patients are young and would require multiple scans for evaluation over the years. Magnetic resonance imaging allows the accurate depiction of fistulous tracts with the advantage of being radiation-free. T2-weighted sequences and intravenous contrast are widely used to delineate fistula anatomy and identification of associated abscess collections in patients with CD. However, whether magnetic resonance imaging is superior to CT for the detection of EVF remains controversial, and further prospective studies are needed[154]. Endoscopy has a very low sensitivity for detecting a fistulous tract but can be used to determine the underlying etiology of the fistula.
Initial management depends upon whether symptoms are present at the time of presentation. Asymptomatic patients can be managed just with medical therapy to control CD. Studies have shown that long-term remission can be achieved in up to 35% of patients[144,155,156]. Antibiotics are necessary in patients who are initially present with infections. Medical management is also recommended in patients who are not suitable for surgery due to a poor condition, intolerance to anesthesia, or terminal disease. Despite the potential promise of medical treatment, most of the patients with EVF require some form of surgical management. Retrospective reviews have noted that around 80%-85% of the patients needed surgical management[157]. Taxonera et al[144] noted that 78 of the 79 patients who underwent surgical management noted improvement in the symptoms. Although an open approach is preferred in most cases, the laparoscopic approach and bladder-preserving procedures can be considered in some limited presentations.
Hyams et al[158] in 1943, presented the first documented case of obstruction of the ureter and kidney associated with CD. Hydronephrosis is noted to occur when inflammation, fistulas, abscess formation, or fibrosis occurs in the bowel adjacent to the ureters. Obstruction is usually noted on the right side at the level of the linea terminalis (75%-100%)[159-163]. Hydronephrosis in CD can also occur secondary to a renal stone obstructing the ureteropelvic junction[164].
Overall the incidence of hydronephrosis varies from around 3.1% to 6%[160,165-167]. In a retrospective review, 4 of 62 (6%) of the patients with CD were noted to have hydronephrosis. Three of them had right-side hydronephrosis with the mean duration between the onset of CD and diagnosis of hydronephrosis being 5.6 years[160].
The primary goal of managing hydronephrosis in CD is ureteral drainage to prevent damage to the obstructed kidney. Some minimally invasive options include percutaneous nephrostomy or indwelling ureteral stenting. This is done alongside medical therapy. Ben-Ami et al[160] noted that 3 out of the 4 patients showed a good response to pulse steroids while Angelberger et al[161] noted success in one patient. The surgical approach is usually the last-line modality and involves resection of the affected bowel along with/without ureterolysis[167].
MONITORING OF KIDNEY FUNCTION FOR IBD PATIENTS
In terms of kidney function monitoring, there is a general agreement on the validity of using serum creatinine levels alongside estimating GFR using either the MDRD or CKD-EPI equations. Additionally, there is widespread acknowledgment of the importance of including a blood ionogram and analyzing urine samples for the protein-to-creatinine ratio. Recommendations are made to integrate these monitoring practices at the onset of IBD diagnosis, before introducing new treatments, and annually for screening extraintestinal manifestations (EIMs) and assessing treatment tolerance. Specifically, it is suggested to evaluate kidney function three months post-initiation of mesalamine therapy, followed by assessments every six months, while annual monitoring is considered adequate for patients receiving biologics[168].
CONCLUSION
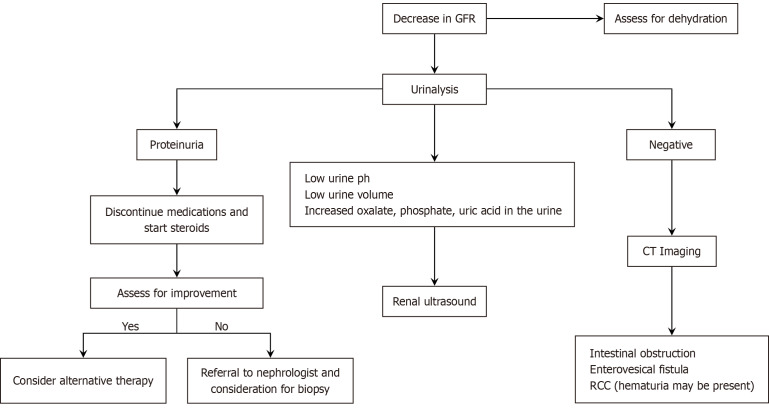
Among patients with IBD, the renal and urological complications are difficult to diagnose as the associated symptoms in these patients can be subtle. Thus, a high index of suspicion is warranted to diagnose these early as just like other EIMs, the presence of renal manifestations can lead to reduced quality of life. Currently, there is a lack of international guidelines regarding the standardized timing of kidney function monitoring. Furthermore, there is no consensus regarding how to approach AKI among patients with IBD. We provide a diagnostic workup that may be beneficial in managing patients with decreased GFR among patients with IBD (Figure 3). Patients with IBD are also at risk of repetitive kidney injury due to episodes of dehydration and various causes discussed above. Thus, a multidisciplinary discussion between gastroenterologists and nephrologists will be beneficial in preventing worse outcomes among these patients.
Figure 3.
A proposed clinical strategy for the workup of inflammatory bowel disease patients with decreased glomerular filtration rate. CT: Computed tomography; GFR: Glomerular filtration rate; RCC: Renal cell carcinoma.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Wang YD S-Editor: Lin C L-Editor: A P-Editor: Zhang L
Contributor Information
Anmol Singh, Department of Medicine, Tristar Centennial Medical Center, Nashville, TN 37203, United States.
Tejasvini Khanna, Department of Medicine, Maulana Azad Medical College, New Delhi 110002, India.
Diksha Mahendru, Department of Medicine, Dayanand Medical College and Hospital, Ludhiana 141001, Punjab, India.
Jasraj Kahlon, Department of Internal Medicine, Abrazo Medical Center, Phoenix, AZ 85015, United States.
Vikash Kumar, Department of Medicine, The Brooklyn Hospital Center, Brooklyn, NY 11201, United States.
Aalam Sohal, Department of Hepatology, Liver Institute Northwest, Seattle, WA 98105, United States. aalamsohal@gmail.com.
Juliana Yang, Division of Gastroenterology and Hepatology, University of Texas Medical Branch, Galveston, TX 77555, United States.
References
- 1.Dharni K, Singh A, Sharma S, Midha V, Kaur K, Mahajan R, Dulai PS, Sood A. Trends of inflammatory bowel disease from the Global Burden of Disease Study (1990-2019) Indian J Gastroenterol. 2024;43:188–198. doi: 10.1007/s12664-023-01430-z. [DOI] [PubMed] [Google Scholar]
- 2.Monsén U, Sorstad J, Hellers G, Johansson C. Extracolonic diagnoses in ulcerative colitis: an epidemiological study. Am J Gastroenterol. 1990;85:711–716. [PubMed] [Google Scholar]
- 3.Malik TF, Aurelio DM. Extraintestinal Manifestations of Inflammatory Bowel Disease. 2023 Mar 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 4.Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2011;7:235–241. [PMC free article] [PubMed] [Google Scholar]
- 5.Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93:504–514. doi: 10.1111/j.1572-0241.1998.156_b.x. [DOI] [PubMed] [Google Scholar]
- 6.Shield DE, Lytton B, Weiss RM, Schiff M Jr. Urologic complications of inflammatory bowel disease. J Urol. 1976;115:701–706. doi: 10.1016/s0022-5347(17)59341-6. [DOI] [PubMed] [Google Scholar]
- 7.van Sommeren S, Janse M, Karjalainen J, Fehrmann R, Franke L, Fu J, Weersma RK. Extraintestinal manifestations and complications in inflammatory bowel disease: from shared genetics to shared biological pathways. Inflamm Bowel Dis. 2014;20:987–994. doi: 10.1097/MIB.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Zhang Y, Ye Z, Yang S, Zhou C, He P, Zhang Y, Hou FF, Qin X. Inflammatory Bowel Disease With Chronic Kidney Disease and Acute Kidney Injury. Am J Prev Med. 2023;65:1103–1112. doi: 10.1016/j.amepre.2023.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Jose FA, Garnett EA, Vittinghoff E, Ferry GD, Winter HS, Baldassano RN, Kirschner BS, Cohen SA, Gold BD, Abramson O, Heyman MB. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63–68. doi: 10.1002/ibd.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greuter T, Bertoldo F, Rechner R, Straumann A, Biedermann L, Zeitz J, Misselwitz B, Scharl M, Rogler G, Safroneeva E, Ali RAR, Braegger C, Heyland K, Mueller P, Nydegger A, Petit LM, Schibli S, Furlano RI, Spalinger J, Schäppi M, Zamora S, Froehlich F, Herzog D, Schoepfer AM, Vavricka SR Swiss IBD Cohort Study Group. Extraintestinal Manifestations of Pediatric Inflammatory Bowel Disease: Prevalence, Presentation, and Anti-TNF Treatment. J Pediatr Gastroenterol Nutr. 2017;65:200–206. doi: 10.1097/MPG.0000000000001455. [DOI] [PubMed] [Google Scholar]
- 11.Guariso G, Gasparetto M, Visonà Dalla Pozza L, D'Incà R, Zancan L, Sturniolo G, Brotto F, Facchin P. Inflammatory bowel disease developing in paediatric and adult age. J Pediatr Gastroenterol Nutr. 2010;51:698–707. doi: 10.1097/MPG.0b013e3181da1db8. [DOI] [PubMed] [Google Scholar]
- 12.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 13.Ranganathan P, Jayakumar C, Santhakumar M, Ramesh G. Netrin-1 regulates colon-kidney cross talk through suppression of IL-6 function in a mouse model of DSS-colitis. Am J Physiol Renal Physiol. 2013;304:F1187–F1197. doi: 10.1152/ajprenal.00702.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CJ, Wang PC, Huang TC, Taniguchi A. Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20061458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ducasa G, Hazime H, Fernandez I, Brito N, Santander A, Burgueno J, Abreu M. Chronic colitis in mice induces kidney injury: translational implications for IBD. Inflamm Bowel Dis. 2022;28:S38–S38. [Google Scholar]
- 16.Yang Y, Ludvigsson JF, Olén O, Sjölander A, Carrero JJ. Absolute and Relative Risks of Kidney and Urological Complications in Patients With Inflammatory Bowel Disease. Am J Gastroenterol. 2024;119:138–146. doi: 10.14309/ajg.0000000000002473. [DOI] [PubMed] [Google Scholar]
- 17.Vajravelu RK, Copelovitch L, Osterman MT, Scott FI, Mamtani R, Lewis JD, Denburg MR. Inflammatory Bowel Diseases Are Associated With an Increased Risk for Chronic Kidney Disease, Which Decreases With Age. Clin Gastroenterol Hepatol. 2020;18:2262–2268. doi: 10.1016/j.cgh.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S, Chun J, Han KD, Soh H, Choi K, Kim JH, Lee J, Lee C, Im JP, Kim JS. Increased end-stage renal disease risk in patients with inflammatory bowel disease: A nationwide population-based study. World J Gastroenterol. 2018;24:4798–4808. doi: 10.3748/wjg.v24.i42.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang HM, Baek HS, Kim JE, Kim JY, Lee YH, Cho HY, Choe YH, Kang B, Choe BH, Choi BS, Cho MH. Renal involvement in children and adolescents with inflammatory bowel disease. Korean J Pediatr. 2018;61:327–331. doi: 10.3345/kjp.2018.06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmud N, Stinson J, O'Connell MA, Mantle TJ, Keeling PW, Feely J, Weir DG, Kelleher D. Microalbuminuria in inflammatory bowel disease. Gut. 1994;35:1599–1604. doi: 10.1136/gut.35.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H, Liu P, Gong S, Liu X, Hill MA, Liu Z, Xu M, Xu C. Inflammatory bowel disease increases the levels of albuminuria and the risk of urolithiasis: a two-sample Mendelian randomization study. Eur J Med Res. 2023;28:167. doi: 10.1186/s40001-023-01128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulou AC, Goumas KE, Dandakis DC, Tyrmpas I, Panagiotaki M, Georgouli A, Soutos DC, Archimandritis A. Microproteinuria in patients with inflammatory bowel disease: is it associated with the disease activity or the treatment with 5-aminosalicylic acid? World J Gastroenterol. 2006;12:739–746. doi: 10.3748/wjg.v12.i5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dincer MT, Dincer ZT, Bakkaloglu OK, Yalin SF, Trabulus S, Celik AF, Seyahi N, Altiparmak MR. Renal Manifestations in Inflammatory Bowel Disease: A Cohort Study During the Biologic Era. Med Sci Monit. 2022;28:e936497. doi: 10.12659/MSM.936497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401–412. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Gkentzis A, Kimuli M, Cartledge J, Traxer O, Biyani CS. Urolithiasis in inflammatory bowel disease and bariatric surgery. World J Nephrol. 2016;5:538–546. doi: 10.5527/wjn.v5.i6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambruzs JM, Larsen CP. Renal Manifestations of Inflammatory Bowel Disease. Rheum Dis Clin North Am. 2018;44:699–714. doi: 10.1016/j.rdc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Corica D, Romano C. Renal Involvement in Inflammatory Bowel Diseases. J Crohns Colitis. 2016;10:226–235. doi: 10.1093/ecco-jcc/jjv138. [DOI] [PubMed] [Google Scholar]
- 28.Cury DB, Moss AC, Schor N. Nephrolithiasis in patients with inflammatory bowel disease in the community. Int J Nephrol Renovasc Dis. 2013;6:139–142. doi: 10.2147/IJNRD.S45466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganji-Arjenaki M, Nasri H, Rafieian-Kopaei M. Nephrolithiasis as a common urinary system manifestation of inflammatory bowel diseases; a clinical review and meta-analysis. J Nephropathol. 2017;6:264–269. doi: 10.15171/jnp.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Pollok R, Goldsmith D. Renal and Urological Disorders Associated With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2023;29:1306–1316. doi: 10.1093/ibd/izac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onime A, Agaba EI, Sun Y, Parsons RB, Servilla KS, Massie LW, Tzamaloukas AH. Immunoglobulin A nephropathy complicating ulcerative colitis. Int Urol Nephrol. 2006;38:349–353. doi: 10.1007/s11255-006-0061-y. [DOI] [PubMed] [Google Scholar]
- 32.Parmar MS. Kidney stones. BMJ. 2004;328:1420–1424. doi: 10.1136/bmj.328.7453.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M. Oxalate toxicity in renal cells. Urol Res. 2005;33:329–339. doi: 10.1007/s00240-005-0485-3. [DOI] [PubMed] [Google Scholar]
- 34.Nazzal L, Puri S, Goldfarb DS. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrol Dial Transplant. 2016;31:375–382. doi: 10.1093/ndt/gfv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oikonomou K, Kapsoritakis A, Eleftheriadis T, Stefanidis I, Potamianos S. Renal manifestations and complications of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1034–1045. doi: 10.1002/ibd.21468. [DOI] [PubMed] [Google Scholar]
- 36.Mutalib M. Renal involvement in paediatric inflammatory bowel disease. Pediatr Nephrol. 2021;36:279–285. doi: 10.1007/s00467-019-04413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alameddine Z, Abi Melhem R, Dimachkie R, Rabah H, Chehab H, El Khoury M, Qaqish F, Stefanov D, El-Sayegh S. Risk of Nephrolithiasis in Patients with Inflammatory Bowel Disease Receiving Biologic Treatment. J Clin Med. 2023;12 doi: 10.3390/jcm12196114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdulrhman A, Alsweed A, Alotaibi MR, Aldakhil AY, Alahmadi SF, Albishri SM, Alhmed NI. Urolithiasis in patients with inflammatory bowel disease: A systematic review and meta-analysis of 13,339,065 individuals. Medicine (Baltimore) 2023;102:e33938. doi: 10.1097/MD.0000000000033938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ene MA, Jinga V, Geavlete PA, Ene CV, Bulai CA, Serboiu CS, Geavlete BF. Stone free-rate experience in post-interventional patients undergoing citrates and pyridoxine administration. Off J Romanian Soc Pharm Sci. 2023;71:573–580. [Google Scholar]
- 40.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 41.Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, Hawkins PN. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356:2361–2371. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 42.Cucino C, Sonnenberg A. The comorbid occurrence of other diagnoses in patients with ulcerative colitis and Crohn's disease. Am J Gastroenterol. 2001;96:2107–2112. doi: 10.1111/j.1572-0241.2001.03943.x. [DOI] [PubMed] [Google Scholar]
- 43.Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol. 2008;103:776–787. doi: 10.1111/j.1572-0241.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- 44.Tada Y, Ishihara S, Ito T, Matsui K, Sonoyama H, Oka A, Kusunoki R, Fukuba N, Mishima Y, Oshima N, Moriyama I, Yuki T, Kawashima K, Sato S, Adachi K, Ikeuchi H, Kinoshita Y. Successful use of maintenance infliximab for nephropathy in a patient with secondary amyloidosis complicating Crohn's disease. Intern Med. 2013;52:1899–1902. doi: 10.2169/internalmedicine.52.0340. [DOI] [PubMed] [Google Scholar]
- 45.Wester AL, Vatn MH, Fausa O. Secondary amyloidosis in inflammatory bowel disease: a study of 18 patients admitted to Rikshospitalet University Hospital, Oslo, from 1962 to 1998. Inflamm Bowel Dis. 2001;7:295–300. doi: 10.1097/00054725-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Saverymuttu SH, Hodgson HJ, Chadwick VS, Pepys MB. Differing acute phase responses in Crohn's disease and ulcerative colitis. Gut. 1986;27:809–813. doi: 10.1136/gut.27.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Beer FC, Mallya RK, Fagan EA, Lanham JG, Hughes GR, Pepys MB. Serum amyloid-A protein concentration in inflammatory diseases and its relationship to the incidence of reactive systemic amyloidosis. Lancet. 1982;2:231–234. doi: 10.1016/s0140-6736(82)90321-x. [DOI] [PubMed] [Google Scholar]
- 48.Tosca Cuquerella J, Bosca-Watts MM, Anton Ausejo R, Tejedor Alonso S, Mora De Miguel F, Minguez Perez M. Amyloidosis in Inflammatory Bowel Disease: A Systematic Review of Epidemiology, Clinical Features, and Treatment. J Crohns Colitis. 2016;10:1245–1253. doi: 10.1093/ecco-jcc/jjw080. [DOI] [PubMed] [Google Scholar]
- 49.Guardiola-Arévalo A, Alcántara-Torres M, Valle-Muñoz J, Lorente-Poyatos RH, Romero-Gutiérrez M, Rodríguez-Merlo R, Pérez-Martínez A, Carrobles-Jiménez JM. Amyloidosis and Crohn´s disease. Rev Esp Enferm Dig. 2011;103:268–274. [PubMed] [Google Scholar]
- 50.Béji S, Kaaroud H, Ben Moussa F, Goucha R, Abderrahim E, El Younsi F, Ben Maïz H. [Renal amyloidosis complicating the outcome of chronic inflammatory colitis] Presse Med. 2004;33:862–865. doi: 10.1016/s0755-4982(04)98773-8. [DOI] [PubMed] [Google Scholar]
- 51.Saitoh O, Kojima K, Teranishi T, Nakagawa K, Kayazawa M, Nanri M, Egashira Y, Hirata I, Katsu Ki KI. Renal amyloidosis as a late complication of Crohn's disease: a case report and review of the literature from Japan. World J Gastroenterol. 2000;6:461–464. doi: 10.3748/wjg.v6.i3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldstein RC, Schwabe AD. Prophylactic colchicine therapy in familial Mediterranean fever. A controlled, double-blind study. Ann Intern Med. 1974;81:792–794. doi: 10.7326/0003-4819-81-6-792. [DOI] [PubMed] [Google Scholar]
- 53.Swarowsky B. [Case report: secondary amyloidosis in ulcerative colitis--successful treatment with colchicine] Z Gastroenterol. 1997;35:XVIII. [PubMed] [Google Scholar]
- 54.Menges M, Steffen HM. [Secondary amyloidosis in ulcerative colitis--successful treatment with colchicine] Z Gastroenterol. 1996;34:753–756. [PubMed] [Google Scholar]
- 55.Meyers S, Janowitz HD, Gumaste VV, Abramson RG, Berman LJ, Venkataseshan VS, Dickman SH. Colchicine therapy of the renal amyloidosis of ulcerative colitis. Gastroenterology. 1988;94:1503–1507. doi: 10.1016/0016-5085(88)90692-0. [DOI] [PubMed] [Google Scholar]
- 56.Garrido Serrano A, Guerrero Igea FJ, Hierro Guilmain C, Ruiz Lupiáñez E, Palomo Gil S. [Good response to colchicine in amyloidosis secondary to inflammatory bowel disease] Gastroenterol Hepatol. 2001;24:196–198. doi: 10.1016/s0210-5705(01)70148-3. [DOI] [PubMed] [Google Scholar]
- 57.Naves JE, Domènech E. [Systemic amyloidosis in inflammatory bowel disease] Gastroenterol Hepatol. 2012;35:259–265. doi: 10.1016/j.gastrohep.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Tan AU Jr, Cohen AH, Levine BS. Renal amyloidosis in a drug abuser. J Am Soc Nephrol. 1995;5:1653–1658. doi: 10.1681/ASN.V591653. [DOI] [PubMed] [Google Scholar]
- 59.Boscá MM, Pérez-Baylach CM, Solis MA, Antón R, Mayordomo E, Pons S, Mínguez M, Benages A. Secondary amyloidosis in Crohn's disease: treatment with tumour necrosis factor inhibitor. Gut. 2006;55:294–295. doi: 10.1136/gut.2005.082057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Said Y, Debbeche R, Hamzaoui L, Lounissi M, Trabelsi S, Bouzaidi S, Salem M, Abdelmoula L, Najjar T. Infiximab for treatment of systemic amyloidosis associated with Crohn's disease. J Crohns Colitis. 2011;5:171–172. doi: 10.1016/j.crohns.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Fernández-Nebro A, Olivé A, Castro MC, Varela AH, Riera E, Irigoyen MV, García de Yébenes MJ, García-Vicuña R. Long-term TNF-alpha blockade in patients with amyloid A amyloidosis complicating rheumatic diseases. Am J Med. 2010;123:454–461. doi: 10.1016/j.amjmed.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Fidalgo C, Calado J, Cravo M. Secondary amyloidosis in a patient with long duration Crohn's disease treated with infliximab. BioDrugs. 2010;24 Suppl 1:15–17. doi: 10.2165/11586250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.Fitchen JH. Amyloidosis and granulomatous ileocolitis. Regression after surgical removal of the involved bowel. N Engl J Med. 1975;292:352–353. doi: 10.1056/NEJM197502132920709. [DOI] [PubMed] [Google Scholar]
- 64.Ravid M, Shapira J, Kedar I, Feigl D. Regression of amyloidosis secondary to granulomatous ileitis following surgical resection and colchicine administration. Acta Hepatogastroenterol (Stuttg) 1979;26:513–515. [PubMed] [Google Scholar]
- 65.Mandelstam P, Simmons DE, Mitchell B. Regression of amyloid in Crohn's disease after bowel resection. A 19-year follow-up. J Clin Gastroenterol. 1989;11:324–326. doi: 10.1097/00004836-198906000-00016. [DOI] [PubMed] [Google Scholar]
- 66.Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, Fontana M, Moon JC, Pinzani M, Gillmore JD, Hawkins PN, Pepys MB. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. 2015;373:1106–1114. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]
- 67.Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N, Yoshizaki K, Shimoyama T, Kishimoto T. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989–96; discussion 947. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Cañas-Ventura A, Rodríguez E, Andreu M, Márquez L. Tocilizumab in amyloidosis-associated kidney disease secondary to inflammatory bowel diseases. Dig Dis Sci. 2013;58:2736–2737. doi: 10.1007/s10620-013-2776-9. [DOI] [PubMed] [Google Scholar]
- 69.Brulhart L, Nissen MJ, Chevallier P, Gabay C. Tocilizumab in a patient with ankylosing spondylitis and Crohn's disease refractory to TNF antagonists. Joint Bone Spine. 2010;77:625–626. doi: 10.1016/j.jbspin.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Algaba A, Guerra I, Castaño A, de la Poza G, Castellano VM, López M, Bermejo F. Risk of cancer, with special reference to extra-intestinal malignancies, in patients with inflammatory bowel disease. World J Gastroenterol. 2013;19:9359–9365. doi: 10.3748/wjg.v19.i48.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP, Simon T, Maynadié M, Hermine O, Faivre J, Carrat F CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 72.Gakis G. The role of inflammation in bladder cancer. Adv Exp Med Biol. 2014;816:183–196. doi: 10.1007/978-3-0348-0837-8_8. [DOI] [PubMed] [Google Scholar]
- 73.Bourrier A, Carrat F, Colombel JF, Bouvier AM, Abitbol V, Marteau P, Cosnes J, Simon T, Peyrin-Biroulet L, Beaugerie L CESAME study group. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther. 2016;43:252–261. doi: 10.1111/apt.13466. [DOI] [PubMed] [Google Scholar]
- 74.Feng D, Bai Y, Liu S, Yang Y, Han P, Wei W. Risk of renal cancer in patients with inflammatory bowel disease: A pooled analysis of population-based studies. Urol Oncol. 2021;39:93–99. doi: 10.1016/j.urolonc.2020.10.078. [DOI] [PubMed] [Google Scholar]
- 75.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 76.Kappelman MD, Farkas DK, Long MD, Erichsen R, Sandler RS, Sørensen HT, Baron JA. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12:265–73.e1. doi: 10.1016/j.cgh.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madanchi M, Zeitz J, Barthel C, Samaras P, Scharl S, Sulz MC, Biedermann L, Frei P, Vavricka SR, Rogler G, Scharl M. Malignancies in Patients with Inflammatory Bowel Disease: A Single-Centre Experience. Digestion. 2016;94:1–8. doi: 10.1159/000447259. [DOI] [PubMed] [Google Scholar]
- 78.Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010;105:1480–1487. doi: 10.1038/ajg.2009.760. [DOI] [PubMed] [Google Scholar]
- 79.Greuter T, Vavricka S, König AO, Beaugerie L, Scharl M Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Malignancies in Inflammatory Bowel Disease. Digestion. 2020;101 Suppl 1:136–145. doi: 10.1159/000509544. [DOI] [PubMed] [Google Scholar]
- 80.Yandian F, Caravaca-Fontán F, Herrera Hernandez LP, Soler MJ, Sethi S, Fervenza FC. Kidney Diseases Associated With Inflammatory Bowel Disease: Impact of Chronic Histologic Damage, Treatments, and Outcomes. Kidney Int Rep. 2024;9:383–394. doi: 10.1016/j.ekir.2023.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9:265–270. doi: 10.2215/CJN.04660513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forshaw MJ, Guirguis O, Hennigan TW. IgA nephropathy in association with Crohn's disease. Int J Colorectal Dis. 2005;20:463–465. doi: 10.1007/s00384-004-0696-z. [DOI] [PubMed] [Google Scholar]
- 83.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akiyama M, Shimomura K, Yoshimoto H, Sako M, Kodama M, Abe K, Gunji M, Kang D, Takaki T, Wada Y, Iyoda M, Honda K. Crohn's disease may promote inflammation in IgA nephropathy: a case-control study of patients undergoing kidney biopsy. Virchows Arch. 2022;481:553–563. doi: 10.1007/s00428-022-03373-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kiryluk K, Novak J, Gharavi AG. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med. 2013;64:339–356. doi: 10.1146/annurev-med-041811-142014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H Jr, Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci U S A. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suárez-Fueyo A, Bradley SJ, Klatzmann D, Tsokos GC. T cells and autoimmune kidney disease. Nat Rev Nephrol. 2017;13:329–343. doi: 10.1038/nrneph.2017.34. [DOI] [PubMed] [Google Scholar]
- 88.Sugihara T, Kobori A, Imaeda H, Tsujikawa T, Amagase K, Takeuchi K, Fujiyama Y, Andoh A. The increased mucosal mRNA expressions of complement C3 and interleukin-17 in inflammatory bowel disease. Clin Exp Immunol. 2010;160:386–393. doi: 10.1111/j.1365-2249.2010.04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coppo R. The pathogenetic potential of environmental antigens in IgA nephropathy. Am J Kidney Dis. 1988;12:420–424. doi: 10.1016/s0272-6386(88)80038-6. [DOI] [PubMed] [Google Scholar]
- 90.Coppo R, Basolo B, Rollino C, Roccatello D, Martina G, Amore A, Piccoli G. Dietary gluten and primary IgA nephropathy. N Engl J Med. 1986;315:1167–1168. doi: 10.1056/nejm198610303151819. [DOI] [PubMed] [Google Scholar]
- 91.Hayashi R, Ueno Y, Tanaka S, Onishi K, Takasago T, Wakai M, Naito T, Sasaki K, Doi S, Masaki T, Chayama K. Clinical characteristics of inflammatory bowel disease patients with immunoglobulin A nephropathy. Intest Res. 2021;19:430–437. doi: 10.5217/ir.2020.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han S, Shang L, Lu Y, Wang Y. Gut Microbiome Characteristics in IgA Nephropathy: Qualitative and Quantitative Analysis from Observational Studies. Front Cell Infect Microbiol. 2022;12:904401. doi: 10.3389/fcimb.2022.904401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang J, Kim CJ, Go YS, Lee HY, Kim MG, Oh SW, Cho WY, Im SH, Jo SK. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020;98:932–946. doi: 10.1016/j.kint.2020.04.048. [DOI] [PubMed] [Google Scholar]
- 94.Inoue T, Iijima H, Tajiri M, Shinzaki S, Shiraishi E, Hiyama S, Mukai A, Nakajima S, Iwatani H, Nishida T, Mizushima T, Yasui T, Isaka Y, Kanto T, Tsujii M, Miyoshi E, Wada Y, Takehara T. Deficiency of N-acetylgalactosamine in O-linked oligosaccharides of IgA is a novel biologic marker for Crohn's disease. Inflamm Bowel Dis. 2012;18:1723–1734. doi: 10.1002/ibd.22876. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, Karaliukas R, Kang HS, Turner JR, Fu YX. Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest. 2004;113:826–835. doi: 10.1172/JCI20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hubert D, Beaufils M, Meyrier A. [Immunoglobulin A glomerular nephropathy associated with inflammatory colitis. Apropos of 2 cases] Presse Med. 1984;13:1083–1085. [PubMed] [Google Scholar]
- 97.Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, Floege J, Hetzel G, Jardine AG, Locatelli F, Maes BD, Mercer A, Ortiz F, Praga M, Sørensen SS, Tesar V, Del Vecchio L NEFIGAN Trial Investigators. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389:2117–2127. doi: 10.1016/S0140-6736(17)30550-0. [DOI] [PubMed] [Google Scholar]
- 98.Smerud HK, Bárány P, Lindström K, Fernström A, Sandell A, Påhlsson P, Fellström B. New treatment for IgA nephropathy: enteric budesonide targeted to the ileocecal region ameliorates proteinuria. Nephrol Dial Transplant. 2011;26:3237–3242. doi: 10.1093/ndt/gfr052. [DOI] [PubMed] [Google Scholar]
- 99.Rehnberg J, Symreng A, Ludvigsson JF, Emilsson L. Inflammatory Bowel Disease Is More Common in Patients with IgA Nephropathy and Predicts Progression of ESKD: A Swedish Population-Based Cohort Study. J Am Soc Nephrol. 2021;32:411–423. doi: 10.1681/ASN.2020060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joher N, Gosset C, Guerrot D, Pillebout E, Hummel A, Boffa JJ, Faguer S, Rabant M, Higgins S, Moktefi A, Delmas Y, Karras A, Lapidus N, Amiot A, Audard V, El Karoui K. Immunoglobulin A nephropathy in association with inflammatory bowel diseases: results from a national study and systematic literature review. Nephrol Dial Transplant. 2022;37:531–539. doi: 10.1093/ndt/gfaa378. [DOI] [PubMed] [Google Scholar]
- 101.Elaziz MMA, Fayed A. Patterns of renal involvement in a cohort of patients with inflammatory bowel disease in Egypt. Acta Gastroenterol Belg. 2018;81:381–385. [PubMed] [Google Scholar]
- 102.Marques da Costa P, Correia L, Correia LA. The Complexity of Renal Involvment in IBD-C3 Glomerulopathy in Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:e4–e5. doi: 10.1093/ibd/izy070. [DOI] [PubMed] [Google Scholar]
- 103.Zhao L, Ren G, Fan R, Feng X, Liu Z, Cheng Z, Zhang T. Spectrum and prognosis of renal histopathological lesions in patients with inflammatory bowel disease: a cross-sectional study from a single center in China. Clin Exp Med. 2022;22:629–635. doi: 10.1007/s10238-021-00766-0. [DOI] [PubMed] [Google Scholar]
- 104.Farooq SM, Stillie R, Svensson M, Svanborg C, Strieter RM, Stadnyk AW. Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2009;329:123–129. doi: 10.1124/jpet.108.145862. [DOI] [PubMed] [Google Scholar]
- 105.Ranganathan P, Jayakumar C, Manicassamy S, Ramesh G. CXCR2 knockout mice are protected against DSS-colitis-induced acute kidney injury and inflammation. Am J Physiol Renal Physiol. 2013;305:F1422–F1427. doi: 10.1152/ajprenal.00319.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thuluvath PJ, Ninkovic M, Calam J, Anderson M. Mesalazine induced interstitial nephritis. Gut. 1994;35:1493–1496. doi: 10.1136/gut.35.10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.World MJ, Stevens PE, Ashton MA, Rainford DJ. Mesalazine-associated interstitial nephritis. Nephrol Dial Transplant. 1996;11:614–621. doi: 10.1093/oxfordjournals.ndt.a027349. [DOI] [PubMed] [Google Scholar]
- 108.Larchet M, Guillot M, Mandard JC, Boutard P, Alibert L, Delmas P, Duhamel JF. [Crohn's enteritis and chronic tubulo-interstitial nephropathy in an adolescent] Arch Fr Pediatr. 1988;45:649–651. [PubMed] [Google Scholar]
- 109.Fraser JS, Muller AF, Smith DJ, Newman DJ, Lamb EJ. Renal tubular injury is present in acute inflammatory bowel disease prior to the introduction of drug therapy. Aliment Pharmacol Ther. 2001;15:1131–1137. doi: 10.1046/j.1365-2036.2001.01041.x. [DOI] [PubMed] [Google Scholar]
- 110.Riley SA, Lloyd DR, Mani V. Tests of renal function in patients with quiescent colitis: effects of drug treatment. Gut. 1992;33:1348–1352. doi: 10.1136/gut.33.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bailly E, Von Tokarski F, Beau-Salinas F, Picon L, Miquelestorena-Standley E, Rousseau G, Jonville-Bera AP, Halimi JM. Interstitial Nephritis Secondary to Vedolizumab Treatment in Crohn Disease and Safe Rechallenge Using Steroids: A Case Report. Am J Kidney Dis. 2018;71:142–145. doi: 10.1053/j.ajkd.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 112.Manenti L, De Rosa A, Buzio C. Mesalazine-associated interstitial nephritis: twice in the same patient. Nephrology Dial Transplant. 1997;12:2031–2031. [PubMed] [Google Scholar]
- 113.Witte T, Olbricht CJ, Koch KM. Interstitial nephritis associated with 5-aminosalicylic acid. Nephron. 1994;67:481–482. doi: 10.1159/000188024. [DOI] [PubMed] [Google Scholar]
- 114.Arend LJ, Springate JE. Interstitial nephritis from mesalazine: case report and literature review. Pediatr Nephrol. 2004;19:550–553. doi: 10.1007/s00467-004-1411-6. [DOI] [PubMed] [Google Scholar]
- 115.Corrigan G, Stevens PE. Review article: interstitial nephritis associated with the use of mesalazine in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1–6. doi: 10.1046/j.1365-2036.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 116.Waters AM, Zachos M, Herzenberg AM, Harvey E, Rosenblum ND. Tubulointerstitial nephritis as an extraintestinal manifestation of Crohn's disease. Nat Clin Pract Nephrol. 2008;4:693–697. doi: 10.1038/ncpneph0955. [DOI] [PubMed] [Google Scholar]
- 117.Calviño J, Romero R, Pintos E, Losada E, Novoa D, Güimil D, Mardaras J, Sanchez-Guisande D. Mesalazine-associated tubulo-interstitial nephritis in inflammatory bowel disease. Clin Nephrol. 1998;49:265–267. [PubMed] [Google Scholar]
- 118.Kaufman J, Dhakal M, Patel B, Hamburger R. Community-acquired acute renal failure. Am J Kidney Dis. 1991;17:191–198. doi: 10.1016/s0272-6386(12)81128-0. [DOI] [PubMed] [Google Scholar]
- 119.Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11:555–565. doi: 10.1097/01.ccx.0000184300.68383.95. [DOI] [PubMed] [Google Scholar]
- 120.Makris K, Spanou L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin Biochem Rev. 2016;37:85–98. [PMC free article] [PubMed] [Google Scholar]
- 121.Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7:52. doi: 10.1038/s41572-021-00284-z. [DOI] [PubMed] [Google Scholar]
- 122.Oikonomou KA, Kapsoritakis AN, Stefanidis I, Potamianos SP. Drug-induced nephrotoxicity in inflammatory bowel disease. Nephron Clin Pract. 2011;119:c89–94; discussion c96. doi: 10.1159/000326682. [DOI] [PubMed] [Google Scholar]
- 123.de Jong DJ, Tielen J, Habraken CM, Wetzels JF, Naber AH. 5-Aminosalicylates and effects on renal function in patients with Crohn's disease. Inflamm Bowel Dis. 2005;11:972–976. doi: 10.1097/01.mib.0000185402.65288.19. [DOI] [PubMed] [Google Scholar]
- 124.Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2007;13:629–638. doi: 10.1002/ibd.20099. [DOI] [PubMed] [Google Scholar]
- 125.Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51:536–539. doi: 10.1136/gut.51.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stange EF, Modigliani R, Peña AS, Wood AJ, Feutren G, Smith PR. European trial of cyclosporine in chronic active Crohn's disease: a 12-month study. The European Study Group. Gastroenterology. 1995;109:774–782. doi: 10.1016/0016-5085(95)90384-4. [DOI] [PubMed] [Google Scholar]
- 127.Sandborn WJ, Tremaine WJ, Lawson GM. Clinical response does not correlate with intestinal or blood cyclosporine concentrations in patients with Crohn's disease treated with high-dose oral cyclosporine. Am J Gastroenterol. 1996;91:37–43. [PubMed] [Google Scholar]
- 128.Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles' heel of immunosuppressive therapy. Kidney Int. 1996;50:1089–1100. doi: 10.1038/ki.1996.415. [DOI] [PubMed] [Google Scholar]
- 129.Bennett WM. The nephrotoxicity of immunosuppressive drugs. Clin Nephrol. 1995;43 Suppl 1:S3–S7. [PubMed] [Google Scholar]
- 130.Shang MH, Yuan WJ, Zhang SJ, Fan Y, Zhang Z. Intrarenal activation of renin angiotensin system in the development of cyclosporine A induced chronic nephrotoxicity. Chin Med J (Engl) 2008;121:983–988. [PubMed] [Google Scholar]
- 131.Choi JY, Kim DK, Kim YW, Yoo TH, Lee JP, Chung HC, Cho KH, An WS, Lee DH, Jung HY, Cho JH, Kim CD, Kim YL, Park SH. The Effect of Mycophenolate Mofetil versus Cyclosporine as Combination Therapy with Low Dose Corticosteroids in High-risk Patients with Idiopathic Membranous Nephropathy: a Multicenter Randomized Trial. J Korean Med Sci. 2018;33:e74. doi: 10.3346/jkms.2018.33.e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials. Arthritis Rheum. 2000;43:2383–2390. doi: 10.1002/1529-0131(200011)43:11<2383::AID-ANR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 133.den Broeder AA, Assmann KJ, van Riel PL, Wetzels JF. Nephrotic syndrome as a complication of anti-TNFalpha in a patient with rheumatoid arthritis. Neth J Med. 2003;61:137–141. [PubMed] [Google Scholar]
- 134.Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D'Haens G, Panaccione R, Loftus EV Jr, Sankoh S, Fox I, Parikh A, Milch C, Abhyankar B, Feagan BG. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017;66:839–851. doi: 10.1136/gutjnl-2015-311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Simpson N, Seenan JP, Patel R, Kipgen D. Acute interstitial nephritis secondary to vedolizumab. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, Menon S, Lamba M, Zwillich S. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:318–328. [PubMed] [Google Scholar]
- 137.Vincenti F, Silva HT, Busque S, O'Connell PJ, Russ G, Budde K, Yoshida A, Tortorici MA, Lamba M, Lawendy N, Wang W, Chan G. Evaluation of the effect of tofacitinib exposure on outcomes in kidney transplant patients. Am J Transplant. 2015;15:1644–1653. doi: 10.1111/ajt.13181. [DOI] [PubMed] [Google Scholar]
- 138.Namour F, Anderson K, Nelson C, Tasset C. Filgotinib: A Clinical Pharmacology Review. Clin Pharmacokinet. 2022;61:819–832. doi: 10.1007/s40262-022-01129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pérez Fernández M, Piteiro Bermejo AB, Peña Esparragoza JK, Blasco Martínez A, Aracil Moreno I, Mancha Ramos J, Moreno Barrio F. Nephrotic syndrome in relation to treatment with ustekinumab. Nefrologia (Engl Ed) 2019;39:100–102. doi: 10.1016/j.nefro.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 140.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 141.Lomaestro BM. Fluoroquinolone-induced renal failure. Drug Saf. 2000;22:479–485. doi: 10.2165/00002018-200022060-00006. [DOI] [PubMed] [Google Scholar]
- 142.Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 143.Golabek T, Szymanska A, Szopinski T, Bukowczan J, Furmanek M, Powroznik J, Chlosta P. Enterovesical fistulae: aetiology, imaging, and management. Gastroenterol Res Pract. 2013;2013:617967. doi: 10.1155/2013/617967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taxonera C, Barreiro-de-Acosta M, Bastida G, Martinez-Gonzalez J, Merino O, García-Sánchez V, Gisbert JP, Marín-Jiménez I, López-Serrano P, Gómez-García M, Iglesias E, Lopez-Sanroman A, Chaparro M, Saro C, Bermejo F, Pérez-Carazo L, Plaza R, Olivares D, Alba C, Mendoza JL, Fernández-Blanco I. Outcomes of Medical and Surgical Therapy for Entero-urinary Fistulas in Crohn's Disease. J Crohns Colitis. 2016;10:657–662. doi: 10.1093/ecco-jcc/jjw016. [DOI] [PubMed] [Google Scholar]
- 145.Vagianos C, Malgarinos G, Spyropoulos C, Triantafillidis JK. Entero-vesical fistulas in CROHN'S disease: A case series report and review of the literature. Int J Surg Case Rep. 2017;41:477–480. doi: 10.1016/j.ijscr.2017.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Solem CA, Loftus EV Jr, Tremaine WJ, Pemberton JH, Wolff BG, Sandborn WJ. Fistulas to the urinary system in Crohn's disease: clinical features and outcomes. Am J Gastroenterol. 2002;97:2300–2305. doi: 10.1111/j.1572-0241.2002.05983.x. [DOI] [PubMed] [Google Scholar]
- 147.Akram W, Shah SK, Sohail M, Rehman U, Rahim M. Recurrent Urinary Tract Infection in a Patient With Asymptomatic Crohn's Disease. Cureus. 2020;12:e9962. doi: 10.7759/cureus.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Daniels IR, Bekdash B, Scott HJ, Marks CG, Donaldson DR. Diagnostic lessons learnt from a series of enterovesical fistulae. Colorectal Dis. 2002;4:459–462. doi: 10.1046/j.1463-1318.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 149.El-Haddad HM, Kassem MI, Sabry AA, Abouelfotouh A. Surgical protocol and outcome for sigmoidovesical fistula secondary to diverticular disease of the left colon: A retrospective cohort study. Int J Surg. 2018;56:115–123. doi: 10.1016/j.ijsu.2018.05.742. [DOI] [PubMed] [Google Scholar]
- 150.Schwaibold H, Popiel C, Geist E, Hartung R. Oral intake of poppy seed: a reliable and simple method for diagnosing vesico-enteric fistula. J Urol. 2001;166:530–531. doi: 10.1016/s0022-5347(05)65976-9. [DOI] [PubMed] [Google Scholar]
- 151.Kwon EO, Armenakas NA, Scharf SC, Panagopoulos G, Fracchia JA. The poppy seed test for colovesical fistula: big bang, little bucks! J Urol. 2008;179:1425–1427. doi: 10.1016/j.juro.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 152.Yang CH, Liu KH, Chen TC, Chang PL, Yeh TS. Enterovesical fistula caused by a bladder squamous cell carcinoma. World J Gastroenterol. 2009;15:4215–4217. doi: 10.3748/wjg.15.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li S, Chen Z, Zhang Q, Huang C, Wang Z, Du S. Four cases of enterovesical fistula and the importance of CT in the diagnosis. BJR Case Rep. 2017;3:20150124. doi: 10.1259/bjrcr.20150124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ravichandran S, Ahmed HU, Matanhelia SS, Dobson M. Is there a role for magnetic resonance imaging in diagnosing colovesical fistulas? Urology. 2008;72:832–837. doi: 10.1016/j.urology.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 155.Zhang W, Zhu W, Li Y, Zuo L, Wang H, Li N, Li J. The respective role of medical and surgical therapy for enterovesical fistula in Crohn's disease. J Clin Gastroenterol. 2014;48:708–11. doi: 10.1097/MCG.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 156.Yamamoto T, Keighley MR. Enterovesical fistulas complicating Crohn's disease: clinicopathological features and management. Int J Colorectal Dis. 2000;15:211–217. doi: 10.1007/s003840000233. [DOI] [PubMed] [Google Scholar]
- 157.Widia F, Firman M, Irdam GA, Syaiful RA. A six years' experience with 41 cases of enterovesical fistula in a Tertiary National Hospital in Indonesia: A retrospective study. Ann Med Surg (Lond) 2022;73:103102. doi: 10.1016/j.amsu.2021.103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hyams JA, Weinberg SR, Alley JL. Chronic ileitis with concomitant ureteritis: Case report. Am J Surg. 1943;61:117–120. [Google Scholar]
- 159.Jansen R, Zaslau S. Hydronephrosis and Ureteral Obstruction in Crohn’s Disease. Op J Urology. 2013;3:219–221. [Google Scholar]
- 160.Ben-Ami H, Ginesin Y, Behar DM, Fischer D, Edoute Y, Lavy A. Diagnosis and treatment of urinary tract complications in Crohn's disease: an experience over 15 years. Can J Gastroenterol. 2002;16:225–229. doi: 10.1155/2002/204614. [DOI] [PubMed] [Google Scholar]
- 161.Angelberger S, Fink KG, Schima W, Szlauer R, Vogelsang H, Reinisch W, Gangl A, Novacek G. Complications in Crohn's disease: right-sided ureteric stenosis and hydronephrosis. Inflamm Bowel Dis. 2007;13:1056–1057. doi: 10.1002/ibd.20130. [DOI] [PubMed] [Google Scholar]
- 162.Present DH, Rabinowitz JG, Banks PA, Janowitz HD. Obstructive hydronephrosis. A frequent but seldom recognized complication of granulomatous disease of the bowel. N Engl J Med. 1969;280:523–528. doi: 10.1056/NEJM196903062801002. [DOI] [PubMed] [Google Scholar]
- 163.Mooney RA, Sant GR. Obstructive uropathy in granulomatous bowel disease. Br J Surg. 1973;60:525–527. doi: 10.1002/bjs.1800600707. [DOI] [PubMed] [Google Scholar]
- 164.Ueno Y, Tanaka S, Kanao H, Yoshioka K, Hatakeyama T, Shimamoto M, Miyanaka Y, Hiyama T, Ito M, Kitadai Y, Yoshihara M, Chayama K. A case of Crohn's disease with hydronephrosis caused by ureteropelvic junction obstruction. Eur J Gastroenterol Hepatol. 2006:18 :1015–1018. doi: 10.1097/01.meg.0000224472.59051.7e. [DOI] [PubMed] [Google Scholar]
- 165.Matsumiya K, Miyake O, Hosomi M, Oka T, Takaha M, Nakayama H, Kikkawa N, Terada A, Murai M. [Hydronephrosis caused by Crohn's disease: a case report--review of 41 cases with urinary tract complication reported in Japan] Hinyokika Kiyo. 1989;35:863–869. [PubMed] [Google Scholar]
- 166.Sato S, Sasaki I, Naito H, Funayama Y, Fukushima K, Shibata C, Masuko T, Ogawa H, Ueno T, Hashimoto A, Matsuno S. Management of urinary complications in Crohn's disease. Surg Today. 1999;29:713–717. doi: 10.1007/BF02482314. [DOI] [PubMed] [Google Scholar]
- 167.Siminovitch JM, Fazio VW. Ureteral obstruction secondary to Crohn's disease: a need for ureterolysis? Am J Surg. 1980;139:95–98. doi: 10.1016/0002-9610(80)90236-6. [DOI] [PubMed] [Google Scholar]
- 168.Guillo L, Delanaye P, Flamant M, Figueres L, Karam S, Lemoine S, Benezech A, Pelletier AL, Amiot A, Caron B, Stefanescu C, Boschetti G, Bouguen G, Rahier JF, Gornet JM, Hugot JP, Bonnet J, Vuitton L, Nachury M, Vidon M, Uzzan M, Serrero M, Dib N, Seksik P, Hebuterne X, Bertocchio JP, Mariat C, Peyrin-Biroulet L. Kidney function monitoring in inflammatory bowel disease: The MONITORED consensus. Dig Liver Dis. 2022;54:309–315. doi: 10.1016/j.dld.2021.11.008. [DOI] [PubMed] [Google Scholar]