Abstract
Chimpanzees infected with the primary isolate DH012 mount potent neutralizing antibodies. This DH012 neutralizing activity is highly strain specific. Immune sera from guinea pigs immunized with recombinant DH012 gp120 could also neutralize this primary isolate. The neutralizing activity in chimpanzee and guinea pig sera against wild-type DH012 appears to be independent of a linear epitope in the V3 region of gp120. Interestingly, the neutralization escape mutant derived from growing DH012 in the presence of the potent neutralizing chimpanzee serum is at least 50-fold more sensitive than wild-type DH012 to neutralization by guinea pig immune sera. The unusually potent neutralizing activity against the DH012 neutralization-resistant virus is due to the presence of anti-V3 antibodies in guinea pig sera. These results suggested that recombinant gp120 could induce neutralizing antibodies against primary isolate DH012. The V3 of wild-type DH012 is poorly immunogenic in infected chimpanzees and is not accessible to neutralizing V3 antibodies. It is likely that this cryptic V3 region became exposed when the virus escaped the neutralizing activity of the chimpanzee serum.
Human immunodeficiency virus type 1 (HIV-1) infection generally provokes a vigorous antibody response to the envelope glycoproteins gp120 and gp41. The envelope glycoproteins are synthesized as a precursor protein, gp160, which is subsequently processed to gp120 and gp41 inside the cell. Most of the antibodies bind well to the envelope glycoproteins without exhibiting neutralizing activities. The immunodominant epitope of gp41 is located in the sequence immediately N-terminal to a cysteine miniloop (40). However, this gp41 immunodominant epitope is not a neutralization epitope, possibly due to its inaccessibility on the mature oligomeric native envelope (40). Thus, the antibodies, which are specific for this immunodominant region, are not able to neutralize the virus (37, 53). The antibody response to gp120 is predominantly directed to the CD4 binding sites, the V2 and V3 regions (6), and the epitopes containing the N- or C-terminal residues of gp120. Most antibodies directed to these regions do not neutralize primary isolates, probably due to the poor accessibility of these epitopes on the native oligomeric envelope (6).
Neutralization epitopes that are accessible on laboratory-adapted HIV-1 strains but are poorly represented on primary isolates include the V3 region (16, 27, 38), the CD4 binding site (19, 21, 29, 33, 48, 49), and the V2 region (13, 26, 47) of gp120. The V3 loop, a disulfide cross-bridged loop in the third variable domain of gp120, has been described as the principal neutralization determinant for laboratory-adapted HIV-1 strains, such as HIV-1 LAI. This region could account for most of the neutralizing activity raised by the envelope immunogens, gp120 (36), gp160 (39), and principal neutralization determinant-containing peptides (17, 32, 35, 38) derived from certain cell-line-adapted viruses, such as HIV-1 LAI and RF. However, anti-V3 antibodies inconsistently neutralize primary isolates (1, 11, 12, 14, 15, 34, 44, 46, 51). In natural infection, antisera that can neutralize a broad spectrum of HIV-1 primary isolates are rarely detected. Likewise, vaccine sera are generally ineffective against primary isolates (2, 3, 5, 10, 18, 24, 25).
In efforts to better understand HIV-1 primary isolate infection in the chimpanzee model, DH012 was selected for the study based on its excellent replication characteristics in chimpanzee peripheral blood mononuclear cells (PBMCs) (42). This virus was isolated from an AIDS patient in 1991. The DH012 virus exhibits neutralization characteristics similar to those of other primary isolates in that HIV-1-positive sera either fail to neutralize the virus or display modest titers in the 10 to 100 range (Thomas J. Matthews, unpublished results). The DH012 virus used in this experiment had been passed twice in human PBMCs and four times in chimpanzee PBMCs. Although this virus replicated in MT4 and C8166 cell lines, it did not grow in a panel of cell lines commonly used to support replication of cell-line-adapted isolates, such as MT2, CEM, MOLT4, AA5, and SupT1. DH012 replicates efficiently in infected chimpanzees (42), which develop potent neutralizing antibodies against DH012. Previous studies indicated that the neutralizing activity in chimpanzee serum is directed to a complicated conformational epitope involving all the variable regions of gp120 (8). In this study we characterized the neutralizing activity in the chimpanzee sera against both wild-type and neutralization-resistant DH012 variants and attempted to induce this potent neutralizing activity by immunizing guinea pigs with a baculovirus-expressed DH012 gp120.
Strain-specific neutralizing activity.
The neutralizing activity of the chimpanzee sera appears to be strain specific. Both of the chimpanzee sera, C1206 (15wk) and C1206 (31wk), exhibit potent neutralizing activity against DH012, as shown in Table 1. C1206 (15wk) and C1206 (31wk) are chimpanzee sera taken 15 and 31 weeks after infection, respectively. Despite the highly potent activity against DH012, the chimpanzee sera failed to neutralize other viral strains, such as HIV-1 MN, IIIB, and SF2. Broadening of this neutralizing activity did not occur in a serum sample taken 31 weeks after infection (Table 1). The strain-specific neutralization suggests that the neutralizing antibodies in the chimpanzee sera are directed to the variable regions of gp120.
TABLE 1.
Strain-specific neutralization of DH012 antiseraa
| Serum | Neutralization titer for isolate:
|
|||
|---|---|---|---|---|
| DH012 | MN | SF2 | IIIB | |
| C1206 (15wk) | 3,323 | <10 | <10 | <10 |
| C1206 (31wk) | 12,000 | <10 | <10 | <10 |
| NC75 | 36 | <10 | <10 | <10 |
| NC76 | 35 | <10 | <10 | <10 |
Twenty microliters of serially diluted virus stock was incubated for 60 min at ambient temperature with 20 μl of various concentrations of immune sera in RPMI 1640 containing 10% fetal bovine serum and antibiotics in a 96-well microtiter plate. Twenty microliters of MT4 or CEM cells at 6 × 105 cells/ml was added to each well. Cultures were incubated at 37°C in a humidified CO2 incubator. Fresh medium (180 μl) was added to the cultures at day 2. The cells were fed at day 4 by replacing 120 μl of culture supernatant with fresh medium. On day 7 postinfection culture supernatants were harvested and assayed for reverse transcriptase activity, as described previously (7), to monitor viral replication. The numbers in the table represent the serum dilution that was required to reduce virus infectivity by 90%. C1206 (15wk) and C1206 (31wk) are chimpanzee sera taken 15 and 31 weeks after infection, respectively. NC75 and NC76 are sera from guinea pigs immunized with DH012 gp120. Preimmune sera from the chimpanzee and guinea pigs were tested and did not exhibit any significant neutralization titer.
Induction of neutralizing antibodies by recombinant DH012 gp120.
The fact that the chimpanzee serum exhibits such potent neutralizing activity raised the possibility of reproducing this activity by immunizing animals with DH012 gp120. To test this possibility, DH012 gp120 was prepared with a baculovirus expression system. A 6-histidine tag was added to the C terminus of gp120 to facilitate purification of DH012 gp120 under mild conditions. Sera from two guinea pigs immunized with DH012 gp120 can also neutralize the DH012 virus, although the neutralizing activity was approximately 2 logs lower than that of the chimpanzee sera. The guinea pig sera also exhibited strain-specific neutralizing activity (Table 1). The ability of the guinea pig sera to neutralize DH012 supports the notion that neutralization antibodies against primary isolates can be induced with appropriate antigens (23). One of the possible reasons for the weak neutralizing activity in guinea pig sera is that DH012 gp120 only retains a fraction of the native gp120 structure that is required to induce the potent neutralizing activity observed in the sera of DH012-infected chimpanzees. It has been well documented that monomeric recombinant gp120 is conformationally and antigenically different from the native oligomeric envelope. Many anti-monomeric envelope antibodies do not interact with oligomeric envelopes efficiently (30, 31, 40). Sera from HIV-1-positive individuals also contain antibodies that react predominantly to conformational epitopes, including those presented on the native envelope glycoproteins (29, 45). Therefore, it might be possible to increase the potency of the neutralizing activity against DH012 by creating an immunogen that more closely mimics the native DH012 envelope structure.
Neutralization of DH012 viruses is not dependent on a linear V3 epitope.
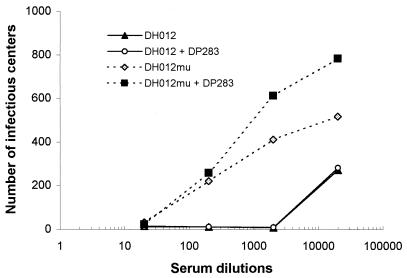
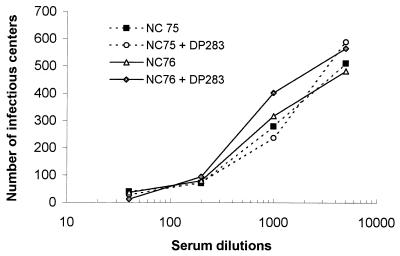
To further characterize the neutralizing activity in chimpanzee and guinea pig sera, a DH012 neutralization-resistant variant (DH012mu) was derived by growing the virus in the presence of escalating doses of neutralizing serum C1206 (15wk) from chimpanzees. The neutralization titer of this chimpanzee serum against DH012mu is lower than 100, whereas the neutralization titer against wild-type DH012 (90% inhibition) is 3,323 (Fig. 1). Although we have shown that multiple variable regions of gp120 are involved in DH012 neutralization, the target of the potent neutralizing activity in chimpanzee serum remains undetermined (8). Since the V3 region could be the target of neutralizing antibodies in both T-cell-adapted and primary isolates (1, 11, 23, 41, 51), the role of the V3 region in the potent neutralization of DH012 was tested by adding a DH012 V3 peptide to the neutralization assays. Addition of the V3 peptide to neutralization assays was shown to abrogate the neutralizing activity directed to the V3 region of T-cell-line-adapted strains, such as HIV-1 IIIB (1, 32, 44, 51). The DH012 V3 peptide DP283 does not significantly affect the neutralizing activity of DH012 chimpanzee serum against either wild-type DH012 or the neutralization-resistant mutant. In addition, we have previously showed that a chimeric virus with the DH012 V3 region in the genetic background of AD8 (AD8-DH120B) is not sensitive to the chimpanzee sera (8). The chimpanzee sera do not neutralize the HIV-1 primary isolate AD8. This argues against the possibility of the involvement of a conformational V3 epitope in DH012 neutralization. These results suggest that the V3 region alone is not sufficient to confer the neutralization sensitivity of DH012 to chimpanzee sera. The same assays also were carried out to test the role of the V3 region in the neutralization of DH012 by guinea pig sera derived from animals immunized with DH012 gp120. Both guinea pig sera neutralized the DH012 virus (Fig. 2). The DH012 V3 peptide DP283 did not significantly affect the DH012 neutralizing activity in the guinea pig sera. Thus, a linear epitope in the V3 loop is unlikely to determine the neutralization sensitivity of DH012 to both chimpanzee and guinea pig sera.
FIG. 1.
DH012 neutralization by the chimpanzee serum C1206 (15wk) in the presence of V3 peptides. A multinuclear activation of a galactosidase indicator assay (MAGI assay) (22) was used to study the neutralizing activity of C1206 (15wk). The MAGI assay involves the use of an incomplete single-cycle replication system to detect HIV infection of HeLa-CD4/β-galactosidase cells. The presence of the HIV-1 transactivation protein tat in HIV-1-infected HeLa-CD4/β-galactosidase cells will turn on the β-galactosidase expression. Therefore, the presence of β-galactosidase is an indication of active HIV-1 replication in the cells. HeLa-CD4/β-galactosidase cells were plated on a 48-well plate at 10,000 cells/well and cultured in Dulbecco's modified Eagle's medium containing 500 μg of G418/ml and 250 μg of hygromycin/ml for 1 day. The cells were infected with virus dilutions in the presence of various dilutions of chimpanzee neutralizing serum and were incubated for 2 days at 37°C. The cells were fixed with a solution containing 1% formaldehyde and 0.2% glutaraldehyde before staining. The infected cells were stained blue by adding 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside at 0.4 mg/ml to the culture. The number of infected cells (infectious centers) was counted by using an AlphaImager (Alpha Innotech). DP283 is a peptide derived from the V3 region of DH012 (CYNTRKGITLGPGRVFYTTGEIVG). DP283 was added to the cultures at 20 μg/ml. DH012mu is the neutralization-resistant mutant derived from culturing DH012 in the presence of escalating concentrations of the chimpanzee C1206 (15wk) serum. The viruses were initially grown in MT4 cells in the presence of C1206 (15wk) at a 1,000-fold dilution. The concentration of C1206 (15wk) was subsequently elevated to 1/500, 1/200, and 1/100. DH12mu is the virus that grows in the presence of C1206 (15wk) at 100-fold dilution.
FIG. 2.
Neutralization of DH012 by guinea pig immune sera in the presence of V3 peptides. Guinea pigs NC75 and NC76 were immunized with baculovirus recombinant DH012 gp120 in the presence of the adjuvant Alum. The animals were boosted three times with the same antigen and adjuvant. The interval between each boost was 3 weeks. DH012 gp120 was prepared by using an Invitrogen Bac-N-Blue expression kit according to the procedure in the brochure provided by the manufacturer. DH012 gp120 contains the entire gp120 sequence plus 6 histidines in the C terminus. The 6-histidine tag was added to the recombinant protein to facilitate affinity purification under mild conditions. The sera obtained from the immunized guinea pigs 2 weeks after the last boost were tested against DH012 virus with or without the presence of the DH012 V3 peptide DP283 (20 μg/ml). The multinuclear activation of a galactosidase indicator assay described in the legend to Fig. 1 was used to evaluate the neutralizing activity of the guinea pig sera.
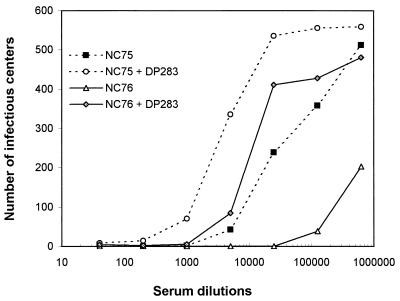
DH012 mutants that are resistant to chimpanzee serum are highly sensitive to guinea pig immune sera.
In contrast to the wild type, DH012mu is very sensitive to the guinea pig immune sera (Fig. 3). The neutralization titers (90% inhibition) of the guinea pig sera, NC75 and NC76, against DH012mu are 8,700 and 184,000, respectively. This potent neutralizing activity is due at least in part to the antibodies that target the V3 region. Addition of the V3 peptide DP283 decreased the neutralization titer (90% inhibition) of NC75 and NC76 to 1,050 and 4,380, respectively. In other words, more than 88 and 95% of the neutralizing activity in NC75 and NC76, respectively, was removed by the V3 peptide DP283. These results suggest that DH012 gp120 induced potent anti-V3-neutralizing antibodies in the guinea pigs. The ability of DH012 gp120 to induce anti-V3 antibodies agrees with previous reports that soluble gp120 can induce anti-V3 antibodies that are responsible at least in part for their neutralizing activity (5, 28). The V3 region does not appear to be accessible in the wild-type DH012 virus, since the virus is not very sensitive to the guinea pig sera. In contrast, the neutralization-resistant mutant DH012mu evolved an envelope with the V3 region accessible to neutralizing antibodies. We have identified a key mutation outside of the V3 region in DH012mu that accounts for the accessibility of the V3 loop (our unpublished data).
FIG. 3.
V3 plays a key role in neutralization of DH012mu by guinea pig sera. Guinea pig sera NC75 and NC76 were tested against the neutralization-resistant mutant DH012mu in the presence or absence of the V3 peptide (20 μg/ml) in a multinuclear activation of a galactosidase indicator assay. The detailed protocol of the multinuclear activation of a galactosidase indicator assay is described in the legend to Fig. 1.
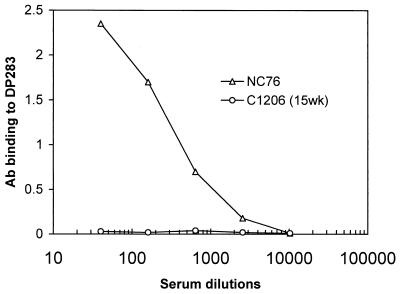
The anti-V3 peptide antibody is absent in chimpanzee serum but is present in guinea pig sera.
To determine the role of anti-V3 antibody in the neutralization of DH012 and DH012mu, the V3 peptide DP283 was used in an enzyme-linked immunosorbent assay (ELISA) to measure the binding between the peptide and the antibodies in chimpanzee and guinea pig sera (Fig. 4). The chimpanzee that was infected with DH012 did not induce antibodies that bind to DP283. The results strengthen the notion that the V3 region is not accessible in the native DH012 envelope glycoproteins. On the other hand, the guinea pig immune serum NC76 contains a high titer of V3 binding antibodies (Fig. 4).
FIG. 4.
Anti-V3 peptide antibodies (Ab) are present in the guinea pig serum but not in the chimpanzee serum. The V3 peptide DP283 was coated on ELISA plates. The detailed procedure of the ELISA was previously described (7). The binding of NC76 and chimpanzee serum C1206 (15WK) to the V3 peptide was detected with horseradish peroxidase-labeled anti-guinea pig and anti-human Fc antibodies (Sigma Immunochemicals, St. Louis, Mo.), respectively.
These results suggest that DH012mu escapes the neutralizing antibodies in chimpanzee serum at the expense of exposing the V3 region. This envelope change is not due to four consecutive passages of DH012 in MT4 cells during the development of the neutralization-resistant mutants. The viruses that had four passages in MT4 cells without the presence of the chimpanzee immune serum did not change their sensitivity to either the chimpanzee sera or the guinea pig sera (data not shown). The V3 region of gp120 was shown to be a key determinant for chemokine receptor usage (4, 9, 20, 43, 50, 52). Despite the apparent change in accessibility of the V3 regions, the neutralization-resistant virus DH012mu did not change its chemokine receptor usage (data not shown). Both DH012 and DH012mu can use either CCR5 or CXCR4 to enter the cells. The envelope sequence of the neutralization-resistant DH012 virus has been determined. Three mutations were observed in the V1/V2, bridging sheet, and V3 regions of DH012mu. Although all three mutations contributed to the neutralization sensitivity of the DH012 viruses, the mutation in the bridging sheet accounts for the change in accessibility of the V3 region (our unpublished data).
Baculovirus-expressed DH012 gp120 appears to be able to induce at least two types of neutralizing antibodies. One of the neutralization activities reacts with the V3 region of gp120, and the other neutralizing activity is not involved in the linear V3 epitope. The neutralizing antibodies induced by infection of chimpanzees with the wild-type virus are very potent and strain specific. Broadening of this restricted neutralizing activity appears to be slow, since the sera taken from weeks 15 and 31 postinfection exhibited a similar neutralization profile. This neutralizing antibody response might be induced with recombinant baculovirus gp120. However, the recombinant protein-induced neutralizing activity is much weaker against the wild-type DH012 virus. It is likely that the native structure of the envelope glycoprotein is needed to induce the potent neutralizing activity. Alternatively, it is possible that the neutralization activities in the guinea pig sera are qualitatively different from that of chimpanzee sera.
Neutralization of HIV-1 primary isolates with anti-V3 antisera has been demonstrated in a simian/human immunodeficiency virus model that contains the envelope of the dualtropic virus HIV-1 89.6 (23). The ability of V3-immune sera to neutralize the virus suggests that the V3 region of simian/human immunodeficiency virus 89.6 is accessible to neutralizing antibodies during the virus infection. On the contrary, the data presented here indicate that anti-V3 antibodies lack neutralizing activity against wild-type DH012. It is likely that the accessibility of the V3 region is varied among HIV-1 primary isolates. Accessibility and sequence variation of the V3 region might pose a tremendous challenge to developing vaccines that are based on inducing antibodies against the V3 region.
Acknowledgments
This work was supported by NIH grant AI40856 (C.-H.C.).
We thank Malcolm A. Martin for providing the chimpanzee sera.
REFERENCES
- 1.Beddows S, Louisirirotchanakul S, Cheingsong-Popov R, Easter-brook P J, Simmonds P, Weber J. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J Gen Virol. 1998;79:77–82. doi: 10.1099/0022-1317-79-1-77. [DOI] [PubMed] [Google Scholar]
- 2.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Gorse G J, Excler J L, Duliege A M, Tartaglia J, Cox W I, McNamara J, Hwang K L, Bradney A, Montefiori D, Weinhold K J. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF2 recombinant vaccines in uninfected volunteers. NIAID AIDS Vaccine Evaluation Group. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Belshe R B, Graham B S, Keefer M C, Gorse G J, Wright P, Dolin R, Matthews T, Weinhold K J, Bolognesi D P, Sposto R, et al. Neutralizing antibodies to HIV-1 in seronegative volunteers immunized with recombinant gp120 from the MN strain of HIV-1. NIAID AIDS Vaccine Evaluation Clinical Trial Network. JAMA. 1994;272:475–480. doi: 10.1001/jama.272.6.475. [DOI] [PubMed] [Google Scholar]
- 4.Bieniasz P D, Fridel R A, Aramori I, Ferguson S S G, Caron M G, Cullen B R. HIV-1 induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bures R, Gaitan A, Zhu T, Graziosi C, McGrath K M, Tartaglia J, Caudrelier P, El Habib R, Klein M, Lazzarin A, Stablein D M, Corey L, Greenberg M L, Schwartz D H, Montefiori D C. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by soluble gp160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 2000;16:2019–2035. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- 6.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11:S87–S98. [PubMed] [Google Scholar]
- 7.Chen C H, Matthews T J, Bolognesi D P, Greenberg M L. A molecular clasp in the HIV-1 TM protein determines the anti-HIV activity of gp41 derivatives: implications for viral fusion. J Virol. 1995;69:3771–3777. doi: 10.1128/jvi.69.6.3771-3777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho M W, Lee M K, Chen C H, Matthews T J, Martin M A. Characterization of a highly potent neutralizing antiserum against a primary HIV-1 isolate elicited in a virus-infected chimpanzee. J Virol. 2000;74:9749–9754. doi: 10.1128/jvi.74.20.9749-9754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocchi F, DeVivco A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Jitters jeopardize AIDS vaccine trials. Science. 1993;262:980–981. doi: 10.1126/science.8235635. [DOI] [PubMed] [Google Scholar]
- 11.Conley A J, Gorny M K, Kessler II J A, Boots L J, Ossorio-Castro M, Koenig S, Lineberger D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447–52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Souza M P, Livnat D, Bradac J A, Bridges S the AIDS Clinical Trials Group Antibody Selection Group and Collaborating Investigators. Evaluation of monoclonal antibodies to HIV-1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 13.Fung M S C, Sun C, Gordon W L, Liou R S, Chang T W, Sun W N C, Daar E S, Ho D D. Identification and characterization of a neutralization site within the second variable region of HIV-1 gp120. J Virol. 1992;66:848–856. doi: 10.1128/jvi.66.2.848-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorny M K, Mascola J R, Israel Z R, VanCott T C, Williams C, Balfe P, Hioe C, Brodine S, Burda S, Zolla-Pazner S. A human monoclonal antibody specific for the V3 loop of HIV type 1 clade E cross-reacts with other HIV type 1 clades. AIDS Res Hum Retrovir. 1998;14:213–221. doi: 10.1089/aid.1998.14.213. [DOI] [PubMed] [Google Scholar]
- 15.Gorny M K, VanCott T C, Hioe C, Israel Z R, Michael N L, Conley A J, Williams C, Kessler II J A, Chigurupati P, Burda S, Zolla-Pazner S. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and interclade cross reactivity. J Immunol. 1997;159:5114–5122. [PubMed] [Google Scholar]
- 16.Goudsmit J, Boucher C A, Meloen R H, Epstein L G, Smit L, van der Hoek L, Bakker M. Human antibody response to a strain specific HIV-1 gp120 epitope associated with cell fusion inhibition. AIDS. 1988;2:157. [PubMed] [Google Scholar]
- 17.Goudsmit J, Debouck C, Meleon R H, Smit L, Bakker M, Asher D M, Wolff A V, Gibbs C J, Gajdusek D C. HIV-1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci USA. 1988;85:4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham B S, McElrath M J, Connor R I, Schwartz D H, Gorse G J, Keefer M C, Mulligan M J, Matthews T J, Wolinsky S M, Montefiori D C, Vermund S H, Lambert J S, Corey L, Belshe R B, Dolin R, Wright P F, Kober B T, Wolff M C, Fast P E. Analysis of intercurrent human immunodeficiency virus infection in phase I and phase II trials of candidate vaccines. AIDS Vaccine Evaluation Group and the Correlates of HIV Immune Protection Group. J Infect Dis. 1998;177:310–319. doi: 10.1086/514209. [DOI] [PubMed] [Google Scholar]
- 19.Ho D D, McKeating J A, Li X L, Moudgil T, Daar E S, Sun N C, Robinson J E. Conformational epitope on gp120 important in CD4 binding and HIV-1 neutralization identified by a human monoclonal antibody. J Virol. 1991;65:489–493. doi: 10.1128/jvi.65.1.489-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman T L, Steohens E B, Narayan O, Dom R W. HIV type 1 envelope determinants for use of the CCR2b, CCR3, STRL33, and APJ coreceptors. Proc Natl Acad Sci USA. 1998;95:11360–11365. doi: 10.1073/pnas.95.19.11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang C Y, Nara P, Chamat S, Caralli V, Rymskamp T, Haigwood N, Newman R, Kohler H. Evidence for non-V3-specific neutralizing antibodies that interfere with gp120/CD4 binding in HIV-1 infected humans. Proc Natl Acad Sci USA. 1991;88:6171–6175. doi: 10.1073/pnas.88.14.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimpton J, Emerman M. Detection of replication competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao H X, Etemad-Moghadam B, Montefiorie D C, Sun Y, Sodroski J, Scearce R M, Doms R W, Thomasch J R, Robinson S, Letvin N L, Haynes B F. Induction of antibodies in Guinea pigs and Rhesus monkeys against the human immunodeficiency virus type 1 envelope: neutralization of nonpathogenic and pathogenic primary isolate simian/human immunodeficiency virus strains. J Virol. 2000;74:254–263. doi: 10.1128/jvi.74.1.254-263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Doloin R, Graham C S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 25.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retrovir. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 26.McKeating J A, Shotton C, Cordell J, Graham S, Balfe P, Sullivan N, Charles M, Page M, Bolmstedt A, Olofsson S. Characterization of neutralizing antibodies to linear and conformational dependent epitopes within the first and second variable regions of HIV-1 gp120. J Virol. 1993;67:4932–4944. doi: 10.1128/jvi.67.8.4932-4944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meloen R H, Liskamp R M, Goudsmit J. Specificity and function of the individual amino acids of an important determinant of HIV-1 that induces neutralizing activity. J Gen Virol. 1989;70:1505. doi: 10.1099/0022-1317-70-6-1505. [DOI] [PubMed] [Google Scholar]
- 28.Montefiori D C, Graham B S, Zhou J, Zhou J, Bucco R A, Schwartz D H, Cavacini L A, Posner M R the N. N. A. V. C. T. Network. V3 specific neutralizing antibodies in sera from HIV gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding site of gp120. J Clin Investig. 1993;92:840–847. doi: 10.1172/JCI116658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 of HIV-1 are highly prevalent in the sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore J P, Sodroski J. Antibody cross competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palker T J, Clark M E, Langloid A J, Matthews T J, Weinhold K J, Randall R R, Bolognesi D P, Haynes B F. Type-specific neutralization of the HIV with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci USA. 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinter A, Honnen W J, Racho M E, Tilley S A. A potent neutralizing human monoclonal antibody against a unique epitope overlapping the CD4-binding site of HIV-1 gp120 that is broadly conserved across North America and Africa virus isolates. AIDS Res Hum Retrovir. 1993;9:985–996. doi: 10.1089/aid.1993.9.985. [DOI] [PubMed] [Google Scholar]
- 34.Poignard P, Klasse P J, Sattentau Q J. Antibody neutralization of HIV-1. Immunol Today. 1996;17:239–246. doi: 10.1016/0167-5699(96)10007-4. [DOI] [PubMed] [Google Scholar]
- 35.Putney S D, Matthews T J, Robey W G, Lynn D L, Robert-Guroff M, Mueller W T, Langloid A J, Ghrayeb J, Petteway S R J R, Weinhold K J, Fischinger P J, Wong-Staal F, Gallo R C, Bolognesi D P. HTLVIII/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986;234:1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- 36.Robey W G, Arthur L O, Matthews T J, Langloid A, Copland T D, Lerch N W, Oroszlan S, Bolognesi D P, Gildon R V, Fischinger P J. Prospect for prevention of human immunodeficiency virus: purified 120 kDa glycoprotein induces neutralizing antibodies. Proc Natl Acad Sci USA. 1986;83:7023–7027. doi: 10.1073/pnas.83.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson W E, Jr, Gorny M K, Xu J Y, Mitchell W M, Zolla-Pazner S. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol. 1991;65:4169–4176. doi: 10.1128/jvi.65.8.4169-4176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rusche J R, Javaherian K, McDanal C, Petro J, Lynn D L, Grimaila R, Langlois A, Gallo R C, Arthur L O, Fischinger P J, Bolognesi D P, Putney S D, Matthews T J. Antibodies that inhibit fusion of HIV-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci USA. 1988;85:3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rusche J R, Lynn D L, Robert-Guroff M, Langloid A J, Lyerly H K, Carson H, Krohn K, Ranki A, Gallo R C, Bolognesi D P, Putney S D, Matthews T J. Humoral immune response to the entire HIV envelope glycoprotein made in insect cells. Proc Natl Acad Sci USA. 1987;84:6924–6928. doi: 10.1073/pnas.84.19.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sattentau Q J, Zolla-Pazner S, Poignard P. Epitopes exposure on functional oligomeric gp41 molecules. Virology. 1995;206:713–717. doi: 10.1016/s0042-6822(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 41.Schreiber M, Wachsmuth C, Muller H, Odemuyiwa S, Schmitz H, Meyer S, Meyer B, Schneider-Mergener J. The V3-directed immune response in natural human immunodeficiency virus type 1 infection is predominantly directed against a variable, discontinuous epitope presented by the gp120 V3 domain. J Virol. 1997;71:9198–9205. doi: 10.1128/jvi.71.12.9198-9205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata R, Hoggan M D, Broscius C, Englund G, Theodore T S, Buckler-White A, Arthur L O, Israel Z, Schultz A, Lane H C, Martin M. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69:4453–4462. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speck R F, Wherly K, Platt E J, Atchison R E, Charo I F, Kabat D, Chesebro B, Goldsmith M A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptor determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spenlehauer C, Saragosti S, Fleury H J, Kirn A, Aubertin A M, Moog C. Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell-line-adapted strains of human immunodeficiency virus type 1. J Virol. 1998;72:9855–9864. doi: 10.1128/jvi.72.12.9855-9864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steimer K S, Scandella C J, Skiles P V, Haigwood N L. Neutralization of divergent HIV-1 isolates by conformation dependent human antibodies to gp120. Science. 1991;254:105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan N, Sun Y, Li J, Hoffman W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell-line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan N, Thali M, Furman C, Ho D D, Sodroski J. Effect of amino acid changes in the V1/V2 region of HIV-1 gp120 glycoprotein on subunit association, syncytium formation, and neutralization by a neutralizing antibody. J Virol. 1993;67:3674–3679. doi: 10.1128/jvi.67.6.3674-3679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thali M, Furman C, Ho D D, Robinson J, Tilley S, Pinter A, Sodroski J. Discontinuous conserved neutralization epitopes overlapping the CD4-binding region of HIV-1 gp120 envelope glycoprotein. J Virol. 1992;66:5635–5641. doi: 10.1128/jvi.66.9.5635-5641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thali M, Olshevsky U, Furman C, Gabuzda D, Posner M, Sodroski J. Characterization of a discontinuous HIV-1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J Virol. 1991;65:6188–6193. doi: 10.1128/jvi.65.11.6188-6193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interaction between HIV-1 and its co-receptor CCR5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 51.VanCott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory adapted isolates of HIV type 1. AIDS Res Hum Retrovir. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 52.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Raffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerad C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 53.Xu J Y, Gorny M K, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991;65:4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]