Abstract
Cynomolgus macaques (Macaca fascicularis) infected with influenza virus A/Hong Kong/156/97 (H5N1) developed acute respiratory distress syndrome and fever associated with a necrotizing interstitial pneumonia. Reverse transcription PCR, virus isolation, and immunohistochemistry showed that the respiratory tract is the major target of the virus.
Influenza A virus infections are an important cause of disease in humans and several animal species, including birds, pigs, and horses. It is well documented that aquatic birds are the reservoir for the introduction of new subtypes into the human population (26). Three influenza A virus subtypes (H1N1, H2N2, H3N2) with hemagglutinin and neuraminidase gene segments of avian origin have been associated with pandemic outbreaks and annually recurring disease in humans in the past century.
In 1997, influenza A viruses of the H5N1 subtype were isolated from patients in the Hong Kong area (3, 5, 25). These viruses showed >99% homology with avian influenza viruses that had caused mass mortality in chicken farms in the same area (3, 14, 22, 24). Although infection with influenza H5N1 viruses proved to have a subclinical course in some exposed individuals (1, 12), 18 infected individuals were hospitalized upon developing severe clinical symptoms. The main clinical presentation of the infection in these patients was a severe primary viral pneumonia (27), which was fatal in 6 of the 18 cases. It was speculated that the high pathogenicity was caused by a wider tissue tropism of influenza A (H5N1) viruses (27), as had been found in chickens (24). Although mouse models have provided insight into the pathogenesis of influenza A (H5N1) viruses in a mammalian host (6, 9, 10, 13), this cannot be extrapolated to humans directly. Therefore, we decided to study the pathogenesis of influenza A (H5N1) virus infection in primates. To this end, we infected cynomolgus macaques (Macaca fascicularis) with influenza virus A/HongKong/156/97 (H5N1), the isolate obtained from the index case of the 1997 outbreak in Hong Kong (2, 5).
This virus was propagated three times in tertiary monkey kidney (tMK) cells obtained from cynomolgus macaques, and subsequently a virus stock was prepared in Madin Darby Canine Kidney (MDCK) cells (passage MK3MDCK2) as previously described (21). All experiments were conducted under biosafety level 3 conditions.
Five days before infection, four adolescent, colony-bred cynomolgus macaques were placed in a negatively pressurized glove box in pairs of one male and one female. The monkeys were infected with 2.5 × 104 50% tissue culture infective dose (TCID50) of influenza virus A/HK/156/97, which was suspended in 5 ml of phosphate buffered saline. Four milliliters was applied intratracheally, 0.5 ml on the tonsils and 0.25 ml on each of the conjunctivas. Just before infection and on days 3 and 5 postinfection (p.i.), the monkeys were anesthetized with ketamine and 10 ml of EDTA-treated blood was collected from an inguinal vein. From two monkeys (no. 3 and no. 4), nasal swabs (NS), pharyngeal swabs (PS), and bronchoalveolar lavage specimens (BAL) were collected on the time points p.i. for virological analyses. The two other monkeys (no. 1 and no. 2) were not intubated at any time before or during the experiment. They were euthanatized on day 4 p.i., and monkeys no. 3 and no. 4 were euthanatized on day 7 p.i. by exsanguination under ketamine anesthesia.
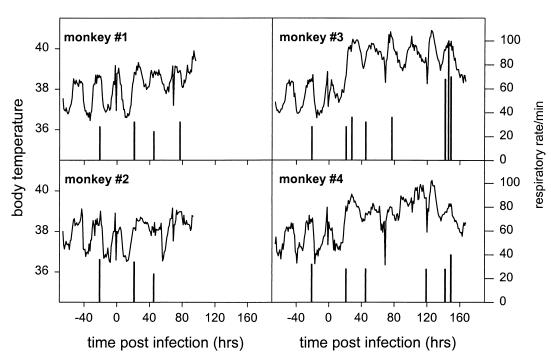
From 5 days before infection onwards, the monkeys were checked for the presence of clinical signs by a veterinarian every day. The body temperatures of the monkeys were measured by telemetry (IMAG, Wageningen, The Netherlands) every 3 min. To this end, a transponder was placed in their abdomens 30 days before infection. The respiratory rate was determined visually at regular intervals. Three of the four monkeys developed a fever within the first 2 days p.i. (Fig. 1). Monkeys no. 3 and no. 4 developed a fever of >40°C 24 and 106 h p.i., respectively. During the late stage of the infection, monkey no. 3 showed signs of acute respiratory distress syndrome. The respiratory rate of this monkey increased from 30 to 100. This monkey also became lethargic, lost its appetite, developed cyanotic ear tips, and was coughing on days 6 and 7 p.i. The clinical signs—fever and severe respiratory illness—in these monkeys were the most frequently presenting clinical signs observed in humans infected with avian H5N1 virus (27). The experimental influenza virus disease in monkeys was more severe with strain A/HK/156/97 (H5N1) than with a human influenza virus A (H3N2) strain previously tested (20). Forty-eight cynomolgus macaques had been infected by the intratracheal route with cell culture-passaged influenza virus A/Netherlands/18/94 (H3N2) with a dose 400 times higher than the dose of A/HK/156/97 that was used. Of these monkeys, 12 were not vaccinated against influenza or treated with anti-influenza virus drugs at the time of infection; none of these 12 animals developed clinical signs, although the virus replicated in the respiratory tract to moderate titers.
FIG. 1.
Recordings of body temperatures (lines) and respiratory rates (bars) of cynomolgus macaques upon intratracheal infection with 2.5 × 104 TCID50 of influenza virus A/HK/156/97. Body temperatures were recorded by telemetry using a transponder which was brought in 30 days prior to the infection. Respiratory rates were monitored by observing the animals without disturbing them.
Necropsies were carried out according to a standard protocol. Samples for virological examination were stored at −70°C, and samples for histological examination were stored in 10% neutral-buffered formalin. Tissue samples of trachea, lung, heart, spleen, liver, kidney, cerebellum, cerebrum, and tracheobronchial lymph nodes (TBLN) were homogenized in minimal essential medium containing 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 4% bovine serum albumin (fraction V; Gibco-BRL), and 4 μg of trypsin (Gibco-BRL) (infection medium) using Potter tissue grinders. After low-speed centrifugation, the infectious virus titers of the homogenized tissue samples, PS, NS, and BAL specimens were determined and expressed as TCID50/gram of tissue or as TCID50/milliliter as described previously (19). The identity of the virus that was isolated was confirmed in a hemagglutination inhibition assay using a rabbit antiserum directed against influenza virus A/Duck/HongKong/205/77 (H5N3) using standard procedures (18). In addition, a reverse transcription (RT)-PCR was performed on homogenized tissue samples with primers derived from conserved regions in the matrix gene segment as described previously (8). After analysis by agarose gel electrophoresis and ethidium bromide staining, Southern blot hybridization was carried out with a biotin-labeled probe for confirmation (8).
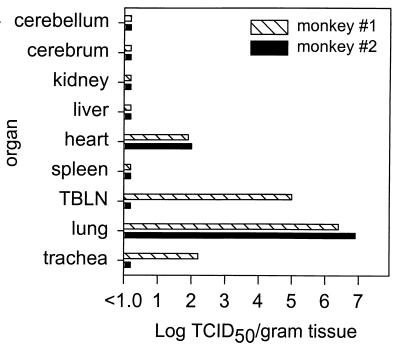
High virus titers (up to 107 TCID50/g of tissue) were demonstrated in the lungs of the two monkeys, which had been euthanatized 4 days p.i. (Fig. 2). From the trachea and the TBLN of monkey no. 1 infectious virus was isolated, and in the heart tissues of monkeys no. 1 and no. 2 infectious virus proved to be present. Since virus-infected cells were not demonstrated in heart tissue (see below), it cannot be excluded that this organ was contaminated with virus originating from the lungs, which were severely damaged as a result of the infection. In the organs of monkeys no. 3 and no. 4, which were euthanatized 7 days p.i., no virus could be isolated from any of the organs. Influenza virus A/HK/156/97 was readily isolated from the BAL specimens obtained from monkeys no. 3 and no. 4 on days 3 and 5 p.i., from the PS obtained from both monkeys on day 5 p.i., and from the NS obtained on days 3 and 7 p.i. from monkey no. 4 (data not shown). The results obtained with the virus isolation procedures were confirmed by RT-PCR: all samples that were positive by virus isolation were also found to be positive by RT-PCR (Table 1). In addition, some tissue samples that were negative by virus isolation tested positive by PCR. The cerebellums of monkeys no. 1 and no. 2 and the cerebrum of monkey no. 2 were positive by RT-PCR (Table 1). Trachea, lung, and TBLN samples obtained from the monkeys that were euthanatized on day 7 p.i. were still positive by RT-PCR but were negative by virus isolation. Furthermore, positive results were obtained with the plasma of monkey no. 4 collected 7 days p.i. and the spleens of all four monkeys. This indicates that virus or virus-infected cells were taken up in the bloodstream and subsequently trapped in the spleen. Liver and kidney tissues tested negative by virus isolation and RT-PCR (Table 1).
FIG. 2.
Infectious virus titers in the indicated organs obtained from monkeys no. 1 (hatched bars) and no. 2 (black bars) 4 days p.i. The results are expressed as TCID50 per gram of organ tissue.
TABLE 1.
Detection of influenza virus in tissue samples obtained from monkeys infected with influenza virus A/HK/156/97 by different methodsa
| Monkey no. | Dpi | Trachea
|
Lung
|
TBLN
|
Tonsil
|
Spleen
|
Heart
|
Liver
|
Kidney
|
Cerebellum
|
Cerebrum
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VI | PCR | IHC | VI | PCR | IHC | VI | PCR | IHC | VI | PCR | IHC | VI | PCR | IHC | VI | PCR | IHC | VI | PCR | IHC | VI | PCR | IHC | VI | PCR | IHC | VI | PCR | IHC | ||
| 1 | 4 | + | + | − | + | + | + | + | + | − | ND | ND | − | − | + | − | + | + | − | − | − | − | − | − | − | − | + | − | − | − | − |
| 2 | 4 | − | + | − | + | + | + | − | + | − | ND | ND | − | − | + | − | + | + | − | − | − | − | − | − | − | − | + | − | − | + | − |
| 3 | 7 | − | + | − | − | + | + | − | + | − | ND | ND | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 4 | 7 | − | + | − | − | + | + | − | + | − | ND | ND | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
VI, virus isolation; IHC, immunohistochemistry; Dpi, days postinfection; ND, not done.
The formalin-fixed tissue samples were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin or with an avidin-biotin complex immunoperoxidase method (11), using a monoclonal antibody (HB-65; American Type Culture Collection) to the nucleoprotein of influenza virus A. As a secondary antibody a biotin-labeled goat antibody preparation directed against mouse immunoglobulin G (Lab Vision, Fremont, Calif.) was used. After incubation with avidin-peroxidase, diamino-benzidine was used as a substrate to produce a dark brown precipitate. In addition, lung samples were stained with the Gram stain for the presence of bacteria. The monkeys euthanatized at 4 days p.i. had focal pulmonary consolidation, involving the right medial lobe in monkey no. 2 and the left caudal lobe in monkey no. 1. The monkeys euthanatized at 7 days p.i. had extensive pulmonary consolidation in both lungs with a cranioventral distribution. Histologically, the lesions in all four monkeys were diagnosed as a necrotizing bronchointerstitial pneumonia, with extensive loss of alveolar and bronchiolar epithelium, and flooding of the alveoli with edema fluid, fibrin, erythrocytes, cell debris, neutrophils, and macrophages. In addition, monkey no. 4 had a large area of necrosis in the central part of the right lung. Histologically, this consisted of a well-demarcated area of coagulation necrosis, which has been recorded in some cases on influenza A virus pneumonia in pigs and humans (7, 16), and may be due to a compromised blood supply due to vascular damage and thrombosis. Lesions in tissues outside the respiratory tract consisted of a mild suppurative tonsillitis in monkeys no. 3 and no. 4, extensive lymphocytic necrosis in spleen and lymph nodes in monkey no. 4, and multifocal renal tubular necrosis in no. 4. No bacteria were seen in the hematoxylin- and eosin- or Gram-stained sections of pulmonary tissue in any of the monkeys, excluding a possible involvement of secondary bacterial infections (23). The lung damage caused by influenza viral infection causes hypoxia and clinical signs of acute respiratory distress syndrome (4), which were most apparent in monkey no. 3. Although the increased pulmonary damage in monkeys no. 3 and no. 4 compared to that in monkeys no. 1 and no. 2 indicates that infection with influenza virus A/HK/156/97 caused a progressive pneumonia in these monkeys, it cannot be excluded that the BALs performed in monkeys no. 3 and no. 4 influenced the natural course of the infection by spreading the virus more efficiently in the lungs or by abrading the lung mucosa.
Positive staining by immunohistochemistry in tissues of the monkeys euthanatized at 4 days p.i. was limited to the respiratory tract. The cell types involved were alveolar macrophages, type 1 and type 2 pneumocytes, bronchiolar and bronchial epithelial cells, neutrophils, and unidentified mononuclear cells (Fig. 3). This corresponds to the cell predilection of influenza virus in human infection (15). In the monkeys euthanatized at 7 days p.i., positive staining was also found in the tonsils, where sloughed epithelial cells in the crypts and dendritic cells in some germinal centers stained positive. In the respiratory tract, fewer cells stained positive and the staining was weaker than at 4 days p.i., which corresponded to the failure to isolate influenza virus from pulmonary tissue at 7 days p.i. Furthermore, virus was isolated from the BAL sample at days 3 and 5 p.i. only and not at day 7 p.i., indicating that the peak of virus replication was around day 4 p.i.
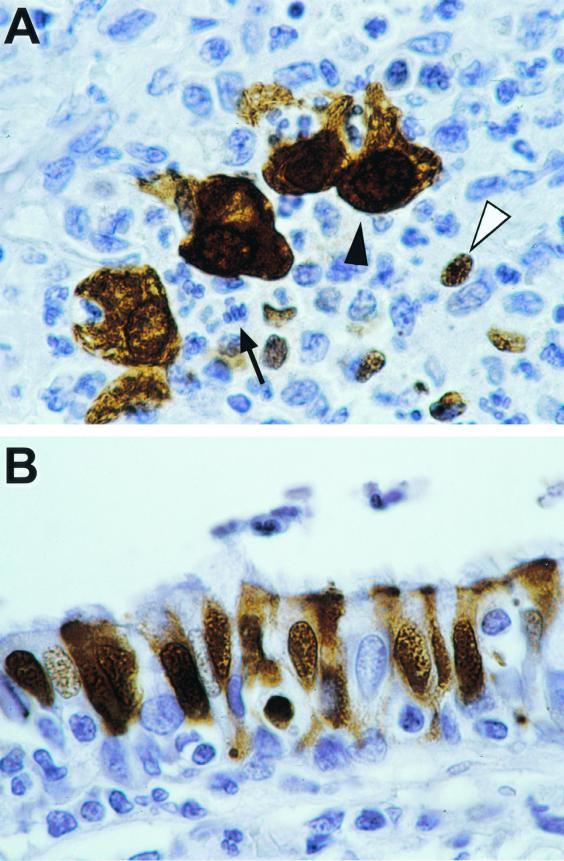
FIG. 3.
Demonstration by immunohistochemistry of virus-infected cells in the respiratory tract of monkey no. 1 4 days after intratracheal infection with 2.5 × 104 TCID50 of influenza virus A/HK/156/97. Specifically stained cells have a dark brown granular precipitate in the nucleus and cytoplasm. Hematoxylin counterstain was used. Original magnification, ×100. (A) Lung with bronchointerstitial pneumonia. The alveolar lumina are not apparent due to flooding with inflammatory cells. There is specific staining for influenza virus nucleoprotein of alveolar macrophages (black arrowhead) and small mononuclear cells (white arrowhead), while most neutrophils (black arrow) do not show specific staining. (B) Bronchus. There is specific staining for influenza virus nucleoprotein in bronchial epithelial cells.
No infected cells were demonstrated in the spleen, heart, or brains by immunohistochemistry in any of these animals. Thus, immunohistochemistry revealed that, in contrast to the situation with mice, the virus did not replicate outside the respiratory tract in the monkeys. In mouse models, influenza virus A/HK/156/97 was found by two studies (10, 13) but not by another (9) to replicate in the brains. In contrast, influenza virus A/HK/483/97 (H5N1) was consistently found to replicate in heart and brain tissues of experimentally infected mice. Recently it was shown in ddY mice that human influenza A (H5N1) viruses can replicate in adipose tissue (17).
In the cynomolgus macaques, however, no evidence was found by immunohistochemistry that influenza virus A/HK/156/97 was adipotropic. In some human cases gastrointestinal manifestations or liver dysfunction were found, and it has been suggested that these clinical symptoms were caused by a wider tissue tropism of H5N1 viruses (27). These symptoms, however, were found in only 33 and 60% of the cases, respectively. Furthermore, definitive evidence of virus replication in organs other than the respiratory tract of humans is still lacking.
Collectively, our data show that infection of cynomolgus macaques with influenza virus A/HK/156/97 leads to viral replication in the respiratory tract resulting in severe lung damage. Since the clinical signs in the cynomolgus macaques resembled those found in humans infected with the avian influenza H5N1 viruses, we conclude that infection of cynomolgus macaques with influenza H5N1 viruses may serve as a model for these infections in humans. It should, however, be realized that due to ethical and practical constraints there is a limit to the number of animals that can be used. The model may be used to study factors involved in the pathogenesis of these influenza A viruses, and it would be of interest to test in cynomolgus macaques different influenza H5N1 viruses with different pathogenic potential for mice. Furthermore, the model will be useful for the evaluation of novel vaccines and antiviral drugs against these viruses.
Acknowledgments
We thank Frida van der Ham for excellent technical assistance and Ger van der Water for continuous support.
This work was supported by the Foundation for Respiratory Virus Infections, Notably Influenza (SRVI). R.A.M.F. is a fellow of the Royal Dutch Academy of Arts and Science.
REFERENCES
- 1.Buxton Bridges C, Katz J M, Seto W H, Chan P K, Tsang D, Ho W, Mak K H, Lim W, Tam J S, Clarke M, Williams S G, Mounts A W, Bresee J S, Conn L A, Rowe T, Hu-Primmer J, Abernathy R A, Lu X, Cox N J, Fukuda K. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181:344–348. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 2.Claas E C, de Jong J C, van Beek R, Rimmelzwaan G F, Osterhaus A D. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine. 1998;16:977–978. doi: 10.1016/s0264-410x(98)00005-x. [DOI] [PubMed] [Google Scholar]
- 3.Claas E C, Osterhaus A D, van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 4.Craighead J E. Pathology and pathogenesis of human viral disease. San Diego, Calif: Academic Press; 2000. Influenza viruses; pp. 35–46. [Google Scholar]
- 5.de Jong J C, Claas E C, Osterhaus A D, Webster R G, Lim W L. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dybing J K, Schultz-Cherry S, Swayne D E, Suarez D L, Perdue M L. Distinct pathogenesis of Hong Kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses. J Virol. 2000;74:1443–1450. doi: 10.1128/jvi.74.3.1443-1450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finckh E S, Bader L. Pulmonary damage from Hong Kong influenza. Aust N Z J Med. 1974;4:16–22. doi: 10.1111/j.1445-5994.1974.tb03140.x. [DOI] [PubMed] [Google Scholar]
- 8.Fouchier R A M, Bestebroer T M, Herfst S, van der Kemp L, Rimmelzwaan G F, Osterhaus A D M E. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J Clin Microbiol. 2000;38:4096–4101. doi: 10.1128/jcm.38.11.4096-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley A J, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gubareva L V, McCullers J A, Bethell R C, Webster R G. Characterization of influenza A/HongKong/156/97 (H5N1) virus in a mouse model and protective effect of zanamivir on H5N1 infection in mice. J Infect Dis. 1998;178:1592–1596. doi: 10.1086/314515. [DOI] [PubMed] [Google Scholar]
- 11.Haines D M, Chelack B J. Technical considerations for developing enzyme immunohistochemical staining procedures on formalin-fixed paraffin-embedded tissues for diagnostic pathology. J Vet Diagn Investig. 1991;3:101–112. doi: 10.1177/104063879100300128. [DOI] [PubMed] [Google Scholar]
- 12.Katz J M, Lim W, Bridges C B, Rowe T, Hu-Primmer J, Lu X, Abernathy R A, Clarke M, Conn L, Kwong H, Lee M, Au G, Ho Y Y, Mak K H, Cox N J, Fukuda K. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180:1763–1770. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 13.Lu X, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J M. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulder J, Hers J F. Influenza. Groningen, The Netherlands: Wolters-Noordhoff; 1979. [Google Scholar]
- 16.Nayak D P, Twiehaus M J, Kelley G W, Underdahl N R. Immunocytologic and histopathologic development of experimental swine influenza infection in pigs. Am J Vet Res. 1965;26:1271–1283. [PubMed] [Google Scholar]
- 17.Nishimura H, Itamura S, Iwasaki T, Kurata T, Tashiro M. Characterization of human influenza A (H5N1) virus infection in mice: neuro-, pneumo- and adipotropic infection. J Gen Virol. 2000;81:2503–2510. doi: 10.1099/0022-1317-81-10-2503. [DOI] [PubMed] [Google Scholar]
- 18.Palmer D F, Dowdle W R, Coleman M T, Schild G C. Advanced laboratory techniques for influenza diagnosis: procedural guide. Atlanta, Ga: U.S. Department of Health, Education and Welfare; 1975. Hemagglutination-inhibition test; pp. 25–62. [Google Scholar]
- 19.Rimmelzwaan G F, Baars M, Claas E C, Osterhaus A D. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods. 1998;74:57–66. doi: 10.1016/s0166-0934(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 20.Rimmelzwaan G F, Baars M, van Beek R, van Amerongen G, Lövgren-Bengtsson K, Claas E C, Osterhaus A D. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997;78:757–765. doi: 10.1099/0022-1317-78-4-757. [DOI] [PubMed] [Google Scholar]
- 21.Rimmelzwaan G F, Claas E C, van Amerongen G, de Jong J C, Osterhaus A D. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine. 1999;17:1355–1358. doi: 10.1016/s0264-410x(98)00390-9. [DOI] [PubMed] [Google Scholar]
- 22.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti K G, Norwood M, Senne D, Sims L, Takada A, Webster R G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 23.Soto P J, Broun G O, Wyatt J P. Asian influenza pneumonitis. A structural and virologic analysis. Am J Med. 1959;27:18–25. doi: 10.1016/0002-9343(59)90057-9. [DOI] [PubMed] [Google Scholar]
- 24.Suarez D L, Perdue M L, Cox N, Rowe T, Bender C, Huang J, Swayne D E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 26.Webster R G, Bean W J, Gorman O T, Chambers T M, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuen K Y, Chan P K, Peiris M, Tsang D N, Que T L, Shortridge K F, Cheung P T, To W K, Ho E T, Sung R, Cheng A F. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]