Abstract
Adenosquamous carcinoma of the pancreas (ASCP) is a rare and aggressive variant of pancreatic cancer, characterized by both adenocarcinoma and squamous cell carcinoma components. It presents significant diagnostic and therapeutic challenges due to its atypical histology and poor prognosis. A 72-year-old male presented with abdominal pain, lighter-colored stools, and intermittent nausea. Initial imaging revealed a complex mass in the distal pancreatic body and tail. Elevated lipase levels and subsequent endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) suggested an atypical pancreatic lesion with keratinizing squamous cells.
Further investigation through fiberoptic bronchoscopy and EBUS-guided transbronchial needle aspiration (TBNA) confirmed carcinoma with squamous differentiation. Genetic testing identified KRAS G12D and PIK3CA mutations. The multidisciplinary tumor board recommended systemic chemotherapy with mFOLFIRINOX and G-CSF support. The patient underwent twelve cycles of mFOLFIRINOX with dose adjustments for thrombocytopenia and effective management of chemotherapy-related side effects. Restaging CT scans showed a decrease in tumor size and stable metastatic nodes. The patient showed a partial biochemical response with decreasing CA 19-9 levels and disease stabilization on imaging. This case demonstrates the critical role of a multidisciplinary approach in managing rare pancreatic malignancies. ASCP requires a comprehensive diagnostic and therapeutic strategy involving advanced imaging, histopathological confirmation, and personalized chemotherapy. Integrating advanced diagnostic techniques, molecular profiling, and a multidisciplinary approach is essential for improving patient outcomes and providing comprehensive care for this challenging malignancy. Addressing the psychological aspects and offering compassionate care are vital for supporting patients through their treatment journey.
Keywords: Adenosquamous carcinoma, Pancreatic cancer, EUS-FNB, KRAS mutation, mFOLFIRINOX, Molecular profiling
Introduction
Pancreatic cancer remains one of the most formidable challenges in oncology, characterized by a high mortality rate and often late-stage diagnosis [1,2]. Among the various histological types, pancreatic ductal adenocarcinoma (PDAC) is the most common yet rarer forms, such as adenosquamous carcinoma of the pancreas (ASCP), pose unique diagnostic and therapeutic challenges [3,4]. ASCP is distinguished by the presence of both adenocarcinoma and squamous cell carcinoma components, which contribute to its aggressive clinical behavior and poorer prognosis compared to other pancreatic neoplasms [5].
The incidence of ASCP is relatively low, accounting for less than 4% of all pancreatic cancers [4,6]. Its clinical presentation can be nonspecific, often mimicking more common gastrointestinal disorders, complicating early detection [7]. Advanced imaging techniques and endoscopic procedures play a crucial role in diagnosing these tumors, but the histopathological confirmation remains the gold standard for definitive diagnosis [3]. The presence of squamous differentiation within a pancreatic tumor often raises differential diagnostic considerations, including metastasis from a primary squamous cell carcinoma of another site [8].
Despite advances in imaging and molecular diagnostics, the optimal management of ASCP is not well-defined due to its rarity [9]. Treatment strategies are typically extrapolated from those used for PDAC, with surgical resection being the primary curative approach for localized disease [10]. However, the aggressive nature of ASCP often necessitates multimodal treatment, including chemotherapy and potentially radiation therapy, especially in metastatic cases [11]. Recent developments in molecular profiling have shed light on the genetic mutations associated with pancreatic cancers, offering potential avenues for targeted therapies and personalized treatment plans [1,12].
Here, we present a rare and complex case of metastatic ASCP, highlighting the diagnostic process, therapeutic interventions, and the role of a multidisciplinary approach in managing such challenging malignancies.
Case presentation
Initial presentation
A 72-year-old male with a past medical history of benign prostatic hypertrophy, coronary artery disease, hyperlipidemia, and hypertension presented to the Emergency Department with 1 day of bilateral lower abdominal pain radiating to the right upper quadrant associated with lighter-colored stools and intermittent nausea. His social history was low-risk; he was a nonsmoker and consumed 1 alcoholic beer 3 to 4 times a week.
On physical examination, the patient had upper abdominal tenderness to palpation. Laboratory studies revealed an elevated lipase level of 972 U/L. Other laboratory findings, including hemoglobin (14.1 g/dL), mean corpuscular volume (MCV) (89.3 fL), platelet count (136 × 109/L), electrolytes, and liver function tests (LFTs), were within normal limits, showing no evidence of anemia, leukocytosis, or thrombocytopenia.
Imaging and initial findings
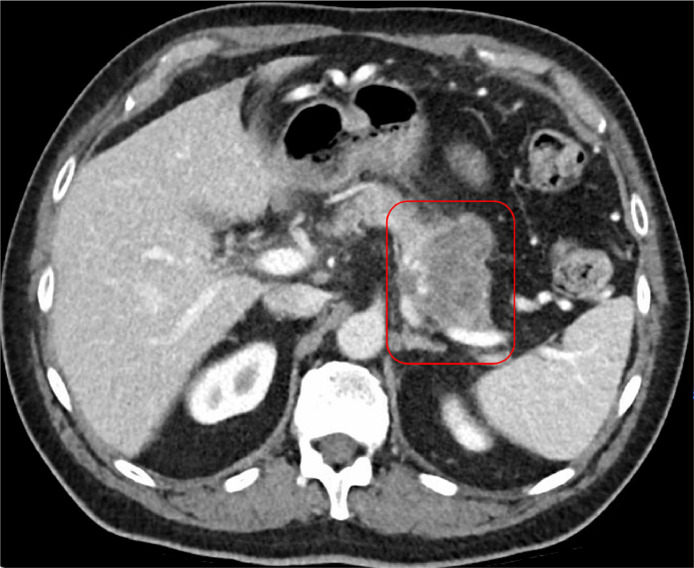
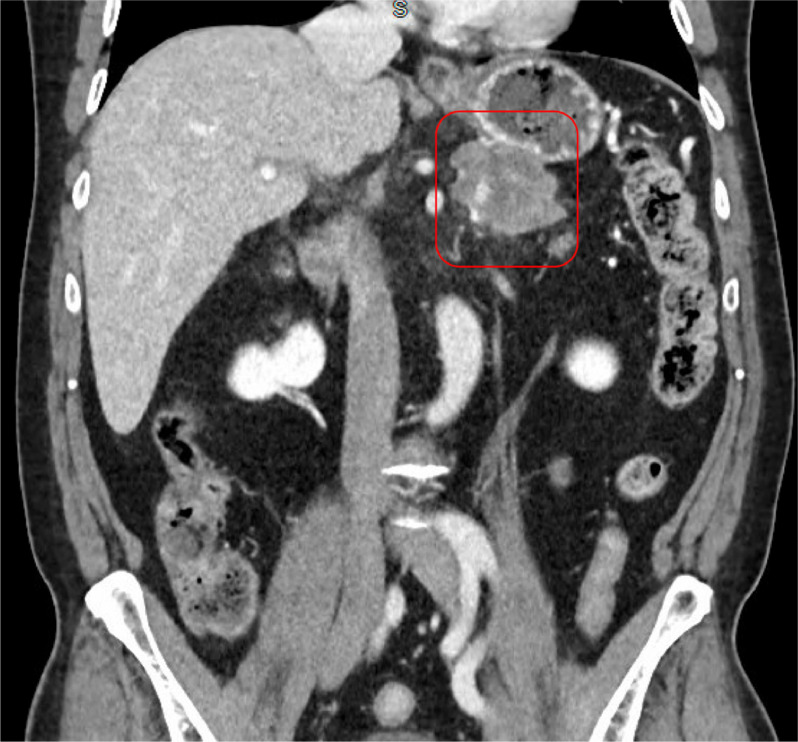
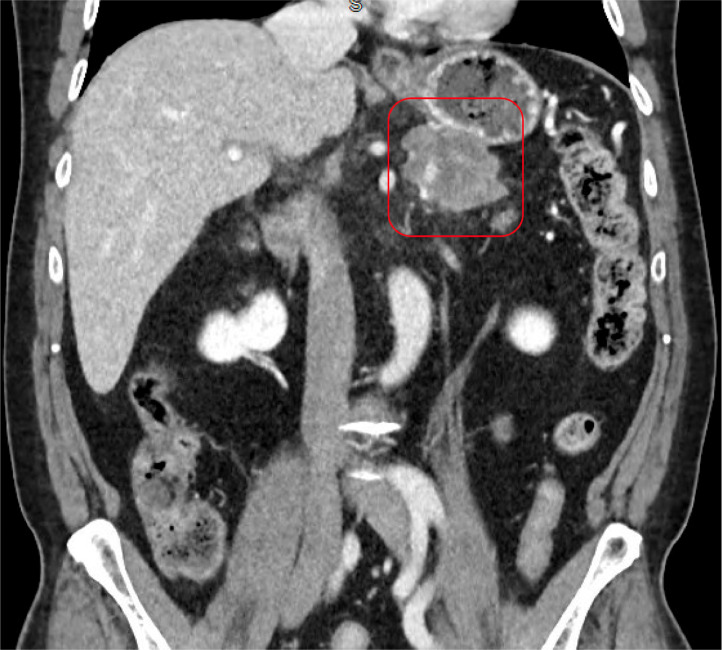
A contrast-enhanced CT scan of the abdomen and pelvis revealed a complex, solid-appearing mass within the distal pancreatic body and tail, measuring 6.0 × 5.5 × 4.3 cm (Fig. 1, Fig. 2). There were no signs of acute pancreatitis. Additionally, a calcified gallstone measuring 1.4 cm was noted without any pericholecystic inflammatory changes or bile duct dilation. Mild wall thickening of the gastric antrum with adjacent fat induration was also observed. A right upper quadrant ultrasound confirmed the presence of cholelithiasis.
Fig. 1.
CT abdomen and pelvis with IV contrast (axial view) shows a complex solid-appearing mass within the distal pancreatic body and tail measuring 6.0 × 5.5 × 4.3 cm (red box), which is highly concerning for pancreatic carcinoma.
Fig. 2.
CT abdomen and pelvis with IV contrast (coronal view) shows a complex solid-appearing mass within the distal pancreatic body and tail measuring 6.0 × 5.5 × 4.3 cm (red box) adjacent to the stomach.
Gastroenterology consultation
The gastroenterology service was consulted, and a CA 19-9 level was measured at 21.89 U/mL (0-37 U/mL). An esophagogastroduodenoscopy was performed with fine-needle biopsy (FNB) and endoscopic ultrasound (EUS). The procedure revealed a well-circumscribed, hypoechoic lesion in the pancreatic tail measuring approximately 5 cm in diameter, with no evidence of peripancreatic lymphadenopathy or liver metastases. The pancreatic tail mass was biopsied, but pathology results were inconclusive, showing atypical, detached keratinizing squamous cells, abundant keratin debris, and blood, with no pancreatic parenchyma identified. The patient was discharged on the day of his esophagogastroduodenoscopy.
Image A.
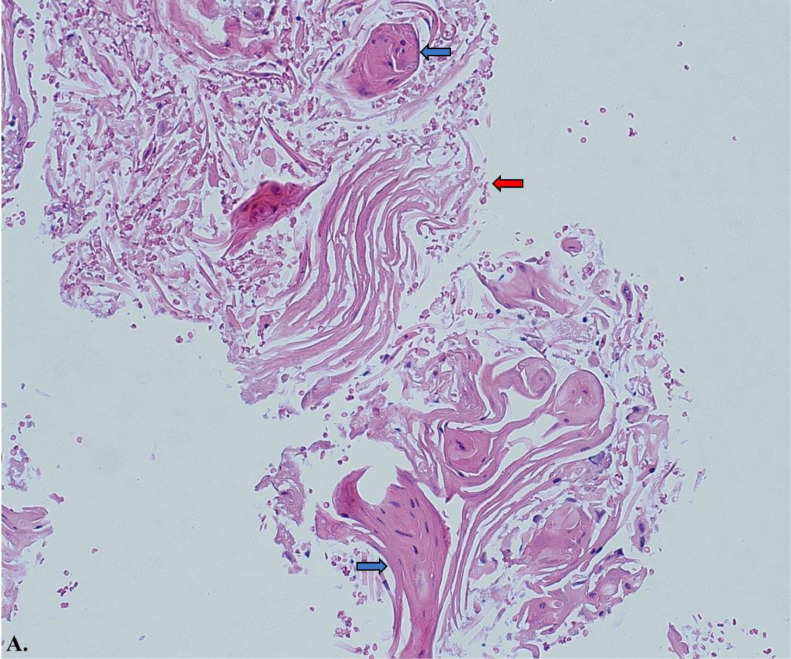
Histology slide from fine needle aspiration (FNA) of pancreatic tail with hematoxylin and eosin stain at 20x magnification. The samples consist predominantly of keratin debris and dyskeratotic squamous cells, with no pancreatic parenchyma identified. The red arrow highlights regions of keratin debris, while the blue arrows depict areas of parakeratosis.
Follow-up and multidisciplinary evaluation
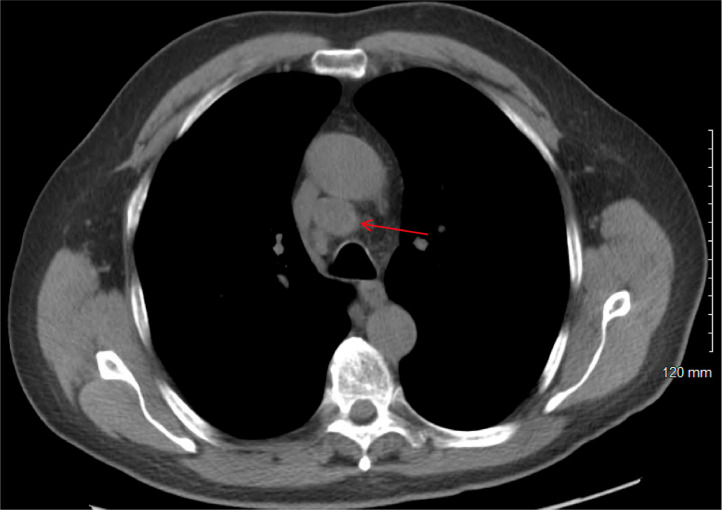
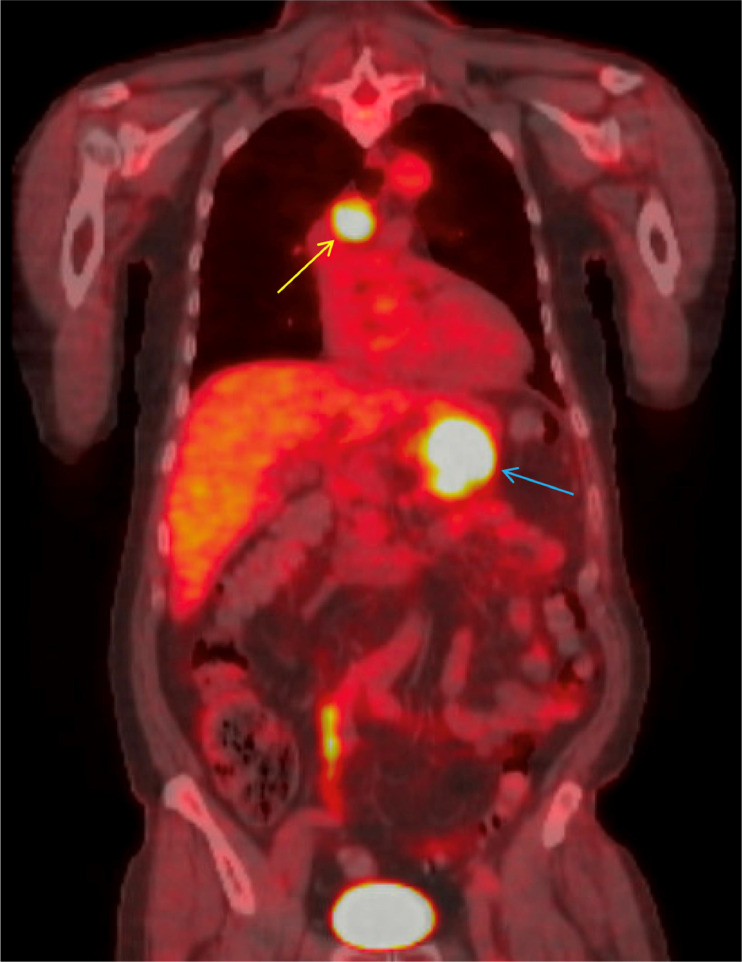
The patient was later evaluated by the hepato-pancreato-biliary (HPB) surgery service. He complained of dull abdominal pain, constipation, and back pain but denied jaundice or scleral icterus. A distal pancreatectomy and splenectomy were planned for both diagnostic and potentially curative purposes. Preoperative imaging included a CT chest without contrast and a PET scan from the skull base to mid-thigh. The CT chest showed an enlarged right paratracheal lymph node (Fig. 3), an enlarged right hilar lymph node, multiple small lung nodules (mostly 1-2 mm), and a pancreatic mass. The PET scan (Fig. 4) revealed a hypermetabolic mass in the pancreatic tail measuring up to 6.5 cm (SUV max 28.4), a 2.3 cm metastatic precarinal lymph node (SUV max 9.1), and several punctate calcified pulmonary micronodules.
Fig. 3.
CT chest without contrast (axial view) showing an enlarged right paratracheal lymph node measuring 2.3 × 2.0 cm.
Fig. 4.
PET CT skull base to mid-thigh (coronal view) with avid mass at the tail of the pancreas measuring 6.5 cm (blue arrow) with intense hypermetabolic activity (SUV max 28.4) and metastatic precarinal lymph node (yellow arrow) measuring 2.3 cm (SUV max 9.1).
Bronchoscopy and EBUS
A fiberoptic bronchoscopy and EBUS-guided transbronchial needle aspiration (TBNA) of the 4R lymph node were performed. The lymph node measured 2.5 × 11 mm. The bronchial mucosa appeared normal, with no endobronchial lesions. Five passes with a 21-gauge needle were made, and samples were sent for cytopathology. Pathology revealed carcinoma with squamous differentiation, characterized by atypical detached keratinizing squamous cells positive for p40 and negative for p16, consistent with metastatic pancreatic adenosquamous carcinoma.
Image B.
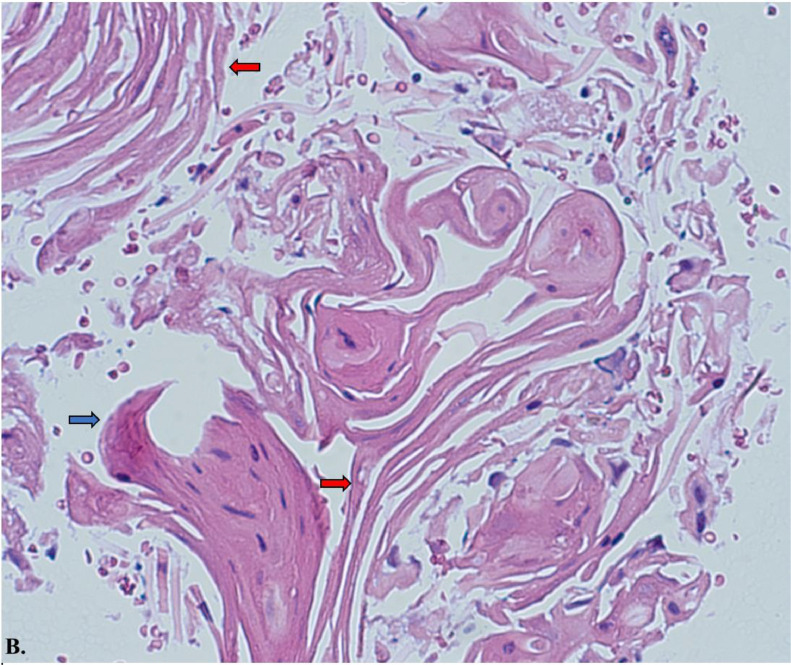
Histology slide from fine needle aspiration (FNA) of pancreatic tail with hematoxylin and eosin stain at 40x magnification. The samples consist predominantly of keratin debris and dyskeratotic squamous cells, with no pancreatic parenchyma identified. The red arrow highlights regions of keratin debris, while the blue arrows depict areas of parakeratosis.
Pathology
Fine needle aspiration (FNA) samples from the pancreatic tail revealed predominantly keratin debris and dyskeratotic squamous cells, with no pancreatic parenchyma identified. The presence of keratin debris and parakeratosis was evident, as shown in Images A and B (Hematoxylin and eosin stains, 20x and 40x magnification, respectively).
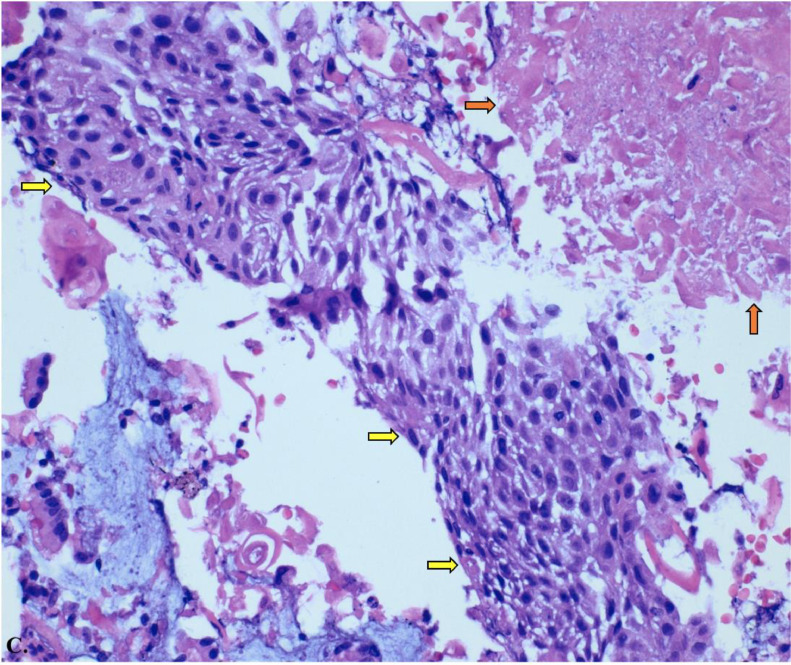
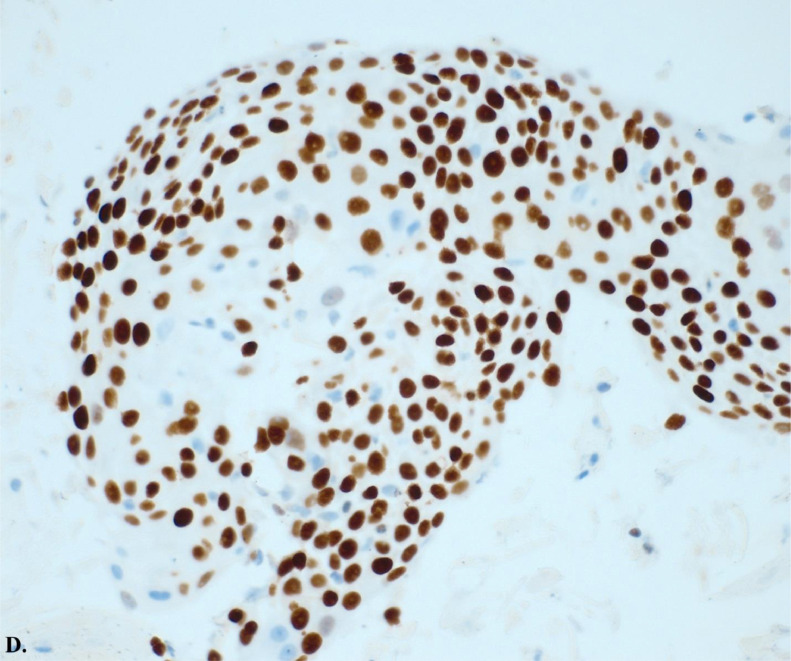
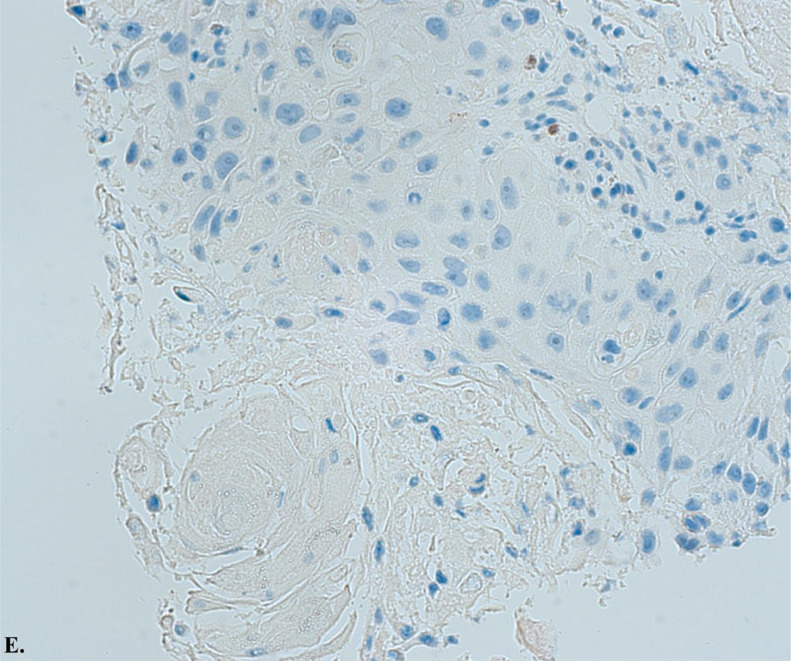
Ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA) of the lymph node showed a predominantly necrotic malignant epithelial neoplasm with squamous differentiation. Images C illustrates hematoxylin and eosin stain of carcinoma with squamous differentiation. Images D and E illustrate positive P40 immunostaining, a highly specific prognostic marker for squamous cell carcinoma, and negative P16 expression, indicating an unlikely association with human papillomavirus (HPV). These histopathological findings confirm the diagnosis of Adenosquamous Carcinoma with metastatic squamous.
Image C.
Histology slide from ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA) of a lymph node with hematoxylin and eosin stain at 20x magnification of carcinoma with squamous differentiation. The yellow arrows show regions of the squamous cell tumor, and the orange arrows highlight keratin and necrotic debris in the background.
Image D.
Histology slide from ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA) of a lymph node with P40 immunostaining at 20x magnification of carcinoma with squamous differentiation. This image shows positive P40 immunostaining, a highly specific prognostic marker for squamous cell carcinoma.
Image E.
Histology slide from ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA) of a lymph node with P16 immunostaining at 20x magnification of carcinoma with squamous differentiation. This image demonstrates negative P16 expression, indicating that the cancer is unlikely to be associated with human papillomavirus (HPV).
Oncology consultation and management
The oncology team diagnosed the patient with metastatic pancreatic cancer (Stage IV), likely Adenosquamous histology. Genetic testing identified a KRAS G12D mutation and a PIK3CA pathogenic variant, with MSI stability, no NTRK fusion, and BRCA 1/2 undetected. A multidisciplinary tumor conference recommended systemic chemotherapy with mFOLFIRINOX and G-CSF support. The patient also sought a second opinion at a tertiary institution, where the diagnosis of a primary Adenosquamous carcinoma was confirmed, and the same chemotherapy regimen was advised.
Chemotherapy course
A mediport was placed, and the patient began mFOLFIRINOX chemotherapy. The treatment was well-tolerated overall, with dose reductions for thrombocytopenia. The management of chemotherapy-induced side effects, such as nausea and diarrhea, was critical in maintaining the patient's quality of life and ensuring treatment adherence. He completed twelve cycles of mFOLFIRINOX with effective management of side effects.
Follow-Up imaging
Restaging CT scans showed a decrease in tumor size and stable metastatic nodes. Follow-up imaging revealed that the pancreatic mass had decreased in size and the metastatic lymph nodes remained stable (Fig. 5).
Fig. 5.
CT chest, abdomen and pelvis with IV contrast (coronal view) post chemotherapy with pancreatic tail mass (red box) measuring 4.5 × 3.4 cm and questionable slight interval increase in stomach involvement.
Discussion
Adenosquamous carcinoma of the pancreas (ASCP) is a rare and particularly aggressive variant of pancreatic cancer [13]. It combines adenocarcinoma and squamous cell carcinoma components [14]. This dual histological nature presents unique diagnostic and therapeutic challenges, setting ASCP apart from the more common pancreatic ductal adenocarcinoma (PDAC) [15].
Diagnostic challenges
The initial presentation of ASCP can be nonspecific, often mimicking more common gastrointestinal disorders [16]. In this case, the patient presented with abdominal pain and gastrointestinal disturbances, symptoms that could easily be attributed to benign conditions. Elevated lipase levels were an early indication of pancreatic pathology, but a definitive diagnosis required advanced imaging and histopathological confirmation. Contrast-enhanced CT, PET scans, and EUS were instrumental in identifying and characterizing the complex mass in the pancreatic body and tail, providing detailed visualization necessary for assessing potential metastases [17].
Histopathological examination through fine-needle biopsy (FNB) revealed atypical keratinizing squamous cells, an uncommon finding for pancreatic tumors, prompting further investigation [18]. Using bronchoscopy and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) to confirm squamous differentiation was critical. EBUS-TBNA provided a minimally invasive means to obtain additional tissue samples from the suspicious lymph nodes identified on imaging. The 4R lymph node, which measured 2.5 × 11 mm, was sampled, revealing carcinoma with squamous differentiation. This thorough approach highlighted the necessity of repeated and comprehensive tissue sampling when initial results are inconclusive [19,20]. Genetic testing provided additional diagnostic clarity, identifying KRAS G12D and PIK3CA mutations, which are significant in understanding the tumor's behavior and potential response to therapy [5,21].
Therapeutic strategies
The management of ASCP requires a comprehensive and well-coordinated multidisciplinary approach [5]. This case emphasized the critical role of various specialties in formulating an effective treatment plan, including gastroenterology, surgery, radiology, pathology, and oncology. Systemic chemotherapy with mFOLFIRINOX was chosen based on the aggressive nature of the tumor and its metastatic spread. mFOLFIRINOX, known for its efficacy in treating advanced pancreatic cancers, was administered with necessary dose adjustments to accommodate the patient's tolerance and manage side effects such as thrombocytopenia [8,12].
Regular monitoring through imaging and tumor markers, including CA 19-9, was essential in evaluating the treatment's effectiveness. The patient's partial biochemical response and disease stabilization on imaging demonstrated the importance of continuous assessment and adaptability in therapeutic strategies.
Multidisciplinary approach and molecular profiling
This case illustrates the necessity of a multidisciplinary approach in managing ASCP. Integrating advanced diagnostic techniques and molecular profiling provided a comprehensive understanding of the tumor's characteristics, guiding personalized therapeutic decisions. The presence of KRAS and PIK3CA mutations informed the prognosis and potential treatment responses, showcasing the value of genetic insights in modern oncology [4,11].
The combination of advanced imaging, detailed histopathological analysis, and genetic profiling allowed for a tailored and effective treatment strategy. This case demonstrates the importance of a coordinated effort among various medical specialties to address the complexities of ASCP. By leveraging a team-based approach and incorporating detailed molecular profiling, healthcare providers can better navigate the challenges of rare and aggressive malignancies like ASCP, ultimately enhancing patient care and survival rates.
Mental health and psychological support for patients with terminal or advanced cancers
Patients diagnosed with terminal or advanced cancers such as adenosquamous carcinoma of the pancreas often face immense psychological and emotional challenges [22]. The mental toll of grappling with a life-limiting illness can manifest as anxiety, depression, and existential distress, impacting their overall well-being and quality of life [23]. It is crucial for physicians and surgeons to recognize the profound psychological burden these patients carry and to integrate compassionate care into their medical practice [24]. Regular psychological assessments, timely referrals to mental health professionals, and including palliative care teams can provide a holistic approach to patient care [25]. Physicians and surgeons can offer reassurance, listen actively to patients' fears and concerns, and provide clear, honest communication about treatment options and prognosis [22].
Creating a supportive environment where patients feel heard and understood can alleviate some emotional burdens [26]. Encouraging family involvement and facilitating support groups can also provide a network of empathy and understanding. Compassionate care, which respects patients' dignity and emotional needs, can enhance their resilience and coping mechanisms [27]. Ultimately, the value of compassion in the treatment of terminal or advanced cancers cannot be overstated [26]. It fosters a therapeutic alliance between patients and their healthcare providers, promotes better mental health outcomes, and ensures that the care provided is medically effective and emotionally supportive [27]. By embracing a compassionate approach, healthcare providers affirm the humanity of their patients, offering solace and strength in the face of profound challenges [23].
Conclusion
Adenosquamous carcinoma of the pancreas represents a particularly challenging malignancy due to its aggressive nature and complex histological features. Effective management requires a high index of suspicion, comprehensive diagnostic workup, and a well-coordinated multidisciplinary approach. Advanced imaging techniques, including CT, PET scans, and endoscopic ultrasound (EUS), are crucial for identifying and characterizing pancreatic masses. Histopathological confirmation through fine-needle biopsy and molecular profiling is essential for accurate diagnosis and treatment planning. Systemic chemotherapy, particularly regimens like mFOLFIRINOX, is a cornerstone of treatment for metastatic disease. Integrating advanced diagnostic techniques, molecular profiling, and a multidisciplinary approach is vital for improving patient outcomes and providing comprehensive care for this challenging malignancy.
Patient consent
We confirm that we have obtained written, informed consent from the patient for the publication of this case report. The patient has been thoroughly informed about the details that will be published and understands the implications of the publication. The written consent is stored securely and is available for review by the editorial team upon request.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ansari D, Tingstedt B, Andersson B, Holmquist F, Sturesson C, Williamsson C, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. 2016;12(16):1929–1946. doi: 10.2217/fon-2016-0010. [DOI] [PubMed] [Google Scholar]
- 2.Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):493–502. doi: 10.1038/s41575-021-00457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhillon J, Betancourt M. Pancreatic ductal adenocarcinoma. Monogr Clin Cytol. 2020;26:74–91. doi: 10.1159/000455736. [DOI] [PubMed] [Google Scholar]
- 4.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borazanci E, Millis SZ, Korn R, Han H, Whatcott CJ, Gatalica Z, et al. Adenosquamous carcinoma of the pancreas: molecular characterization of 23 patients along with a literature review. World J Gastrointest Oncol. 2015;7(9):132–140. doi: 10.4251/wjgo.v7.i9.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, et al. Advances in the epidemiology of pancreatic cancer: trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1–11. doi: 10.1016/j.canlet.2021.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Del Arco H, Chakiba-Brugère C, Salabert L, Béchade D. Adenosquamous carcinoma of the pancreas. Clin Med Insights Oncol. 2019;13 doi: 10.1177/1179554919886587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ettrich TJ, Seufferlein T. Systemic therapy for metastatic pancreatic cancer. Curr Treat Options Oncol. 2021;22(11):106. doi: 10.1007/s11864-021-00895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goral V. Pancreatic cancer: pathogenesis and diagnosis. Asian Pac J Cancer Prev. 2015;16(14):5619–5624. doi: 10.7314/apjcp.2015.16.14.5619. [DOI] [PubMed] [Google Scholar]
- 10.Marcus R, Maitra A, Roszik J. Recent advances in genomic profiling of adenosquamous carcinoma of the pancreas. J Pathol. 2017;243(3):271–272. doi: 10.1002/path.4959. [DOI] [PubMed] [Google Scholar]
- 11.Moslim MA, Lefton MD, Ross EA, Mackrides N, Reddy SS. Clinical and histological basis of adenosquamous carcinoma of the pancreas: a 30-year experience. J Surg Res. 2021;259:350–356. doi: 10.1016/j.jss.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Sugiura T, Okamura Y, Yamamoto Y, Ashida R, Ohgi K, et al. Long-term outcomes after an aggressive resection of adenosquamous carcinoma of the pancreas. Surg Today. 2019;49(10):809–819. doi: 10.1007/s00595-019-01807-8. [DOI] [PubMed] [Google Scholar]
- 13.Limaiem F, Hajri M, Ben Farhat L. Adenosquamous carcinoma of the pancreas: a rare and aggressive variant of pancreatic cancer. Clin Case Rep. 2022;10(8):e6181. doi: 10.1002/ccr3.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madura JA, Jarman BT, Doherty MG, Yum MN, Howard TJ. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999;134(6):599–603. doi: 10.1001/archsurg.134.6.599. [DOI] [PubMed] [Google Scholar]
- 15.Paramythiotis D, Kyriakidis F, Karlafti E, Didangelos T, Oikonomou IM, Karakatsanis A, et al. Adenosquamous carcinoma of the pancreas: two case reports and review of the literature. J Med Case Rep. 30 2022;16(1):395. doi: 10.1186/s13256-022-03610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simone CG, Zuluaga Toro T, Chan E, Feely MM, Trevino JG, George TJ., Jr Characteristics and outcomes of adenosquamous carcinoma of the pancreas. Gastrointest Cancer Res. 2013;6(3):75–79. [PMC free article] [PubMed] [Google Scholar]
- 17.Tarabay J, Li X, Chandan VS. Adenosquamous carcinoma of the pancreas. Clin Res Hepatol Gastroenterol. 2020;44(6):796–798. doi: 10.1016/j.clinre.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Trikudanathan G, Dasanu CA. Adenosquamous carcinoma of the pancreas: a distinct clinicopathologic entity. South Med J. 2010;103(9):903–910. doi: 10.1097/SMJ.0b013e3181ebadbd. [DOI] [PubMed] [Google Scholar]
- 19.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/s0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Z, Liu W. Pancreatic cancer: a review of risk factors, diagnosis, and treatment. Technol Cancer Res Treat. 2020;19 doi: 10.1177/1533033820962117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoffel EM, Brand RE, Goggins M. Pancreatic cancer: changing epidemiology and new approaches to risk assessment, early detection, and prevention. Gastroenterology. 2023;164(5):752–765. doi: 10.1053/j.gastro.2023.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Back AL. Patient-clinician communication issues in palliative care for patients with advanced cancer. J Clin Oncol. 2020;38(9):866–876. doi: 10.1200/jco.19.00128. [DOI] [PubMed] [Google Scholar]
- 23.Badr H. Psychosocial interventions for patients with advanced cancer and their families. Am J Lifestyle Med. 2016;10(1):53–63. doi: 10.1177/1559827614530966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ann-Yi S, Bruera E. Psychological aspects of care in cancer patients in the last weeks/days of life. Cancer Res Treat. 2022;54(3):651–660. doi: 10.4143/crt.2022.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolsteren EEM, Deuning-Smit E, Chu AK, van der Hoeven YCW, Prins JB, van der Graaf WTA, et al. Psychosocial aspects of living long term with advanced cancer and ongoing systemic treatment: a scoping review. Cancers (Basel) 2022;14(16) doi: 10.3390/cancers14163889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodin G, An E, Shnall J, Malfitano C. Psychological interventions for patients with advanced disease: implications for oncology and palliative care. J Clin Oncol. 2020;38(9):885–904. doi: 10.1200/jco.19.00058. [DOI] [PubMed] [Google Scholar]
- 27.Kang KA, Han SJ, Lim YS, Kim SJ. Meaning-centered interventions for patients with advanced or terminal cancer: a meta-analysis. Cancer Nurs. 2019;42(4):332–340. doi: 10.1097/ncc.0000000000000628. [DOI] [PubMed] [Google Scholar]