Abstract
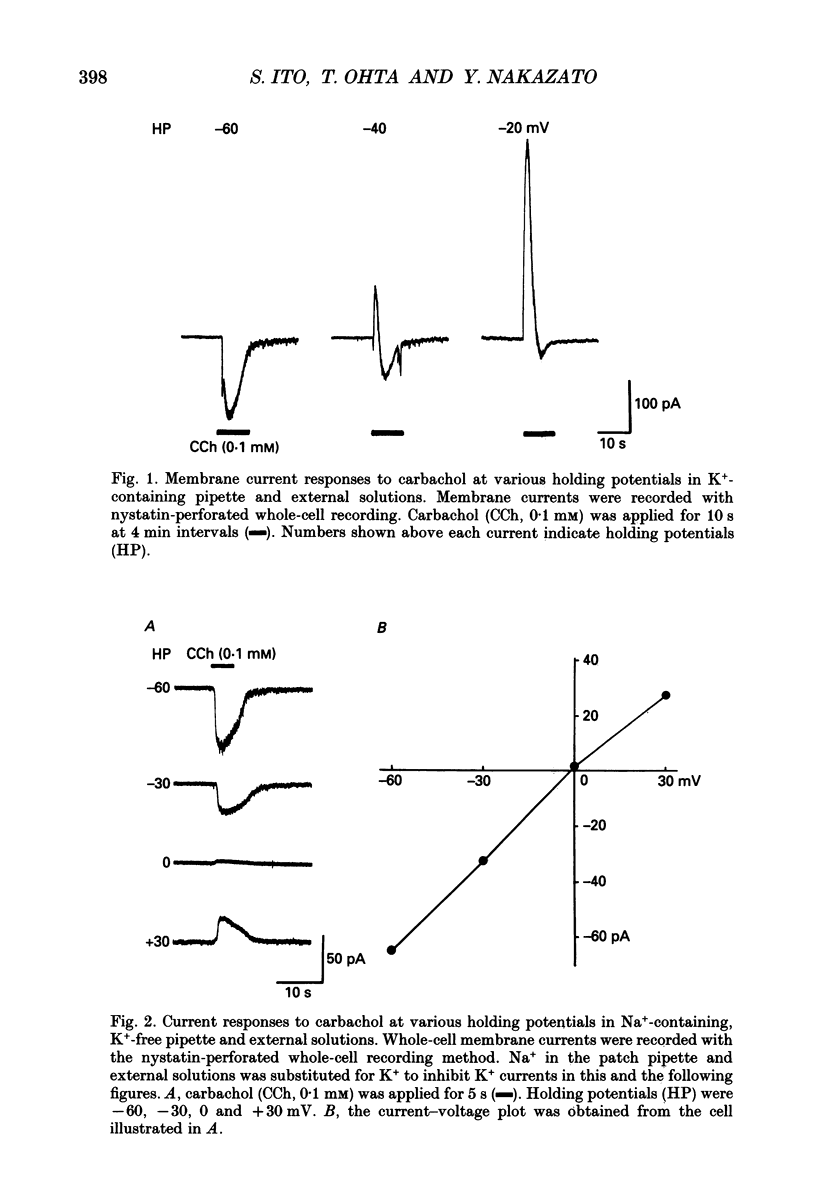
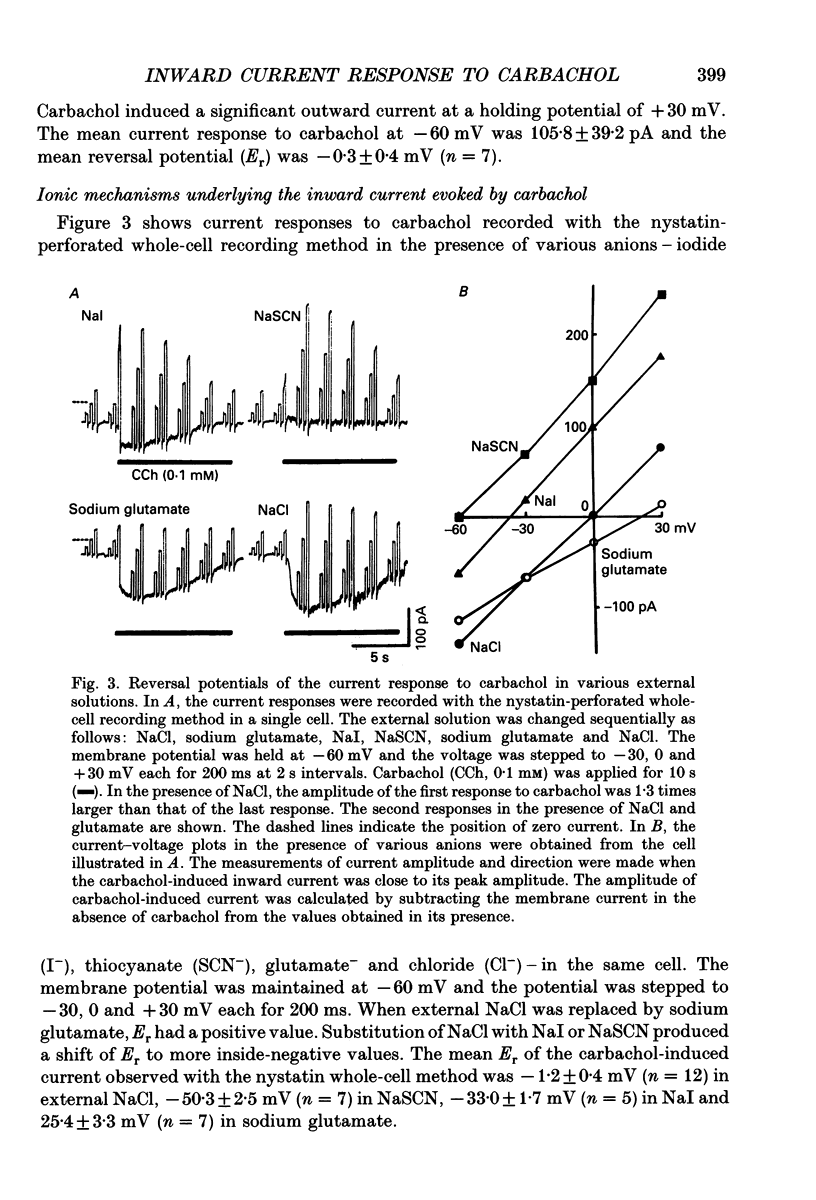
1. Carbachol (0.1 mM or 10 microM)-evoked inward currents were studied with standard and perforated whole-cell patch clamp techniques in smooth muscle cells isolated from rat small intestine. The intracellular free Ca2+ concentration was monitored simultaneously with the fura-2 method. 2. With a K(+)-containing pipette solution, carbachol produced an inward current at -60 mV and a large outward current at -20 mV. 3. When NaCl was substituted for KCl in the external and pipette solutions, carbachol elicited inward currents at holding potentials more inside-negative than 0 mV. The reversal potential of the carbachol-induced current altered when external chloride (-0.9 mV) was replaced by iodide (-21.2 mV), thiocyanate (-27.0 mV) and glutamate (18.2 mV). The carbachol-induced current at -60 mV was slightly decreased by the replacement of external NaCl with Tris-Cl. 4. The carbachol-induced inward current at -60 mV was accompanied by an increase in the intracellular concentration of free Ca2+. Both responses to carbachol were observed 2 min after exposure of the cells to a Ca(2+)-free solution containing 2 mM EGTA. 5. Intracellular application of heparin inhibited the inward current and Ca2+ transient responses to carbachol but not those to caffeine (10 mM). An inward current and Ca2+ transient were elicited after the patch membrane was ruptured at -60 mV, using a patch pipette containing inositol 1,4,5-trisphosphate (InsP3). 6. It is concluded that the carbachol-induced inward current is due to increases in membrane Cl- and Na+ conductances. Ca2+ released from InsP3-sensitive stores may play a role in increasing both conductances.
Full text
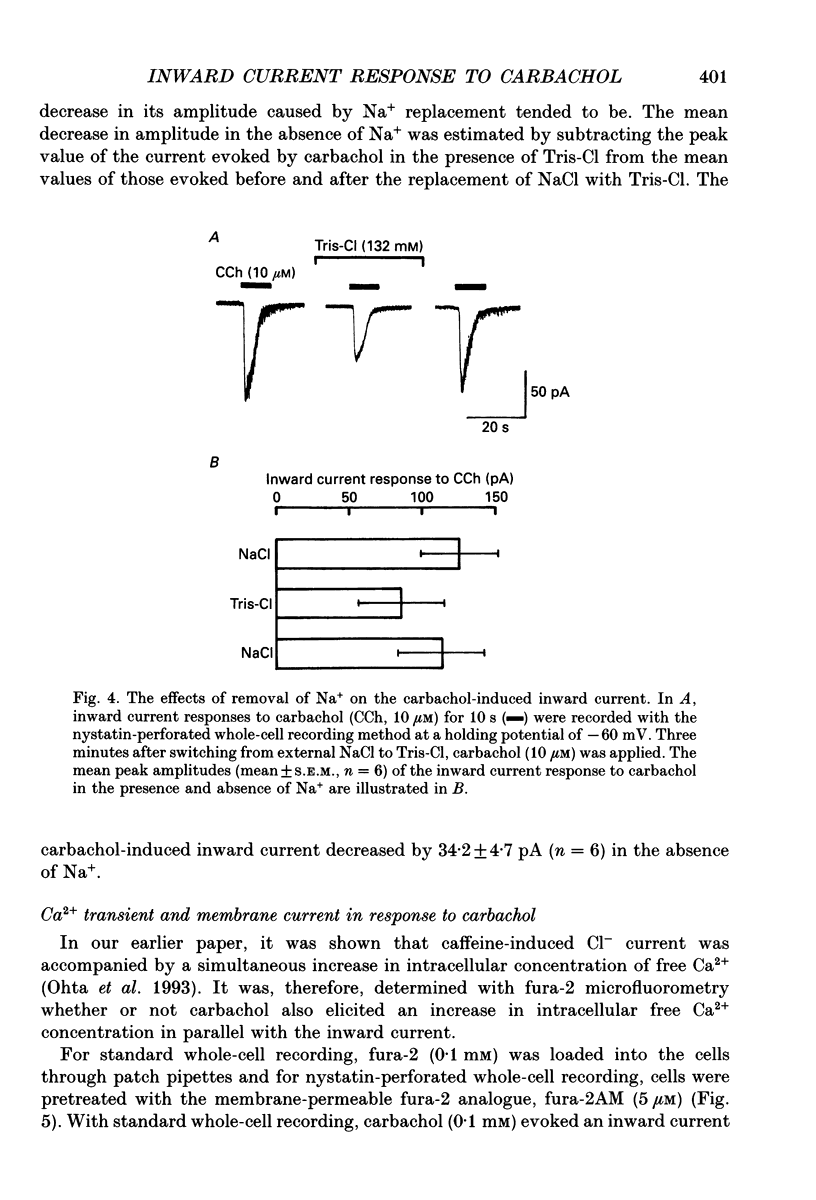
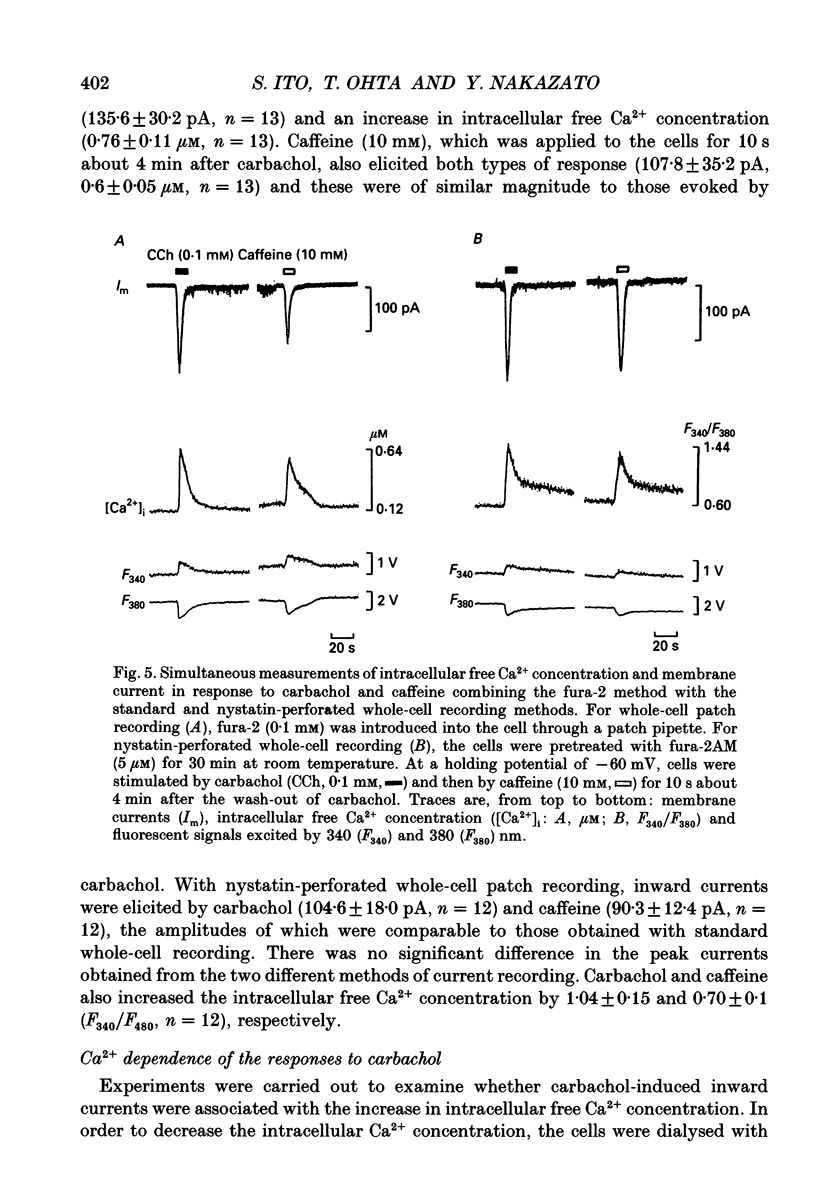
PDF



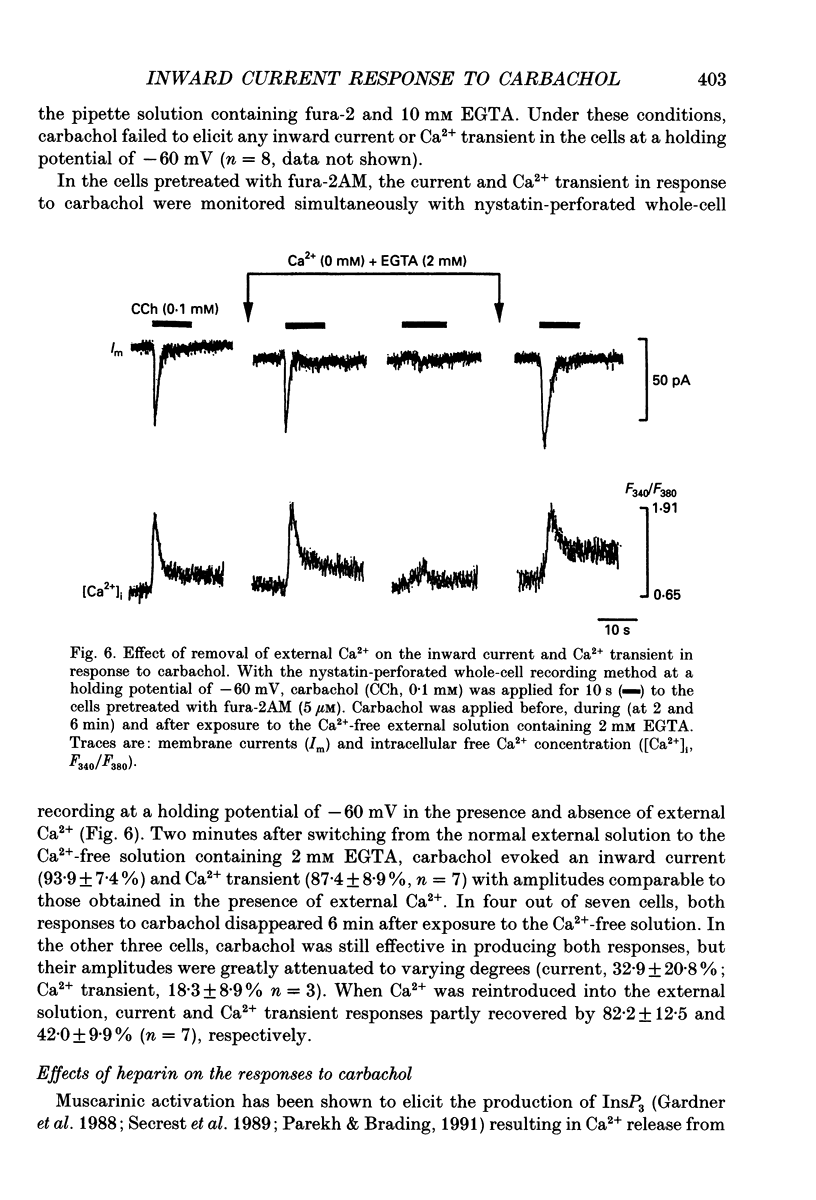
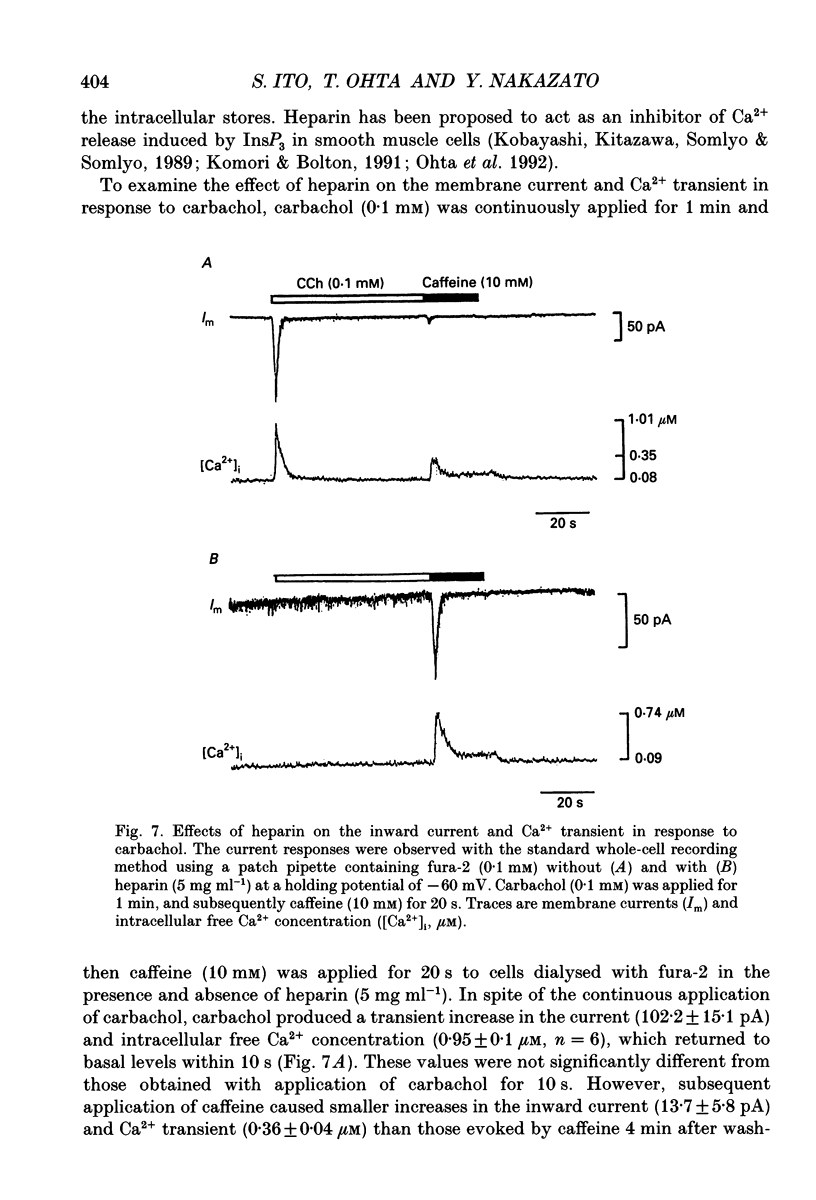
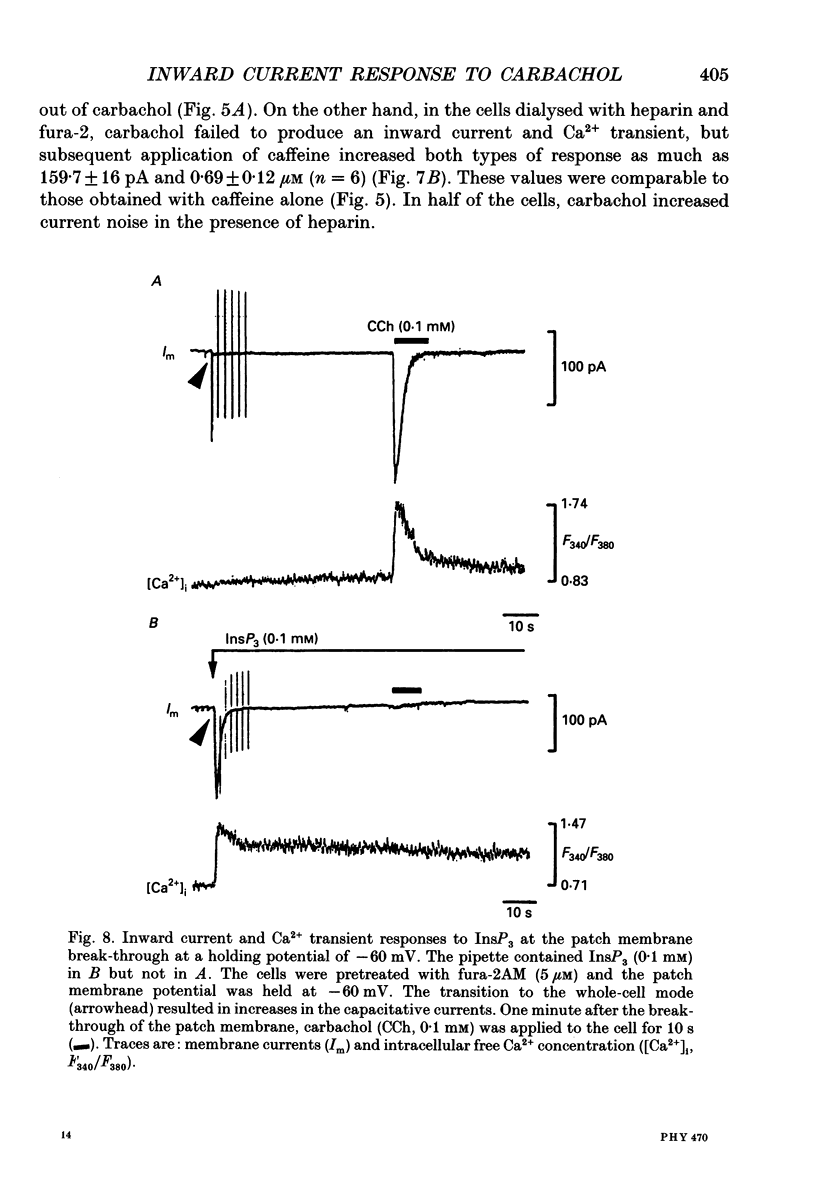




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Potassium, chloride and non-selective cation conductances opened by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Apr;423:551–568. doi: 10.1113/jphysiol.1990.sp018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Action of noradrenaline on single smooth muscle cells freshly dispersed from the rat anococcygeus muscle. J Physiol. 1987 Aug;389:513–525. doi: 10.1113/jphysiol.1987.sp016669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988 Oct;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A. L., Choo L. K., Mitchelson F. Comparison of the effects of some muscarinic agonists on smooth muscle function and phosphatidylinositol turnover in the guinea-pig taenia caeci. Br J Pharmacol. 1988 May;94(1):199–211. doi: 10.1111/j.1476-5381.1988.tb11516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991 Feb;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990 Aug;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Ito S., Nakazato Y. Chloride currents activated by caffeine in rat intestinal smooth muscle cells. J Physiol. 1993 Jun;465:149–162. doi: 10.1113/jphysiol.1993.sp019670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Ito S., Noto T., Tachibana R., Nakazato Y., Ohga A. The inhibitory action of cyclic AMP on responses to carbachol dependent on calcium stores in rat gastric smooth muscle. J Physiol. 1992;453:367–384. doi: 10.1113/jphysiol.1992.sp019233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Bolton T. B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991 Sep;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Mironneau C., Mironneau J. Noradrenaline activates a calcium-activated chloride conductance and increases the voltage-dependent calcium current in cultured single cells of rat portal vein. Br J Pharmacol. 1989 May;97(1):139–146. doi: 10.1111/j.1476-5381.1989.tb11934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B., Brading A. F. The sources of calcium for carbachol-induced contraction in the circular smooth muscle of guinea-pig stomach. Br J Pharmacol. 1991 Oct;104(2):412–418. doi: 10.1111/j.1476-5381.1991.tb12444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrest R. J., Schoepp D. D., Cohen M. L. Comparison of contractions to serotonin, carbamylcholine and prostaglandin F2 alpha in rat stomach fundus. J Pharmacol Exp Ther. 1989 Sep;250(3):971–978. [PubMed] [Google Scholar]
- Sims S. M. Cholinergic activation of a non-selective cation current in canine gastric smooth muscle is associated with contraction. J Physiol. 1992 Apr;449:377–398. doi: 10.1113/jphysiol.1992.sp019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F., Sanders K. M. Cholinergic stimulation activates a non-selective cation current in canine pyloric circular muscle cells. J Physiol. 1990 Oct;429:223–236. doi: 10.1113/jphysiol.1990.sp018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Hogg R. C., Large W. A. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. J Physiol. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Large W. A. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J Physiol. 1991 Apr;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]


