Abstract
1. Electrical communication between circular muscle, longitudinal muscle and interstitial cells of Cajal (ICC) was investigated; the hypothesis was tested that the resting membrane potential (RMP) gradient in the circular muscle of canine colon is caused by electrical coupling to neighbouring cells. 2. Isolated longitudinal muscle exhibited spike-like action potentials at a RMP of -45 mV with a frequency and amplitude of 20 cycles/min and 12 mV, respectively. 3. The circular muscle (CM), devoid of longitudinal muscle, myenteric plexus and submuscular ICC-smooth-muscle network, was electrically quiescent at a uniform RMP of -62 mV across the entire circular muscle layer. 4. Preparations consisting of only the submuscular ICC network and a few adjacent layers of circular muscle cells exhibited slow wave-type action potentials at a RMP of about -80 mV. 5. In ICC-CM preparations, consisting of the submuscular ICC network and circular muscle, a RMP gradient of 10 mV was observed near the submucosal border, whereas the RMP was constant at -62 mV in the myenteric half of the circular muscle. 6. In full thickness (FT) preparations, a RMP gradient of 23 mV was observed. The RMP decreased gradually from -71 mV at the submucosal border to -48 mV at the myenteric border of the circular muscle. 7. Coupling of longitudinal muscle to circular muscle caused circular muscle cells at the myenteric surface to depolarize by 14 mV and longitudinal muscle cells to hyperpolarize by 3 mV. 8. In the ICC-CM preparations, the slow wave amplitudes did not decay exponentially away from the ICC network indicating that slow waves propagated actively into the circular muscle; in the FT preparations there was an apparent exponential decay but this was due to the RMP gradient. 9. Spike-like action potentials (SLAPs) superimposed on the plateau phase of slow waves did not decay exponentially away from the myenteric border suggesting that SLAPs were generated within the circular muscle layer. 10. In summary, circular muscle cells possess a uniform intrinsic RMP of -62 mV. The RMP gradient in situ is caused by electrical coupling of circular muscle cells to longitudinal muscle cells and the submuscular network of ICC. In situ, slow wave-type action potentials propagate actively into the circular muscle layer, and, dependent on the level of excitation, circular muscle cells actively generate spikes.
Full text
PDF
















Images in this article
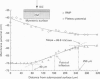
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barajas-López C., Den Hertog A., Huizinga J. D. Ionic basis of pacemaker generation in dog colonic smooth muscle. J Physiol. 1989 Sep;416:385–402. doi: 10.1113/jphysiol.1989.sp017767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-López C., Huizinga J. D. Different mechanisms of contraction generation in circular muscle of canine colon. Am J Physiol. 1989 Mar;256(3 Pt 1):G570–G580. doi: 10.1152/ajpgi.1989.256.3.G570. [DOI] [PubMed] [Google Scholar]
- Barajas-López C., Huizinga J. D. Heterogeneity in spontaneous and tetraethylammonium induced intracellular electrical activity in colonic circular muscle. Pflugers Arch. 1988 Jul;412(1-2):203–210. doi: 10.1007/BF00583751. [DOI] [PubMed] [Google Scholar]
- Bauer A. J., Publicover N. G., Sanders K. M. Origin and spread of slow waves in canine gastric antral circular muscle. Am J Physiol. 1985 Dec;249(6 Pt 1):G800–G806. doi: 10.1152/ajpgi.1985.249.6.G800. [DOI] [PubMed] [Google Scholar]
- Bauer A. J., Reed J. B., Sanders K. M. Slow wave heterogeneity within the circular muscle of the canine gastric antrum. J Physiol. 1985 Sep;366:221–232. doi: 10.1113/jphysiol.1985.sp015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. J., Sanders K. M. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol. 1985 Dec;369:283–294. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin I., Huizinga J. D., Daniel E. E. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol. 1988 Jul 1;273(1):42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Berezin I., Huizinga J. D., Daniel E. E. Structural characterization of interstitial cells of Cajal in myenteric plexus and muscle layers of canine colon. Can J Physiol Pharmacol. 1990 Nov;68(11):1419–1431. doi: 10.1139/y90-216. [DOI] [PubMed] [Google Scholar]
- Burke E. P., Reed J. B., Sanders K. M. Role of sodium pump in membrane potential gradient of canine proximal colon. Am J Physiol. 1988 Apr;254(4 Pt 1):C475–C483. doi: 10.1152/ajpcell.1988.254.4.C475. [DOI] [PubMed] [Google Scholar]
- Chow E., Huizinga J. D. Myogenic electrical control activity in longitudinal muscle of human and dog colon. J Physiol. 1987 Nov;392:21–34. doi: 10.1113/jphysiol.1987.sp016767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie H. L., Kirk D. Electrical activity of human colonic smooth muscle in vitro. J Physiol. 1978 Oct;283:319–330. doi: 10.1113/jphysiol.1978.sp012502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy T. Y. Electrical activities of the muscle layers of the canine colon. J Physiol. 1983 Sep;342:67–83. doi: 10.1113/jphysiol.1983.sp014840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elden L., Bortoff A. Electrical coupling of longitudinal and circular intestinal muscle. Am J Physiol. 1984 May;246(5 Pt 1):G618–G626. doi: 10.1152/ajpgi.1984.246.5.G618. [DOI] [PubMed] [Google Scholar]
- Huizinga J. D. Action potentials in gastrointestinal smooth muscle. Can J Physiol Pharmacol. 1991 Aug;69(8):1133–1142. doi: 10.1139/y91-166. [DOI] [PubMed] [Google Scholar]
- Huizinga J. D., Barajas-Lopez C., Chow E. Generation of spiking activity in circular muscle cells of the canine colon. Can J Physiol Pharmacol. 1987 Oct;65(10):2147–2150. doi: 10.1139/y87-337. [DOI] [PubMed] [Google Scholar]
- Huizinga J. D., Barajas-López C. Ionic and cellular basis for slow-wave-type and spike-like action potentials. Prog Clin Biol Res. 1990;327:605–615. [PubMed] [Google Scholar]
- Huizinga J. D., Farraway L., Den Hertog A. Effect of voltage and cyclic AMP on frequency of slow-wave-type action potentials in canine colon smooth muscle. J Physiol. 1991 Oct;442:31–45. doi: 10.1113/jphysiol.1991.sp018780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga J. D., Liu L. W., Blennerhassett M. G., Thuneberg L., Molleman A. Intercellular communication in smooth muscle. Experientia. 1992 Oct 15;48(10):932–941. doi: 10.1007/BF01919140. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Burke E. P., Sanders K. M. Participation of Ca currents in colonic electrical activity. Am J Physiol. 1989 Sep;257(3 Pt 1):C451–C460. doi: 10.1152/ajpcell.1989.257.3.C451. [DOI] [PubMed] [Google Scholar]
- Liu L. W., Daniel E. E., Huizinga J. D. Excitability of canine colon circular muscle disconnected from the network of interstitial cells of Cajal. Can J Physiol Pharmacol. 1992 Feb;70(2):289–295. doi: 10.1139/y92-036. [DOI] [PubMed] [Google Scholar]
- Sabourin P. J., Kingma Y. J., Bowes K. L. Simultaneous measurement of electrical activity from two colonic smooth muscle layers using a dual sucrose gap apparatus. IEEE Trans Biomed Eng. 1990 May;37(5):509–514. doi: 10.1109/10.55641. [DOI] [PubMed] [Google Scholar]
- Sakai T., Terada K., Kitamura K., Kuriyama H. Ryanodine inhibits the Ca-dependent K current after depletion of Ca stored in smooth muscle cells of the rabbit ileal longitudinal muscle. Br J Pharmacol. 1988 Dec;95(4):1089–1100. doi: 10.1111/j.1476-5381.1988.tb11743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio R., Barajas-Lopez C., Daniel E. E., Berezin I., Huizinga J. D. Slow-wave activity in colon: role of network of submucosal interstitial cells of Cajal. Am J Physiol. 1991 Apr;260(4 Pt 1):G636–G645. doi: 10.1152/ajpgi.1991.260.4.G636. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol. 1987 Mar;252(3 Pt 1):C290–C299. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987 Feb;252(2 Pt 1):C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Taylor A. B., Kreulen D., Prosser C. L. Electron microscopy of the connective tissues between longitudinal and circular muscle of small intestine of cat. Am J Anat. 1977 Nov;150(3):427–441. doi: 10.1002/aja.1001500305. [DOI] [PubMed] [Google Scholar]