Abstract
BACKGROUND
Surgical resection and liver transplantation (LT) are the most effective curative options for hepatocellular carcinoma (HCC). However, few patients with huge HCC (> 10 cm in diameter), especially those with portal vein tumor thrombus (PVTT), can receive these treatments. Selective internal radiation therapy (SIRT) can be used as a conversion therapy for them because it has the dual benefit of shrinking tumors and increasing residual hepatic volume. However, in patients with huge HCC, high lung absorbed dose often prevents them from receiving SIRT.
CASE SUMMARY
A 35-year-old man was admitted because of emaciation and pain in the hepatic region for about 1 month. The computed tomography scan showed a 20.2 cm × 19.8 cm tumor located in the right lobe–left medial lobes with right portal vein and right hepatic vein invasion. After the pathological type of HCC was confirmed by biopsy, two conversions were presented. The first one was drug-eluting bead transarterial chemoembolization plus hepatic arterial infusion chemotherapy and lenvatinib and sintilimab, converted to SIRT, and the second one was sequential SIRT with continued systemic treatment. The tumor size significantly decreased from 20.2 cm × 19.8 cm to 16.2 cm × 13.8 cm, then sequentially to 7.8 cm × 6.8 cm. In the meantime, the ratio of spared volume to total liver volume increased gradually from 34.4% to 55.7%, then to 62.9%. Furthermore, there was visualization of the portal vein, indicating regression of the tumor thrombus. Finally, owing to the new tumor in the left lateral lobe, the patient underwent LT instead of resection without major complications.
CONCLUSION
Patients with inoperable huge HCC with PVTT could be converted to SIRT first and accept surgery sequentially.
Keywords: Hepatocellular carcinoma, Two conversions, Liver transplantation, Yttrium-90 resin microspheres, Transarterial chemoembolization, Hepatic arterial infusion chemotherapy, Case report
Core Tip: We report a patient with > 20 cm hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT) who successfully received liver transplantation (LT) after two conversions. He was first converted to selective internal radiation therapy (SIRT) eligibility with transarterial chemoembolization and infusion chemotherapy combined with systemic therapy and then converted to LT with SIRT and continued systemic therapy. This is significant because few patients with huge (> 10 cm in diameter) HCC and PVTT can receive curative treatments or curative treatments after one conversion.
INTRODUCTION
Surgical resection and liver transplantation (LT) are curative treatments for hepatocellular carcinoma (HCC) that can provide patients with long-term survival. However, because of insufficient surgical margins, insufficient future liver remnant (FLR) or portal vein tumor thrombus (PVTT), patients with huge (> 10 cm in diameter) HCC often are not eligible for these treatments[1-3]. Locoregional therapies, such as selective internal radiation therapy (SIRT), transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC), are the predominant conversion therapies and offer alternatives for downstaging treatment[4]. SIRT with yttrium-90 (90Y) is a form of brachytherapy capitalizing on targeted delivery of β-particle-emitting microspheres, with indications depending on tumor size, type and stage, as well as the patient’s condition, liver function and comorbidities[5]. The continuous advancements in treatment strategies and radiation dosimetry have paved the way for the incorporation of SIRT in all HCC stages with different intent, including palliation, downstaging, bridging or curative[6]. Classically, TACE is the treatment of choice for downstaging and bridging for HCC but there is a growing trend in favor of SIRT, especially in the intermediate stage or in unresectable HCC due to tumor size or the number of nodules[7,8]. HAIC is recommended as the preferred treatment for HCC, especially in patients with PVTT[9,10]. However, the efficacy of HAIC mainly relies on the chemosensitivity of the tumor and drugs lodged in the tumor for a short period[11]. In addition, the combination treatment of tyrosine kinase inhibitors (TKIs) with programmed death-1 (PD-1) inhibitors shows a synergistic effect that not only modulates the immune microenvironment but also promotes normal function of immunocompetent cells[12-14].
We present a case of huge HCC (20.2 cm × 19.8 cm) with PVTT who received LT after two conversions, one with drug-eluting bead (DEB)-TACE plus HAIC combined with lenvatinib and anti-PD-1 antibody sintilimab, and the other with SIRT and systemic treatments.
CASE PRESENTATION
Chief complaints
A 35-year-old man presented with emaciation and pain in the hepatic region for 1 month.
History of present illness
The patient had mild pain in the right upper quadrant that lasted for 1 month. At the same time, he was emaciated, with weight loss of 3 kg, but there was no fever, nausea, vomiting or other symptoms. After that, the patient presented to the hospital for computed tomography (CT) examination that revealed a liver tumor, and led to further treatment.
History of past illness
The patient had a history of hepatitis B without regular treatment.
Personal and family history
The patient had no specific personal medical history and there was no similar family history.
Physical examination
There was mild pressing pain in the hepatic region and other physical examinations were normal.
Laboratory examinations
Blood tests revealed a-fetoprotein (AFP) level of 279.95 ng/mL (reference range, < 8.78 ng/mL) and other results were within normal limits.
Imaging examinations
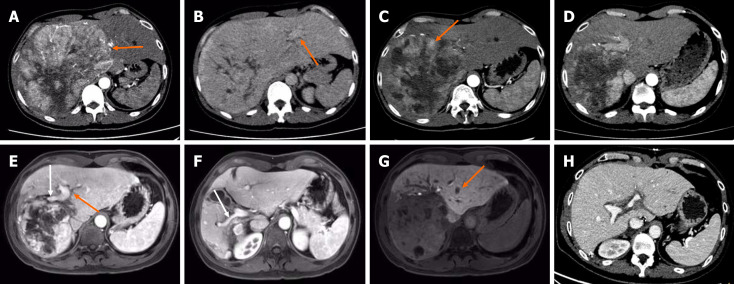
CT indicated a huge tumor measuring 20.2 cm × 19.8 cm located in the right lobe–left medial lobe of the liver (Figure 1A), with right portal vein and right hepatic vein invasion (Figure 1B).
Figure 1.
Imaging characteristics of the patient. A: The arterial phase showed tumor had marked enhancement and abundant arterial blood supply (orange arrow); B: The portal venous phase showed the visualization of the left portal vein (orange arrow), but an absence of the right portal vein and the right hepatic vein, indicating a vascular invasion. Enhanced computed tomography (CT) showed hepatocellular carcinoma located in the right-left lobe of liver at initial diagnosis; C: After 4 cycles of drug-eluting bead transarterial chemoembolization plus hepatic arterial infusion chemotherapy, the tumor size was reduced but the residual tumor was still active (orange arrow); D: Four months after the first selective internal radiation therapy (SIRT), the residual liver volume increased progressively and the tumor volume significantly decreased; E-G: Two months after the second SIRT, gadoxetic acid-enhanced magnetic resonance imaging showed a further compensatory increase in left liver volume, a decrease in tumor volume, and the visualization of the left portal vein (E, orange arrow), the right anterior portal vein branch (E, white arrow) and the right posterior portal vein branch (F, white arrow), indicating the regression of the tumor thrombus and the recanalization of the portal vein flow. However, a new tumor was found in the left liver (G, orange arrow) at hepatobiliary phase; H: Five months after transplantation, the transplanted liver was well without tumor recurrence. A-D and H were enhanced CT scan.
FINAL DIAGNOSIS
The patient was diagnosed with advanced stage HCC [Barcelona Clinic Liver Cancer (BCLC) stage C] by biopsy and imaging examination and Child–Pugh classification was A.
TREATMENT
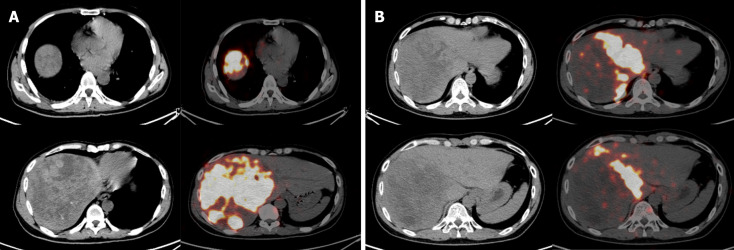
Based on huge tumor and vascular invasion, a combination of DEB-TACE, HAIC, lenvatinib and sintilimab was recommended by a multidisciplinary tumor board. Lenvatinib and sintilimab were used as described in our previous study[15]. DEB-TACE was performed in extrahepatic arteries including the right phrenic, right adrenal and left hepatic artery. HAIC was performed through the right hepatic artery as previously reported[11]. After four cycles of DEB-TACE plus HAIC, tumor size decreased from 20.2 cm × 19.8 cm to 16.2 cm × 13.8 cm as shown in follow-up imaging (Figure 1C). AFP level decreased from 279.95 ng/mL to 66.93 ng/mL. After tumor debulking and the decrease in tumor activity after combined therapy, the patient successfully reached the SIRT treatment criteria. Considering the large tumor burden, 6.0 mCi technetium-99m macroaggregated albumin was infused only in the right hepatic artery, the main tumor feeding artery, for simulation, which confirmed the absence of extrahepatic uptake and showed a lung shunt rate of 10.6%. For SIRT with resin microspheres (SIR-Spheres®, Sirtex Medical), the prescribed activity was 3.3 GBq, with estimated tumor and lung absorbed doses of 100 Gy and 15.4 Gy, respectively. Single-photon emission CT (SPECT/CT) examination after 90Y injection showed good deposition in the tumor (Figure 2A). Lenvatinib, sintilimab, and antiviral therapy were continued. Four months after SIRT, the tumor size decreased from 16.2 cm × 13.8 cm to 10.2 cm × 9.8 cm, and the left liver volume increased from 840 mL to 1675 mL, meaning hypertrophy of the FLR (from 34.4% to 54.7%) (Figure 1D).
Figure 2.
The single-photon emission/computed tomography scan after the yttrium-90 injection. A: After the first yttrium-90 (90Y) injection, the microspheres in the tumor were well distributed in the tumor; B: After the second 90Y injection, the localization of the microspheres in the tumor was as expected.
Five months after the first SIRT, a second SIRT was performed on the middle hepatic artery. The prescribed activity was 0.3 GBq, with estimated tumor and lung absorbed doses of 120 Gy and 3.3 Gy, respectively. SPECT/CT showed that 90Y resin microspheres were delivered within the targeted tissue (Figure 2B). Two months after the second SIRT, the tumor size decreased from 10.2 cm × 9.8 cm to 7.8 cm × 6.8 cm, and the ratio of FLR volume further increased from 1675 mL to 1712 mL (from 54.7% to 62.9%). There was patency of the portal venous network, indicating complete involution of PVTT (Figure 1E and F). However, a new tumor was found in the left lateral lobe of the liver (Figure 1G). Despite the excellent response to SIRT (downgrading from BCLC C to B) and decrease of AFP to normal level (3.83 ng/mL), the discovery of a new tumor in the left lateral lobe led to the change of treatment strategy from planned resection to LT according to the Hangzhou criteria[16]. The explant showed a tumor with a maximum diameter of 10.5 cm in the right lobe of the liver with a small volume of viable tumor tissue, and a tumor nodule (1.8 cm in maximum diameter) in the left lateral lobe.
OUTCOME AND FOLLOW-UP
Follow-up imaging 5 months after transplantation demonstrated no evidence of recurrence (Figure 1H). The follow-up is ongoing.
DISCUSSION
LT remains the most effective curative treatment option for HCC, since it treats liver cirrhosis and reduces the risk of new HCC lesions[17]. Only about 30% of patients are suitable for curative procedures like LT at the time of diagnosis of HCC[18]. Even though several expanded criteria for HCC beyond the Milan criteria have been proposed, many patients still need downstaging to be eligible for LT. In addition, HCC complicated with PVTT has a poor prognosis, which is present in 10%–40% of patients at the time of diagnosis[19,20].
SIRT with 90Y microspheres has emerged as a viable treatment option with a favorable benefit/risk ratio, both as a stand-alone therapy and in conjunction with systemic or regional therapy. Downstaging through 90Y-SIRT in patients beyond surgical resection or transplantation criteria may lead to tumor shrinkage, residual hepatic hyperplasia, and even PVTT regression, enabling surgical resection or LT[21,22]. Seven studies have reported on 90Y-SIRT downstaging of HCC, and pooled analyses showed that 55 of 204 patients were finally transplanted[23]. A 15-year single-center experience showed that 13 patients underwent transplantation following 90Y-SIRT, with 10-year overall and disease-free survival rates of 51.3% and 43.3%, respectively[24]. A multicenter study reported that, for HCC patients with segmental or lobar PVTT, the median OS of the 90Y-SIRT and TKIs groups were 28.2 months and 7.2 months, respectively[25]. However, due to limitations such as excessive tumor load and higher 90Y dose, some patients are not suitable for SIRT initially. It may be that we can perform conversion therapy first to meet the requirements of SIRT.
In recent years, interventional therapy based on TACE and HAIC has been widely recognized[26]. Several studies have reported that various combination therapies can significantly improve the rate of salvage surgery after tumor downstaging in advanced HCC[11,12,27]. In a systematic review, 741 patients in 15 studies received the triple combination of TACE/HAIC, TKIs and immune checkpoint inhibitors, which showed that the conversion rate was 35.9%[28]. Furthermore, the combination therapies may provide more benefits for HCC patients complicated with PVTT[29]. A retrospective study explored the efficacy of TACE–HAIC combined with TKIs or PD-1 (n = 139) vs TACE (n = 604) in patients with HCC and PVTT. The overall response rate was significantly higher in the combination group than in the TACE group (42.1% vs 5.0%)[19].
Here, we report a case of LT after two conversions for a > 20 cm HCC with PVTT. Due to insufficient FLR, the patient was initially unable to undergo surgery. 90Y-SIRT might be a promising conversion treatment, but a high lung shunt might be of concern. The median lung shunt fraction (LSF) in patients with HCC was reported to be 11.47%[30]. Tumor size > 11 cm and hepatic vein invasion are independent predictors for high LSF > 20%[31]. We did not choose 90Y-SRT at the beginning of the treatment because the tumor was > 11 cm with hepatic vein invasion, which predicted high LSF. If 90Y-SIRT was performed with a target tumor dose of 120 Gy and 20% LSF initially, the lung absorbed a dose of 67.73 Gy which would have exceeded the maximum acceptable dose of 30 Gy[31]. Even assuming 10% LSF, the lung absorbed dose would be 30.1 Gy. Therefore, we conducted DEB-TACE plus HAIC along with lenvatinib and sintilimab as the first conversion. After four cycles of DEB-TACE plus HAIC, the tumor size declined from 20.2 cm × 19.8 cm to 16.2 cm × 13.8 cm with weaker tumor activity. Considering that the tumor was still large, the patient was treated with sequential 90Y-SIRT. During the first SIRT, the 90Y resin microspheres were injected only at the major tumor blood supply, the right hepatic artery. The second SIRT was conducted after the tumor shrank notably after the first SIRT. Finally, because a new tumor appeared in the left lateral lobe, the patient was treated with LT instead of resection.
CONCLUSION
The present case has important clinical implications for altering workflow in clinical practice, suggesting that inoperable huge HCC with PVTT could be converted to meet SIRT criteria firstly by the combination of locoregional therapies and systemic therapy, then to curative treatment. The possibility of combining therapies is intriguing, as it could theoretically change the therapeutic perspectives and lead to a higher rate of objective response[32]. This scenario will improve with the availability of the combination of interventional therapies (TACE/HAIC/SIRT) and systemic treatment, which is demonstrated in our case. Furthermore, this strategy may be a promising modality, prompting further research with large sample sizes and comparative analysis with other treatment approaches. This case provides an excellent approach to the treatment of patients with huge HCC and PVTT, which may guide clinicians in adopting strategic conversion therapies to similar cases in clinical practice.
Footnotes
Informed consent statement: Informed Written consent was obtained from the patient. This case was approved by the Institutional Review Board of the Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong Province, China (No. 2024-hg-ks-04).
Conflict-of-interest statement: All the authors have no relevant conflicts of interest to declare for this article.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade C
P-Reviewer: Lei E; Yan X S-Editor: Luo ML L-Editor: A P-Editor: Xu ZH
Contributor Information
Li-Cong Liang, Department of Minimally Invasive Interventional Radiology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Wen-Sou Huang, Department of Minimally Invasive Interventional Radiology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Zhao-Xiong Guo, Department of Minimally Invasive Interventional Radiology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Hong-Ji You, Department of Nuclear Medicine, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Yong-Jian Guo, Department of Minimally Invasive Interventional Radiology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Ming-Yue Cai, Department of Minimally Invasive Interventional Radiology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Li-Teng Lin, Department of Minimally Invasive Interventional Radiology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Guo-Ying Wang, Department of Hepatobiliary Surgery, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou 510120, Guangdong Province, China.
Kang-Shun Zhu, Department of Minimally Invasive Interventional Radiology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China. zhksh010@163.com.
References
- 1.Yang B, Li CL, Guo WH, Qin TQ, Jiao H, Fei ZJ, Zhou X, Duan LJ, Liao ZY. Intra-arterial ethanol embolization augments response to TACE for treatment of HCC with portal venous tumor thrombus. BMC Cancer. 2018;18:101. doi: 10.1186/s12885-018-3989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakayama K, Kamiyama T, Yokoo H, Orimo T, Shimada S, Einama T, Kamachi H, Taketomi A. Huge hepatocellular carcinoma greater than 10 cm in diameter worsens prognosis by causing distant recurrence after curative resection. J Surg Oncol. 2017;115:324–329. doi: 10.1002/jso.24501. [DOI] [PubMed] [Google Scholar]
- 3.Hao Y, Xie F, Zhou Y, Li C, Zhang X, Shen J, Yao M, Sun X, Zhou J, Wen T, Peng W. Neoadjuvant therapy of sequential TACE, camrelizumab, and apatinib for single huge hepatocellular carcinoma (NEO-START): study protocol for a randomized controlled trial. Trials. 2024;25:490. doi: 10.1186/s13063-024-08340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatzidakis A, Müller L, Krokidis M, Kloeckner R. Local and Regional Therapies for Hepatocellular Carcinoma and Future Combinations. Cancers (Basel) 2022;14:2469. doi: 10.3390/cancers14102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramdhani K, Lam MGEH, Braat AJAT, Smits MLJ, El-Haddad G. Hepatic Radioembolization: A Multistep Theragnostic Procedure. PET Clin. 2024;19:431–446. doi: 10.1016/j.cpet.2024.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Qadan M, Fong ZV, Delman AM, Gabr A, Salem R, Shah SA. Review of Use of Y90 as a Bridge to Liver Resection and Transplantation in Hepatocellular Carcinoma. J Gastrointest Surg. 2021;25:2690–2699. doi: 10.1007/s11605-021-05095-x. [DOI] [PubMed] [Google Scholar]
- 7.Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol. 2017;67:173–183. doi: 10.1016/j.jhep.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Arar A, Heglin A, Veluri S, Alnablsi MW, Benjamin JL, Choudhary M, Pillai A. Radioembolization of HCC and secondary hepatic tumors: a comprehensive review. Q J Nucl Med Mol Imaging. 2024 doi: 10.23736/S1824-4785.24.03572-6. [DOI] [PubMed] [Google Scholar]
- 9.Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 10.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Huang W, Zhan M, Guo Y, Liang L, Cai M, Lin L, He M, Lian H, Lu L, Zhu K. Drug-Eluting Bead Transarterial Chemoembolization Combined with FOLFOX-Based Hepatic Arterial Infusion Chemotherapy for Large or Huge Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:1445–1458. doi: 10.2147/JHC.S339379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan L, Lang M, Tian X, Ren S, Li G, Liu Y, Han R, Zhu K, Li H, Wu Q, Cui Y, Zhang W, Fang F, Li Q, Song T. A Retrospective Analysis of Conversion Therapy with Lenvatinib, Sintilimab, and Arterially-Directed Therapy in Patients with Initially Unresectable Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2023;10:673–686. doi: 10.2147/JHC.S404675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Tong S, Hu B, Wan T, Tang H, Zhao F, Jiao T, Li J, Zhang Z, Cai J, Ye H, Wang Z, Chen S, Wang Y, Li X, Wang F, Cao J, Tian L, Zhao X, Chen M, Wang H, Cai S, Hu M, Bai Y, Lu S. Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J Immunother Cancer. 2023;11:e007366. doi: 10.1136/jitc-2023-007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanini B, Ielasi L, Chen R, Abbati C, Tonnini M, Tovoli F, Granito A. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2023;23:279–291. doi: 10.1080/14737140.2023.2181162. [DOI] [PubMed] [Google Scholar]
- 15.Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, Zhou J, Lin L, Cao B, Chen Y, Zhou J, Zhu K. Transarterial Chemoembolization Combined With Lenvatinib Plus PD-1 Inhibitor for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study. Front Immunol. 2022;13:848387. doi: 10.3389/fimmu.2022.848387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 17.Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115–4127. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Titano J, Voutsinas N, Kim E. The Role of Radioembolization in Bridging and Downstaging Hepatocellular Carcinoma to Curative Therapy. Semin Nucl Med. 2019;49:189–196. doi: 10.1053/j.semnuclmed.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, He W, Yang Z, Qiu J, Huang Z, Shi Y, Lin Z, Zheng Y, Chen M, Lau WY, Li B, Yuan Y. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg. 2023;109:1222–1230. doi: 10.1097/JS9.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Zhao M, Qi X, Tang Y, Cheng S. Mechanisms of portal vein tumour thrombus formation and development in patients with hepatocellular carcinoma. J Cell Mol Med. 2023;27:2103–2111. doi: 10.1111/jcmm.17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller FH, Lopes Vendrami C, Gabr A, Horowitz JM, Kelahan LC, Riaz A, Salem R, Lewandowski RJ. Evolution of Radioembolization in Treatment of Hepatocellular Carcinoma: A Pictorial Review. Radiographics. 2021;41:1802–1818. doi: 10.1148/rg.2021210014. [DOI] [PubMed] [Google Scholar]
- 22.Somma F, Stoia V, Serra N, D'Angelo R, Gatta G, Fiore F. Yttrium-90 trans-arterial radioembolization in advanced-stage HCC: The impact of portal vein thrombosis on survival. PLoS One. 2019;14:e0216935. doi: 10.1371/journal.pone.0216935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Lopez V, Miura K, Kuemmerli C, Capel A, Eshmuminov D, Ferreras D, Baroja-Mazo A, Cascales-Campos P, Jiménez-Mascuñán MI, Pons JA, Castellon MI, Sánchez-Bueno F, Robles-Campos R, Ramírez P. Selecting the Appropriate Downstaging and Bridging Therapies for Hepatocellular Carcinoma: What Is the Role of Transarterial Radioembolization? A Pooled Analysis. Cancers (Basel) 2023;15:2122. doi: 10.3390/cancers15072122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aliseda D, Martí-Cruchaga P, Zozaya G, Rodríguez-Fraile M, Bilbao JI, Benito-Boillos A, Martínez De La Cuesta A, Lopez-Olaondo L, Hidalgo F, Ponz-Sarvisé M, Chopitea A, Rodríguez J, Iñarrairaegui M, Herrero JI, Pardo F, Sangro B, Rotellar F. Liver Resection and Transplantation Following Yttrium-90 Radioembolization for Primary Malignant Liver Tumors: A 15-Year Single-Center Experience. Cancers (Basel) 2023;15:733. doi: 10.3390/cancers15030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hur MH, Cho Y, Kim DY, Lee JS, Kim GM, Kim HC, Sinn DH, Hyun D, Lee HA, Seo YS, Lee IJ, Park JW, Kim YJ. Transarterial radioembolization versus tyrosine kinase inhibitor in hepatocellular carcinoma with portal vein thrombosis. Clin Mol Hepatol. 2023;29:763–778. doi: 10.3350/cmh.2023.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JJ, Jin ZC, Zhong BY, Fan W, Zhang WH, Luo B, Wang YQ, Teng GJ, Zhu HD. Locoregional therapies for hepatocellular carcinoma: The current status and future perspectives. United European Gastroenterol J. 2024;12:226–239. doi: 10.1002/ueg2.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen B, Dai H, Yang J, Zhang G, Wen C, Xiang X, Lin R, Huang Y. Transarterial Chemoembolization Followed by Hepatic Arterial Infusion Chemotherapy Combined a Tyrosine Kinase Inhibitor for Treatment of Large Hepatocellular Carcinoma. Curr Cancer Drug Targets. 2023;23:564–571. doi: 10.2174/1568009623666230215142941. [DOI] [PubMed] [Google Scholar]
- 28.Ke Q, Xin F, Fang H, Zeng Y, Wang L, Liu J. The Significance of Transarterial Chemo(Embolization) Combined With Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors for Unresectable Hepatocellular Carcinoma in the Era of Systemic Therapy: A Systematic Review. Front Immunol. 2022;13:913464. doi: 10.3389/fimmu.2022.913464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung JH, Wang SY, Leung HWC, Chan ALF. Comparative efficacy and safety of multimodality treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: patient-level network meta-analysis. Front Oncol. 2024;14:1344798. doi: 10.3389/fonc.2024.1344798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing M, Lahti S, Kokabi N, Schuster DM, Camacho JC, Kim HS. 90Y Radioembolization Lung Shunt Fraction in Primary and Metastatic Liver Cancer as a Biomarker for Survival. Clin Nucl Med. 2016;41:21–27. doi: 10.1097/RLU.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 31.Choi TW, Joo I, Kim HC. Association of dysmorphic intratumoral vessel with high lung shunt fraction in patients with hepatocellular carcinoma. Sci Rep. 2022;12:14248. doi: 10.1038/s41598-022-18697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudo M. A Paradigm Change in the Treatment Strategy for Hepatocellular Carcinoma. Liver Cancer. 2020;9:367–377. doi: 10.1159/000507934. [DOI] [PMC free article] [PubMed] [Google Scholar]