Abstract
Background
While serum periostin and Krebs von den Lungen-6 (KL-6) have been acknowledged as independent markers in idiopathic pulmonary fibrosis (IPF) diagnosis, the clinical combinatory potential of these biomarkers combined with high-resolution computed tomography (HRCT) has yet to be fully explored.
Methods
This retrospective study involved 78 participants, comprising 51 UIP-IPF patients and 27 healthy controls. All subjects underwent clinical and laboratory examinations, particularly the detection of periostin and KL-6 using ELISA with innovative HRCT fibrosis score evaluations at admission and discharge during hospitalization in UIP-IPF patients.
Results
In our cohort of patients with IPF, predominantly male, over an average follow-up period of 195.27 days. Serum levels of periostin and KL-6 were significantly elevated in IPF patients compared to healthy controls (*p < 0.05). Post-treatment, KL-6 levels decreased significantly, while periostin levels increased. Notably, periostin exhibited superior prognostic accuracy over KL-6, with a higher AUC of 0.875 than 0.639 in ROC analysis. An increase in periostin levels correlated with disease progression, as evidenced by worsened HRCT fibrotic scores and decreased survival probability. These findings underscore periostin’s potential as a reliable biomarker for assessing IPF severity and therapeutic response.
Conclusion
Our findings underscore the preeminence of serum periostin over KL-6 in UIP-IPF diagnosis, particularly when conjoined with HRCT fibrosis score.
Keywords: periostin, Krebs von den Lungen-6, idiopathic pulmonary fibrosis, HRCT fibrosis score, diagnostic hematology
Introduction
Idiopathic Pulmonary Fibrosis (IPF) is a progressive, chronic lung disorder marked by abnormal wound healing and excessive extracellular matrix (ECM) deposition, leading to a median survival of only 3 to 5 years.1,2 The clinical course of IPF comprises exertional dyspnea, declining lung function, physical activity constraints, impaired quality of life, and ultimately, mortality.3,4 Despite advances in treatment, such as pirfenidone and nintedanib, these therapies have limitations, including challenges related to dose suspension or discontinuation, which can impact functional progression in real-life scenarios.5 Additionally, pharmacological interactions between nintedanib and pirfenidone, especially during the COVID-19 pandemic, further complicate treatment strategies.6 Furthermore, recent studies have highlighted the role of epithelial–mesenchymal transition (EMT) as a major pathogenic driver in IPF, contributing to fibrosis progression.7 These insights underline the need for effective diagnostic biomarkers to predict disease progression and optimize treatment, beyond the current reliance on high-resolution computed tomography (HRCT).
Periostin, a matricellular protein in the Fasciclin family, plays a significant role in the fibrotic process, being upregulated in IPF tissues and linked to worsening lung function. It is a critical intermediary product in the transformation growth factor-beta (TGF-β) and interleukin-13 (IL-13) pathway and acts as a potent fibrogenic cytokine. It is also induced by bronchial epithelial cells and fibroblasts, with its expression markedly elevated in IPF lung tissues, thus implicating it in the pathogenesis of various inflammatory and fibrotic diseases. By binding multiple integrin molecules to target cells, periostin modulates cell function.8–11 Notably, periostin is abundantly expressed in fibroblast foci, which are regions of active fibrosis, presumably through direct TGF-β induction, pinpointing fibroblasts as the primary source of periostin in IPF patients.12–14 Elevated serum periostin levels in IPF patients have been correlated with deteriorating lung function, advocating for its potential role as an IPF biomarker and a harbinger of grim prognosis.15,16 Uchida et al found that it has further elucidated its role in mitigating bleomycin-induced lung fibrosis.17 Moreover, Alzobaidi et al demonstrated that clinical trials have identified serum periostin as a predictive biomarker of overall survival and time to event in IPF patients.18 Similarly, Krebs von den Lungen-6 (KL-6), a glycoprotein predominantly overexpressed in damaged or regenerated type 2 alveolar epithelium in the lung, reflects the severity of interstitial lung diseases.19–21 It has gained popularity as a diagnostic biomarker for IPF. However, its prognostic accuracy of IPF progression remains contested. Particularly, Katoh et al supported that the superior identification capacity of monomeric periostin compared to KL-6 and SP-D levels further questions the role of KL-6 in early IPF detection.22 Likewise, Alzobaidi et al found that monomeric serum periostin is superior to oligomeric periostin and conventional IPF biomarkers, such as KL-6, SP-D, and lactate dehydrogenase in the early detection of IPF progression.18
Despite this knowledge, the comparative predictive efficacy of periostin and KL-6 in early IPF progression remains unclear. This study aims to clarify the predictive value of periostin and KL-6 and investigates their combined utility with HRCT in determining IPF’s clinical trajectory.
Materials and Methods
Study Design and Participants
This study adhered to international IPF protocols,23 encompassing 78 individuals: 51 IPF patients under anti-fibrotic therapy (pirfenidone or nintedanib) for a maximum of not exceeding 407 days and 27 healthy controls, recruited from the First Affiliated Hospital of Guangzhou Medical University between January 2020 and May 2022. IPF patients were stratified into improved (n = 30) or worsened (n = 21) categories based on severity changes, and informed consent was obtained.23 Clinical assessments included demographics, blood tests, pulmonary function tests (PFTs), and HRCT fibrotic scoring. This study was conducted in full accordance with the principles outlined in the Declaration of Helsinki. Ethical approval was obtained from First Affiliated Hospital of Guangzhou Medical University ethics committee (GYFYY-2016-73), and informed consent was secured from all participants or their legal guardians prior to their inclusion in the study.
Pulmonary Function Testing
Pulmonary function parameters, including forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), diffusing capacity for carbon monoxide (DLCO), and FEV1/FVC%, were presented as the percentage of the predicted values (% pred.) and recorded in compliance with ATS/ERS and Global Lung Function Initiative (GLI) recommendations.24,25
HRCT Imaging and Fibrotic Scoring
In the present study, patients diagnosed with IPF underwent evaluation via High-Resolution Computed Tomography (HRCT) alongside clinical attributes, in alignment with the 2022 IPF guidelines.26 The HRCT scans, executed with 1-mm collimation and 1-mm layer thickness, were conducted in 10-mm increments from the apex to the base of the lung, with patients positioned supine and fully aspirated. All HRCT images were assessed independently by two expert radiologists, who remained blinded to patient identities, treatment assignments, and pulmonary function tests. Also, definitive and possible Usual Interstitial Pneumonia (UIP) patterns were identified by HRCT features, and any disagreements were addressed through subsequent reexamination and consensus building.27
Serum Periostin and KL-6 Analysis
Serum samples procured from study participants were stored at −80 °C in preparation for assessment. In this study, the serum periostin and KL-6 levels were measured via commercial assay kits, adhering to manufacturer’s protocols. Serum periostin was determined using a double-antibody sandwich ELISA (courtesy of Jiangsu Meimian Industrial, Jiangsu, China), a technique that provides high specificity by employing two antibodies that bind to different epitopes of the target protein, and adhered strictly to the protocol stipulated by the manufacturer, which includes steps such as sample and reagent preparation, incubation, washing, and detection. KL-6 was ascertained using the HISCL-5000 (Sysmex Corp., Hyogo, Japan), a fully automated immunoassay platform that ensures consistency and precision in measurement, and system’s methodology relies on chemiluminescent enzyme immunoassay technology. The detection ranges for periostin and KL-6 were established as 2–90 pg/mL and 10–6000 U/mL, respectively. Serum KL-6 ≥500 U/mL was indicative of IPF, with periostin serving as a quantitative marker.28
Severity Changes of Early Idiopathic Pulmonary Fibrosis
IPF severity was evaluated at the initial diagnosis and post-therapy using PFTs and HRCT. Disease progression was quantified by an absolute decrease in FVC% pred. ≥10% and/or DLCO% pred. ≥15%, and disease improvement by an absolute increase in FVC% pred. ≥10% and/or DLCO% pred. ≥15% (post-therapy vs initial diagnosis). In our study, we intertwined serum periostin and KL-6 with HRCT fibrotic scoring changes in IPF patients.This innovative approach allowed disease progression to be defined by an increased fibrotic score >10% and disease improvement by a decreased fibrotic score >10% after anti-fibrotic therapy.
Statistical Analysis
Participant data were expressed as means with standard deviations (SD) for continuous variables and as frequencies or percentages for categorical variables. For comparisons among groups, the chi-square test was employed for categorical data, while one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was used for continuous numeric data to assess differences between multiple groups. Spearman’s rank correlation coefficient was applied to evaluate the strength and direction of associations between non-parametric variables. The diagnostic performance of periostin, KL-6, and the combined periostin-KL-6 was evaluated using Receiver Operating Characteristic (ROC) curves, and the Area Under the Curve (AUC) was calculated to quantify the biomarkers’ ability to distinguish IPF patients from healthy controls. All statistical analyses were conducted using SPSS version 26.0, with graphical representations created using R version 4.1.2 and GraphPad Prism version 9.1.1 (223). A p-value of less than 0.05 was considered to indicate statistical significance.
Results
Clinical Characteristics
Table 1 presented 51 IPF patients, predominantly male (86.3%, n = 44), over an average duration of 195.27 ± 11.18 days, alongside 27 healthy subjects. Treatment led to a nominal reduction in BMI from 24.13 ± 0.45 to 23.81 ± 0.52, despite a longer mean smoking history. A notable 41.1% of the IPF cohort were nonsmokers. Biochemical analyses indicated significantly raised serum levels of periostin and KL-6 in IPF patients compared to controls. Post-treatment, KL-6 levels markedly decreased (from 1680.71 ± 1842.60U/mL to 769.34 ± 678.21 U/mL), whereas periostin levels increased (from 13.02 ± 3.66 pg/mL to 16.77 ± 8.98 pg/mL). Pirfenidone was the preferred anti-fibrotic agent over nintedanib (76.5% vs 23.5%). An increase in post-therapy fibrotic scores (from 108.7 ± 77.6 to 141.3 ± 123.0) was observed. Fluctuations in inflammatory indicators (CRP, IL-2, IL-4, IL-6, IL-10, D-Dimer) also emerged, with statistical significance in certain markers when compared to controls (P = 0.011, 0.346, 0.112, 0.295, 0.391 and 0.048), as detailed in Table 1 and Figure S1. 58.8% of IPF patients showed clinical improvement indicated by HRCT scores, while others worsened. Maher et al’s criteria29 identified 20 of 51 patients as early-stage. PFTs revealed no significant post-therapy changes in IPF patients, though their metrics remained notably lower than healthy controls (P > 0.05).
Table 1.
Clinical Characteristics of Patients at Admission and Post-Therapy
| Admission | Post-Therapy | HC | P value | |
|---|---|---|---|---|
| Participants (n) | 51 | 27 | - | |
| Sex | ||||
| Male (n (%))a | 44 (86.3) | 14 (51.9) | - | |
| Female (n (%))a | 7 (13.7) | 13 (48.1) | - | |
| Age (yrs) (mean ± SD)b | 62.08 ± 9.29 | 62.27 ± 9.34 | 44.37 ± 15.77 | 0.916 |
| BMI (mean ± SD)b | 24.13 ± 0.45 | 23.81 ± 0.52 | 23.21 ± 0.38 | 0.638 |
| Smoking (yrs) (mean ± SD)b | 30.00 ± 6.17 | 30.86 ± 6.17 | 24.71 ± 5.38 | 0.794 |
| Never smoked (n (%)) | 21 (41.1) | 20 (74.0) | - | |
| KL-6 (U/mL) (mean ± SD)b | 1680.71 ± 1842.60 *** ### | 769.34 ± 678.21 ^^^ | 317.22 ± 71.38 | < 0.001 |
| Periostin (pg/mL) (mean ± SD) b | 13.02 ± 3.66 *** ### | 16.77 ± 8.98 ^^^ | 7.07 ± 2.57 | < 0.0001 |
| Hospital stay (days) (mean ± SD) b | - | 195.27 ± 11.18 | - | - |
| Medication | ||||
| Pirfenidone (n (%)) | 39 (76.5) | - | - | |
| Nintedanib (n (%)) | 12 (23.5) | - | - | |
| Fibrotic score (mean ± SD)b | 108.7 ± 77.60 * | 141.3 ± 123.02 | - | < 0.05 |
| Serum indications | ||||
| TNF-α (nmol/l) (mean ± SD)b | 1.91 ± 1.08 | 1.99 ± 1.29 | 6.52 ± 2.10 | 0.733 |
| IFN-γ (ng/mL) (mean ± SD)b | 2.01 ± 1.37 | 2.35 ± 1.49 | 4.08 ± 11.10 | 0.243 |
| IgG (g/L) (mean ± SD)b | 14.71 ± 4.99 | 15.05 ± 15.10 | 15.84 ± 3.65 | 0.878 |
| IgA (g/L) (mean ± SD)b | 3.04 ± 1.30 | 2.71 ± 1.39 | 3.36 ± 1.28 | 0.221 |
| IgM (g/L) (mean ± SD)b | 1.09 ± 0.68 | 1.05 ± 0.73 | 1.08 ± 0.67 | 0.792 |
| CRP (mg/L) (mean ± SD)b | 1.02 ± 1.87 * # | 6.02 ± 13.68 ^ | 6.08 ± 1.67 | 0.011 |
| IL-2 (U/L) (mean ± SD)b | 1.20 ± 1.10 * # | 1.01 ± 0.83 ^ | 0.96 ± 0.67 | 0.036 |
| IL-4 (U/L) (mean ± SD)b | 1.87 ± 1.78 * # | 1.41 ± 1.09 ^ | 3.06 ± 1.23 | 0.011 |
| IL-6 (U/L) (mean ± SD)b | 8.90 ± 8.52 * # | 7.19 ± 7.85 ^ | 9.96 ± 9.67 | 0.024 |
| IL-10 (U/L) (mean ± SD)b | 2.52 ± 1.72 * # | 2.81 ± 1.62 ^ | 3.96 ± 1.02 | 0.041 |
| D-D (U/L) (mean ± SD)b | 647.27 ± 626.78 * # | 1227.02 ± 1967.63 ^ | 439.96 ± 384.67 | 0.048 |
| Blood cell ratio | ||||
| Leukocyte (x109/L) (mean ± SD)b | 0.59 ± 0.28 | 0.56 ± 0.21 | 0.49 ± 0.11 | 0.578 |
| Neutrophil (x109/L) (mean ± SD)b | 4.67 ± 2.17 | 4.61 ± 2.20 | 4.62 ± 2.19 | 0.914 |
| Lymphocyte (x109/L) (mean ± SD)b | 1.75 ± 0.65 | 1.75 ± 0.73 | 2.01 ± 0.92 | 0.824 |
| Eosinophils (x109/L) (mean ± SD)b | 0.22 ± 0.16 | 0.24 ± 0.23 | 0.21 ± 0.19 | 0.643 |
| Basophil (x109/L) (mean ± SD)b | 0.03 ± 0.04 | 0.04 ± 0.05 | 0.03 ± 0.02 | 0.448 |
| Severity changes in HRCT | ||||
| Improved (n (%)) | - | 30 (58.8) | - | - |
| Worsened (n (%)) | - | 21 (41.2) | - | - |
| PFTs | ||||
| FEV1% pred. (mean ± SD)b | 78.84 ± 22.50 | 80.31 ± 18.14 | 86.58 ± 17.94 | 0.716 |
| FVC% pred. (mean ± SD)b | 75.22 ± 20.13 | 75.23 ± 18.11 | 88.32 ± 15.96 | 0.998 |
| FEV1/FVC% pred. (mean ± SD)b | 80.00 ± 4.29 | 80.94 ± 5.31 | 85.31 ± 3.92 | 0.332 |
| DLCO% pred. (mean ± SD)b | 49.00 ± 18.87 | 47.92 ± 18.37 | 83.17 ± 17.98 | 0.771 |
| PaO2 (mean ± SD)b | 133.20 ± 40.31 | 137.34 ± 69.49 | 97.53 ± 2.11 | 0.714 |
Notes: Post-hoc Test (Tukey’s): *Admission vs Post-therapy; #Admission vs HC; ^Post-therapy vs HC. aChi-square test used to determine statistical significance for categorical variables; bANOVA was used to determine statistical significance for continuous variables.
Abbreviations: Admission, patients with initial diagnosis; Post-therapy, patients with anti-fibrotic therapy; HC, healthy controls; BMI, body mass index; KL-6, Krebs von den Lungen-6; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; CRP, C-reactive protein; IL-2, interleukin-2; IL-4, interleukin-4; IL-6, interleukin-6; IL-10, interleukin-10; D-D, dimer-dimer; HRCT, high-resolution computed tomography; IPF, idiopathic pulmonary fibrosis; PFTs, Pulmonary function tests; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; DLCO, diffuse lung carbon monoxide.
Serum Periostin Dynamics and HRCT Fibrotic Score
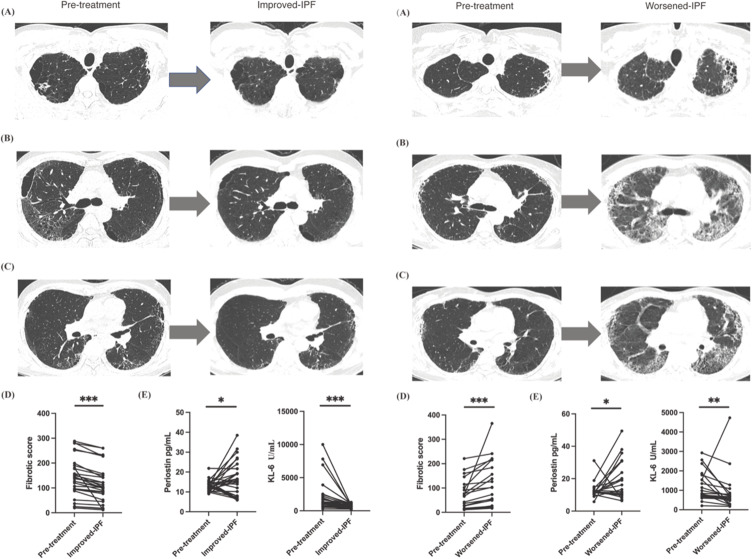
Our analysis of Figure 1 Left A–C presents a 57-year-old male with IPF who exhibited radiological improvement post 115 days of anti-fibrotic therapy, evidenced by a decline in periostin from 14.39 to 9.47 pg/mL. Specifically, diminution of honeycombing in the superior lung fields and a reduction of ground-glass opacities and reticular patterns in the right lung were noted, correlating with a significant fibrotic score decrease from 87.5 to 56.3. This improvement corresponded with an increase in FVC% pred. from 96.63 to 99.28, suggesting fibrotic regression. Conversely, as depicted in Figure 1 Right A–C, a 48-year-old female IPF patient experienced radiological and clinical deterioration after 136 days of therapy, with periostin levels rising from 18.88 to 24.42 pg/mL. This was characterized by an expanded honeycomb pattern and increased ground-glass opacity, culminating in a fibrotic score surge from 70 to 365 and a decrease in FVC% pred. from 77.37 to 71.03, indicating progression of fibrosis. These cases highlight the complex relationship between radiological and serological markers in IPF progression and therapeutic response. Our study revealed a statistically significant association between changes in fibrotic scores, periostin levels, and treatment outcomes (P < 0.05). Patients with declining fibrotic scores generally showed clinical improvement, whereas those with increasing scores experienced worsening conditions. These findings, along with the observed variability in periostin levels in Figure 1 Left D and E and Right D and E, underscore the necessity for further research to elucidate periostin’s role in IPF management.
Figure 1.
(Left A–C) A 56-year-old male exhibited a reduction in his HRCT total fibrosis score, from 87.5 to 56.3. In patients with idiopathic pulmonary fibrosis (UIP-IPF) who were treated with anti-fibrotic medication for 115 days, HRCT changes at the pulmonary apex, bronchi, and bronchioles showed improvement. This was accompanied by a decrease in periostin levels. The lesion shadow can be diminished through the administration of pharmacological agents, as evidenced in the final images. (Left D and E) Concentration changes of periostin, KL-6 and fibrotic score in the stage of pre-treatment and improved IPF stages. (Right A–C) A 48-year-old female exhibited an increase in HRCT total fibrosis score, from 70 to 365. HRCT changes at levels of pulmonary apex, bronchi, and bronchioles in worsened UIP-IPF patients with increasing periostin when acceptance of anti-fibrotic for 136 days. The lesion shadow became augmented when received medication treatment in the final images. (Right D and E) Concentration changes of periostin, KL-6 and fibrotic score in patients with pre-treatment and worsened-IPF. Pre-treatment, means admission; Improved-IPF, means IPF improved after post-therapy; Worsened -IPF, means IPF worsened after post-therapy. *Means <0.05, **Means <0.01, ***Means <0.001.
Serum Periostin versus KL-6
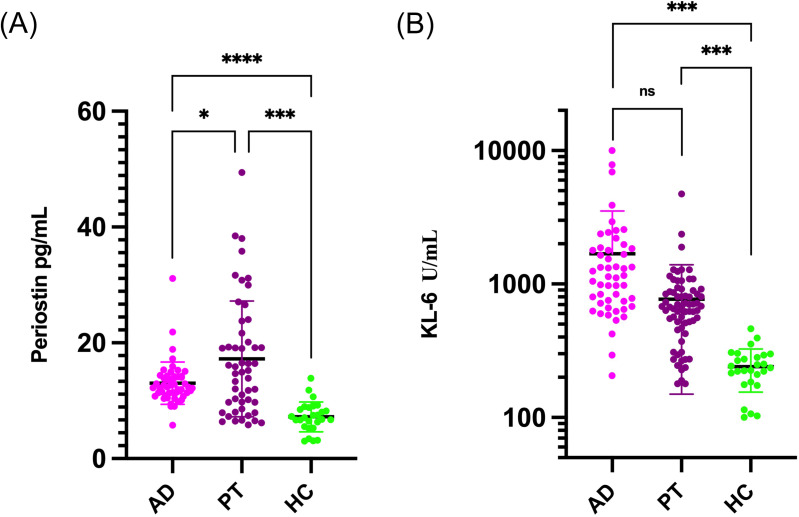
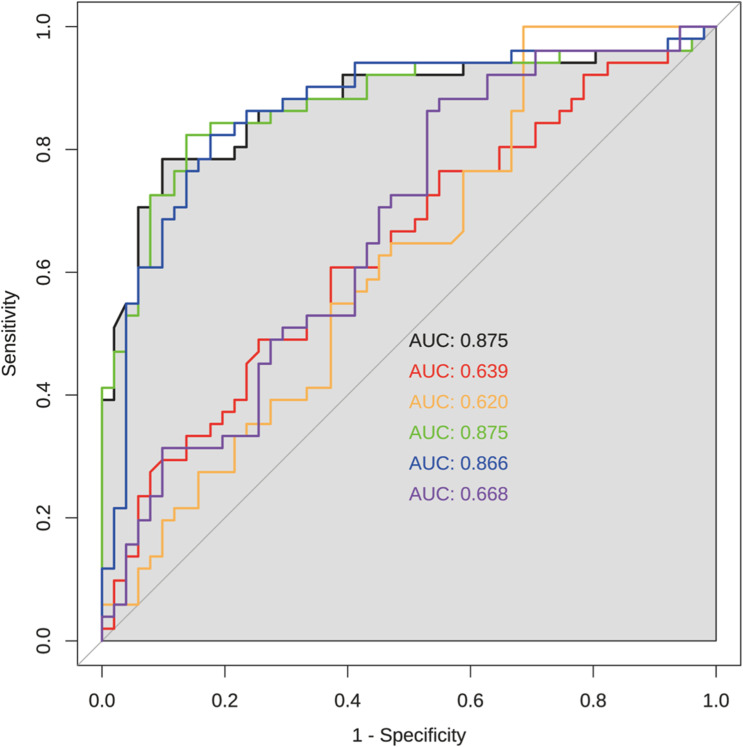
As depicted in Figure 2, periostin demonstrated significant variability between patient groups before and after therapy, as well as between patients and healthy controls, as indicated by scatter analysis (*p < 0.05). In contrast, KL-6 levels did not significantly differentiate between these groups based on logarithmic assessment (ns, p > 0.05), remaining consistent across comparisons (***p < 0.001). The diagnostic efficacy, assessed through ROC curves, underscored periostin’s superiority with the highest AUC of 0.875, both alone and in combination with fibrotic score, and 0.866 when paired with KL-6. Meanwhile, KL-6 alone, and in combination with the fibrotic score, showed significantly lower AUCs of 0.639 and 0.668, respectively (Figure 3).
Figure 2.
Comparison of periostin (A) and KL-6 (B) among admission, post-therapy patients and health controls. *Means <0.05, ***Means <0.001, ****Means <0.0001.
Abbreviatuions: AD, means admission; PT, means post-therapy; HC, means health controls.
Figure 3.
AUC of periostin, KL-6, and fibrotic score in subjects with idiopathic pulmonary fibrosis (IPF). Black line represents periostin; Red line represents KL-6; Orange line represents fibrotic score; Green line represents periostin combined with fibrotic score; Blue line represents periostin combined with KL-6; Purple line represents KL-6 combined with fibrotic score.
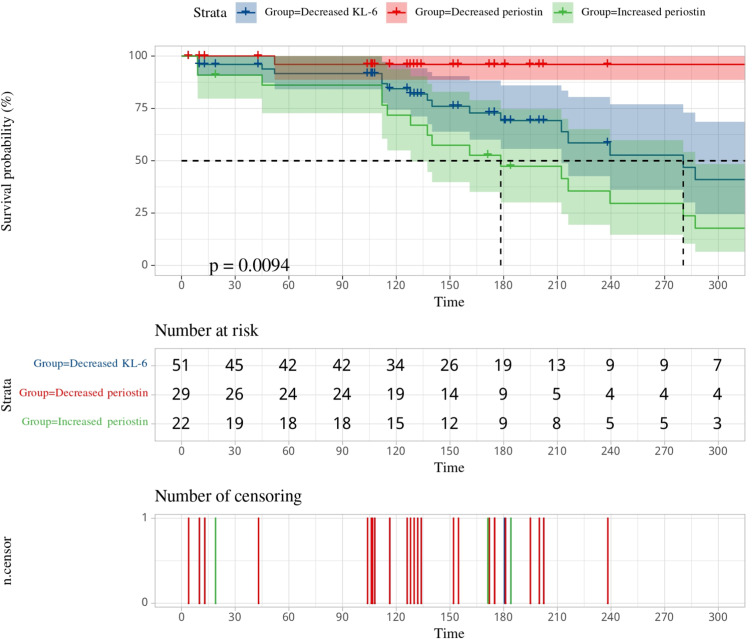
Disease progression correlation with serum biomarkers was further substantiated by contrasting increased periostin levels (n = 22) in 86.4% of patients with disease progression, against 93.1% of improved conditions in those with decreased levels (n = 51) (Table 2). Table 2 also illustrates the predictive value of serum periostin and KL-6 levels in IPF severity status, showing a strong correlation with survival probability (P < 0.01). A focused survival probability analysis based on changes in both biomarkers over 405 days in Figure 4 highlighted a critical trend: within the initial 110 days, a decrease in survival risk was noted across the cohort. Post this period, patients with increased periostin levels displayed a significant decline in survival probability, contrary to those with decreased levels who showed improved survival prospects (p = 0.0094).
Table 2.
Disease Severity Changes Based on Anti-Fibrotic Therapy in IPF Patients
| DecreasedKL-6 (n=51) | Increased Periostin (n=22) | Decreased Periostin (n=29) | P-value | |
|---|---|---|---|---|
| Age (yrs)a | 62.27 ± 9.34 | 61.41 ± 11.23 | 62.93 ± 7.76 | 0.953 |
| Male (n (%))a | 44 (86.3) | 16 (72.73) | 23 (79.3) | 0.813 |
| Female (n (%))a | 7 (13.7) | 6 (27.27) | 6 (20.7) | 0.926 |
| BMIa | 24.13 ± 0.45 | 23.41 ± 4.43 | 24.12 ± 3.04 | 0.953 |
| Smoker (n (%))a | 30 (58.9) | 11 (50) | 18 (62.1) | 0.897 |
| Smoking (yrs)a | 30.00 ± 6.17 | 24.55 ± 9.34 | 32.22 ± 11.14 | 0.953 |
| Severity status by HRCT fibrotic scoring | ||||
| Worsening (n (%))a | 21 (41.2) | 19 (86.4) | 2 (6.9) | < 0.01 |
| Improvement (n (%))a | 30 (58.8) | 3 (13.6) | 27 (93.1) | < 0.01 |
Notes: Data are presented as the mean ± SD or number (%) unless otherwise indicated. [a]ANOVA was used to determine statistical significance for continuous variables.
Abbreviations: KL-6, Krebs von den Lungen-6; BMI, body mass index; HRCT, high-resolution computed tomography; IPF, idiopathic pulmonary fibrosis.
Figure 4.
Survival probability analysis of the IPF patients’ stratification groups.
Assessing Clinical Marker Pre and Post Anti-Fibrotic Therapy
Our investigation into the dynamics of 28 clinical markers in IPF patients, both before and after anti-fibrotic therapy, reveals nuanced interactions as illustrated in Figure S2. Initially, periostin exhibited a significant correlation with PaO2, leukocytes, and CRP, while KL-6 showed a marked correlation with CRP and fibrotic scores. Notably, after treatment, the correlation profile of periostin shifted, revealing strong associations with the fibrotic score and inflammatory markers such as IL-2, IL-10, TNF-α, IFN-γ, and DLCO% pred. In contrast, KL-6 maintained a consistent correlation solely with periostin and the fibrotic score. These findings underline the shifting landscape of biomarker interactions following therapy and highlight periostin’s evolving role in reflecting disease progression.
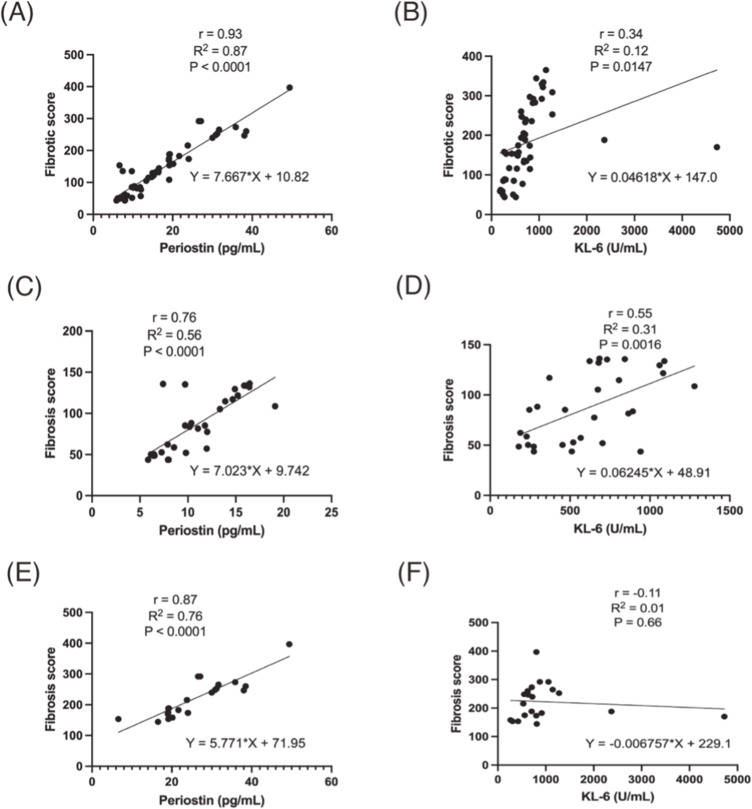
A detailed correlation analysis of the fibrotic score post-treatment highlighted stronger associations with periostin, KL-6, TNF-α, and IFN-γ, as opposed to the pre-treatment associations which were more pronounced with smoking history, KL-6, CRP, and IgG. The treatment-dependent variability in the relationship between periostin and KL-6 prompted further examination through linear regression, as depicted in Figure 5. This regression analysis confirmed a significant positive correlation between the stratified fibrotic scores (≤141.3, >141.3) and each biomarker, with periostin displaying a consistently stronger correlation and model fit than KL-6 (p < 0.0001). These insights affirm periostin’s robust correlation with fibrotic scores from radiological assessments, underscoring its potential as a surrogate marker for the clinical progression in IPF patients. The marked interrelation between periostin levels and the detailed radiological profile of lung fibrosis highlights the biomarker’s clinical relevance.
Figure 5.
Horizontal correlational comparison of fibrotic score with periostin (A, C, E) and KL-6 (B, D, F).
Discussion
Understanding the biomarkers involved in IPF is crucial due to the disease’s progressive nature and poor prognosis. Previous studies have suggested IPF as a progressive, fibrotic disorder often arising from repeated alveolar epithelial injuries and abnormal healing responses, leading to extensive lung damage.1,30–34 This disorder, marked by fibroblast recruitment and ECM production, disrupts the pulmonary architecture,33 highlighting an urgent need for biomarkers that can reliably indicate disease progression, especially in early stages. This study focuses on the roles of periostin and KL-6, which have shown potential in reflecting disease severity and progression.
Consistent with the findings of Katoh et al,22 periostin demonstrated superior prognostic capabilities compared to KL-6. This difference was statistically significant both before and after antifibrotic treatment (p < 0.05), suggesting its potential utility in clinical practice (see Table 1 and Figure 2). Despite discrepancies in the detection of periostin levels across different studies, it may be attributed to the use of various ELISA kits, which may have different specificities and sensitivities for the periostin isoforms – monomeric or oligomeric forms.15,16 The monomeric form of periostin is considered to be the functional form involved in fibrosis, and monomeric periostin ELISA kits sourced from Jiangsu Meimian Industrial preferentially in our study of IPF. In addition, though an increase in the total fibrotic score post-treatment from 108.7 ± 77.6 to 141.3 ± 123.0, over half of our cohort (58.8%) showed improvement in HRCT imaging, a finding that challenges earlier studies where elevated KL-6 levels were consistently associated with IPF severity.19,35,36 Our study also highlights a suite of clinically relevant indicators, such as CRP, fibrotic score, D-Dimer, and interleukins (IL-2, IL-4, IL-6, and IL-10), which collectively paint a comprehensive picture of the IPF landscape, as detailed in Figure S1.
During follow-up, a predominant percentage of patients (76.5%) received pirfenidone therapy, and HRCT fibrotic scores were pivotal in monitoring disease progression (some explanation about medicine in IPF therapy; see section “A brief explanation of Pirfenidone and Nintedanib for the readers’ convenience” in Supplementary Material). In our cohort, 41.2% of IPF patients experienced a worsening of their condition despite exhibiting decreased KL-6 levels. Additionally, we scrutinized disease progression via an analysis of changes in serum periostin levels and HRCT fibrotic scoring. Remarkably, of the 22 patients with an elevated periostin level, a significant majority (86.4%) were in a state of disease worsening. In contrast, among 29 patients with a decreased periostin level, an overwhelming majority (93.1%) demonstrated improvement (P < 0.01). This pattern may reveal that a correlation between rising periostin levels and worsening disease state, while decreasing periostin levels were generally associated with improvement, also aligns with the observations of Ohta et al15 and underscores the dynamic nature of biomarker expression influenced by therapeutic interventions. The correlation between periostin and KL-6, absent at baseline but apparent post-therapy (r = - 0.02; r = 0.44), further supports this notion in Figure S2.
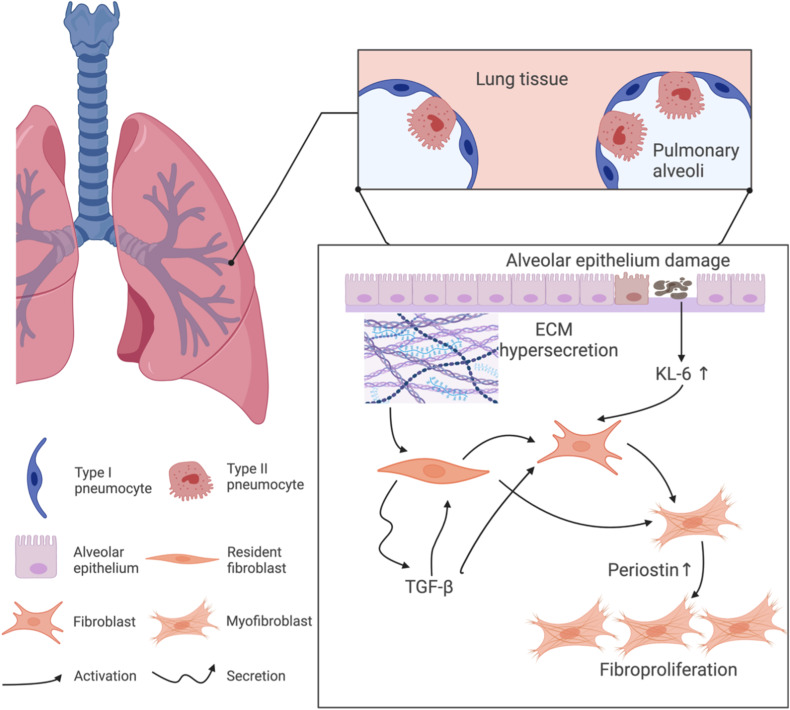
We delved deeper into the roles of periostin and KL-6 within the lung’s microenvironment, Figure 6 outlines their respective contributions to this process. Periostin, primarily produced by fibroblasts in response to TGF-β signaling, plays a critical role in ECM remodeling—a hallmark of fibrotic processes. In addition, its involvement in ECM remodeling and fibroblast proliferation, crucial for fibrotic tissue formation, has been increasingly recognized.1,12,15,26,28 Unlike KL-6, periostin, influenced by TGF-β signaling, directly impacts fibroblast activation and ECM synthesis.1,12,37 TGF-β can directly stimulate resident fibroblasts and induce periostin to engage with cell receptors such as integrin, thereby activating fibroblasts and ultimately catalyzing fibrosis. Furthermore, the intricate cross-talk between TGF-β and periostin enhances the expression of Smad3, a key signal molecule in lung fibrosis as noted by Naik et al.1 Nanri, Y.12 proposed that periostin is upregulated by TGF-β in lung mesenchymal cells and stimulates extracellular matrix deposition, thereby revealing yet another potential avenue for scrutinizing the activity of periostin and fibrosis. This intricate interaction underscores the multifaceted role of periostin in pulmonary fibrosis and is documented in various fibrotic mechanisms, encompassing scleroderma, cancer, bronchopulmonary dysplasia, and asthma.1 Additionally, in contrast to KL-6, periostin, released into the systemic circulation by injured alveolar epithelial cells (AECs), triggers fibroblasts and is secreted by activated fibroblasts, thus potentially reflecting the pulmonary fibrosis alterations in IPF more accurately than KL-6.
Figure 6.
Interplay of periostin and KL-6 in fibroblast proliferation and fibrosis formation.
Emerging research highlights periostin’s crucial role in lung fibrosis, yet its diagnostic and prognostic potential in IPF warrants more exploration, especially when compared to KL-6.1,8,12,16,22,35,38,39 Katoh et al22 posited periostin’s superiority in fibrosis assessment, corroborated by Ohta et al,15 who demonstrated its impact on IPF diagnosis and progression. Interestingly, Nukui et al even have demonstrated that periostin’s expression over KL-6 is not limited to IPF but extends to other fibrotic lung diseases, such as chronic bird-related hypersensitivity pneumonitis, where it serves as a prognostic marker and serum levels correlate with disease severity and progression.40 Similarly, recent studies have highlighted periostin’s involvement in ARDS and COVID-19, where it parallels the fibrotic processes observed in IPF,41 while the dual-specificity protein phosphatase DUSP10/MKP-5 in pulmonary fibrosis has also been mentioned.42
This also appears in our comparative study, confirming periostin’s enhanced prognostic value over KL-6 in all patient cohorts (Figure 2). Despite traditionally using KL-6 for IPF diagnosis, our findings align with Majewski et al,12,35 showing its limitations post-treatment. Conversely, periostin levels correlated with the total fibrotic score, offering a more accurate reflection of disease progression. In Figure 4, analyzing survival probability and two targeted biomarker levels, it emerged that decreased periostin ones fared notably better than the other two groups from day 113 of follow-up (p = 0.0094). Utilizing the ROC curve analysis for these indicators, we found that the AUC for periostin and periostin-KL-6 was 0.875, outperforming that of KL-6 and the fibrotic score alone. In comparing the combined measurements among the three clinical indicators, the AUC for periostin combined with the fibrotic score markedly exceeded that of KL-6 combined with the fibrotic score (AUC: 0.866 vs 0.668). These findings illuminate the potential of serum periostin as a reliable biomarker for predicting the severity changes in early-onset IPF and enhancing the accuracy of IPF disease course diagnosis (Figure 3). This is further substantiated by our use of HRCT fibrotic scores for IPF severity assessment, a technique confirmed by prior research.27,43 Nonetheless, studies associating the HRCT fibrotic score with periostin and/or KL-6, particularly in the course of IPF, are conspicuously absent. We have endeavored to utilize a quantitative stratification of the fibrotic score for IPF, as detailed in Supplementary Materials.27,38,39,43 Our data illustrate that while KL-6 levels decreased, periostin changes align more consistently with fibrosis severity, as evidenced by HRCT (Figures S3 and S4). Post-treatment, periostin not only correlated with HRCT scores more strongly than KL-6 (r: 0.9 vs 0.44) but also demonstrated a higher goodness of fit for disease progression prediction (Figure S2).
HRCT has been widely and accurately used to assess the severity of IPF progression; however, there is almost no study linking the HRCT fibrotic score to periostin and/or KL-6 in early-onset IPF. Therefore, we tried to utilize fibrotic score stratification quantitatively for IPF in Table S1 and radiographic change exemplifications at the level of bronchi in improved and worsened UIP-IPF patients in Figures S3 and S4 to support our findings.27,43 Further, this stratification was also evidenced by levels of pulmonary apex, bronchi, and bronchioles with HRCT. In a 56-year-old male, the HRCT total fibrosis score decreased from 87.5 to 56.3, but periostin levels showed a decreasing trend. Similarly, a 48-year-old woman’s total HRCT fibrosis score increased significantly from 70 to 365, while levels of periostin increased accordingly. Through the analysis of the above cases, we can boldly believe that in the context of patients with KL-6 levels decreased, levels of periostin had altered consistently with the fluctuation of fibrotic score (Figure 1). This is despite periostin being insignificant and not correlated with the HRCT fibrotic score in pre-treatment shown in Figures S4A and S2A. Yet, to our surprise, this biomarker had the highest correlation after antifibrotic treatment and was much greater than KL-6 (r: 0.9 vs 0.44) in Figures S4B and S2B. Comparative correlation analysis and linear regression among these three indicators were formulated to further discern which biomarker held the most promise for diagnosing IPF progression (Table 3 and Figure 5). Both periostin and KL-6 exhibited statistically significant correlations with the fibrotic score (r: 0.93 vs 0.34); however, the goodness of fit was markedly higher in periostin than KL-6 (R2: 0.87 vs 0.12) (Figure 5A and B). Upon setting the mean of the fibrotic score as the threshold, we subdivided the data into two groups for further examination: ≤141.3 and >141.3. Notably, for a fibrotic score of ≤141.3, both periostin and KL-6 demonstrated significant correlations, but KL-6 exhibited suboptimal goodness of fit (Figure 5C and D). In contrast, there was almost no correlation between KL-6 and fibrotic score, while the periostin showed better correlation and goodness of fit in the group of fibrotic score >141.3 (Figure 5E and F).
Table 3.
Linear Regression Analysis Between Periostin, KL-6, and Fibrotic Score
| r | 95% CI | R2 | P-value | |
|---|---|---|---|---|
| Periostin/Fibrotic score | 0.93 | (6.81, 8.52) | 0.87 | < 0.0001 |
| KL-6/Fibrotic score | 0.34 | (0.07, 0.56) | 0.12 | 0.01 |
| Periostin/Fibrotic score ≤ 141.3 | 0.76 | (0.56, 0.88) | 0.56 | < 0.0001 |
| KL-6/Fibrotic score ≤ 141.3 | 0.55 | (0.24, 0.76) | 0.31 | 0.0016 |
| Periostin/Fibrotic score > 141.3 | 0.87 | (0.71, 0.95) | 0.76 | < 0.0001 |
| KL-6/Fibrotic score > 141.3 | −0.11 | (−0.51, 0.34) | 0.01 | 0.66 |
Abbreviations: KL-6, Krebs von den Lungen-6; CI, confidence interval.
In light of this, the enhanced correlation between periostin levels and HRCT fibrotic scores, especially when the fibrotic score is low, could indicate that periostin reflects early and potentially reversible stages of fibrosis, which could be pivotal for timely therapeutic interventions. The superior performance of periostin compared to KL-6 in this respect could be attributable to its direct involvement in extracellular matrix remodeling and fibroblast activation, processes central to the pathophysiology of IPF. These findings suggest that periostin may more accurately depict the ongoing pathological changes within the lungs, thus serving as a critical marker for early detection and management of IPF. Such insights pave the way for the development of targeted therapies aimed at modulating periostin activity, potentially halting or reversing the fibrotic process. Future research could focus on the therapeutic implications of these biomarkers, exploring the benefits of periostin inhibitors and their role in the treatment landscape of IPF.
Conclusion
Our research endeavors have explored the clinical utility of periostin and KL-6, two forefront biomarkers, for the preliminary diagnosis and anti-fibrotic treatment of IPF. To enhance the insights drawn from these biomarkers, we have employed an innovative HRCT fibrotic scoring system to gauge periostin levels in various stages of IPF for the first time. Our findings suggest that periostin serum measurements could hold significant potential in prognosticating the severity trajectory of IPF, particularly during its early onset, as well as in predicting the prognosis of patients with UIP-IPF.
Acknowledgments
The authors would like to express their profound gratitude to Hiplot (https://hiplot-academic.com/) for the expert plotting services they provided. Furthermore, their appreciation goes to Jiangsu Meimian Industrial, Jiangsu, China, for their invaluable technical support during the experiment. Without their contributions, this work would not have been possible.
Funding Statement
This study was supported by the Independent Project of the State Key Laboratory of Respiratory Disease (SKLRD-Z-202305), General guidance project of health science and technology in Guangzhou (20231A011082), Guangdong Zhong Nanshan Medical Foundation (ZNSXS-20220015 and ZNSXS-20220019), Natural Science Foundation of Guangdong Province (2021B1515230008). We are grateful for the generous support that enabled us to undertake this research and extend our sincere appreciation to the institutions involved.
Abbreviations
IPF, idiopathic pulmonary fibrosis; UIP, Usual interstitial pneumonia; HRCT, high-resolution computed tomography; ECM, Extracellular matrix; IL-13, Interleukin-13; BMI, Body mass index; TGF-β, transforming growth factor-β; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusing capacity for carbon monoxide; SP-D, surfactant protein-D; AUC, Area under the curve.
Data Sharing Statement
The present article contains original contributions that have been thoroughly reviewed and discussed. Those seeking further information on the subject matter may direct their queries to the corresponding authors.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Nanri Y, Nunomura S, Terasaki Y, et al. Cross-talk between transforming growth factor-β and periostin can be targeted for pulmonary fibrosis. Am J Respir Cell Mol Biol. 2020;62(2):204–216. doi: 10.1165/rcmb.2019-0245OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song S, Fu Z, Guan R, et al. Intracellular hydroxyproline imprinting following resolution of bleomycin-induced pulmonary fibrosis. Eur Respir J. 2022;59(5):2100864. doi: 10.1183/13993003.00864-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American thoracic society. Idiopathic pulmonary fibrosis: diagnosis and treatment. Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00 [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Cheng ZJ, Xue M, et al. The application of metabolomics toward idiopathic pulmonary fibrosis and potential metabolomic value of diverse samples in interstitial lung diseases. Precision Medical Sciences. 2023;12(3):134–143. doi: 10.1002/prm2.12106 [DOI] [Google Scholar]
- 5.Ruaro B, Salotti A, Reccardini N, et al. Functional progression after dose suspension or discontinuation of nintedanib in idiopathic pulmonary fibrosis: a real-life multicentre study. Pharmaceuticals. 2024;17(1):119. doi: 10.3390/ph17010119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serra López-Matencio JM, Gómez M, Vicente-Rabaneda EF, et al. Pharmacological interactions of nintedanib and pirfenidone in patients with idiopathic pulmonary fibrosis in times of COVID-19 pandemic. Pharmaceuticals. 2021;14(8):819. doi: 10.3390/ph14080819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salton F, Ruaro B, Confalonieri P, et al. Epithelial-mesenchymal transition: a major pathogenic driver in idiopathic pulmonary fibrosis? Medicina. 2020;56(11):608. doi: 10.3390/medicina56110608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpagnano GE, Soccio P, Scioscia G, et al. The potential role of airways periostin in the clinical practice of patients affected by idiopathic pulmonary fibrosis. Rejuvenation Res. 2021;24(4):302–306. doi: 10.1089/rej.2020.2401 [DOI] [PubMed] [Google Scholar]
- 9.Passalacqua G, Mincarini M, Colombo D, et al. IL-13 and idiopathic pulmonary fibrosis: possible links and new therapeutic strategies. Pulm Pharmacol Ther. 2017;45:95–100. doi: 10.1016/j.pupt.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 10.Leppäranta O, Sens C, Salmenkivi K, et al. Regulation of TGF-β storage and activation in the human idiopathic pulmonary fibrosis lung. Cell Tissue Res. 2012;348(3):491–503. doi: 10.1007/s00441-012-1385-9 [DOI] [PubMed] [Google Scholar]
- 11.Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14(7):1239–1249. doi: 10.1359/jbmr.1999.14.7.1239 [DOI] [PubMed] [Google Scholar]
- 12.Naik PK, Bozyk PD, Bentley JK, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1046–L1056. doi: 10.1152/ajplung.00139.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izuhara K, Nunomura S, Nanri Y, et al. Periostin: an emerging biomarker for allergic diseases. Allergy. 2019;74(11):2116–2128. doi: 10.1111/all.13814 [DOI] [PubMed] [Google Scholar]
- 14.Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046 [DOI] [PubMed] [Google Scholar]
- 15.Ohta S, Okamoto M, Fujimoto K, et al. The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis. PLoS One. 2017;12(3):e0174547. doi: 10.1371/journal.pone.0174547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto M, Hoshino T, Kitasato Y, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37(5):1119–1127. doi: 10.1183/09031936.00059810 [DOI] [PubMed] [Google Scholar]
- 17.Uchida M, Shiraishi H, Ohta S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46(5):677–686. doi: 10.1165/rcmb.2011-0115OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzobaidi N, Rehman S, Naqvi M, et al. Periostin: a potential biomarker and therapeutic target in pulmonary diseases. J Pharm Pharm Sci. 2022;25:137–148. doi: 10.18433/jpps32306 [DOI] [PubMed] [Google Scholar]
- 19.d’Alessandro M, Bergantini L, Cameli P, et al. Serum concentrations of KL-6 in patients with IPF and lung cancer and serial measurements of KL-6 in IPF patients treated with antifibrotic therapy. Cancers. 2021;13(4):689. doi: 10.3390/cancers13040689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.d’Alessandro M, Bergantini L, Cameli P, et al. Krebs von den Lungen-6 as a biomarker for disease severity assessment in interstitial lung disease: a comprehensive review. Biomarker Med. 2020;14(8):665–674. doi: 10.2217/bmm-2019-0545 [DOI] [PubMed] [Google Scholar]
- 21.Wakamatsu K, Nagata N, Kumazoe H, et al. Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respir Investig. 2017;55(1):16–23. doi: 10.1016/j.resinv.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 22.Katoh S, Matsumoto N, Tanaka H, et al. Elevated levels of periostin and TGF-β1 in the bronchoalveolar lavage fluid of patients with idiopathic eosinophilic pneumonia. Asian Pac J Allergy Immunol. 2020;38(3):208–213. doi: 10.12932/AP-111018-0414 [DOI] [PubMed] [Google Scholar]
- 23.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline Am J Respir Crit Care Med. 2018;198(5):e44–e68. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson JA, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 25.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 26.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an Update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–e47. doi: 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sverzellati N. Highlights of HRCT imaging in IPF. Respir Res. 2013;14(1):S3. doi: 10.1186/1465-9921-14-S1-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto T, Fujii M, Furusawa H, et al. The usefulness of KL-6 and SP-D for the diagnosis and management of chronic hypersensitivity pneumonitis. Respir Med. 2015;109(12):1576–1581. doi: 10.1016/j.rmed.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 29.Maher TM, Stowasser S, Nishioka Y, et al. Biomarkers of extracellular matrix turnover in patients with idiopathic pulmonary fibrosis given nintedanib (INMARK study): a randomised, placebo-controlled study. Lancet Respir Med. 2019;7(9):771–779. doi: 10.1016/S2213-2600(19)30255-3 [DOI] [PubMed] [Google Scholar]
- 30.Leslie KO. Idiopathic pulmonary fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung: a unifying hypothesis regarding etiology and pathogenesis. Arch Pathol Lab Med. 2012;136(6):591–600. doi: 10.5858/arpa.2011-0511-OA [DOI] [PubMed] [Google Scholar]
- 31.Knudsen L, Ruppert C, Ochs M. Tissue remodelling in pulmonary fibrosis. Cell Tissue Res. 2017;367(3):607–626. doi: 10.1007/s00441-016-2543-2 [DOI] [PubMed] [Google Scholar]
- 32.Confalonieri P, Volpe MC, Jacob J, et al. Regeneration or repair? The role of alveolar epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis (IPF). Cells. 2022;11(13):2095. doi: 10.3390/cells11132095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, Jiang Q, Chen R, et al. Tacrolimus ameliorates bleomycin-induced pulmonary fibrosis by inhibiting M2 macrophage polarization via JAK2/STAT3 signaling. Int Immunopharmacol. 2022;113:109424. doi: 10.1016/j.intimp.2022.109424 [DOI] [PubMed] [Google Scholar]
- 34.Liu M, Xue M, Zhang T, et al. Detection of interstitial pneumonia with autoimmune features and idiopathic pulmonary fibrosis are enhanced by involvement of matrix metalloproteinases levels and clinical diagnosis. J Clin Lab Analysis. 2022;36(11):e24734. doi: 10.1002/jcla.24734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majewski S, Szewczyk K, Żal A, et al. Serial measurements of circulating KL-6, SP-D, MMP-7, CA19-9, CA-125, CCL18, and periostin in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy: an exploratory study. J Clin Med. 2021;10(17):3864. doi: 10.3390/jcm10173864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett D, Salvini M, Fui A, et al. Calgranulin B and KL-6 in bronchoalveolar lavage of patients with IPF and NSIP. Inflammation. 2019;42(2):463–470. doi: 10.1007/s10753-018-00955-2 [DOI] [PubMed] [Google Scholar]
- 37.Izuhara K, Nunomura S, Nanri Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017;74(23):4293–4303. doi: 10.1007/s00018-017-2648-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomaru A, Kobayashi T, Hinneh JA, et al. Oligonucleotide-targeting periostin ameliorates pulmonary fibrosis. Gene Ther. 2017;24(11):706–716. doi: 10.1038/gt.2017.80 [DOI] [PubMed] [Google Scholar]
- 39.Yoshihara T, Nanri Y, Nunomura S, et al. Periostin plays a critical role in the cell cycle in lung fibroblasts. Respir Res. 2020;21(1):38. doi: 10.1186/s12931-020-1299-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nukui Y, Miyazaki Y, Masuo M, et al. Periostin as a predictor of prognosis in chronic bird-related hypersensitivity pneumonitis. Allergol Int. 2019;68(3):363–369. doi: 10.1016/j.alit.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 41.Ntatsoulis K, Karampitsakos T, Tsitoura E, et al. Commonalities between ards, pulmonary fibrosis and COVID-19: the potential of autotaxin as a therapeutic target. Front Immunol. 2021;12:687397. doi: 10.3389/fimmu.2021.687397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xylourgidis N, Min K, Ahangari F, et al. Role of dual-specificity protein phosphatase DUSP10/MKP-5 in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2019;317(5):L678–l689. doi: 10.1152/ajplung.00264.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung JH, Goldin JG. Interpretation of HRCT scans in the diagnosis of IPF: improving communication between pulmonologists and radiologists. Lung. 2018;196(5):561–567. doi: 10.1007/s00408-018-0143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The present article contains original contributions that have been thoroughly reviewed and discussed. Those seeking further information on the subject matter may direct their queries to the corresponding authors.