Abstract
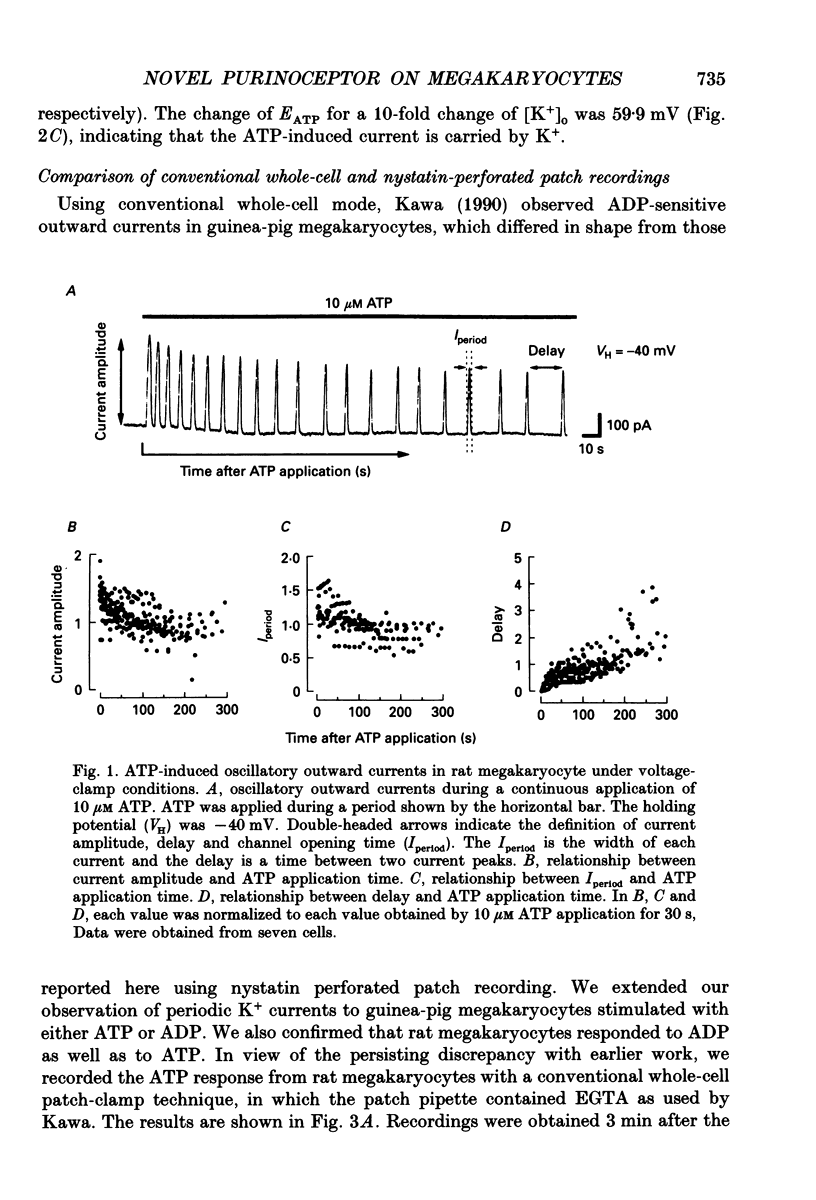
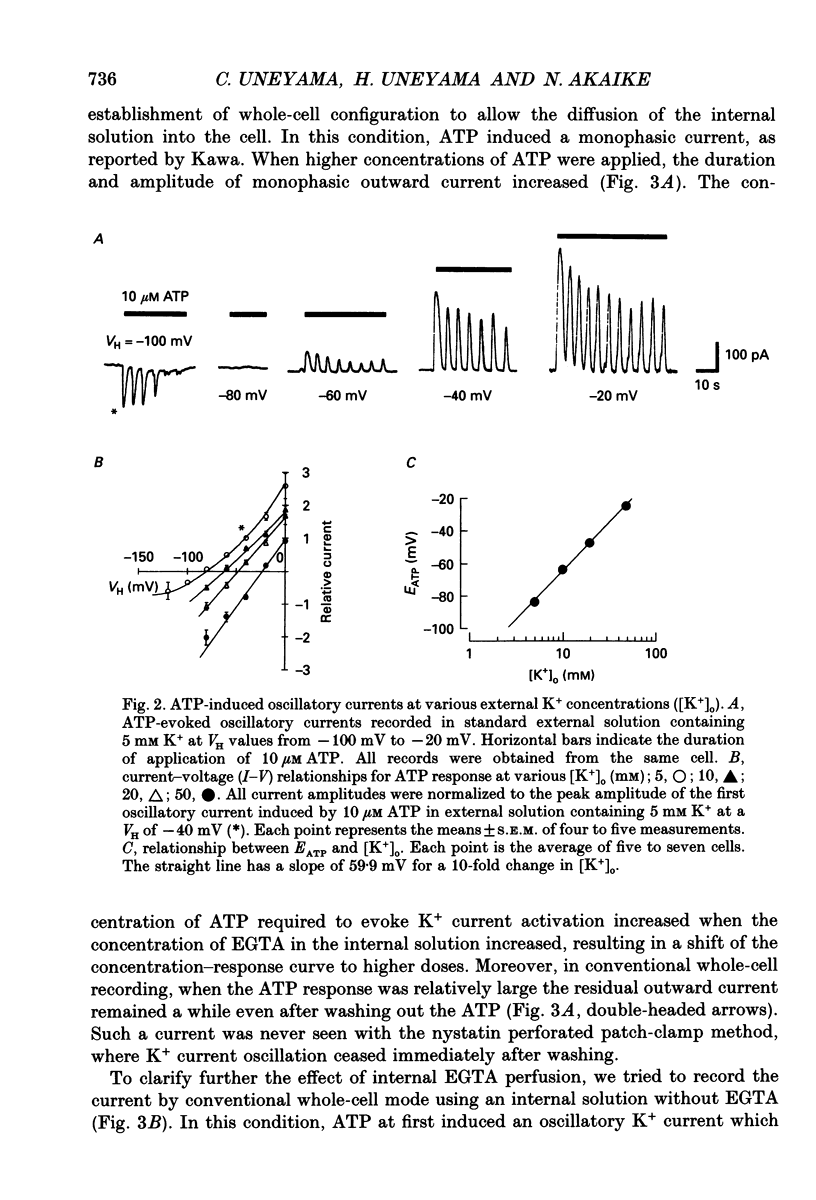
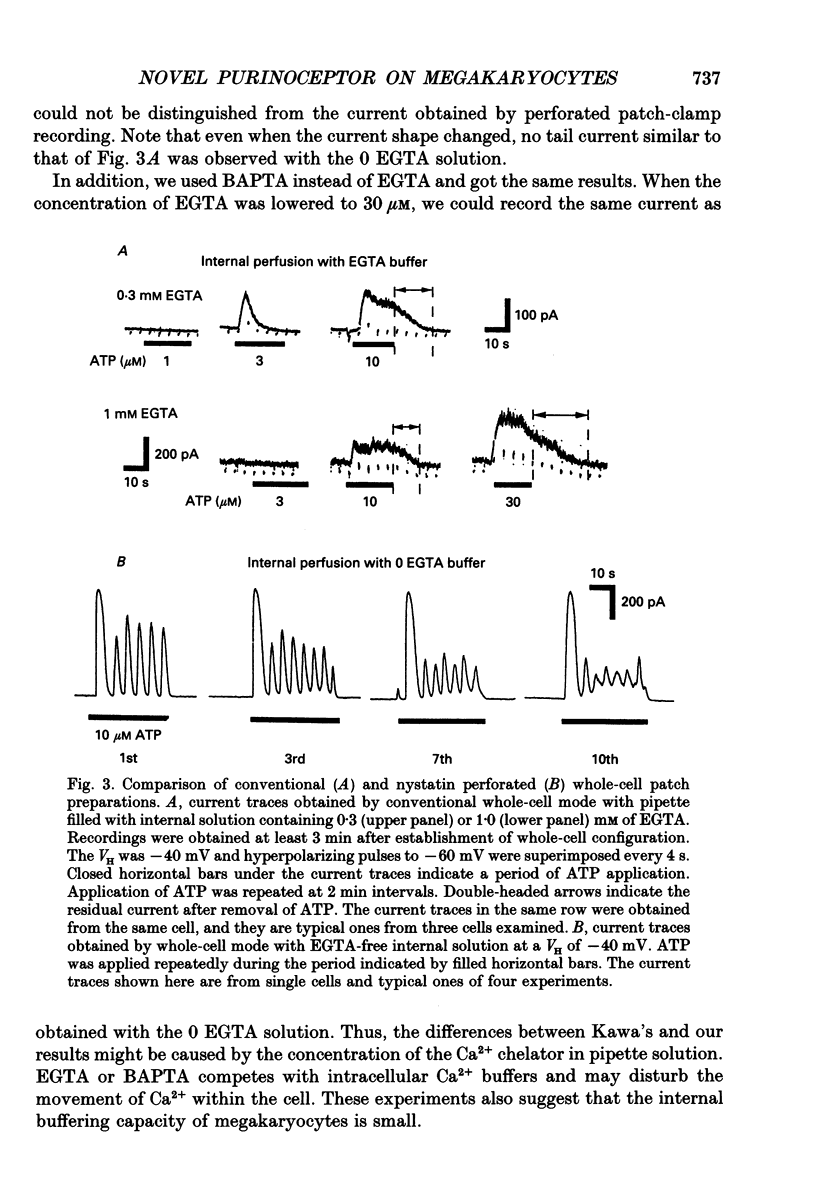
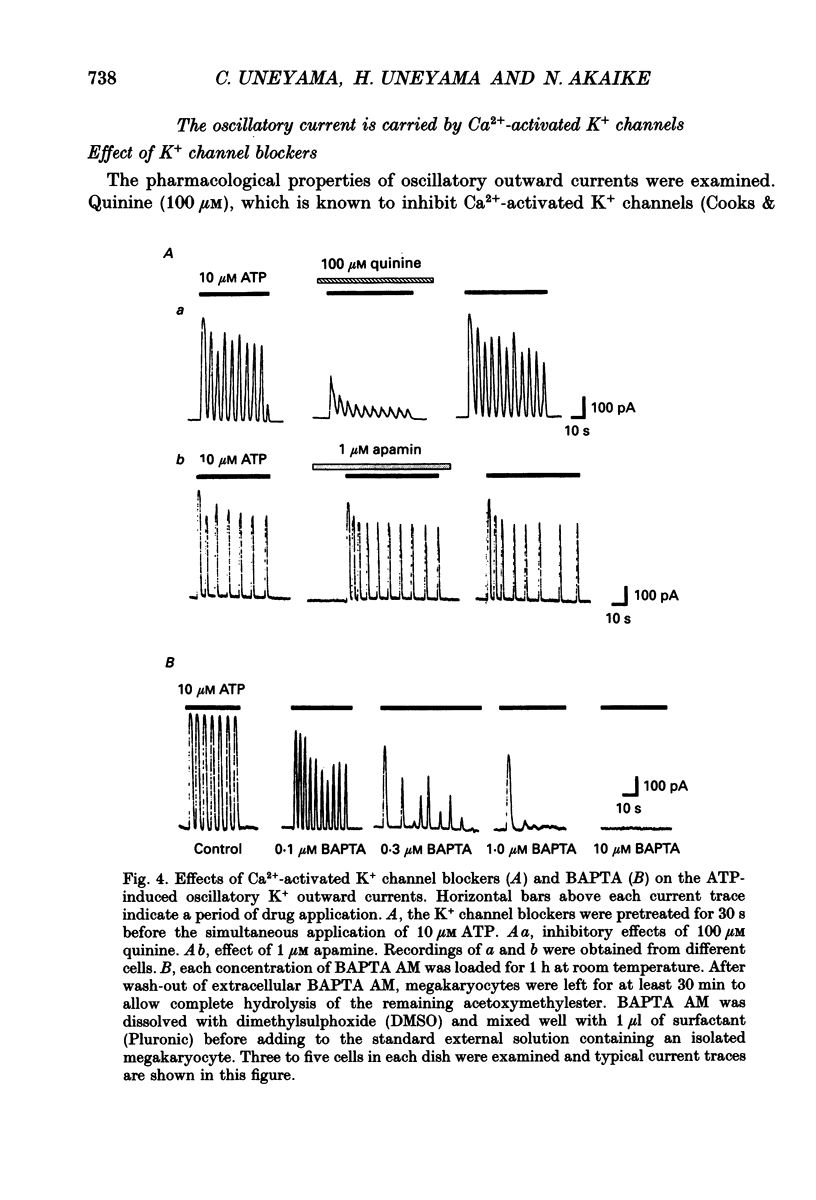
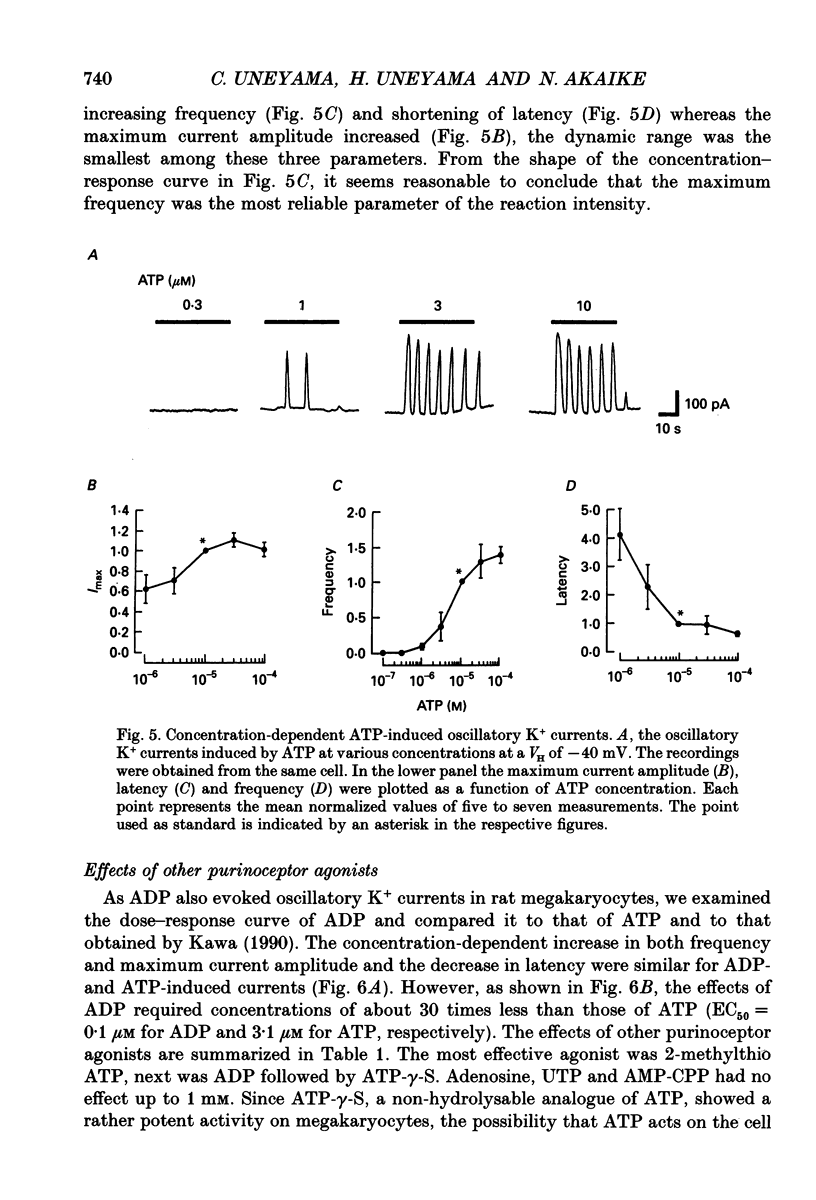
1. The responses of megakaryocytes isolated from rat bone marrow to externally applied adenosine triphosphate (ATP) were investigated in the whole-cell mode by the use of nystatin perforated patch-clamp technique. 2. ATP at 1-100 microM evoked periodic outward currents at a holding potential of -40 mV. The reversal potential of the currents was close to K+ equilibrium potential (EK) and the K+ channel blockers such as quinine and quinidine suppressed the currents, indicating that the outward currents are predominantly carried by K+. 3. Since it has been reported that adenosine diphosphate (ADP) evoked monophasic K+ current using a conventional whole-cell recording, we compared the results obtained by perforated and conventional patch-clamp techniques. The crucial difference between our results and previous results was due to the intracellular perfusion with internal solution containing a high concentration of EGTA by which both current shape and concentration response were modified. 4. The membrane permeable Ca2+ chelator, 1,2-bis(O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (acetoxy methyl ester; BAPTA AM), inhibited the K+ current concentration dependently, suggesting that ATP-induced oscillatory K+ currents are caused by changes in cytoplasmic free Ca2+ concentration ([Ca2+]i). 5. With increasing ATP concentration, the frequency and the maximum amplitude of K+ current oscillation increased and the latency of current, which is the period required to activate the first K+ current after ATP application, decreased. 6. ADP, 2-methylthio-ATP and ATP-gamma-S could also evoke the periodic K+ currents, but adenosine, uridine triphosphate (UTP) and alpha-beta-methylene adenosine 5'-triphosphate (AMP-CPP) failed. 2-Methylthio-ATP was the most potent agonist; next was ADP which showed a 10-30 times stronger effect than ATP. Cross-desensitization was observed between ATP and ADP, but not between ATP or ADP and thrombin. 7. Extracellular Ca2+ was not required for the ATP-induced K+ current activation, indicating that Ca2+ released from intracellular pools induced the oscillatory response. In addition, the agonist potency increased when extracellular Ca2+ concentration ([Ca2+]o) decreased, suggesting that the principal agonists might be ATP4- and ADP3-. 8. The results suggest the presence of a novel subtype of purinoceptor in the megakaryocyte plasma membrane which induces cytoplasmic Ca2+ oscillation and evokes periodic K+ current flux.
Full text
PDF




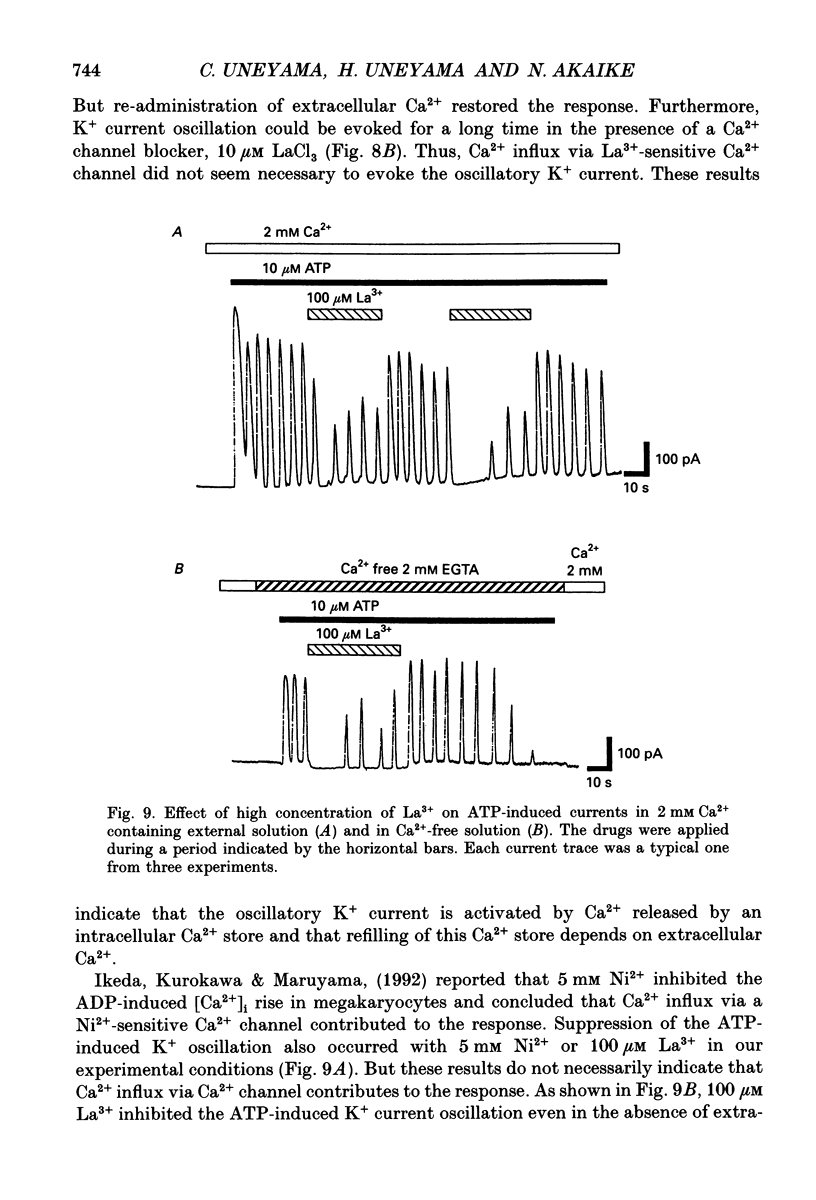




Selected References
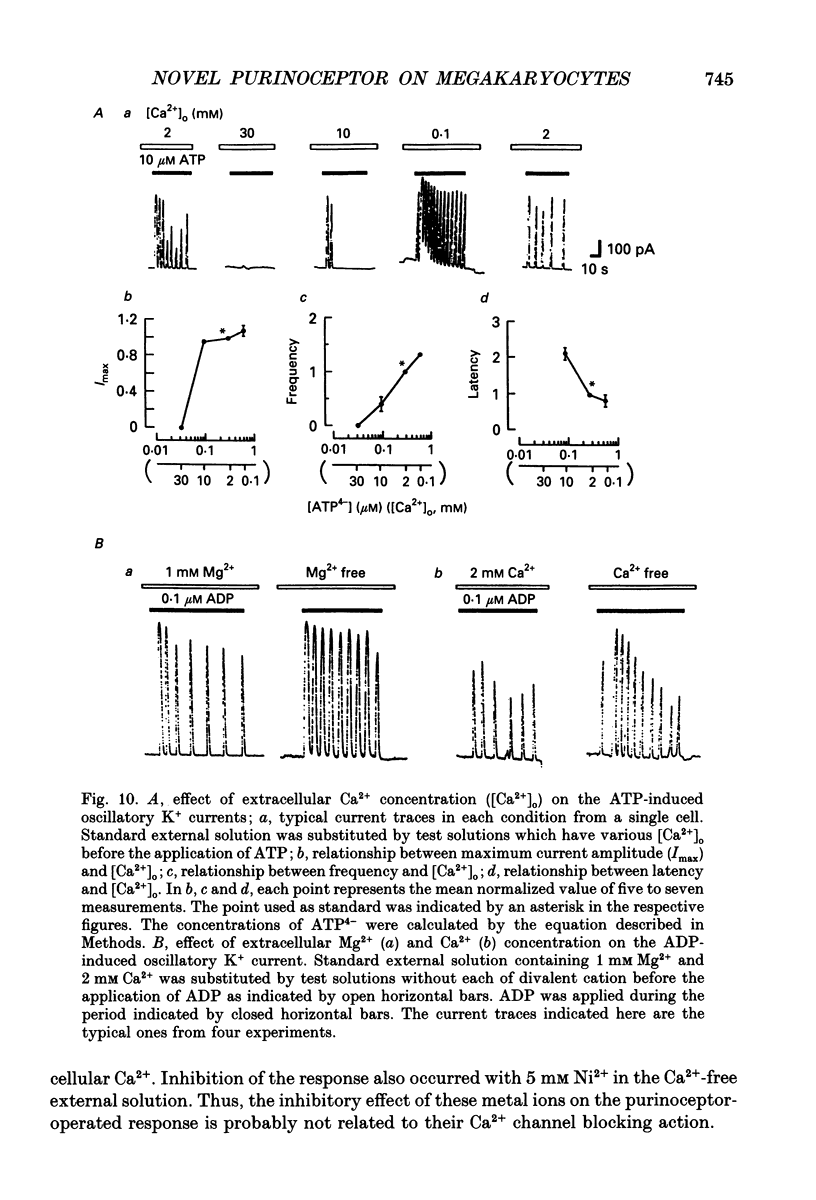
These references are in PubMed. This may not be the complete list of references from this article.
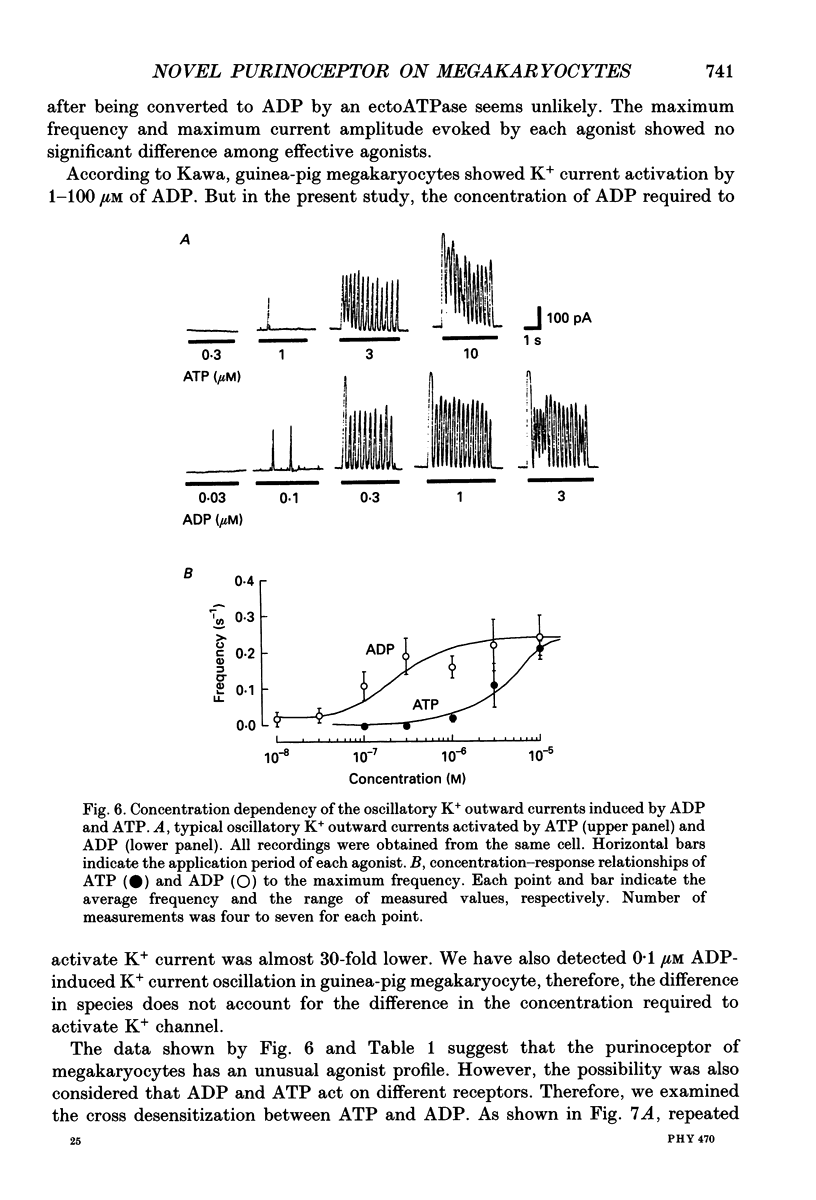
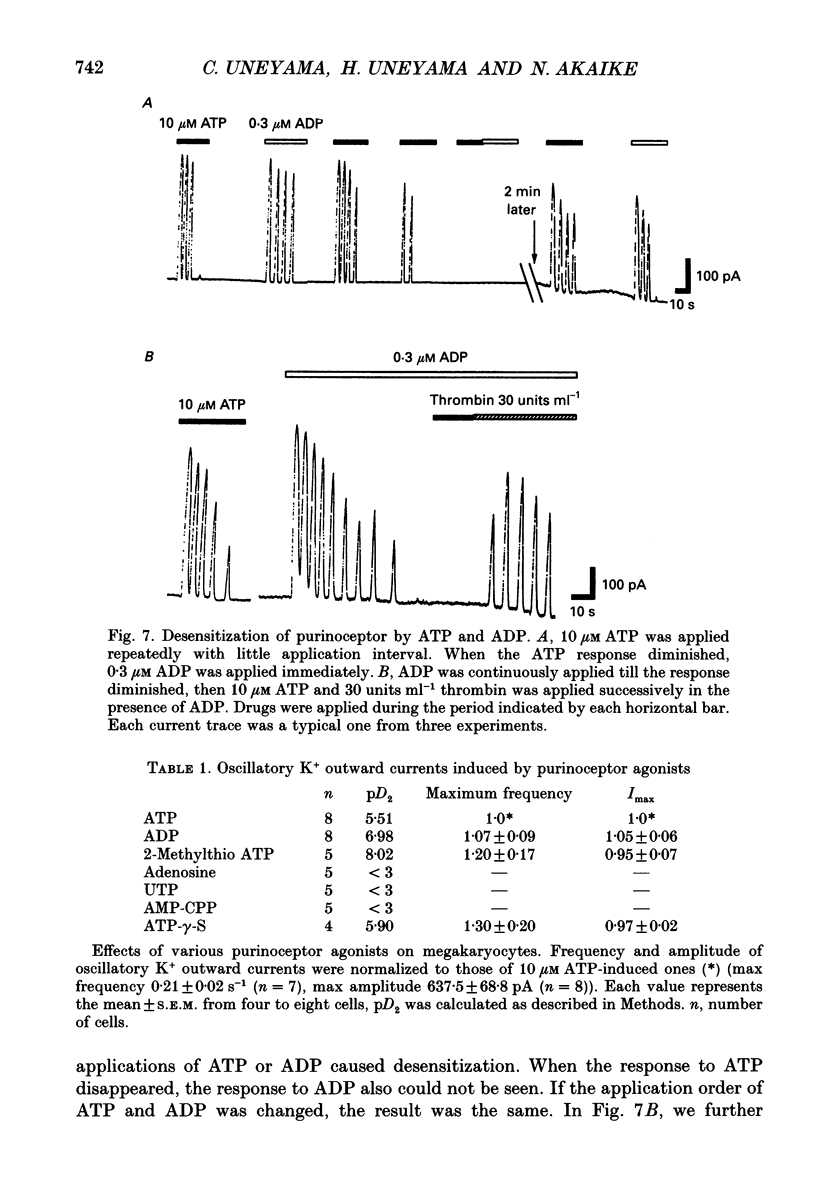
- Cockcroft S., Gomperts B. D. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979 Nov;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
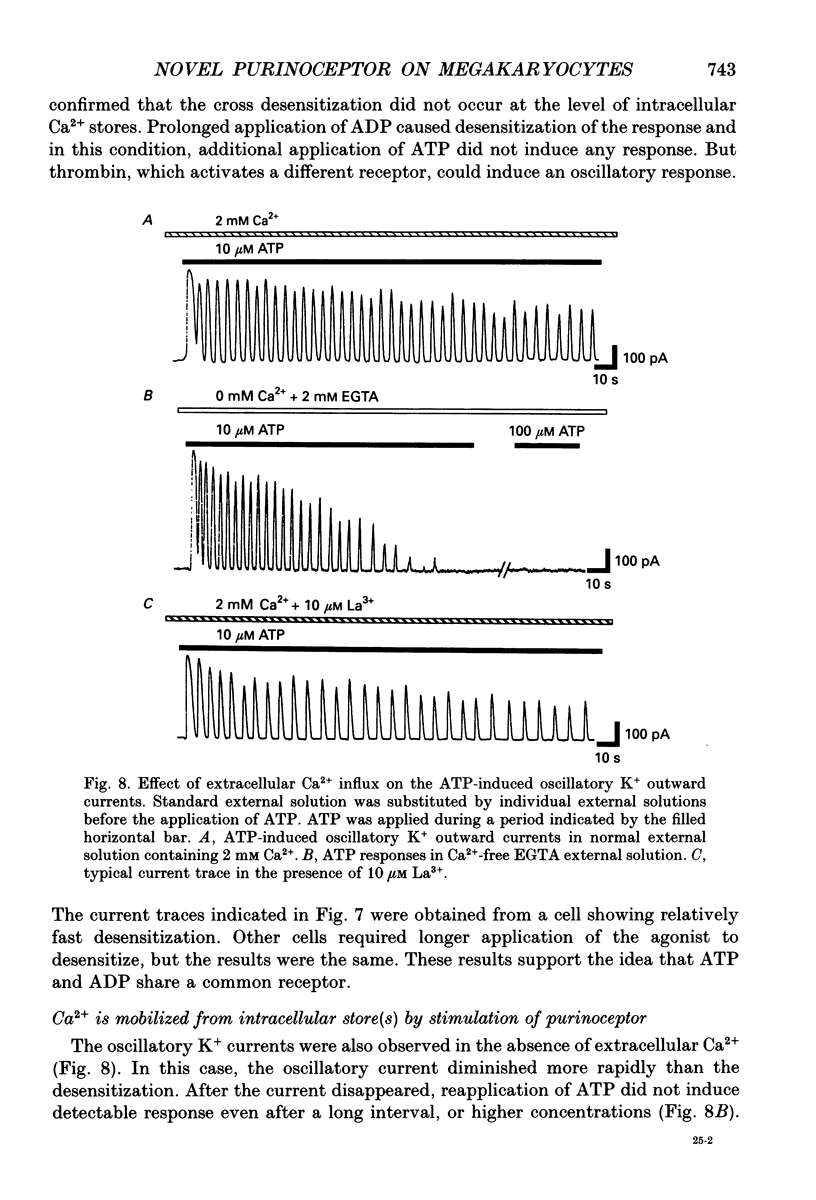
- Dahlquist R., Diamant B. Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharmacol Toxicol (Copenh) 1974 May;34(5):368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Fedorko M. E. The functional capacity of guinea pig megakaryocytes. I. Uptake of 3H-serotonin by megakaryocytes and their physiologic and morphologic response to stimuli for the platelet release reaction. Lab Invest. 1977 Mar;36(3):310–320. [PubMed] [Google Scholar]
- Fedorko M. E. The functional capacity of guinea pig megakaryocytes. II. The uptake of particles and macromolecules and the effect of rabbit antiguinea pig platelet antiserum. Lab Invest. 1977 Mar;36(3):321–328. [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Tsien R. Y. Agonist-induced calcium oscillations in depolarized fibroblasts and their manipulation by photoreleased Ins(1,4,5)P3, Ca++, and Ca++ buffer. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):935–943. doi: 10.1101/sqb.1988.053.01.108. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Kurokawa K., Maruyama Y. Cyclic nucleotide-dependent regulation of agonist-induced calcium increases in mouse megakaryocytes. J Physiol. 1992 Feb;447:711–728. doi: 10.1113/jphysiol.1992.sp019025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Kawa K. Guinea-pig megakaryocytes can respond to external ADP by activating Ca2(+)-dependent potassium conductance. J Physiol. 1990 Dec;431:207–224. doi: 10.1113/jphysiol.1990.sp018327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. M., Martell A. E. Thermodynamic quantities associated with the interaction of adenosine triphosphate with metal ions. J Am Chem Soc. 1966 Feb 20;88(4):668–671. doi: 10.1021/ja00956a008. [DOI] [PubMed] [Google Scholar]
- Leven R. M., Nachmias V. T. Cultured megakaryocytes: changes in the cytoskeleton after ADP-induced spreading. J Cell Biol. 1982 Feb;92(2):313–323. doi: 10.1083/jcb.92.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. A patch-clamp study of mammalian platelets and their voltage-gated potassium current. J Physiol. 1987 Oct;391:467–485. doi: 10.1113/jphysiol.1987.sp016750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L. Characterization of the megakaryocyte secretory response: studies of continuously monitored release of endogenous ATP. Blood. 1983 May;61(5):967–972. [PubMed] [Google Scholar]
- Minta A., Tsien R. Y. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989 Nov 15;264(32):19449–19457. [PubMed] [Google Scholar]
- Murase K., Ryu P. D., Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989 Aug 14;103(1):56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Komune S., Uemura T., Akaike N. Excitatory amino acid response in isolated spiral ganglion cells of guinea pig cochlea. J Neurophysiol. 1991 Mar;65(3):715–723. doi: 10.1152/jn.1991.65.3.715. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Shirasaki T., Wakamori M., Fukuda A., Akaike N. Excitatory amino acid response in isolated nucleus tractus solitarii neurons of the rat. Neurosci Res. 1990 Jun;8(2):114–123. doi: 10.1016/0168-0102(90)90063-k. [DOI] [PubMed] [Google Scholar]
- Nishio H., Ikegami Y., Segawa T. Fluorescence digital image analysis of serotonin-induced calcium oscillations in single blood platelets. Cell Calcium. 1991 Feb-Mar;12(2-3):177–184. doi: 10.1016/0143-4160(91)90019-b. [DOI] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Yada T. Calcium channel and calcium pump involved in oscillatory hyperpolarizing responses of L-strain mouse fibroblasts. J Physiol. 1982 Jun;327:449–461. doi: 10.1113/jphysiol.1982.sp014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Stimulated calcium efflux from fura-2-loaded human platelets. J Physiol. 1987 Dec;393:513–524. doi: 10.1113/jphysiol.1987.sp016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989 Oct 15;264(29):17131–17141. [PubMed] [Google Scholar]
- Schick B. P., Walsh C. J., Jenkins-West T. Sulfated proteoglycans and sulfated proteins in guinea pig megakaryocytes and platelets in vivo. Relevance to megakaryocyte maturation and platelet activation. J Biol Chem. 1988 Jan 15;263(2):1052–1062. [PubMed] [Google Scholar]
- Tsunoda Y., Stuenkel E. L., Williams J. A. Oscillatory mode of calcium signaling in rat pancreatic acinar cells. Am J Physiol. 1990 Jan;258(1 Pt 1):C147–C155. doi: 10.1152/ajpcell.1990.258.1.C147. [DOI] [PubMed] [Google Scholar]
- Uneyama H., Munakata M., Akaike N. 5-HT response of rat hippocampal pyramidal cell bodies. Neuroreport. 1992 Jul;3(7):633–636. doi: 10.1097/00001756-199207000-00023. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


