Abstract
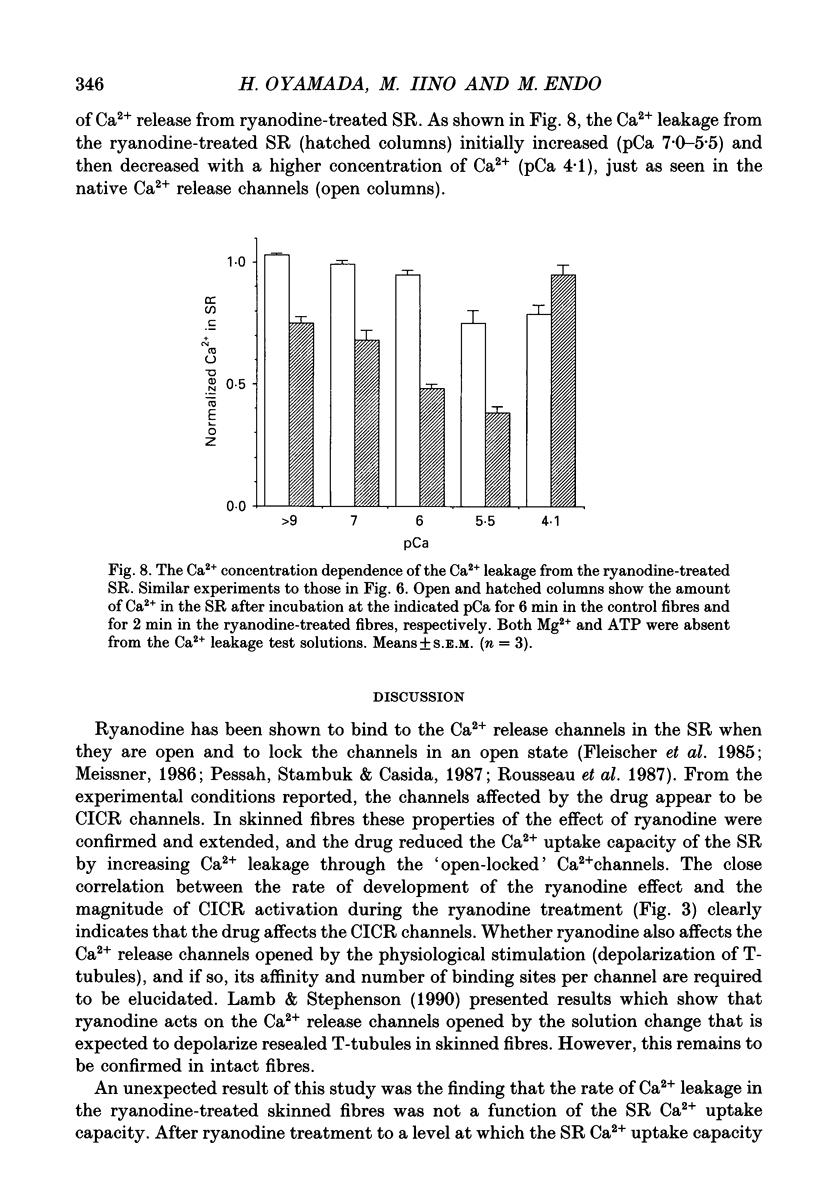
1. We studied the effects of ryanodine on the functions of the sarcoplasmic reticulum (SR) in skinned muscle fibres from Xenopus laevis. 2. Ryanodine treatment decreased the Ca2+ uptake capacity of the SR in a fixed Ca2+ loading condition. The extent of the decrease in the SR Ca2+ uptake capacity was closely correlated with the activity of the Ca(2+)-induced Ca2+ release (CICR) during the ryanodine treatment. This suggests, in agreement with the previous biochemical results, that ryanodine acts on the CICR channels when they are open. 3. The rate of Ca2+ leakage from the SR increased after ryanodine treatment. However, the leakage rate constants were independent of the degree of the loss of SR Ca2+ uptake capacity by the ryanodine treatment. This is inconsistent with the notion that the SR is a single uniform compartment and that the decline in the SR Ca2+ uptake capacity is a result of the increase in the Ca2+ leakage. 4. Partial recovery of the Ca2+ uptake capacity of the ryanodine-treated SR was observed when Ca2+ loading was carried out in the presence of 10 mM procaine. This indicates that procaine partially blocks the open-locked channels. 5. After Ca2+ loading in the presence of procaine, removal of procaine induced a rapid release of Ca2+ from the SR through the open-locked channels. This rapid release was dependent both on the adenine nucleotide concentration and on the Ca2+ concentration. Thus, the 'open-locked' CICR channels are still regulated by Ca2+ and adenine nucleotides.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell K. P., Knudson C. M., Imagawa T., Leung A. T., Sutko J. L., Kahl S. D., Raab C. R., Madson L. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J Biol Chem. 1987 May 15;262(14):6460–6463. [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S. Excitation-contraction coupling and the mechanism of muscle contraction. Annu Rev Physiol. 1991;53:1–16. doi: 10.1146/annurev.ph.53.030191.000245. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Measurement of Ca2+ release in skinned fibers from skeletal muscle. Methods Enzymol. 1988;157:12–26. doi: 10.1016/0076-6879(88)57064-7. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuti K. Mechanism of contracture on cooling of caffeine-treated frog skeletal muscle fibres. J Physiol. 1988 Apr;398:131–148. doi: 10.1113/jphysiol.1988.sp017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuti K. Some properties of the contractile system and sarcoplasmic reticulum of skinned slow fibres from Xenopus muscle. J Physiol. 1986 Apr;373:1–23. doi: 10.1113/jphysiol.1986.sp016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Jenden D. J., Fairhurst A. S. The pharmacology of ryanodine. Pharmacol Rev. 1969 Mar;21(1):1–25. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H., Block B. A., Meissner G. Evidence for a junctional feet-ryanodine receptor complex from sarcoplasmic reticulum. Biochem Biophys Res Commun. 1987 Mar 13;143(2):704–709. doi: 10.1016/0006-291x(87)91411-2. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. J Physiol. 1990 Apr;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Pessah I. N., Stambuk R. A., Casida J. E. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987 Mar;31(3):232–238. [PubMed] [Google Scholar]
- Seiler S., Wegener A. D., Whang D. D., Hathaway D. R., Jones L. R. High molecular weight proteins in cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles bind calmodulin, are phosphorylated, and are degraded by Ca2+-activated protease. J Biol Chem. 1984 Jul 10;259(13):8550–8557. [PubMed] [Google Scholar]
- Su J. Y. Effects of ryanodine on skinned skeletal muscle fibers of the rabbit. Pflugers Arch. 1987 Nov;410(4-5):510–516. doi: 10.1007/BF00586534. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]


