Abstract
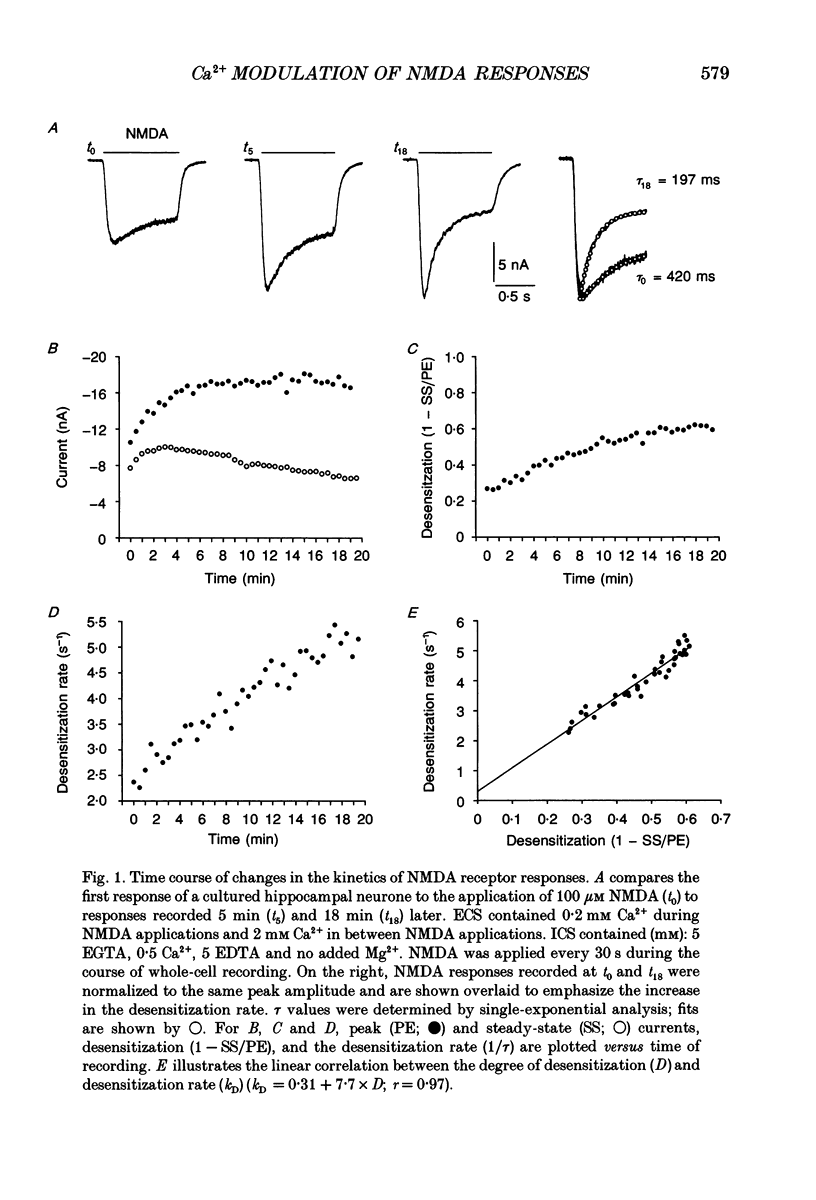
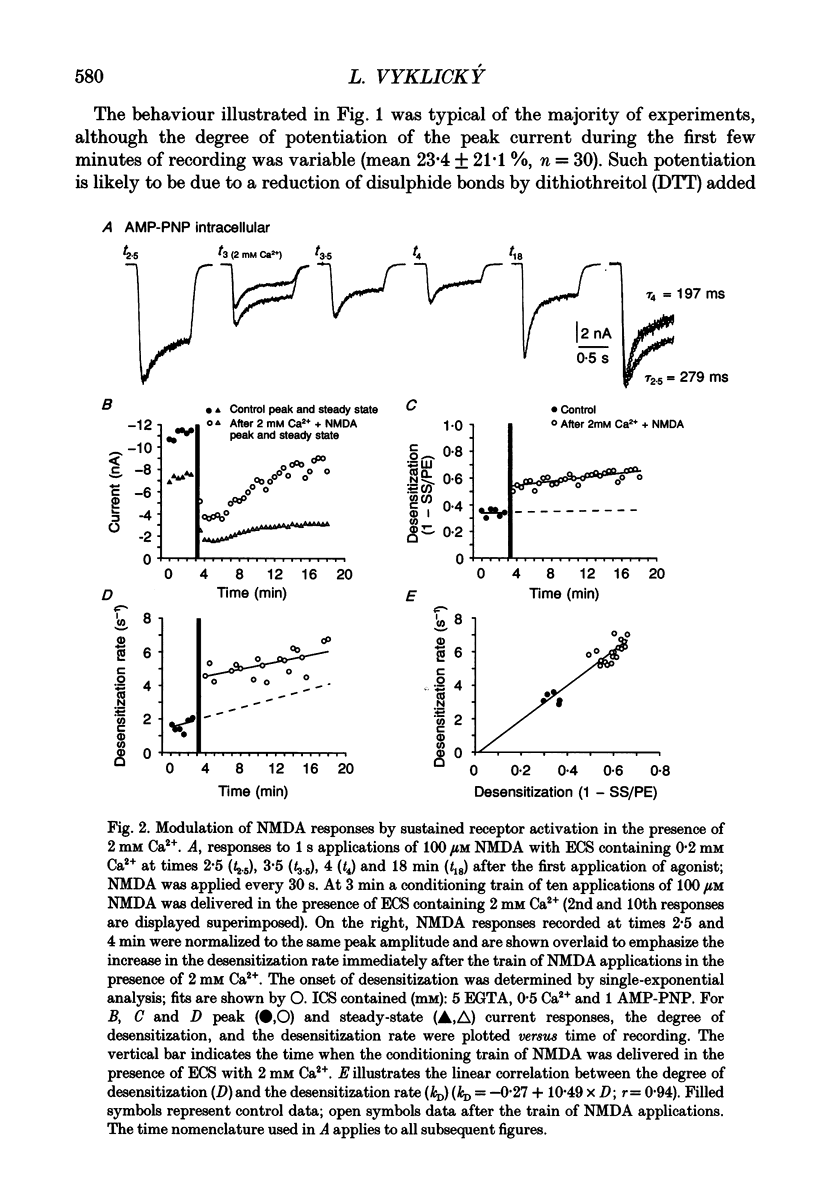
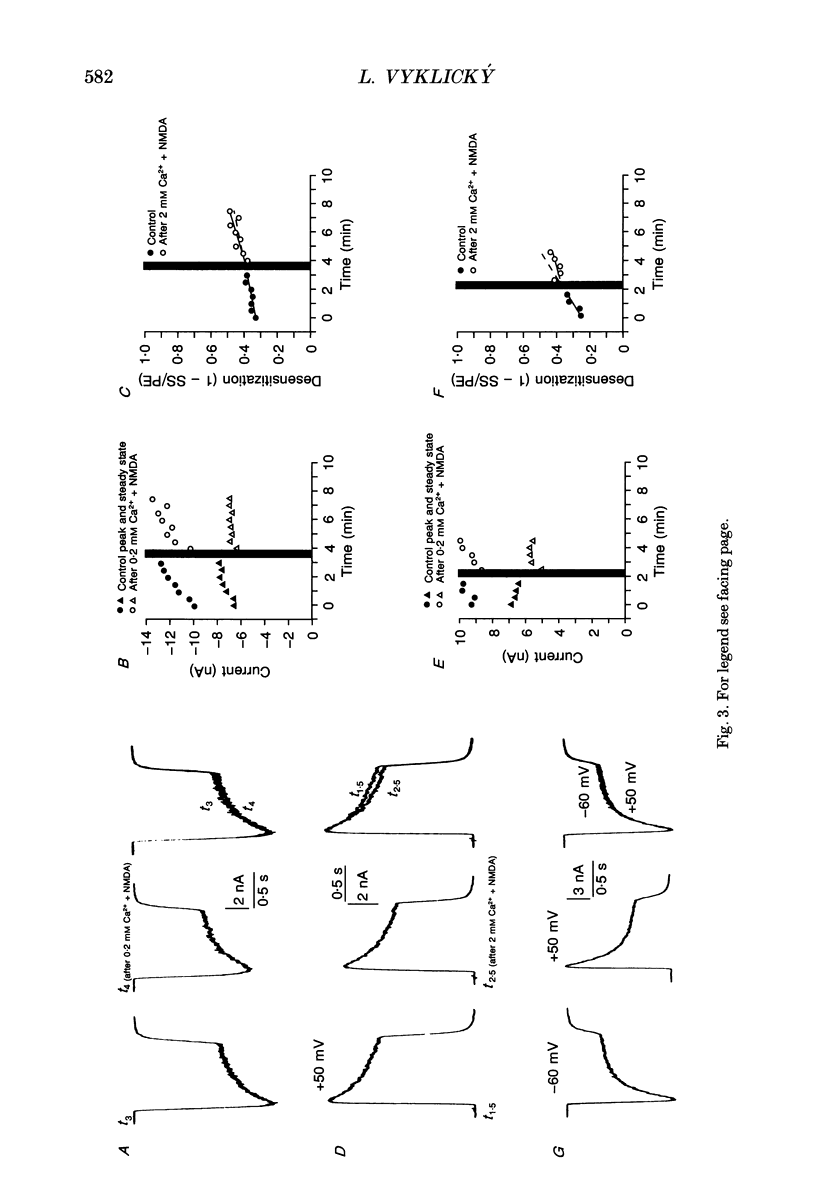
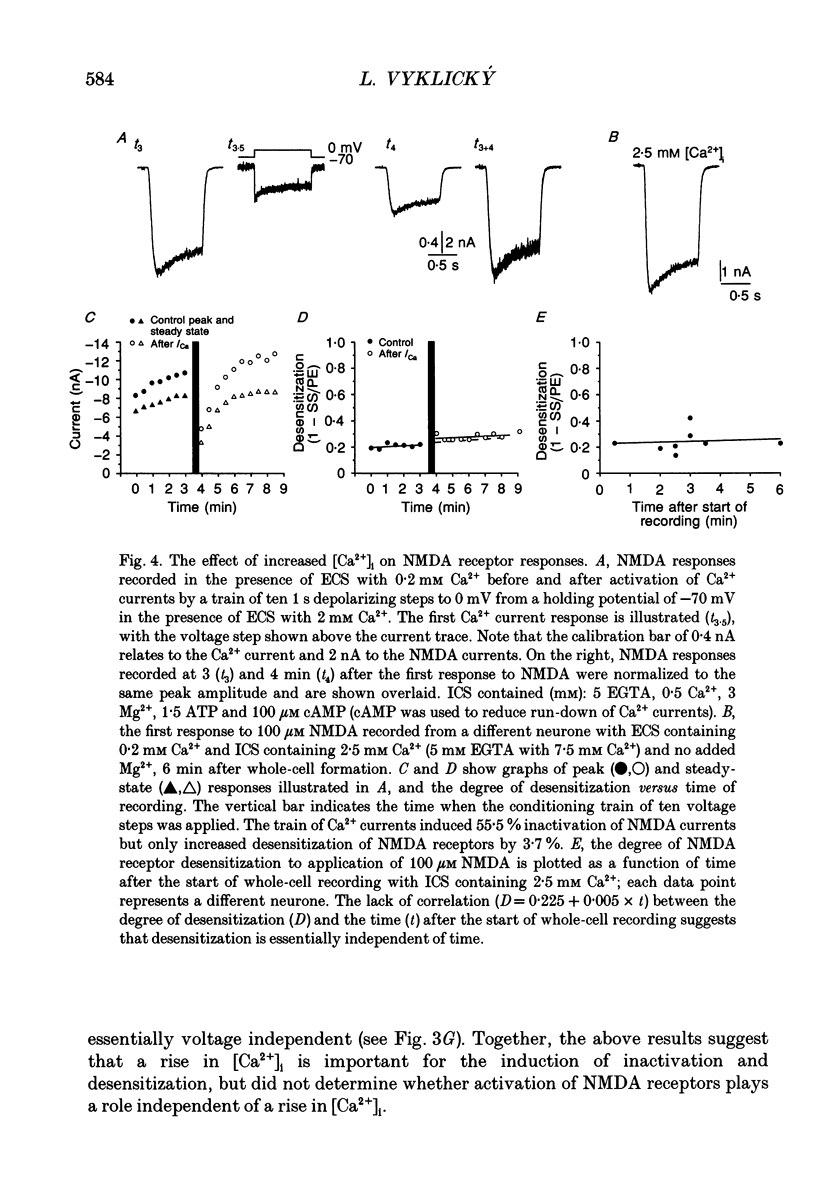
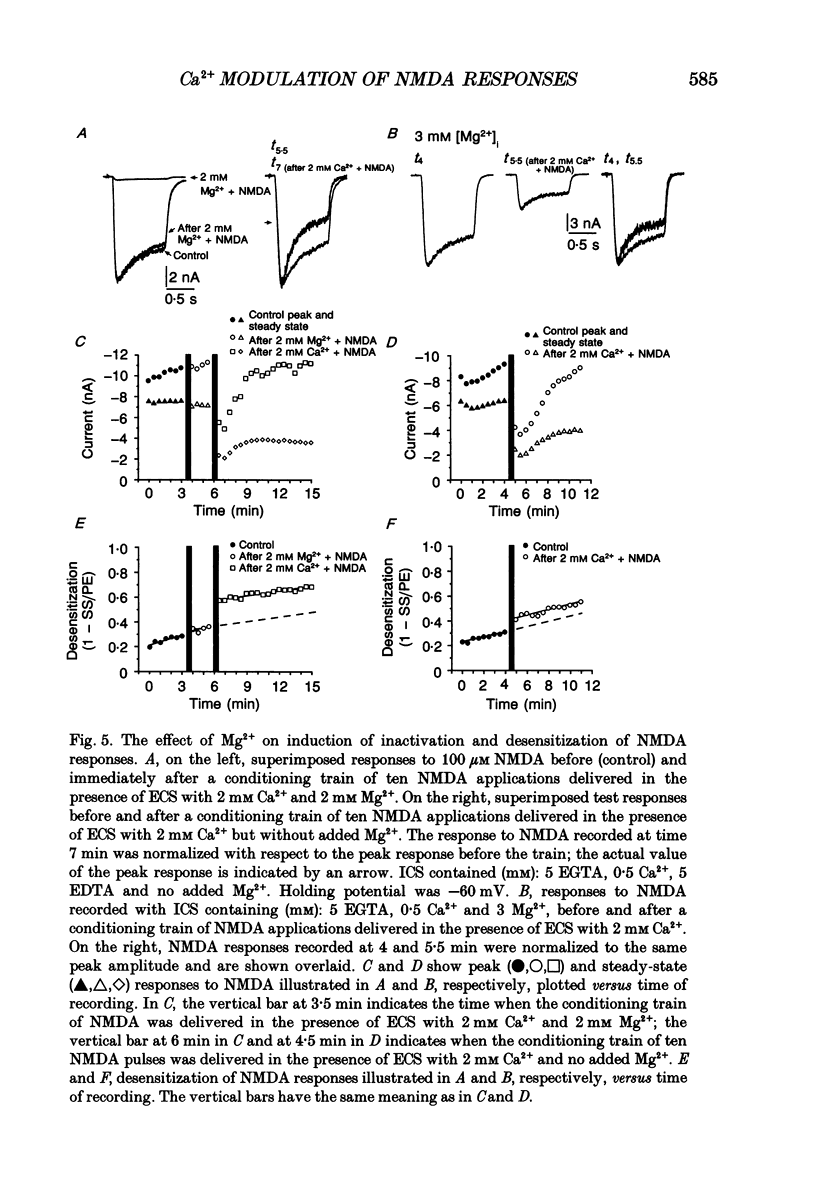
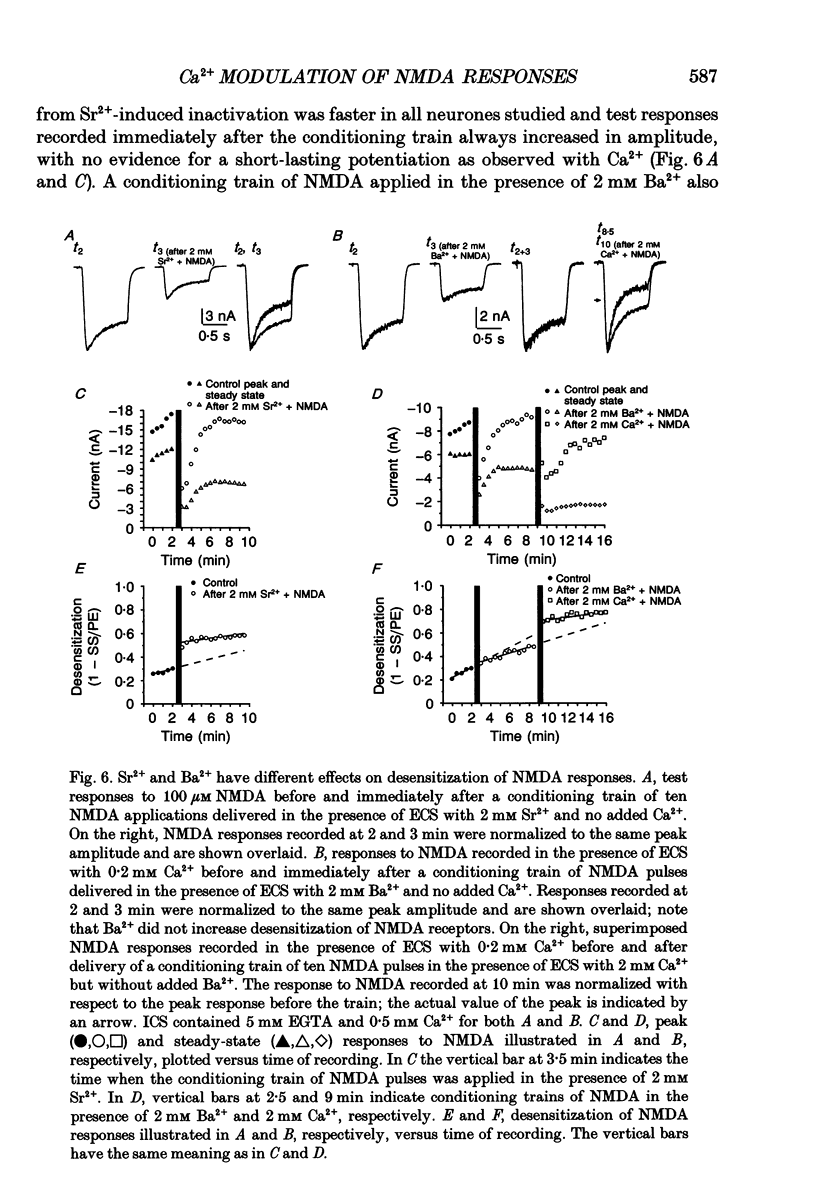
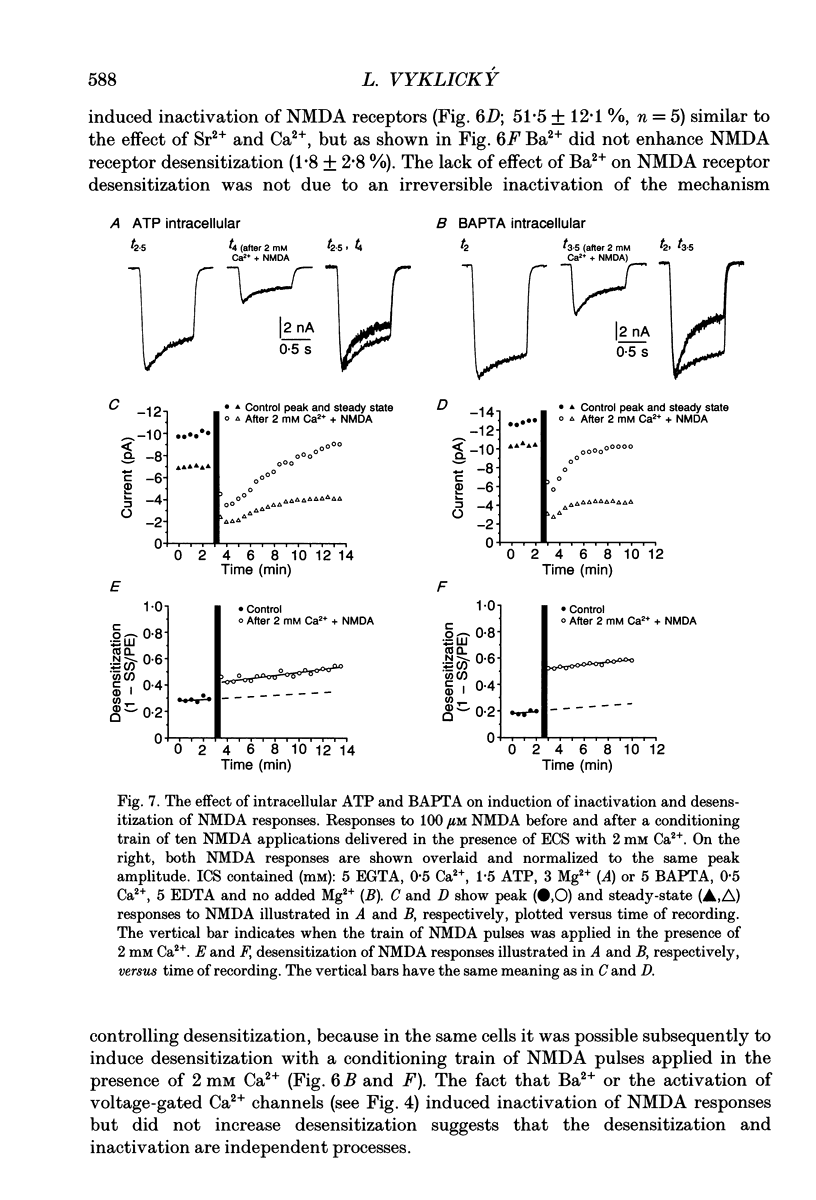
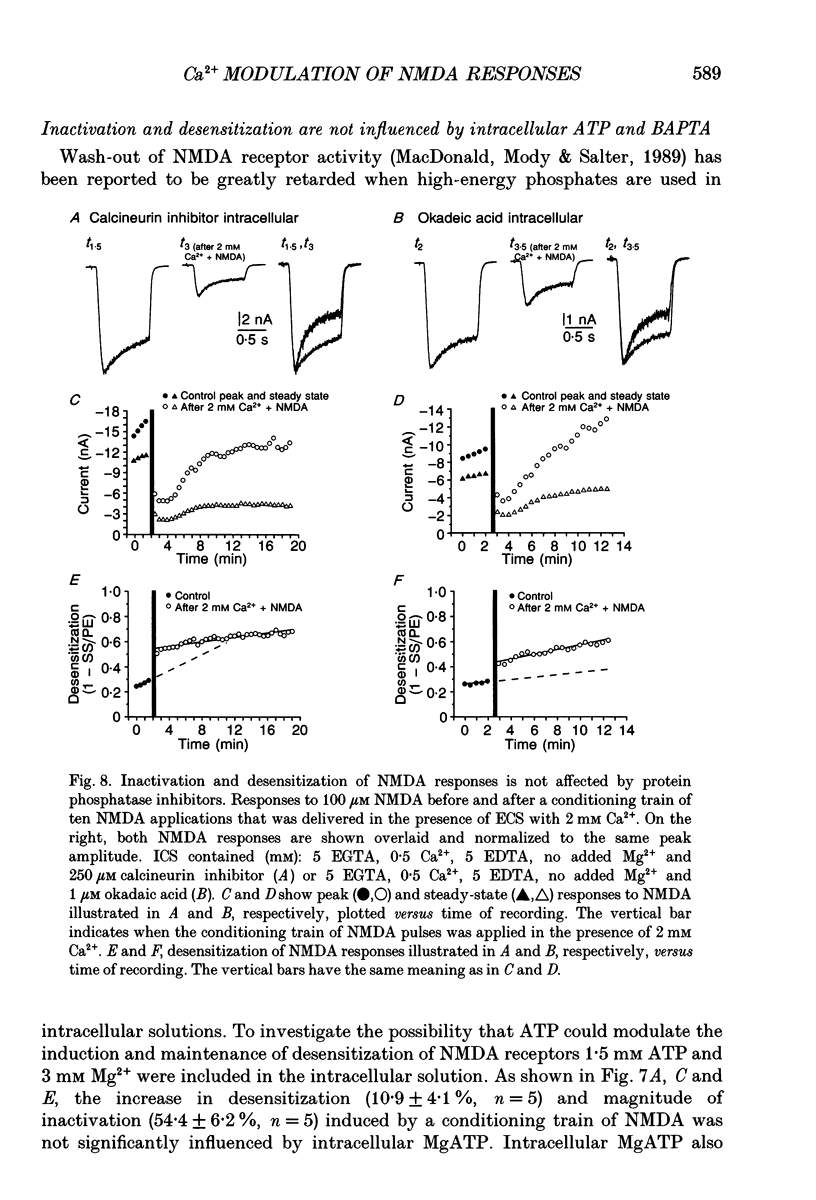
1. Agonist-independent (inactivation) and agonist-induced (desensitization) refractory states of N-methyl-D-aspartate (NMDA) receptors were studied on cultured rat hippocampal neurones using whole-cell and inside-out patch-clamp techniques and a fast perfusion system. 2. Shortly after whole-cell formation, application of 100 microM NMDA in the presence of 10 microM glycine and 0.2 mM [Ca2+]o induced membrane currents that desensitized by 23%. Repeated application of NMDA at 30 s intervals resulted in a progressive increase in the degree and rate of onset of NMDA receptor desensitization. 3. Test responses to NMDA recorded in the presence of 0.2 mM [Ca2+]o were reversibly inactivated by 60% following a train of ten 1 s applications of NMDA delivered at 0.5 Hz in the presence of 2 mM [Ca2+]o; similar results were obtained with 2 mM [Sr2+]o and 2 mM [Ba2+]o. In the presence of Ca2+ or Sr2+, desensitization during the train of responses to NMDA increased by 14 and 19% respectively, while with Ba2+ there was no increase in desensitization. 4. In the presence of 0.2 mM [Ca2+]o at a holding potential of -60 mV, or in the presence of 2 mM [Ca2+]o at a holding potential of +50 mV, a train of ten applications of NMDA failed to induce either inactivation or an increase in desensitization of test responses to NMDA. These results suggest an important role for [Ca2+]o in the induction of both inactivation and desensitization of NMDA receptors. 5. Increasing the intracellular calcium concentration, [Ca2+]i, via repeated activation of voltage-gated Ca2+ channels, resulted in a reversible inactivation of test responses to NMDA by 35% but failed to increase desensitization. In neurones dialysed with intracellular solution containing 2.5 mM Ca2+ NMDA receptor desensitization was similar to that in neurones dialysed with 10 nM Ca2+. 6. Block of NMDA receptor-channels by 2 mM [Mg2+]o during the train application of NMDA prevented the induction of both inactivation and desensitization. In contrast 3 mM [Mg2+]i was ineffective. 7. The magnitude of both inactivation and desensitization of NMDA receptors was not affected by intracellular dialysis of ATP, the non-hydrolysable ATP analogue 5'-adenylylimido-diphosphate (AMP-PNP), different Ca2+ chelators (EGTA or BAPTA), the Ca(2+)-activated protease inhibitor (leupeptin), dithiothreitol, or the phosphatase inhibitors (okadaic acid and a calcineurin inhibitor). 8. Application of 2.5 mM Ca2+ to the cytoplasmic side of inside-out patches induced inactivation of NMDA responses similar in magnitude to the inactivation seen in whole-cell recording.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









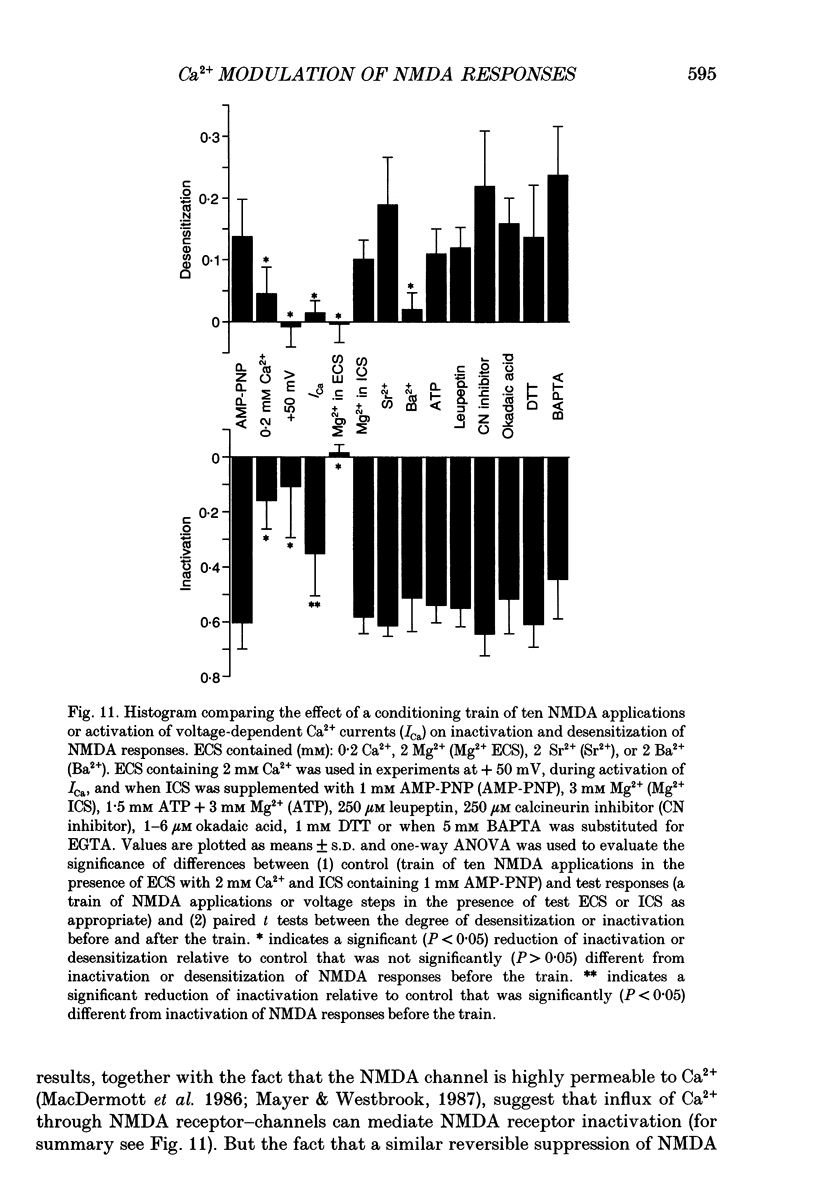





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman E., Lipton S. A., Loring R. H. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989 Mar;2(3):1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
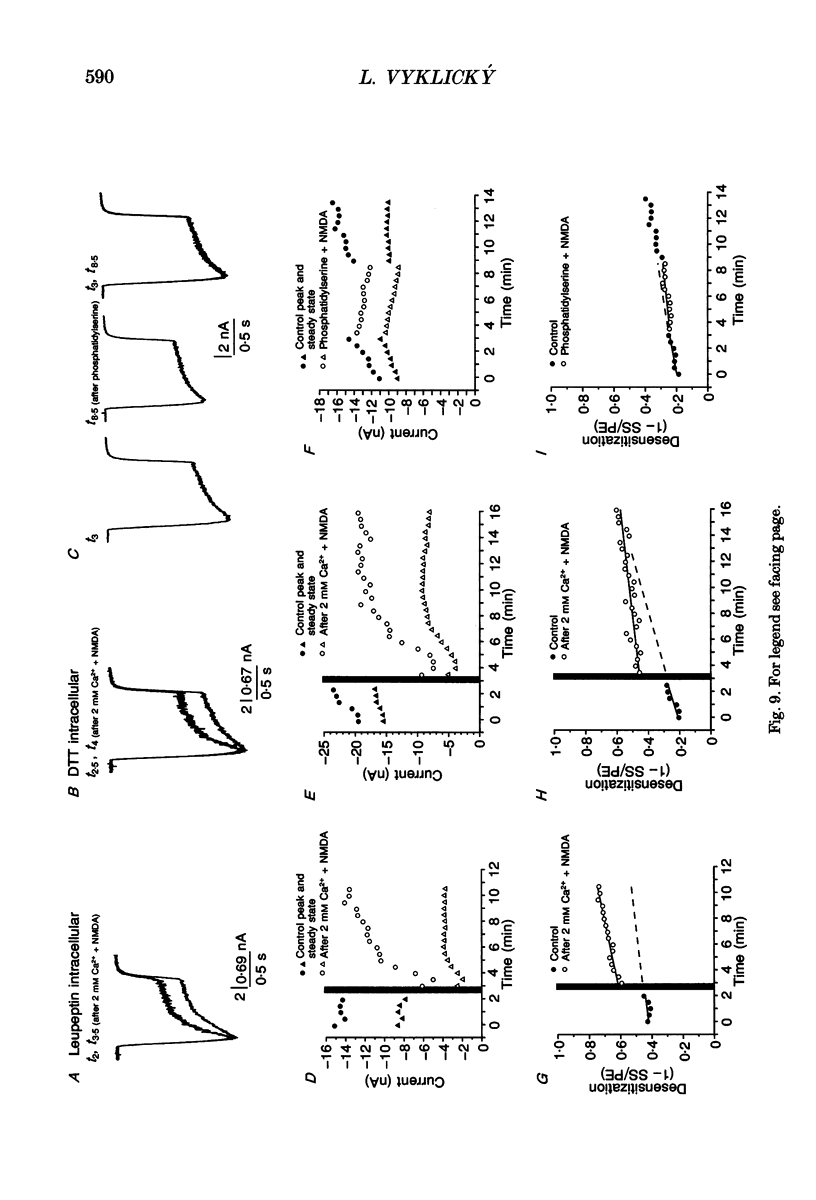
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
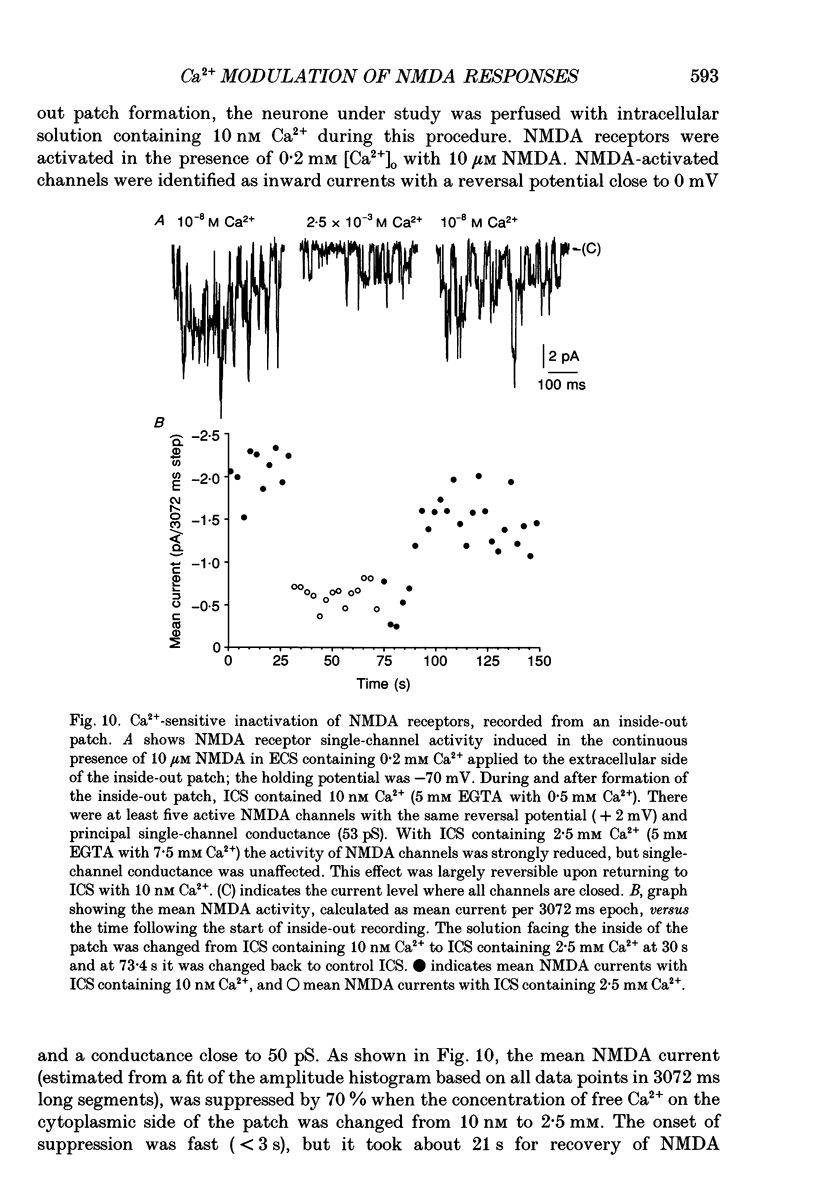
- Bading H., Greenberg M. E. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991 Aug 23;253(5022):912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R. J., Smith K. L., Swann J. W. Calcium modulation of the N-methyl-D-aspartate (NMDA) response and electrographic seizures in immature hippocampus. Neurosci Lett. 1991 Mar 11;124(1):92–96. doi: 10.1016/0304-3940(91)90829-i. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron. 1991 Aug;7(2):319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- Chizhmakov I. V., Kiskin N. I., Krishtal O. A. Two types of steady-state desensitization of N-methyl-D-aspartate receptor in isolated hippocampal neurones of rat. J Physiol. 1992 Mar;448:453–472. doi: 10.1113/jphysiol.1992.sp019051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. D., Clifford D. B., Zorumski C. F. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39(3):787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Halpain S., Greengard P. Activation of NMDA receptors induces rapid dephosphorylation of the cytoskeletal protein MAP2. Neuron. 1990 Sep;5(3):237–246. doi: 10.1016/0896-6273(90)90161-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Perrino B. A., Soderling T. R. Identification of an autoinhibitory domain in calcineurin. J Biol Chem. 1990 Feb 5;265(4):1924–1927. [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate-activated channels. Biophys J. 1990 May;57(5):1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Draetta G. F., Hubbard M. J. Calcineurin. Adv Enzymol Relat Areas Mol Biol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Legendre P., Rosenmund C., Westbrook G. L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993 Feb;13(2):674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Zukin R. S., Bennett M. V. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Leresche N., Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991 Apr;6(4):565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Sather W., Dieudonné S., MacDonald J. F., Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992 May;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Seubert P., Larson J., Oliver M., Jung M. W., Baudry M., Lynch G. Stimulation of NMDA receptors induces proteolysis of spectrin in hippocampus. Brain Res. 1988 Sep 13;460(1):189–194. doi: 10.1016/0006-8993(88)91222-x. [DOI] [PubMed] [Google Scholar]
- Siman R., Noszek J. C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988 Jun;1(4):279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol. 1991 Feb;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklický L., Jr, Vlachová V., Krůsek J. The effect of external pH changes on responses to excitatory amino acids in mouse hippocampal neurones. J Physiol. 1990 Nov;430:497–517. doi: 10.1113/jphysiol.1990.sp018304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. K. Developing selective inhibitors of calpain. Trends Pharmacol Sci. 1990 Apr;11(4):139–142. doi: 10.1016/0165-6147(90)90060-L. [DOI] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J., Fischbach G. D. Calcium-dependent, slow desensitization distinguishes different types of glutamate receptors. Cell Mol Neurobiol. 1989 Mar;9(1):95–104. doi: 10.1007/BF00711446. [DOI] [PMC free article] [PubMed] [Google Scholar]


