Abstract
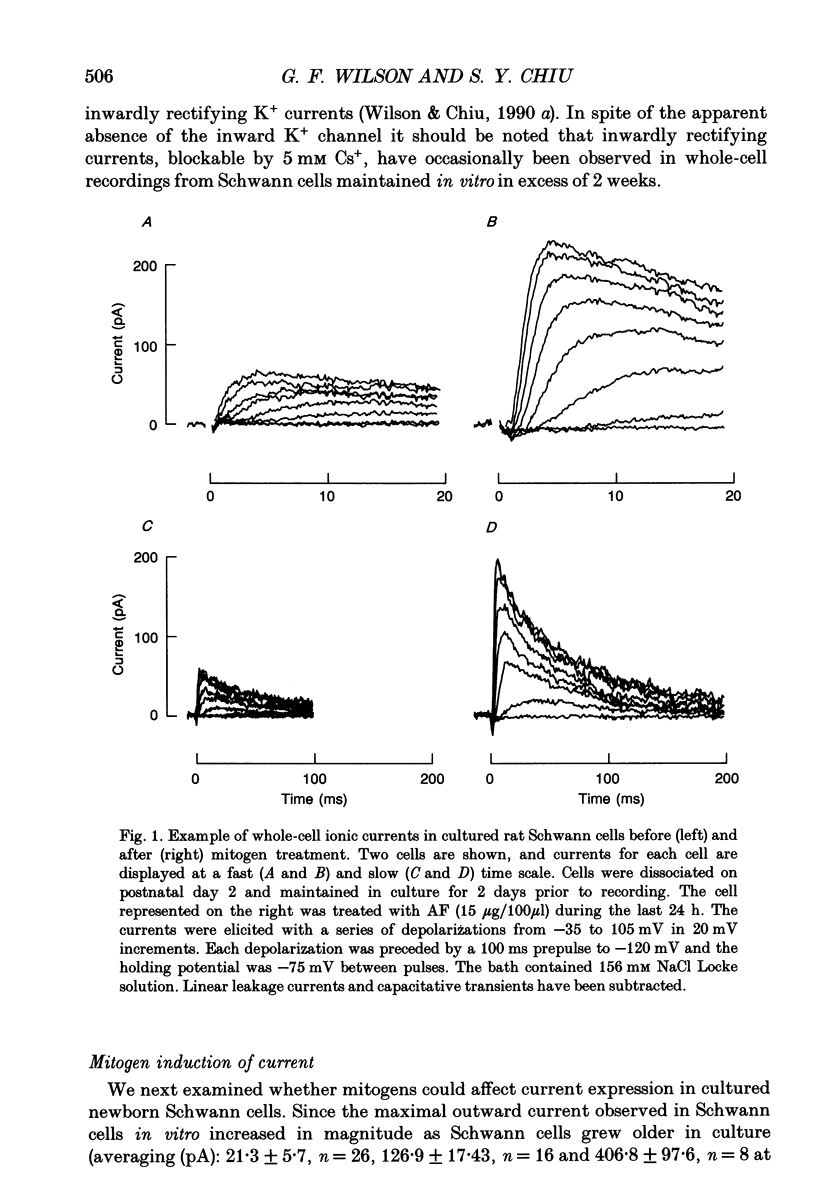
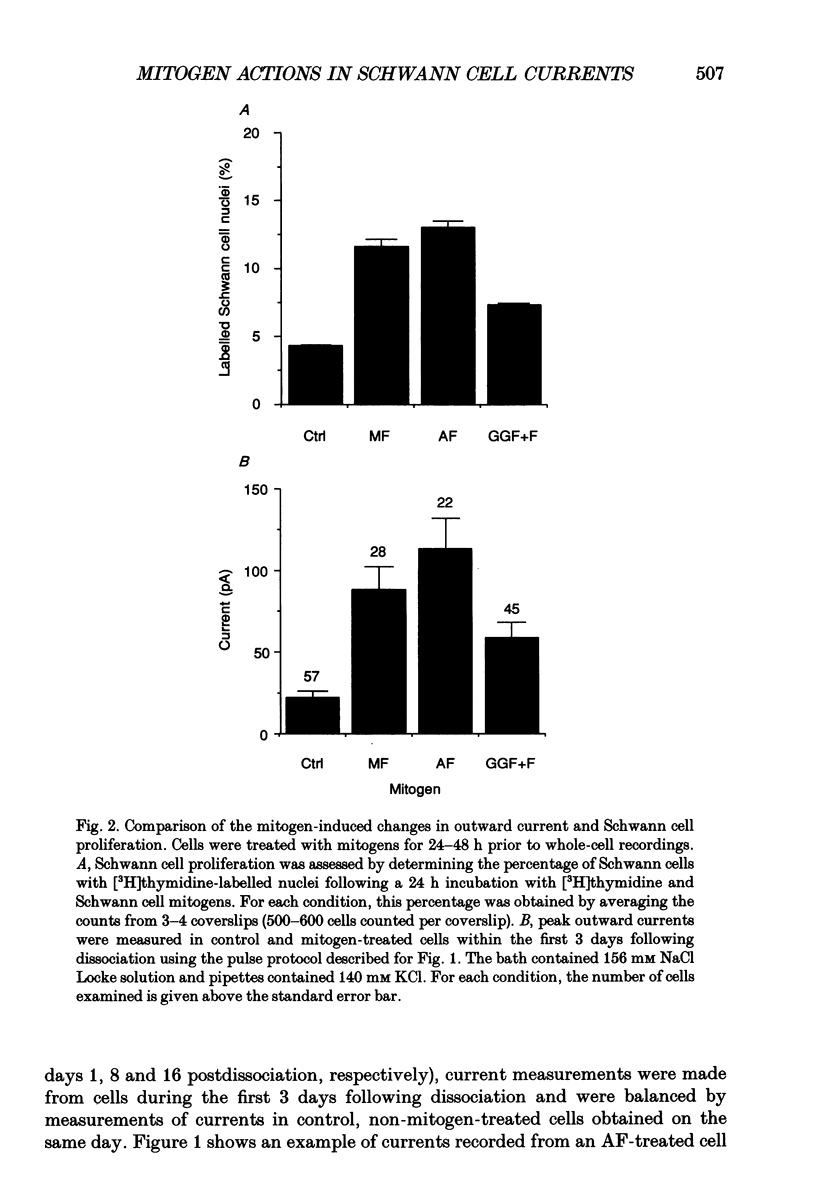
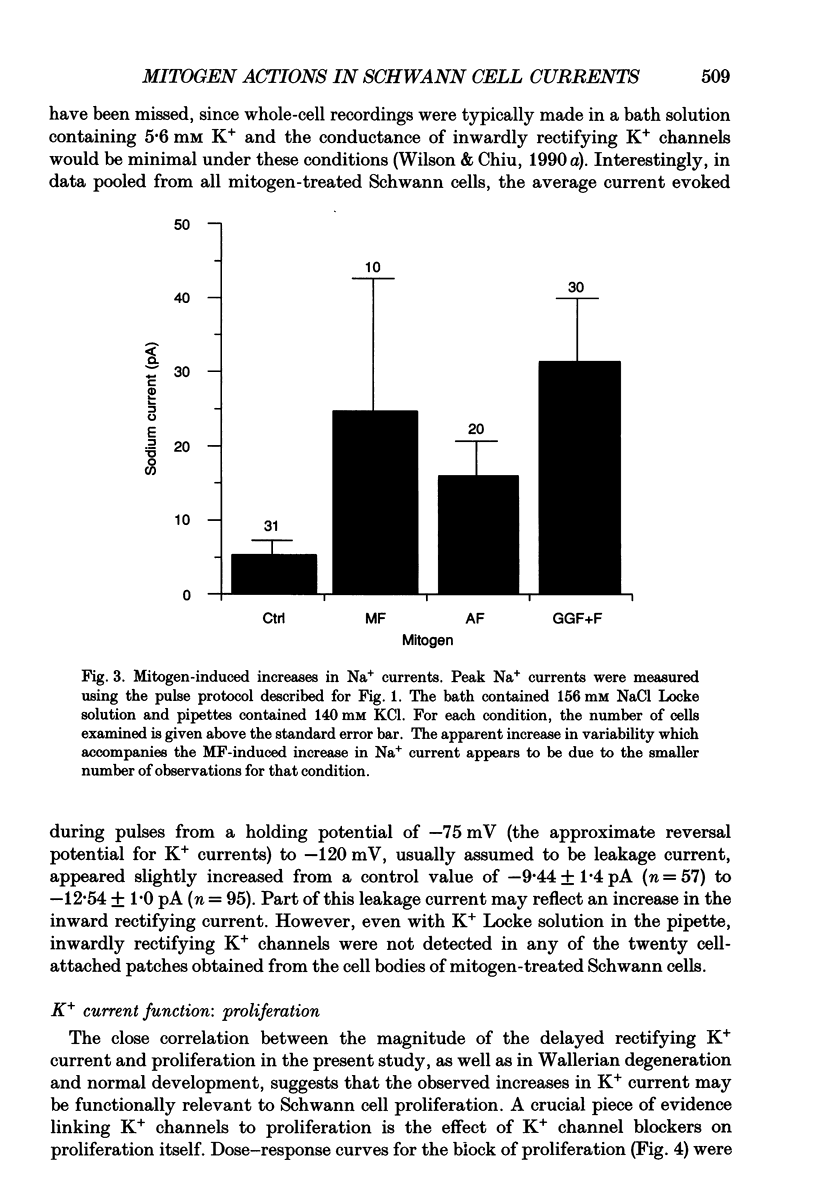
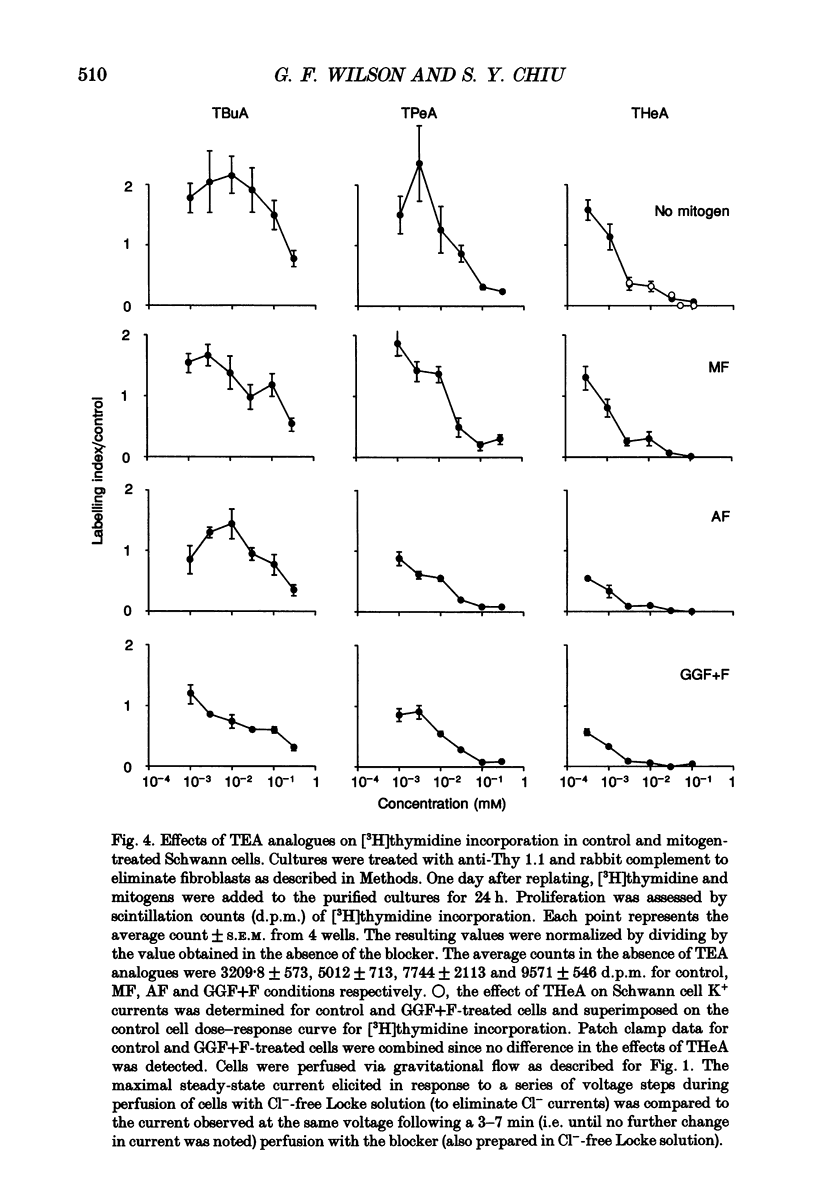
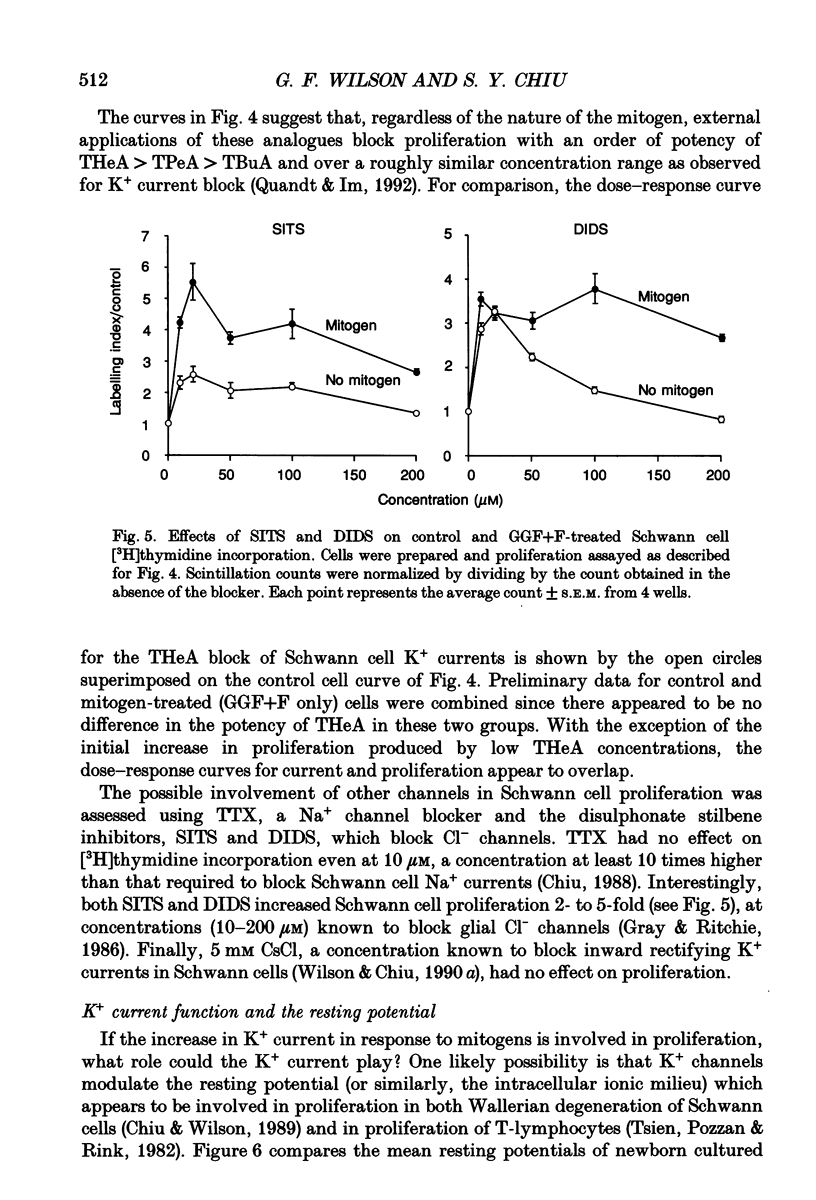
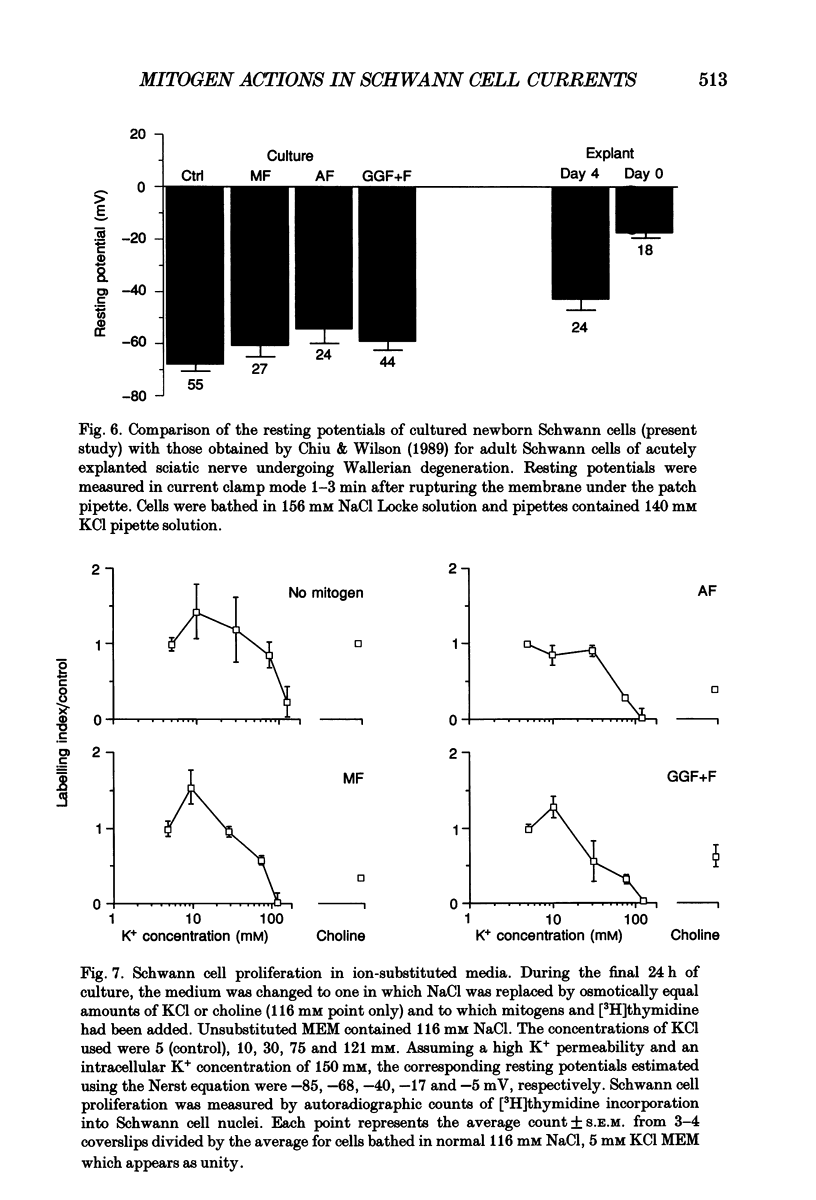
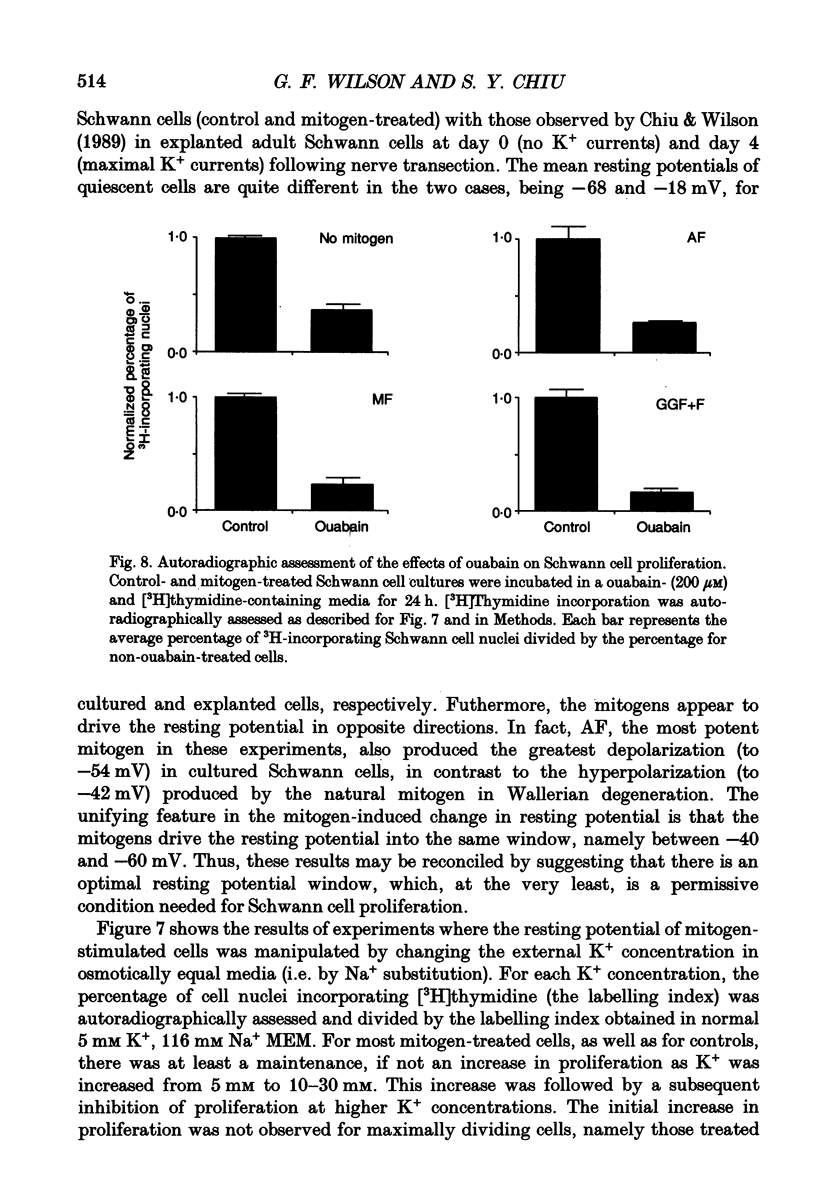
1. Patch clamp studies were carried out in Schwann cells cultured from newborn rat sciatic nerve to determine the effects of mitogens on voltage-gated currents without the confounding influences of axonal contact and myelin present in vivo. The relevance of the various Schwann cell currents to proliferation was assessed using assays of [3H]thymidine incorporation. 2. Treatment of cultured Schwann cells with known mitogens, namely axon fragments (AF), myelin fragments (MF), or glial growth factor in combination with forskolin (GGF+F), increased the magnitudes of delayed rectifying potassium (K+) and sodium (Na+) currents. 3. In both control and mitogen-treated cells, the magnitude of net outward current paralleled clearly the magnitude of the cells' proliferative response. 4. The K+ channel-blocking quaternary ammonium ions, tetrabutylammonium (TBuA), tetrapentylammonium (TPeA) and tetrahexylammonium (THeA), but not the Na+ channel blocker tetrodotoxin (TTX), reduced proliferation in a dose-dependent fashion offering further evidence for a role for K+ channels in Schwann cell proliferation. 5. Voltage-gated chloride (Cl-) currents were observed in both control and mitogen-treated cells. Addition of the Cl- channel blockers, 4-acetamido-4'-isocyanatostilbene-2,2'-disulphonate (SITS) or 4,4'-diisothiocyanatostilbene-2,2'-disulphonate (DIDS), to the culture media enhanced proliferation. 6. The possible intermediary role of the Schwann cell resting potential was explored in ion substitution experiments by increasing the K+ concentration of the media and by adding ouabain. Both manipulations inhibited Schwann cell mitosis. 7. Comparison of the expression of functional ion channels in vitro with that previously described for Schwann cells in vivo suggests a difference in the Schwann cell response to the membrane fragment mitogens and their intact counterparts in regard to the regulation of ion channels. MF up-regulates the number of functional channels, whereas the elaboration of myelin (or a factor related to its presence) in vivo appears to down-regulate channel expression, at the cell soma of myelinating Schwann cells. In addition, axonal contact may be required for normal expression of functional inwardly rectifying K+ channels.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Choquet D., Teillaud J. L., Korn H., Fridman W. H. Ion channel blockers inhibit B cell activation at a precise stage of the G1 phase of the cell cycle. Possible involvement of K+ channels. J Immunol. 1990 Mar 15;144(6):2038–2045. [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1(1):10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Koroshetz W. J., Swartz K. J., Chun L. L., Corey D. P. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990 Apr;4(4):507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Bigbee J. W., Yoshino J. E., DeVries G. H. Morphological and proliferative responses of cultured Schwann cells following rapid phagocytosis of a myelin-enriched fraction. J Neurocytol. 1987 Aug;16(4):487–496. doi: 10.1007/BF01668503. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979 Apr 6;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Lemke G. E., Balzer D. R., Jr Purification and preliminary characterization of a glial growth factor from the bovine pituitary. J Biol Chem. 1980 Sep 25;255(18):8374–8377. [PubMed] [Google Scholar]
- Brown M. J., Asbury A. K. Schwann cell proliferation in the postnatal mouse: timing and topography. Exp Neurol. 1981 Oct;74(1):170–186. doi: 10.1016/0014-4886(81)90157-6. [DOI] [PubMed] [Google Scholar]
- Burns C. P., Rozengurt E. Extracellular Na+ and initiation of DNA synthesis: role of intracellular pH and K+. J Cell Biol. 1984 Mar;98(3):1082–1089. doi: 10.1083/jcb.98.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Changes in excitable membrane properties in Schwann cells of adult rabbit sciatic nerves following nerve transection. J Physiol. 1988 Feb;396:173–188. doi: 10.1113/jphysiol.1988.sp016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Differential expression of sodium channels in acutely isolated myelinating and non-myelinating Schwann cells of rabbits. J Physiol. 1993 Oct;470:485–499. doi: 10.1113/jphysiol.1993.sp019871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Sodium currents in axon-associated Schwann cells from adult rabbits. J Physiol. 1987 May;386:181–203. doi: 10.1113/jphysiol.1987.sp016529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Wilson G. F. The role of potassium channels in Schwann cell proliferation in Wallerian degeneration of explant rabbit sciatic nerves. J Physiol. 1989 Jan;408:199–222. doi: 10.1113/jphysiol.1989.sp017455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984 Feb 2;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Deane K. H., Mannie M. D. An alternative pathway of B cell activation: stilbene disulfonates interact with a Cl- binding motif on AEn-related proteins to stimulate mitogenesis. Eur J Immunol. 1992 May;22(5):1165–1171. doi: 10.1002/eji.1830220509. [DOI] [PubMed] [Google Scholar]
- Gray P. T., Ritchie J. M. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- Griffin J. W., Drucker N., Gold B. G., Rosenfeld J., Benzaquen M., Charnas L. R., Fahnestock K. E., Stocks E. A. Schwann cell proliferation and migration during paranodal demyelination. J Neurosci. 1987 Mar;7(3):682–699. doi: 10.1523/JNEUROSCI.07-03-00682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. Two types of potassium current in rabbit cultured Schwann cells. Proc R Soc Lond B Biol Sci. 1988 Oct 22;235(1278):19–27. doi: 10.1098/rspb.1988.0061. [DOI] [PubMed] [Google Scholar]
- Konishi T. Voltage-dependent potassium channels in mouse Schwann cells. J Physiol. 1989 Apr;411:115–130. doi: 10.1113/jphysiol.1989.sp017564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T. Voltage-gated potassium currents in myelinating Schwann cells in the mouse. J Physiol. 1990 Dec;431:123–139. doi: 10.1113/jphysiol.1990.sp018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff J. H., Yoshino J. E., Bigbee J. W., Lewis B. L., Devries G. H. Differential proliferative responses of cultured Schwann cells to axolemma and myelin-enriched fractions. II. Morphological studies. J Neurocytol. 1985 Aug;14(4):619–635. doi: 10.1007/BF01200801. [DOI] [PubMed] [Google Scholar]
- Pellegrino R. G., Politis M. J., Ritchie J. M., Spencer P. S. Events in degenerating cat peripheral nerve: induction of Schwann cell S phase and its relation to nerve fibre degeneration. J Neurocytol. 1986 Feb;15(1):17–28. doi: 10.1007/BF02057901. [DOI] [PubMed] [Google Scholar]
- Porter S., Clark M. B., Glaser L., Bunge R. P. Schwann cells stimulated to proliferate in the absence of neurons retain full functional capability. J Neurosci. 1986 Oct;6(10):3070–3078. doi: 10.1523/JNEUROSCI.06-10-03070.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G., Roberge F., Chan C. C. Retinal glial cell proliferation and ion channels: a possible link. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):521–529. [PubMed] [Google Scholar]
- Quandt F. N., Im W. B. Tetraalkylammonium ion block of potassium currents in mouse neuroblastoma cells. J Pharmacol Exp Ther. 1992 Mar;260(3):1379–1385. [PubMed] [Google Scholar]
- Raff M. C., Abney E., Brockes J. P., Hornby-Smith A. Schwann cell growth factors. Cell. 1978 Nov;15(3):813–822. doi: 10.1016/0092-8674(78)90266-0. [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B., Dubois J. M. A quantitative analysis of the role of K+ channels in mitogenesis of neuroblastoma cells. Cell Signal. 1991;3(4):333–339. doi: 10.1016/0898-6568(91)90062-y. [DOI] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P., Glaser L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980 Mar;84(3):767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980 Mar;84(3):739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P., Chiu S. Y., Ritchie J. M. Voltage-dependent sodium and potassium channels in mammalian cultured Schwann cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):948–952. doi: 10.1073/pnas.82.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue G., Shuman S., Pleasure D. Schwann cell responses to cyclic AMP: proliferation, change in shape, and appearance of surface galactocerebroside. Brain Res. 1986 Jan 1;362(1):23–32. doi: 10.1016/0006-8993(86)91394-6. [DOI] [PubMed] [Google Scholar]
- Soliven B., Szuchet S., Arnason B. G., Nelson D. J. Forskolin and phorbol esters decrease the same K+ conductance in cultured oligodendrocytes. J Membr Biol. 1988 Oct;105(2):177–186. doi: 10.1007/BF02009170. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Trotter J., Schachner M., Kettenmann H. Channel expression correlates with differentiation stage during the development of oligodendrocytes from their precursor cells in culture. Neuron. 1989 Feb;2(2):1135–1145. doi: 10.1016/0896-6273(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Usowicz M. M., Gallo V., Cull-Candy S. G. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989 Jun 1;339(6223):380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Wilson G. F., Chiu S. Y. Ion channels in axon and Schwann cell membranes at paranodes of mammalian myelinated fibers studied with patch clamp. J Neurosci. 1990 Oct;10(10):3263–3274. doi: 10.1523/JNEUROSCI.10-10-03263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. F., Chiu S. Y. Potassium channel regulation in Schwann cells during early developmental myelinogenesis. J Neurosci. 1990 May;10(5):1615–1625. doi: 10.1523/JNEUROSCI.10-05-01615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. M., Bunge R. P. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 1975 Aug 21;256(5519):662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]


