Abstract
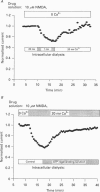
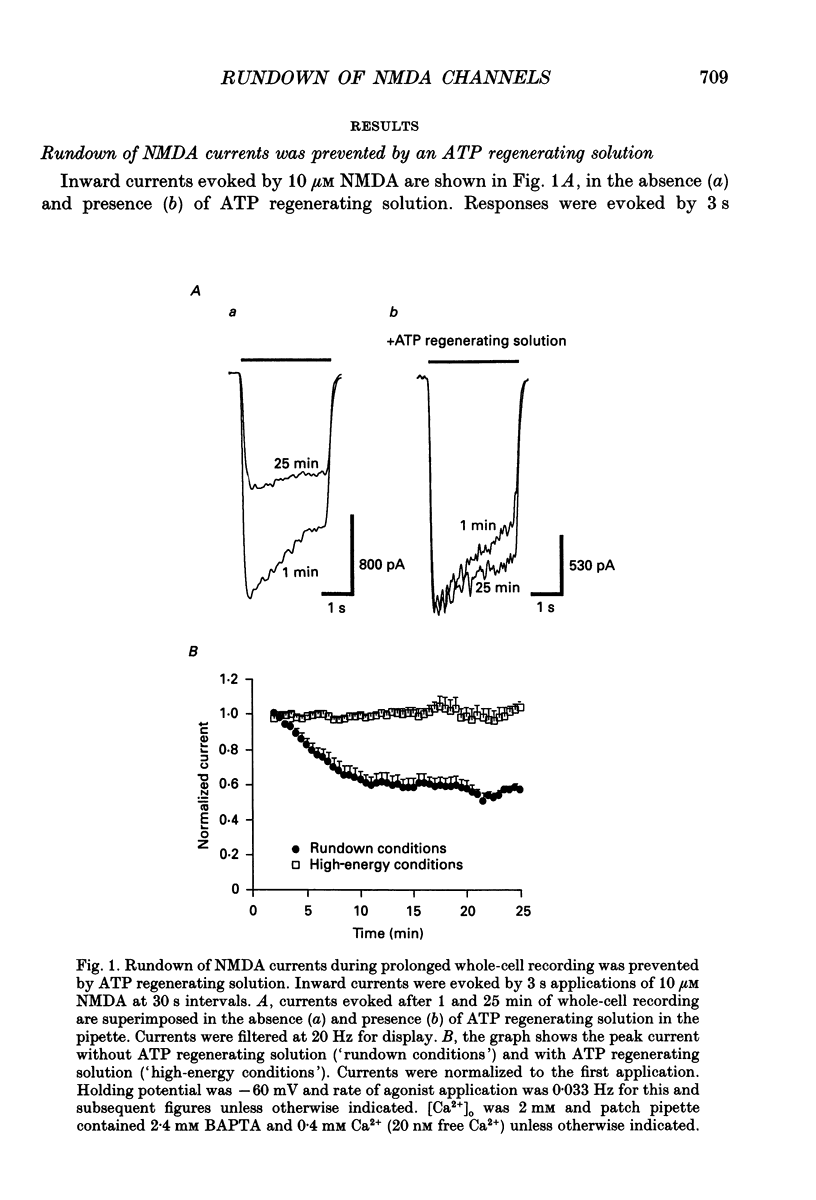
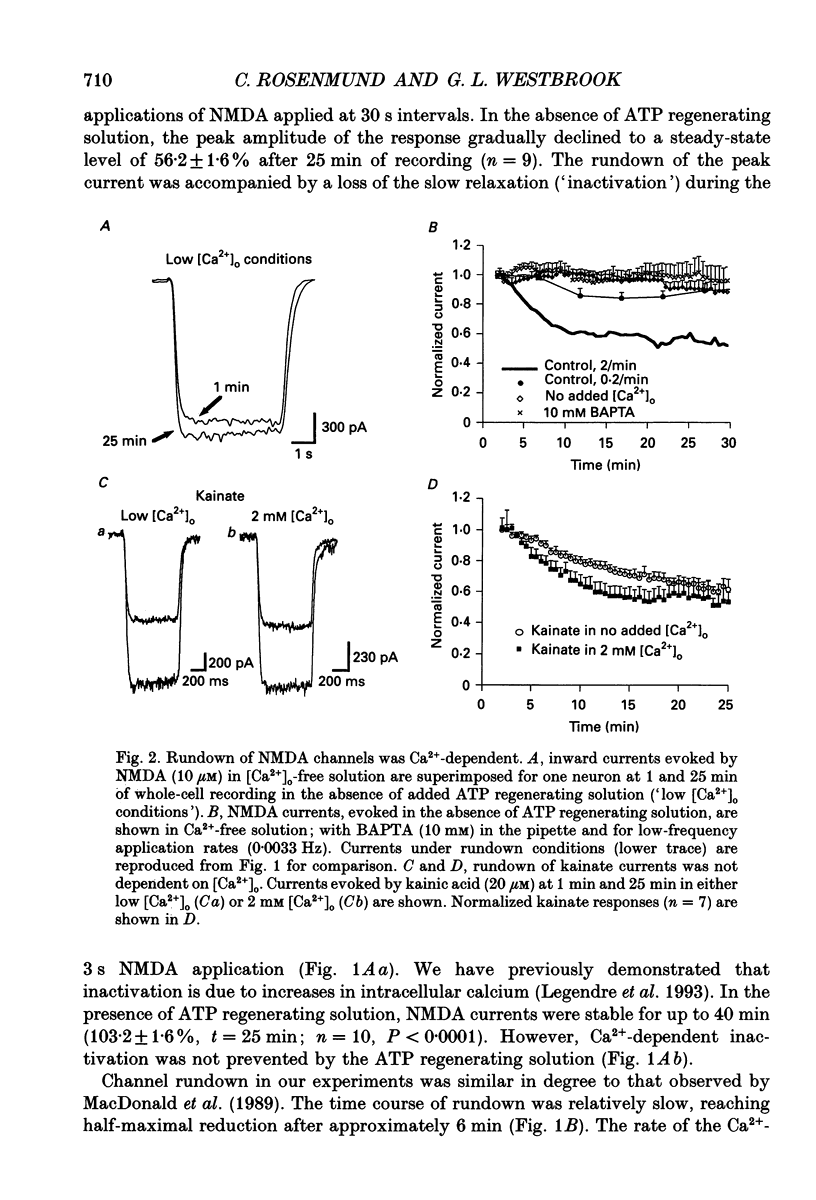
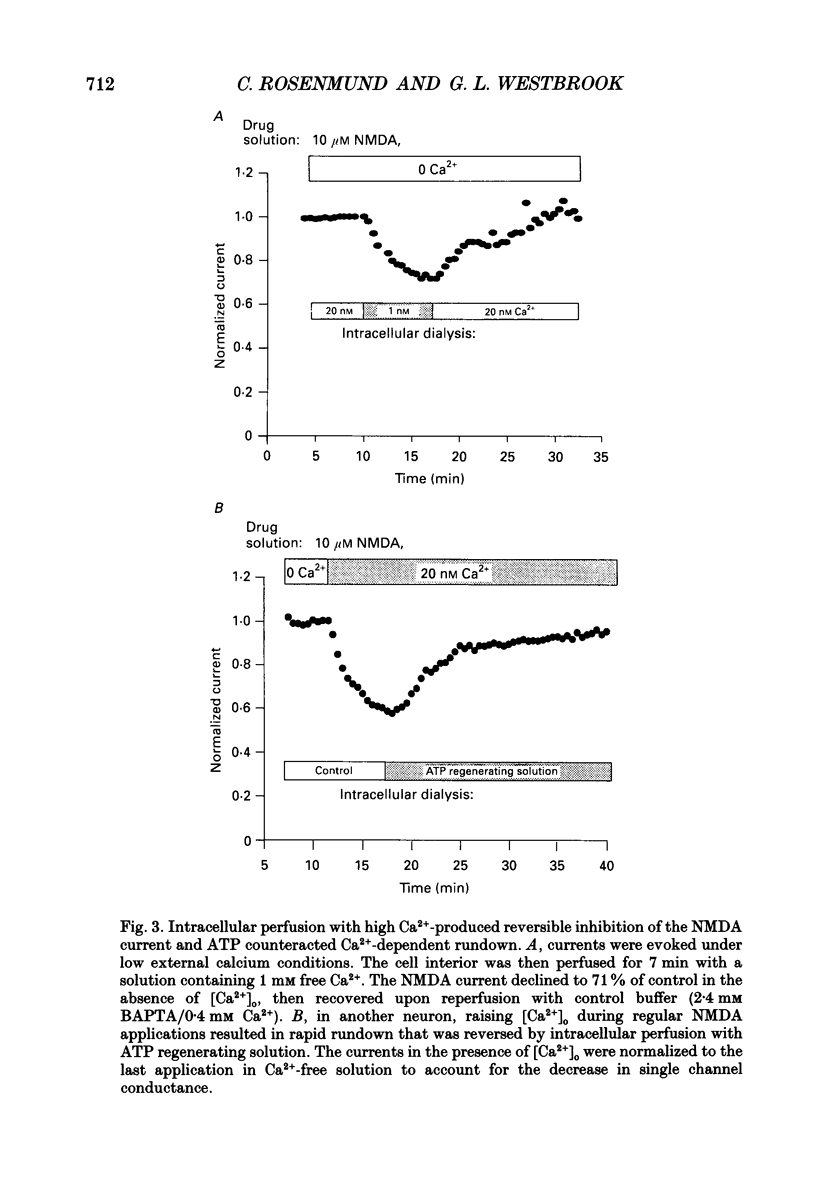
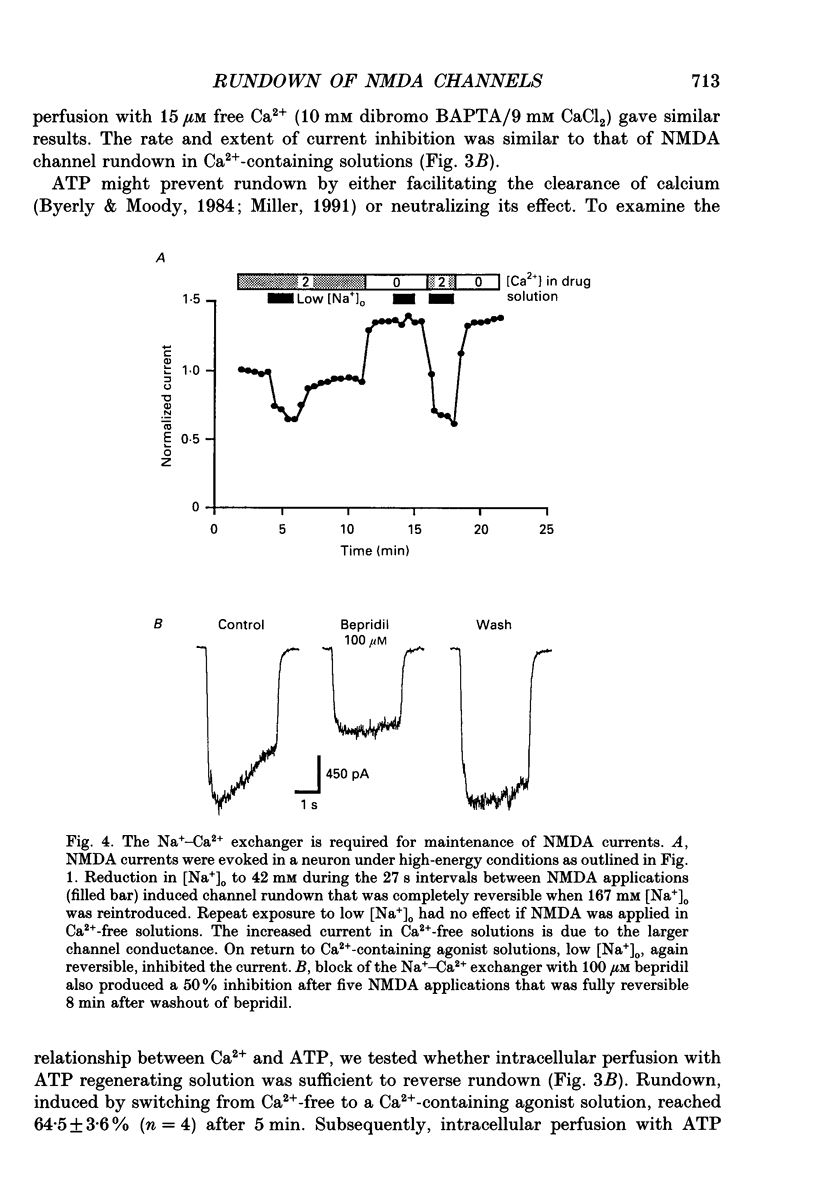
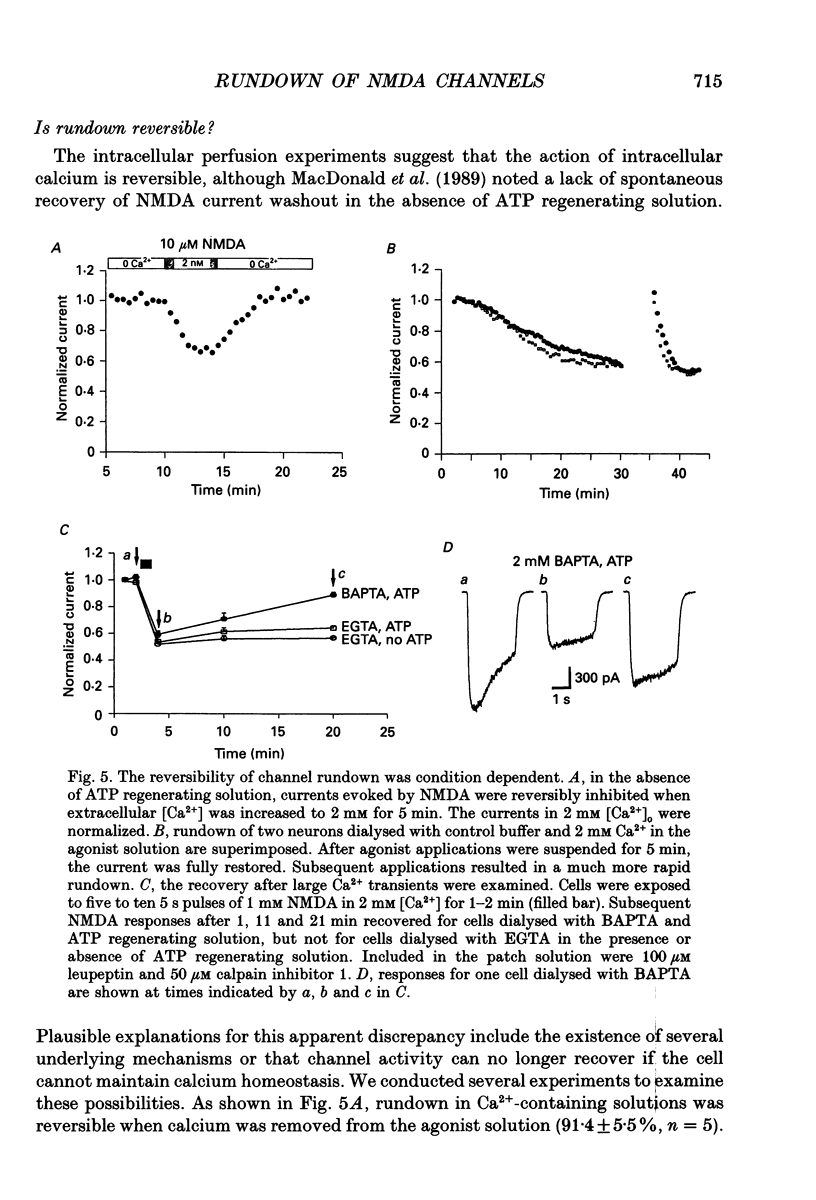
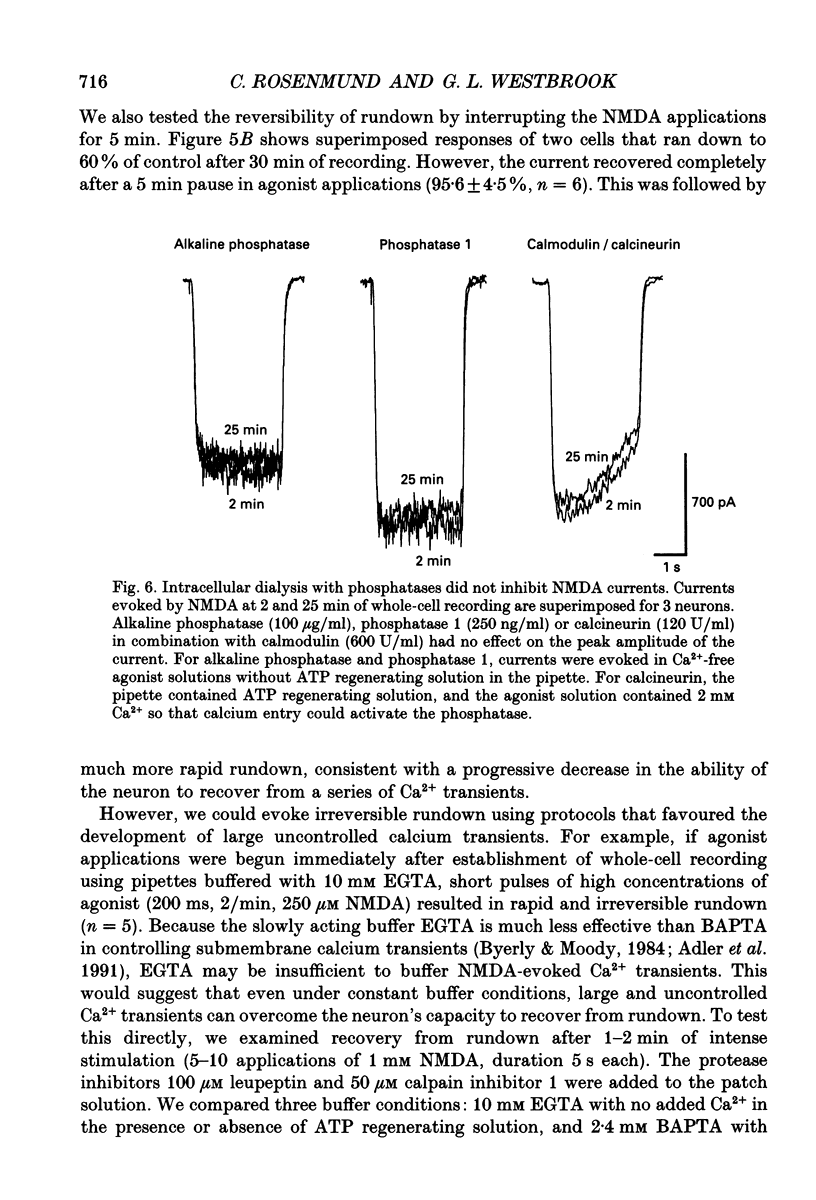
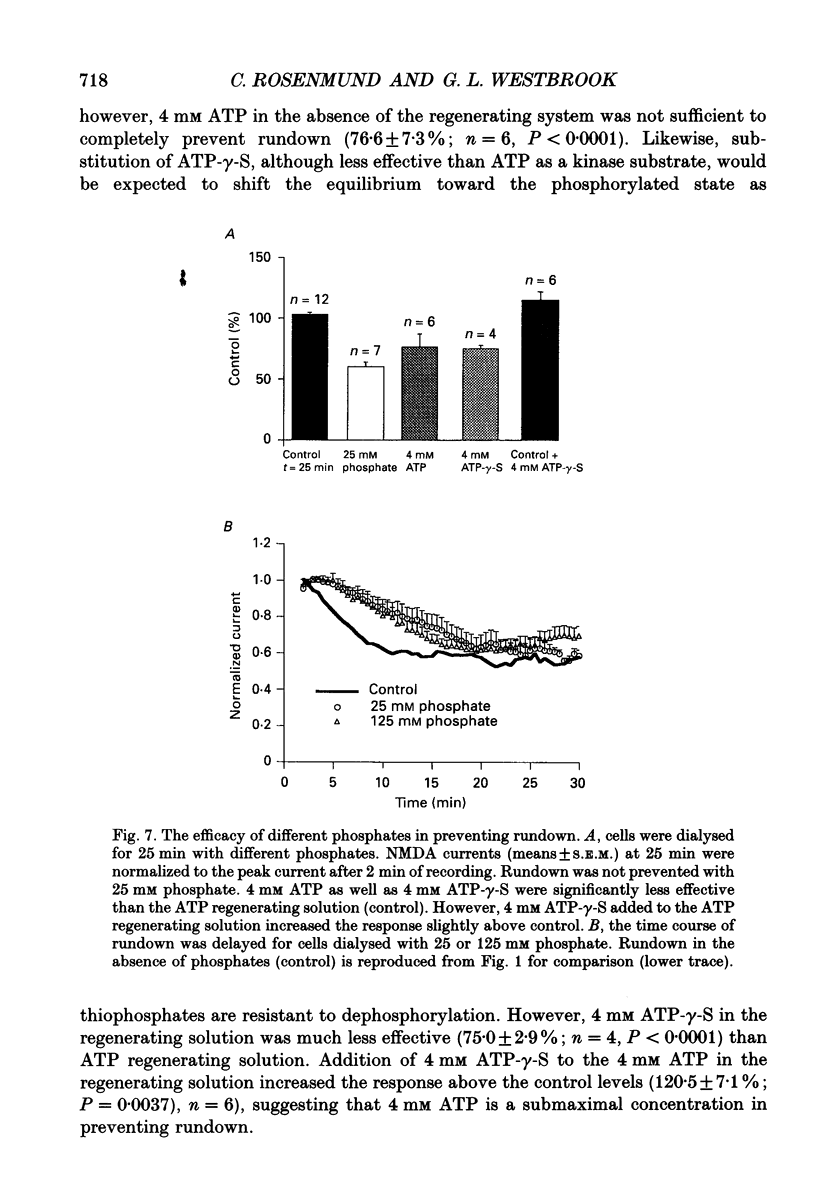
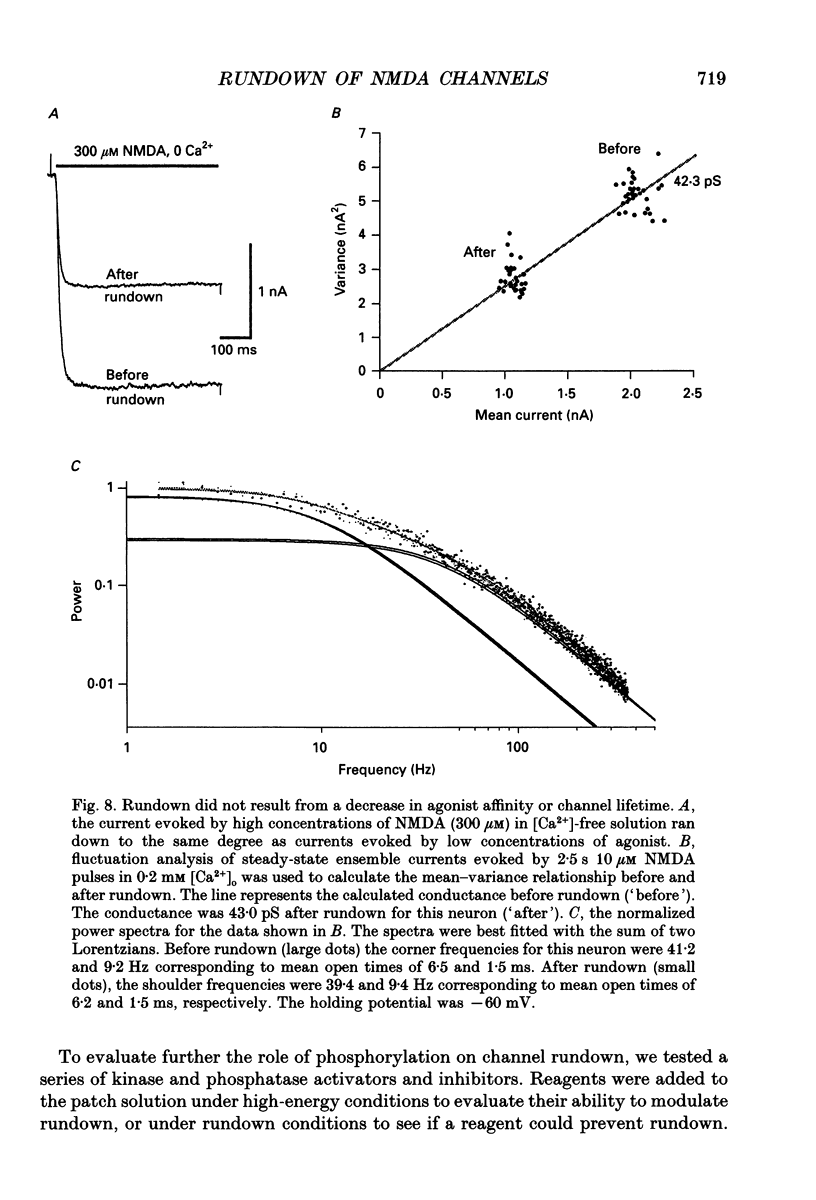
1. N-methyl-D-aspartate (NMDA) channel activity was studied on cultured rat hippocampal neurons in whole-cell voltage-clamp mode. NMDA responses were evoked by rapid application of NMDA and the cytosol was modified using pipette dialysis and intracellular perfusion. 2. In the presence of 2 mM [Ca2+]o with 2.4 mM BAPTA (1,2-bis(O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid) and 0.4 mM Ca2+ in the whole-cell pipette, the response evoked by regular applications of 10 microM NMDA gradually decreased during prolonged whole-cell recording. After 25 min the peak current was reduced to 56 +/- 1.6% of control. Channel 'rundown' could be prevented by inclusion of an ATP regenerating solution in the pipette. 3. Rundown did not occur in Ca(2+)-free medium even in the absence of added ATP regenerating solution. Rundown was also prevented by increasing [BAPTA]i to 10 mM whereas raising [Ca2+]i by inhibiting the Na(+)-Ca2+ exchanger or by perfusing the patch pipette with high [Ca2+]i (15-1000 microM) reversibly inhibited the NMDA current. By contrast, the rundown of kainate responses was Ca(2+)-independent. 4. The rate and reversibility of rundown was use-dependent. Rundown did not occur with infrequent NMDA applications (0.2/min). Following channel rundown in Ca(2+)-containing medium, a 5 min pause in agonist applications or adding ATP regenerating solution by intracellular perfusion resulted in complete recovery. However, rundown did not recover following large currents evoked by 300 microM NMDA or when 10 mM EGTA was used as the intracellular buffer. Protease inhibitors did not prevent irreversible rundown. 5. ATP-gamma-S (4 mM) was less effective than the ATP regenerating solution in preventing rundown. Likewise, intracellular dialysis with alkaline phosphatase, phosphatase 1 or calcineurin did not induce rundown and addition of phosphatase inhibitors also did not block rundown. Thus receptor dephosphorylation did not appear to be primarily responsible for channel rundown. 6. The mean open time and unitary conductance of the NMDA channel were unaffected by rundown as estimated by fluctuation analysis. The conductance was 42.8 +/- 2.9 nS before and 43.7 +/- 2.8 nS after rundown. The mean open times were 17.3 and 4.0 ms before and 15.9 and 4.0 ms after rundown. However the open probability was reduced following rundown as determined by the onset of MK-801 block of steady-state NMDA currents. 7. Our results suggest that an increase in intracellular calcium leads to channel rundown during whole-cell recording by reducing the open probability of the NMDA channel.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
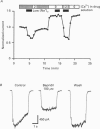
PDF






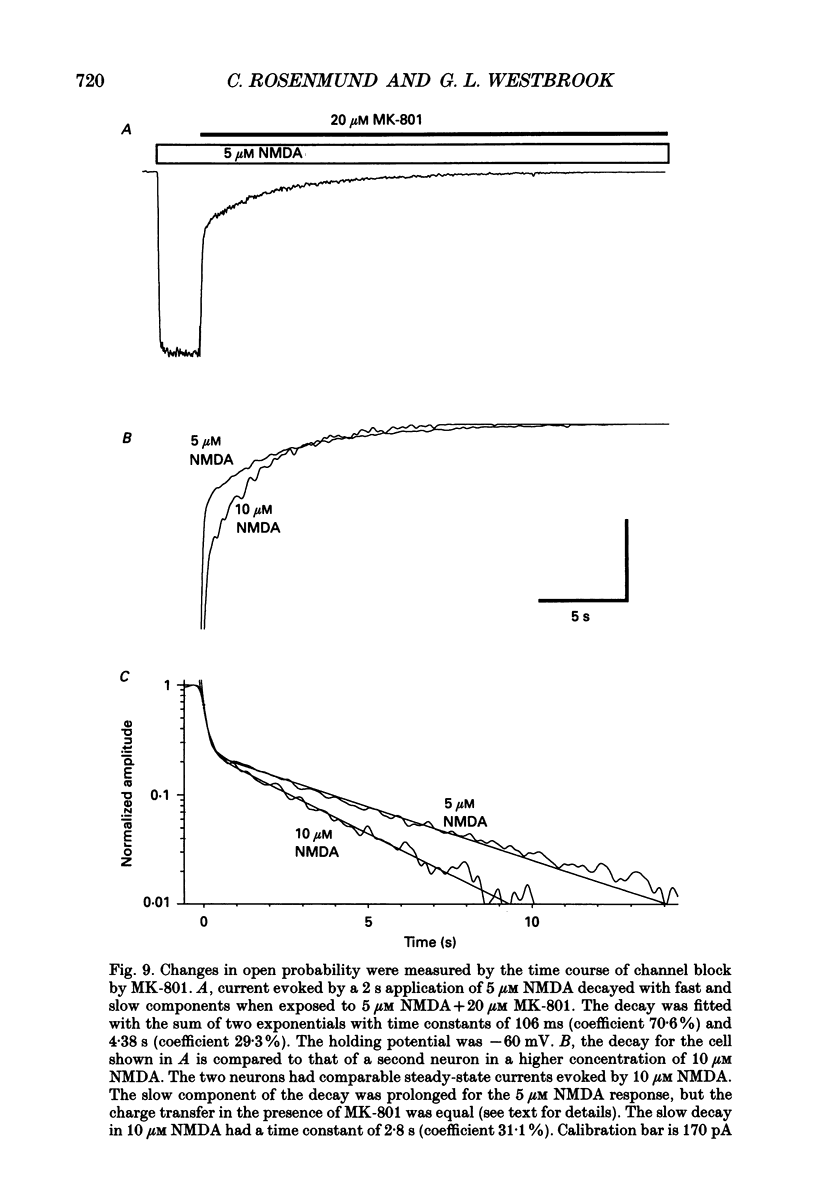


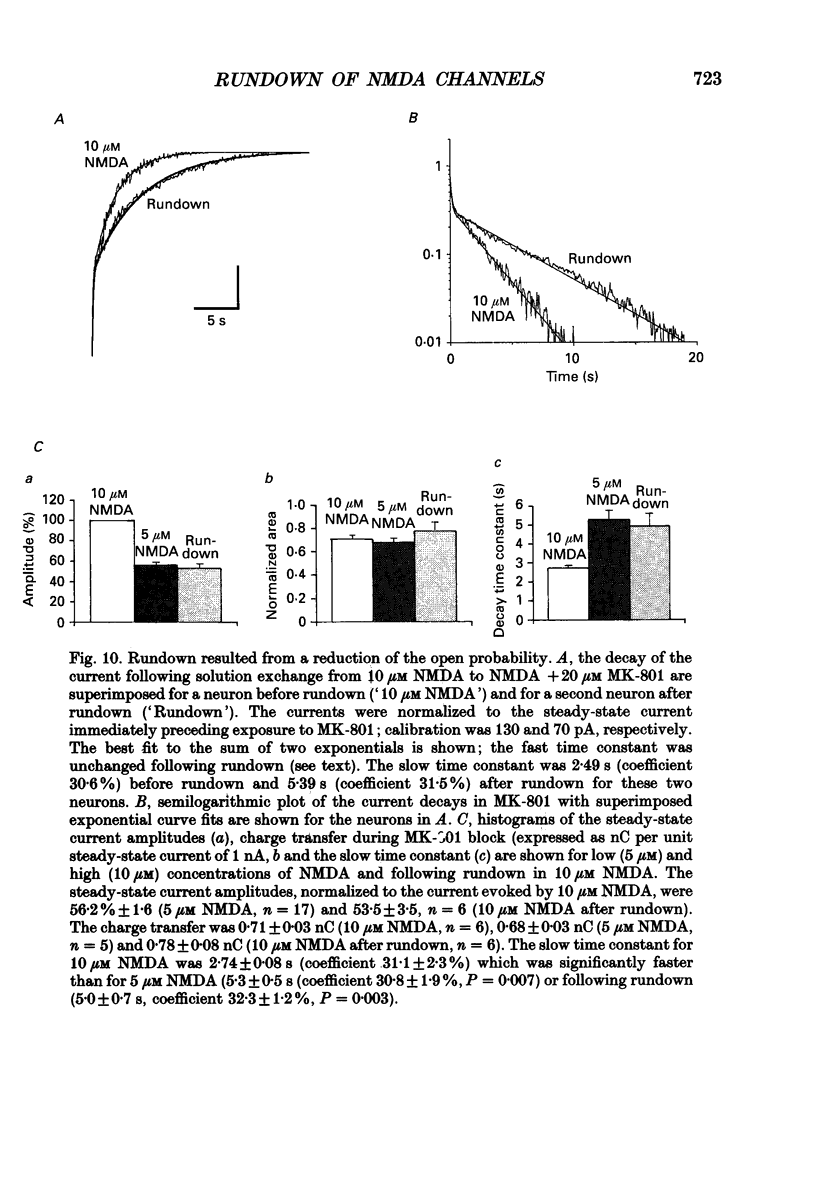






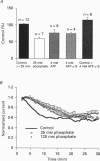
Images in this article
Selected References
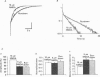
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador M., Dani J. A. Protein kinase inhibitor, H-7, directly affects N-methyl-D-aspartate receptor channels. Neurosci Lett. 1991 Apr 1;124(2):251–255. doi: 10.1016/0304-3940(91)90106-4. [DOI] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Neuronal Ca2+ signalling takes the local route. Curr Opin Neurobiol. 1992 Jun;2(3):302–307. doi: 10.1016/0959-4388(92)90119-6. [DOI] [PubMed] [Google Scholar]
- Behrends J. C., Maruyama T., Tokutomi N., Akaike N. Ca2+-mediated suppression of the GABA-response through modulation of chloride channel gating in frog sensory neurones. Neurosci Lett. 1988 Apr 12;86(3):311–316. doi: 10.1016/0304-3940(88)90502-2. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Calcium transport and buffering in neurons. Trends Neurosci. 1988 Oct;11(10):438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Moody W. J. Intracellular calcium ions and calcium currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984 Jul;352:637–652. doi: 10.1113/jphysiol.1984.sp015314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron. 1991 Aug;7(2):319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. D., Clifford D. B., Zorumski C. F. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39(3):787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Westbrook G. L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991 Oct;7(4):605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Collins A., Somlyo A. V., Hilgemann D. W. The giant cardiac membrane patch method: stimulation of outward Na(+)-Ca2+ exchange current by MgATP. J Physiol. 1992 Aug;454:27–57. doi: 10.1113/jphysiol.1992.sp019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P. F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991 Feb 5;30(5):1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Oxford G. S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985 May;85(5):743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Jen J., Nairn A. C., Stevens C. F. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991 Sep 6;253(5024):1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Perrino B. A., Soderling T. R. Identification of an autoinhibitory domain in calcineurin. J Biol Chem. 1990 Feb 5;265(4):1924–1927. [PubMed] [Google Scholar]
- Hilgemann D. W., Collins A. Mechanism of cardiac Na(+)-Ca2+ exchange current stimulation by MgATP: possible involvement of aminophospholipid translocase. J Physiol. 1992 Aug;454:59–82. doi: 10.1113/jphysiol.1992.sp019254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- Jahr C. E. High probability opening of NMDA receptor channels by L-glutamate. Science. 1992 Jan 24;255(5043):470–472. doi: 10.1126/science.1346477. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Slaughter R. S., King V. F., Garcia M. L. Inhibitors of sodium-calcium exchange: identification and development of probes of transport activity. Biochim Biophys Acta. 1989 May 9;988(2):287–302. doi: 10.1016/0304-4157(89)90022-1. [DOI] [PubMed] [Google Scholar]
- Kano M., Rexhausen U., Dreessen J., Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992 Apr 16;356(6370):601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Korn S. J., Horn R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J Gen Physiol. 1989 Nov;94(5):789–812. doi: 10.1085/jgp.94.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Rosenmund C., Westbrook G. L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993 Feb;13(2):674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Westbrook G. L. The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones. J Physiol. 1990 Oct;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Segal M. Activation of protein kinase C suppresses responses to NMDA in rat CA1 hippocampal neurones. J Physiol. 1992 Nov;457:491–501. doi: 10.1113/jphysiol.1992.sp019389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- Miller R. J. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37(3):255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993 May;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Shirasaki T., Aibara K., Akaike N. Direct modulation of GABAA receptor by intracellular ATP in dissociated nucleus tractus solitarii neurones of rat. J Physiol. 1992 Apr;449:551–572. doi: 10.1113/jphysiol.1992.sp019101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R., Noszek J. C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988 Jun;1(4):279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Tang J. M., Wang J., Quandt F. N., Eisenberg R. S. Perfusing pipettes. Pflugers Arch. 1990 May;416(3):347–350. doi: 10.1007/BF00392072. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Salter M. W., MacDonald J. F. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991 Sep 6;253(5024):1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Zador A., Koch C., Brown T. H. Biophysical model of a Hebbian synapse. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6718–6722. doi: 10.1073/pnas.87.17.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y. I., Uteshev V. V., Sokolova S. N., Motin L. G., Eremjan H. H. Potentiation of glutamate-activated currents in isolated hippocampal neurons. Neuron. 1990 Nov;5(5):597–602. doi: 10.1016/0896-6273(90)90214-z. [DOI] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J., Fischbach G. D. Calcium-dependent, slow desensitization distinguishes different types of glutamate receptors. Cell Mol Neurobiol. 1989 Mar;9(1):95–104. doi: 10.1007/BF00711446. [DOI] [PMC free article] [PubMed] [Google Scholar]