Abstract
Frailty is an age-related syndrome that drives multiple physiological system impairments in some older adults, and its pathophysiological mechanisms remain unclear. We evaluated whether frailty-related biological processes could impair stem cell compartments, specifically the renal stem compartment, given that kidney dysfunctions are frequent in frailty. A well-characterized in vitro nephrosphere model of human adult renal stem/progenitor cells has been instrumental to and was appropriate for verifying this hypothesis in our current research. Evaluating the effects of plasma from older individuals with frailty (frail plasma) on allogeneic renal stem/progenitor cells, we showed significant functional impairment and nuclear DNA damage in the treated cells of the renal stem compartment. The analysis of the frail plasma revealed mitochondrial functional impairment associated with the activation of oxidative stress and a unique inflammatory mediator profile in frail individuals. In addition, the plasma of frail subjects also contained the highest percentage of DNA-damaged autologous circulating hematopoietic progenitor/stem cells. The integration of both molecular and functional data obtained allowed us to discern patterns associated with frailty status, irrespective of the comorbidities present in the frail individuals. The data obtained converged toward biological conditions that in frailty caused renal and hematopoietic impairment of stem cells, highlighting the possibility of concomitant exhaustion of several stem compartments.
Keywords: Aging, Cellular senescence, Comorbidity, Frailty pathophysiology, Mitochondrial impairment
Frailty is a multidimensional geriatric syndrome that can be associated with aging. It is defined as a state of increased vulnerability, resulting from the deterioration of several physiological systems, a decrease in functional reserves, and a diminished ability to maintain homeostasis (1). Frail individuals exhibit a reduced capacity to cope with both internal and external stressors, putting them at high risk of severe adverse outcomes such as falls, fractures, hospitalizations, iatrogenic complications, and mortality (1). One of the most commonly used methods for assessing frailty relies on the Fried phenotype, which defines frailty as the presence of at least 3 out of 5 clinical criteria (2,3). These criteria comprise unintentional weight loss, fatigue in performing daily living activities, reduced walking speed, a low physical activity level, and diminished muscle strength. Frailty is not synonymous with either comorbidity or disability, and adverse outcomes can occur independently of their presence (1,2). The WHO World Report on Aging and Health (4) states that the prevalence of frailty in older adults aged 65 or older is approximately 17% and tends to increase with advancing age.
The underlying pathophysiological mechanisms that lead some older adults to develop frailty remain unclear due to the complex interactions among the different proposed hallmarks of aging during the biological aging process (5,6). In fact, molecular processes, such as increased production of reactive oxygen species (ROS) following age-related mitochondrial dysfunction (7), DNA damage, with the body’s response to such damage (8), and the local production of inflammatory cytokines in the context of “inflammaging” (chronic low-grade inflammation in aging in the absence of manifest infection) (9) are systemic processes that are highly interconnected during aging (10). These interconnections make it difficult to determine the extent to which these processes contribute to biological aging development itself or act as drivers of various age-related diseases. To date, no univocal biological indicators are available to explain the development of frailty and identify frail individuals (11,12). Consequently, several studies aiming to shed light on frailty development are still underway, particularly because early frailty can be reversible (1,2) and therefore manageable.
Among the proposed cellular and molecular hallmarks of aging is stem cell exhaustion (5,13), which is a consequence of the increased vulnerability of stem cells due to the various types of age-related damage they accumulate. Recently, we demonstrated that a significantly higher percentage of CD34+ circulating hematopoietic progenitor/stem cells (cHPSCs), as well as peripheral blood mononuclear cells and CD3+ T cells, have DNA damage in frail older adults, and there is a significantly elevated oxidative stress level in their plasma (14). In the literature, DNA damage in hematopoietic stem cells is associated with their functional impairment (15,16). Pondering these data, we asked whether it was possible to identify specific biological features that could highlight impairments in different stem compartments in a clinical setting of frail older adults. Of note, there is evidence that progressive renal dysfunction is often present in frailty (17–19). Among older adults with moderate-to-severe chronic kidney disease, 20.9% are frail (17), and the prevalence of frailty in dialysis-dependent patients ≥65 years old is 71.4% (20). These observations led us to explore whether impairment of the renal stem cell compartment could be a possible factor involved in frailty development. The availability of a well-characterized in vitro model of human adult renal stem/progenitor cells grown as nephrospheres (NSs) (21–23) in our laboratory has been instrumental to and is appropriate for verifying this hypothesis in our current research. Evaluating the effects of plasma from older individuals with frailty (frail plasma) on allogeneic renal stem/progenitor cells, we showed significant functional impairment and nuclear DNA damage in the treated cells of the renal stem compartment. The analysis of the frail plasma revealed mitochondrial functional impairment associated with the activation of oxidative stress and a unique inflammatory mediator profile in frail individuals. Moreover, the plasma of these frail subjects contained the highest percentage of autologous DNA-damaged cHPSCs. In addition, the integration of both molecular and functional data allowed us to discern patterns associated with frailty status, irrespective of the comorbidities present in the frail individuals.
Method
Study Population
A group of 76 older adults (41 frail and 35 non-frail) aged ≥65 years were recruited from the outpatient services of the Acute Geriatrics Unit, San Gerardo Hospital, IRCCS San Gerardo, Monza, Italy. The exclusion criteria were illiteracy, psychiatric disorders, Parkinson’s disease, cognitive impairment (moderate or worse), multiple sclerosis, neurodegenerative diseases, hip fracture, or other severe trauma affecting mobility. The identification of a frail phenotype in all 76 subjects was based on the 5 operative criteria reported by Fried et al. (2). The comorbid conditions of these older adults are described in Supplementary Methods and for the purpose of overall data integration, the older adults were stratified by the comorbidity cutoff 2, generating subgroups with ≤2 and >2 pathologies (24). Thirty-eight young subjects aged between 25 and 35 years with a healthy lifestyle, no history of past or present drug abuse, alcohol consumption, or smoking, and without functional disability or acute pathologies in progress were recruited as control group. Analytical data of study population are reported in Supplementary Table 1A and B. Whole blood from the recruited individuals was collected into BD Vacutainer tubes with sodium heparin (Becton Dickinson, Franklin Lakes, NJ, USA). The plasma was separated by centrifugation at 1 500g for 15 minutes, scaled in aliquots, and stored at −80°C until analysis.
Renal Tissues
Kidney tissues were obtained from 21 patients (12 males and 9 females), after nephrectomy performed at Urology Unit, IRCCS San Gerardo (Supplementary Table 2) and the normal tissues, from the opposite side of the pathological tissue, were isolated by an experienced pathologist.
Ethics Approval
The study was conducted according to the principles of the Declaration of Helsinki II and approved by the Brianza Ethics Committee, Monza, Italy (code FRA-ARSC), on March 22, 2018. Sensitive information about the recruited individuals was available to researchers anonymously. The participants in the study were volunteers, and all were fully informed about the study protocol and the nature of their participation. Each participant signed an informed consent form, and the documents were stored at San Gerardo Hospital.
Nephrosphere Cultures and Plasma Treatment
Normal kidney tissues were dissociated as described (25). The single-cell suspension obtained was plated allowing adhering for 24–48 hours (21); the cells were then detached enzymatically and plated at low density (10 000 cells/mL) in nonadherent condition in a 6-well plate coated with poly-Hema (Sigma‒Aldrich, St. Louis, MO, USA) to obtain NSs as previously described (21). For the treatment of NS cultures, 10% individual plasma sample from frail, non-frail, or young individuals was added to the SC medium of each growing culture. The cultures grown without plasma addition were the untreated control. After 10 days, the sphere-forming efficiency (SFE) was determined by counting the number of obtained floating NSs under a contrast phase microscope (Olympus Life Science, Tokyo, Japan); this number was divided by the number of plated cells and is expressed as a percentage (21).
Flow Cytometry Analysis of NS Cells
The floating NSs were also collected and dissociated, enzymatically and mechanically by repetitive pipette syringing to generate a single-cell suspension (21). The dissociated NS cells were then centrifuged and suspended in phosphate-buffered saline (PBS) with 5% fetal bovine serum (FBS) for 15 minutes at RT and then fixed with 4% methanol-free formaldehyde (Thermo Fisher Scientific, Waltham, MA, USA) and permeabilized with 0.1% Triton X-100 (Sigma‒Aldrich). After centrifugation, 70% cold methanol was added dropwise to the cellular pellets while vortexing, and the samples were then stored at −20°C for 24 hours (14). After washing, these were stained at 4°C with FITC-conjugated anti-phospho-histone H2AX (Ser139) (γ-H2AX) (Clone JBW301; Millipore, Burlington, MA, USA; 2.5 mg/mL) for 2 hours or with eFluor 660-conjugated anti-human Ki-67 (Clone 20Raj1; Invitrogen, Waltham, MA; 1:200) for 30 minutes. Additional aliquots of the same dissociated NS cells were stained with the FITC Annexin V Apoptosis Detection Kit with propidium iodide (PI; Biolegend, San Diego, CA, USA) following the manufacturer’s instructions. The stained samples were then analyzed, at least 2 × 104 events were acquired, and data on DNA damage, cell proliferation, and live, early apoptotic, late apoptotic, and necrotic cells among NS cells were obtained. A MoFlo Astrios cell sorter equipped with Summit 6.3 software was used, and data were analyzed with Kaluza 2.1 software (Beckman Coulter, Miami, FL, USA) or FCS express 7 (De Novo, Pasadena, CA, USA) for offline analysis.
Immunofluorescence to Detect γ-H2AX in NS Cells
After dissociation of plasma-treated floating NSs, the single cells were subjected to cytospinning for preparation for immunofluorescence staining. The slides were prepared by spinning 20 000 cells, with the specific plasma treatment, at 800g for 15 minutes on a Heraeus Multifuge 3S+ Centrifuge (Thermo Scientific). Cytospinned cells were fixed in freshly buffered 4% paraformaldehyde for 20 minutes. After blocking with PBS containing 0.1% bovine serum albumin (Sigma‒Aldrich), 0.3% Triton X-100 (Sigma‒Aldrich) and 0.1% saponin (Sigma‒Aldrich), the cells were incubated overnight at 4°C with anti-phospho-histone H2AX (Ser139) (Clone JBW301; Millipore; 1:100) primary antibody. After rinsing in PBS, the cells were incubated for 1 hour at room temperature with Alexa Fluor 488-conjugated anti-rabbit IgG secondary antibody (Molecular Probes Invitrogen, Waltham, MA, USA; 1:100). The slides were mounted with ProLong Gold Antifade with DAPI (Molecular Probes Invitrogen) for nuclear counterstaining. Microphotographs were taken using a Zeiss LSM710 confocal microscope equipped with Zen software version 2009, acquiring 10 different microscopic fields (Zeiss, Oberkochen, Germany) for each specific plasma treatment. The number of γ-H2AX+ foci in NS cells, the percentage of nuclei with multiple foci, and the nuclear fluorescence intensity were evaluated with Count Nuclear Foci Plugin-ImageJ software version 1.53c (National Institutes of Health, NIH, Bethesda, MD, USA; http://imagej.nih.gov/ij).
Oxysterols and Cholesterol Precursors in Plasma Samples
The respective levels were determined as described (14,26), in a set of randomly chosen plasma samples from frail, non-frail, and young individuals, using isotope dilution gas chromatography–mass spectrometry (GC–MS) with a HP 6890 Network GC system connected to a quadrupole mass selective detector HP5975B (Agilent Technologies, Santa Clara, CA, USA).
Reactive Oxygen Species Assay
Independent NS cultures were established. Each culture has been grown for 10 days with a set of 10% plasma from frail, non-frail, or young individuals. The dissociated NS cells obtained were suspended in 300 µL of SC medium (21) at room temperature, supplemented with 10 μM of the fluorescent cell-permeable indicator 2ʹ,7ʹ-dichlorofluorescin diacetate (DCFH-DA; Sigma‒Aldrich) and incubated for 30 minutes at 37°C. DCFH-DA was oxidized into fluorescent 2ʹ,7ʹ-dichlorofluorescein (DCF) in the presence of intracellular ROS. Successively, samples were washed with 300 µL of cold PBS by spinning, and then the pellets were suspended in 300 µL of cold PBS. A total of 2 × 104 events were acquired by analyzing DCF fluorescence with the MoFlo Astrios cell sorter at excitation and emission wavelengths of 485 and 528 nm, respectively. Flow cytometry data were analyzed with Kaluza 2.1 software (Beckman Coulter) or FCS express 7 (De Novo). The DCF median fluorescence intensities obtained by treatment were normalized versus the respective untreated NS cells considered equal to 1.
Plasma Cytokines Quantification
The concentrations (pg/mL) of 38 cytokines (Supplementary Table 7A) in the plasma of 38 frail, 34 non-frail, and 36 young individuals were evaluated using the Human Magnetic Luminex Screening Assay, performed as a service by Labospace s.r.l. (Milano, Italy). Details in Supplementary Methods.
Statistical Analysis
Comparisons of clinical data between the frail and non-frail groups were analyzed with Student’s t test, the Mann‒Whitney U test, or the chi-square test, as indicated. Comparisons of the experimental data among the frail, non-frail, and young groups were analyzed by 1-way ANOVA with Tukey’s test or Holm‒Sidak’s test for pairwise multiple comparison. The ORIGINPRO 2016 64BIT software (Origin Lab Corporation, Northampton, MA, USA) was used. Values of p < .05 (with 95% confidence intervals) were considered statistically significant. In the box/dot graphs, corresponding to at least 3 independent experiments, each dot represents a single independent experiment, the boxes indicate the 25°–75° percentile, the continuous horizontal line into the box represents the mean, and the dotted horizontal line represents the median. The variables, first examined individually for their differences among the frail, non-frail, and young groups, were then also examined collectively through principal component analysis (PCA) (27). Details in Supplementary Methods.
Results
Study Population
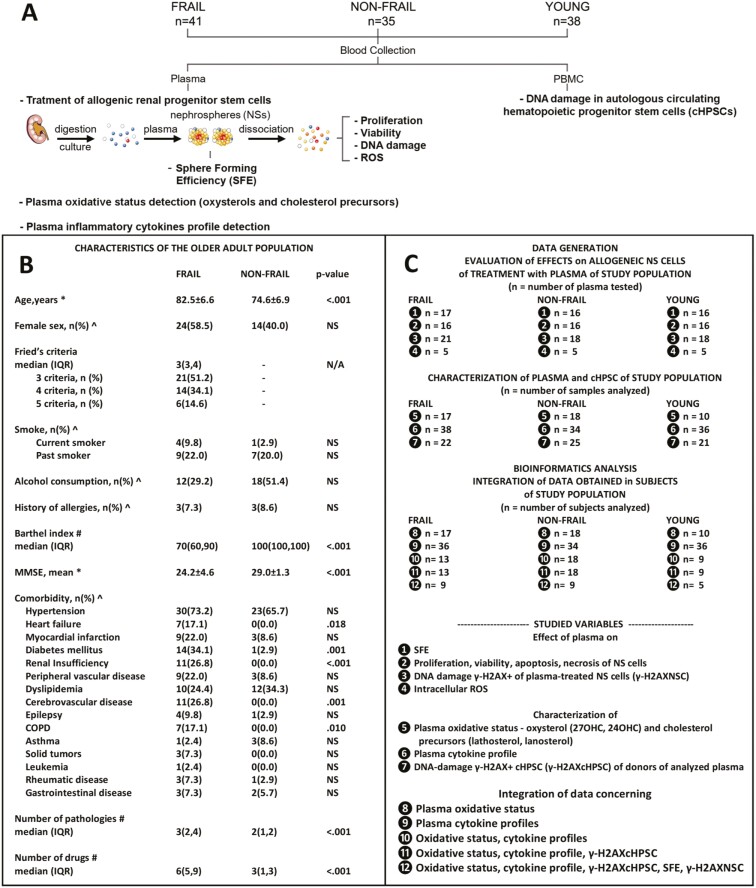
Seventy-six older adults, 41 frail and 35 non-frail, were consecutively recruited based on the frailty phenotype criteria proposed by Fried et al. (2). Thirty-eight young individuals (mean age ± SD, 29.4 ± 2.3 years; males 14, females 24) without functional impairments or diseases were also recruited. The experimental strategy of this study is reported (Figure 1A) and the characteristics of the older adult population are summarized (Figure 1B). Frail participants were on average older than non-frail participants (82.5 vs 74.6 years, respectively), with no significant differences in terms of sex distribution. Both groups displayed a similar percentage of current and former smokers and history of allergies. Although the frail group had a lower percentage of moderate alcohol consumption than the non-frail group (29.2% vs 51.4%, respectively), the difference was not significant. The average Mini-Mental State Examination (MMSE) score was higher among non-frail participants than among frail participants (29.0 vs 24.2), but overall, they did not indicate cognitive impairment. Frail participants had a higher median number of comorbid conditions (24) documented by medical history (3 vs 2) and drugs (6 vs 3). A summary of the experiments and analysis performed on the different plasma samples and individuals is reported in Figure 1C. The specific characteristics of each individual are provided in Supplementary Table 1A and B. Peripheral blood was collected from the recruited individuals, and the separated plasma was scaled in aliquots and stored at −80°C until use.
Figure 1.
A framework of the study. (A) Experimental strategy. Forty-one frail, 35 non-frail older adults, and 38 young subjects (young: mean age ± standard deviation, 29.4 ± 2.3 years; males 14, females 24) were recruited. Collection of peripheral blood samples and separation of plasma and peripheral blood mononuclear cells (PBMCs). Plasma is used to treat human allogeneic renal cells growing as nephrospheres (NSs) that harbor adult renal stem/progenitor cells. Evaluation of the sphere-forming efficiency (SFE) of renal stem/progenitor cells. Evaluation of the proliferation, viability, DNA damage, and ROS production of NS cells. Evaluation of DNA damage in autologous cHPSCs present in PBMCs of plasma donors. Evaluation of plasma oxidative status and plasma inflammatory cytokines. The difficulty to obtain renal tissue did not permit to include pre-frail subjects in this study. (B) Clinical characteristics of older adults. Data are summarized as the mean ± standard deviation, frequency and percentage, or median and interquartile range (IQR). Comparisons between groups were analyzed by *Student’s t test, ^the chi-square test, or #the Mann‒Whitney U test, as indicated. Significance was set for values of p < .05; NS = not significant; N/A = not applicable; MMSE = Mini-Mental State Examination; COPD = chronic obstructive pulmonary disease. (C) Summary of the experiments and analyses performed for data generation. (B, C) Analytical data are provided in Supplementary Table 1A and B.
Effects of Frail, Non-Frail, and Young Plasma on NS Cell Behavior
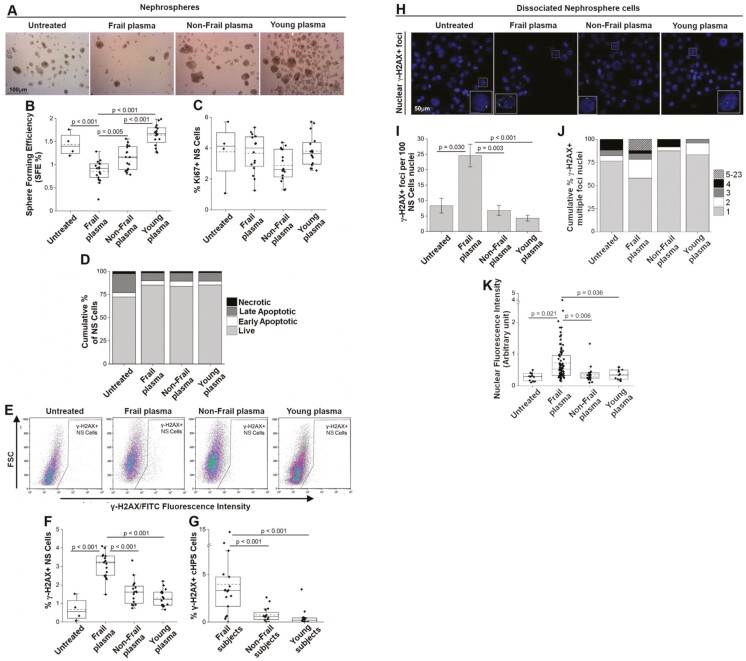
The effects of plasma from enrolled subjects were evaluated on 11 cell cultures established from 10 human allogeneic normal renal tissues obtained after nephrectomy (Supplementary Table 2). The renal cells were grown for 10 days to give rise to NSs, an in vitro model harboring adult renal stem/progenitor cells (21–23). The 11 cultures grew in the presence of 10% plasma from 17 frail, 16 non-frail, or 16 young individuals (Figure 2A). Each plasma was tested on 2–3 cultures and each culture was tested with 3–5 plasmas (Supplementary Table 3). After this treatment, the SFE was obtained (Figure 2B). Sphere-forming efficiency indicates the percentage of plated cells that maintain both the self-renewal capacity, peculiar characteristics of stem and early progenitor cells, and the capacity to produce NSs containing stem/progenitor cells and more mature progenitor cells (21). The NS cultures treated with frail plasma had a significantly lower SFE than did the cultures untreated or treated with plasma from non-frail and young individuals (Figure 2B). As a marker of proliferation activity, Ki-67 expression (28) on NS cells after NS dissociation was evaluated by flow cytometry, and the results showed no significant differences among the various treatments (Figure 2C). Furthermore, analysis by Annexin V/PI staining showed no differences in viability, early or late apoptosis or necrosis among the NS cells treated with the different plasma samples during NS formation (Figure 2D).
Figure 2.
Effects of 10 days of treatment with plasma from frail, non-frail, and young subjects on NS cells. (A) Phase contrast images representative of renal cells grown as NS, untreated or treated with the indicated plasma. Scale bar: 100 μm. Eleven NS cultures were used (Supplementary Table 2), and the treatments were made with 17 frail, 16 non-frail, and 16 young plasma samples (Supplementary Table 3). (B) Sphere-forming efficiency (SFE %) of renal cells grown as NSs and treated with the indicated plasma; data were obtained with a contrast phase microscope. (C) Percentage of NS cells positive for Ki-67 after the indicated plasma treatment. Flow cytometry data are shown (gating strategy in Supplementary Figure 1). (D) Cumulative percentage of live, early, late apoptotic, and necrotic NS cells after the indicated plasma treatments, flow cytometry data of Annexin V/PI staining are shown (representative dot plots in Supplementary Figure 2); color-code: light gray, live; white, early apoptotic; dark gray, late apoptotic; black, necrotic. (E) Representative flow cytometry dot plots of γ-H2AX+ NS cells treated with the same plasma samples as in Figure 2A. FSC = Forward Scatter (gating strategy in Supplementary Figure 1). (F) Percentage of nuclear DNA-damaged (γ-H2AX+) NS cells after the indicated plasma treatments, flow cytometry data. (G) Percentage of DNA-damaged (γ-H2AX+) cHPSCs (Supplementary Table 4) circulating in the subjects donors of the plasma used in panel F, flow cytometry data. (H) Representative cytospin immunofluorescence analysis of nuclear γ-H2AX+ foci in 3 different independent NS cultures grown with 7 frail, 4 non-frail, and 4 young plasma samples. Ten different fields were evaluated for each specific plasma treatment. Scale bars: 50 μm; zoomed inserts: 10 μm. Nuclei stained by DAPI. (I) Mean ± SEM. of γ-H2AX+ foci per 100 nuclei of untreated or treated NS cells. (J) Cumulative % of γ-H2AX+ multiple-focus nuclei. Color-code: light gray, 1 focus; white, 2 foci; dark gray, 3 foci; black, 4 foci; lines upward right, 5–23 foci per nucleus. (K) γ-H2AX fluorescence intensity per nucleus of NS cells. p < .05 obtained with 1-way ANOVA with Tukey’s test for pairwise multiple comparison was considered significant.
These data (Supplementary Table 3) indicated that the plasma of frail patients was able to functionally affect allogeneic renal stem/progenitor cells, decreasing their self-renewal ability (SFE) in an in vitro culture model and that the SFE decrement was not linked to cell proliferation or cell death.
Frail Plasma Treatment Increases DNA Damage in NS Cells
To better understand the functional impairment of plasma-treated allogeneic NS cells, we investigated DNA damage, in literature described as an important cause of stem cell dysfunction (29). The phosphorylation of histone H2AX in Serine 139 (γ-H2AX) represents a single DNA double-strand break (DSB) at a ratio of 1:1 (30) and is a good marker of DNA damage. The γ-H2AX can be detected by flow cytometry (31,32). In the same dissociated NS cells described above, γ-H2AX fluorescent foci were detected (Figure 2E). The highest percentages of DNA-damaged NS cells (γ-H2AX+) in cultures treated with plasma from frail individuals compared to the other groups were significantly different (Figure 2F, Supplementary Table 3). The percentage of DNA-damaged NS cells (Figure 2F) showed an inverse trend with respect to SFE evaluated in the same cells (Figure 2B). Moreover, the DNA damage in the treated allogeneic NS cells (Figure 2F) showed the same trend as that in autologous cHPSCs of a wider population previously described (14), evaluated with the same flow cytometry method. In this wider population (14), the donors of plasma used in the current set of treatments were also present and the percentages of DNA-damaged cHPSCs (γ-H2AX+) circulating in the same plasma of these specific donors were reported (Figure 2G). Therefore, this cohort of frail individuals had the highest percentage of autologous DNA-damaged cHPSCs (γ-H2AX+) (Figure 2G, see also Supplementary Table 4), and their plasma induced the highest percentage of DNA damage (Figure 2F) and the lowest SFE (Figure 2B) in allogeneic NS cells. Instead, non-frail and young subjects had lowest DNA-damaged cHPSCs (Figure 2G), and their own plasma induced lowest DNA damage (Figure 2F) and maintained highest SFE (Figure 2B) in NS cells. In addition, 3 further different NS cultures (Supplementary Table 2) grown in the presence of 10% plasma from 7 frail, 4 non-frail, or 4 young individuals were dissociated, subjected to cytospinning, and immunostained for a better evaluation of cells positive for nuclear γ-H2AX+ foci (Figure 2H). The confocal analysis of cells treated with frail plasma showed a significantly higher mean number of γ-H2AX+ foci per 100 nuclei (Figure 2I) and a higher percentage of nuclei with multiple foci (Figure 2J), which was also confirmed by the highest nuclear γ-H2AX fluorescence intensity following frail plasma treatment (Figure 2K).
These data showed that the plasma of frail patients was able to cause a significant increase in the percentage of allogeneic NS cells with increased DNA damage that matched the reduced self-renewal capacity (SFE) of plasma-treated renal stem/progenitor cells. In addition, there was evidence that a significantly higher percentage of autologous cHPSCs with DNA damage circulated in the same frail plasma.
Oxysterols and Cholesterol Precursors in Frail Plasma
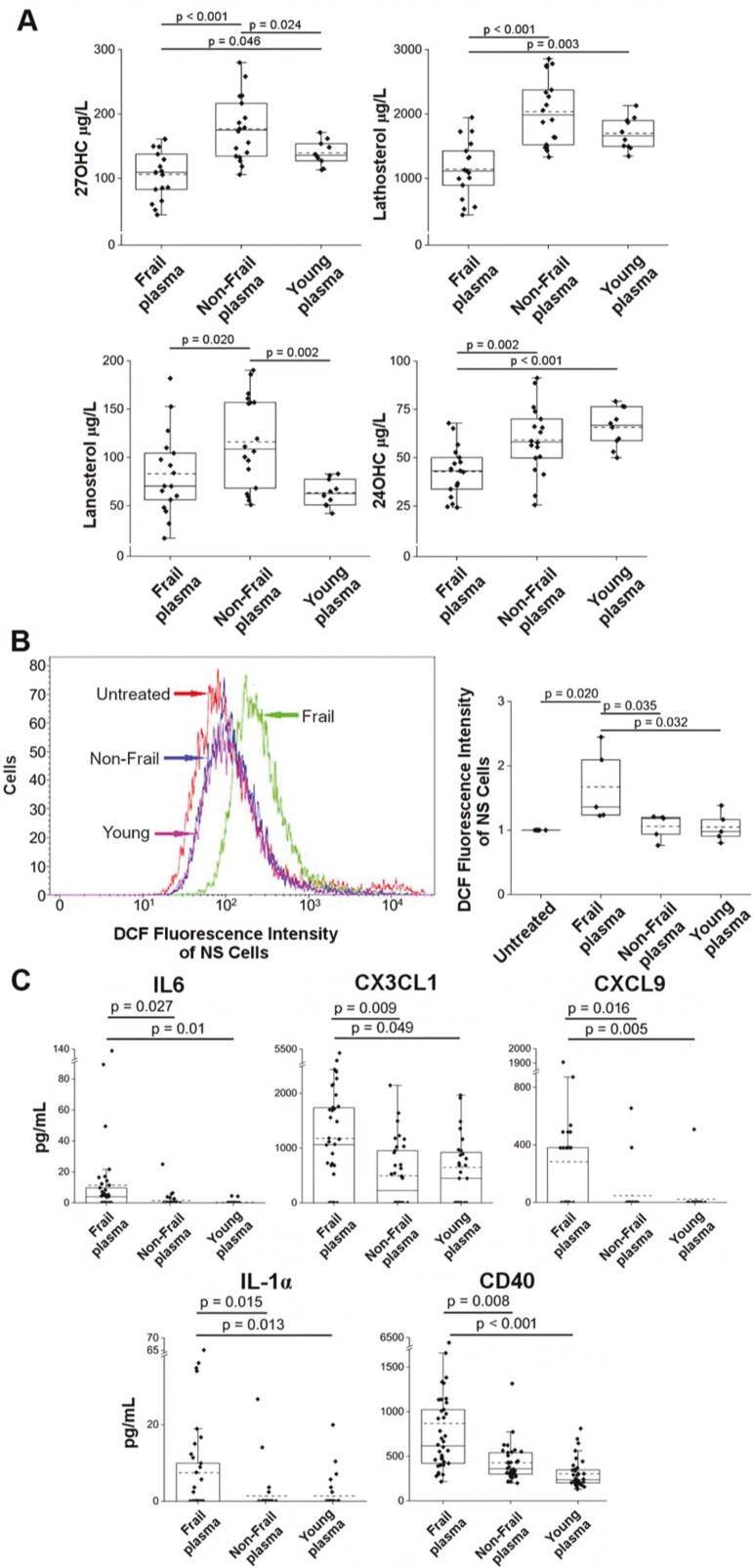
In the plasma of frail individuals, we had previously detected significantly higher levels of 8-hydroxy-2-deoxy guanosine (8-OHdG) (14), a byproduct of oxidative DNA damage; therefore, the highest DNA damage could be related to an abnormally high oxidative status. To document the eventual role of ROS as responsible for DNA damage, 3 additional NS cultures (Supplementary Table 2) were grown for 10 days in the presence of increasing concentrations of hydrogen peroxide (H2O2), and more DNA damage and a decrease in SFE were observed (Supplementary Figure 3). The mitochondria are frequently involved in aging-related dysfunction (7) associated with the altered production of ROS, and we wondered if mitochondrial functional impairment could be detected in the plasma of our frail individuals. 27-Hydroxycholesterol (27-OHC), a marker of mitochondrial cholesterol elimination, whose levels are decreased in the case of mitochondrial impairment (26), and lathosterol and lanosterol, markers of cholesterol synthesis depending on the mitochondrial production of citrate (26), were significantly decreased in the plasma of frail individuals compared to non-frail, suggesting impaired mitochondrial function (26) (Figure 3A). Circulating 24-hydroxycholesterol (24-OHC), which depends on the number of metabolically active neurons, was also decreased, reflecting neuron loss (33). In our frail patients, mitochondrial dysfunction may be responsible for the activation of oxidative stress that manifested as the significant presence of autoxidation forms of cholesterol (26), as previously reported in the same frail plasma (Ref. 14; see also Supplementary Table 5B).
Figure 3.
Plasma oxysterols and cholesterol precursors, intracellular ROS in NS cells, plasma cytokine concentrations. (A) Levels of 27-OHC, lathosterol, lanosterol, and 24-OHC in plasma samples from 17 frail, 18 non-frail, and 10 young individuals (Supplementary Table 5). (B) Left, representative production of intracellular ROS in NS cells with or without treatment with the indicated plasma during growth, flow cytometry analysis of 2ʹ,7ʹ-dichlorofluorescein (DCF) (gating strategy in Supplementary Figure 1). Right, median DCF fluorescence intensity peak in dissociated NS cells of 5 different independent NS cultures, each one treated during growth with individual plasma from a set of 5 frail, 5 non-frail, or 5 young individuals; there were 5 untreated NS cultures (Supplementary Table 6). The DCF median values of plasma-treated cells were normalized to the DCF of corresponding untreated NS cells considered equal to 1. (C) Cytokine concentrations (pg/mL) of IL-6, CX3CL1, CXCL9, IL-1α, and CD40 were significantly higher in frail plasma. Cytokines were evaluated in 38 frail, 34 non-frail, and 36 young subjects by the Human Magnetic Luminex Screening Assay (Supplementary Table 7A). A p value < .05 by 1-way ANOVA with Tukey’s test or Holm‒Sidak’s test for pairwise multiple comparison was considered significant.
These results showed that in the plasma of our frail individuals, there was documentation of mitochondrial dysfunction and activation of oxidative stress that may affect different tissues.
Intracellular ROS Formation in NS Cells
We also investigated whether treatment with plasma itself could cause ROS formation in NS cells. Intracellular ROS were assessed by detecting DCF in 5 NS cultures (Supplementary Table 2) grown in the presence of 10% plasma from 5 frail, 5 non-frail, or 5 young individuals. Flow cytometry analysis, looking at the shift in the DCF fluorescence intensity peak, documented a significant intracellular ROS increase elicited by frail plasma in the treated cultures (Figure 3B, Supplementary Table 6).
Therefore, the plasma of frail individuals was able to elicit intracellular ROS production in NS cells, likely related to a specific frail plasma composition.
Cytokines in Human Plasma Samples of Frail, Non-Frail, and Young Subjects
The above findings provide a strong indication to evaluate components of plasma that may have a role in modulating the oxidative status of frail subjects. The inflammaging described as a characteristic aspect of aging (34) prompted us to evaluate a panel of inflammatory cytokines (9,35). Thirty-eight inflammatory cytokines in human plasma samples from 38 frail, 34 non-frail, and 36 young subjects were analyzed by Luminex assays (Supplementary Table 7A). The results showed that IFNγ, IL5, and IL12p70 were quantifiable only in 3 or fewer plasma samples of the studied groups. Several cytokines showed a trend toward a concentration increase in the plasma of the frail group (CXCL10, G-CSF, GM-CSF, TGF-α, CCL3, CCL7, IL-4, IL-7, IL-8, VEGF, CCL2, CCL22, CXCL1, and CXCL12) in comparison with the non-frail group. Other cytokines were more abundant in the plasma of the non-frail (CCL11, FLT3 LIGAND, CCL4, IL-13) and young (RANTES, PDGF-BB, PDGF-AA, EGF, CD40 LIGAND) groups. In addition, IL-15, IL-1β, IL-2, IL-17, and TNF-β were exclusively represented in some frail plasma samples (Table 1A). Moreover, TNF-α was present in 15 frail and 3 non-frail subjects, and IL-10 was present in 6 frail and 4 non-frail subjects; neither cytokine was observed in young subjects (Table 1A). However, among the data of the studied plasma cytokines, the mean levels of 5 (IL-6, CX3CL1, CXCL9, IL-1α, CD40) were significantly higher in frail subjects than in the others (Figure 3C). Even in the subgroup (Supplementary Table 7B) of randomly selected plasma samples used to treat NS cells, the cytokines that in the overall population were significantly more abundant in frail plasma (IL-6, CX3CL1, CXCL9, IL-1α, CD40), those found exclusively in frail plasma (IL-15, IL-1β, IL-2, IL-17, TNF-β) and those found in both frail and non-frail plasma (TNF-α, IL-10), maintained a higher mean concentration in the frail plasma of the selected samples (Table 1B). Moreover, their presence in frail plasma matched the elevated DNA damage and the reduced SFE in the allogeneic NS cells following treatment, and both the elevated oxidative stress and the elevated DNA damage of autologous cHPSCs in the respective plasma (Supplementary Table 7B). Of note, the literature data report that some of these cytokines (ie, IL-6 (36), CX3CL1 (37), IL-1α (38), CD40 (39), IL-1β (40), IL-17 (41), TNF-α (42), IL-4 (43), and CCL2 (44)) interacting with the respective receptors on different cell types are able to elicit intracellular ROS formation (Table 1B). Although we do not have the receptor profile of our NS cells, it is suggestive from a clinical point of view that “The Human Protein Atlas” database (https://www.proteinatlas.org/) reports the expression of the specific receptors of many of these cytokines on renal cells (Table 1B).
Table 1.
Characteristics of Plasma Cytokines
| (A) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokines | Frail Plasma | Non-Frail Plasma | |||||||
| Positive Plasma (Gender) | pg/mL Median (Range) | Positive Plasma (Gender) | pg/mL Median (Range) | ||||||
| IL-15 | 9 (3F/6M) | 4.7 (3.9–13.0) | - | - | |||||
| IL-1β | 4 (2F/2M) | 26.6 (11.5–50.6) | - | - | |||||
| IL-2 | 2 (2M) | 94.3 (85.6–102.9) | - | - | |||||
| IL-17 | 2 (2M) | 149.8 (12.5–287.1) | - | - | |||||
| TNFβ | 2 (2M) | 134.5 (8.8–260.2) | - | - | |||||
| TNFα | 15 (9F/6M) | 7.8 (4.7–198.7) | 3 (1F/2M) | 6.7 (6.0–7.0) | |||||
| IL-10 | 6 (4F/2M) | 3.9 (2.6–441.6) | 4 (3F/1M) | 42.9 (5.2–410.6) | |||||
| (B) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokines | Frail Plasma | Non-Frail Plasma | Young Plasma | Receptor | Tubule/Glomerule | Cellular Ros | |||
| n Positive Plasma (Gender) | pg/mL Mean ± SD Median |
n Positive Plasma (Gender) | pg/mL Mean ± SD Median |
n Positive Plasma (Gender) | pg/mL Mean ± SD Median |
Expression Level | |||
| IL-6 | 7F/5M | 27 ± 42 12 |
2F/4M | 8 ± 9 5 |
0 | IL6R/IL6ST | High/Low | YES (Ref. 36) | |
| CX3CL1 | 9F/3M | 2 297 ± 1 351 2 057 |
5F/3M | 980 ± 618 663 |
3F/2M | 2 151 ± 1 363 1 916 |
CX3CR1 | High/Low | YES (Ref. 37) |
| CXCL9 | 4F/3M | 652 ± 557 490 |
2F | 518 ± 194 518 |
1M | 508 508 |
CXCR3 | -/- | - |
| IL-1α | 6F/4M | 21 ± 19 13 |
2F/2M | 12 ± 11 9 |
2M | 15 ± 7 15 |
IL1R1 | High/High | YES (Ref. 38) |
| CD40 | 13F/8M | 1 114 ± 1 266 923 |
10F/12M | 482 ± 233 430 |
11F/8M | 366 ± 179 304 |
CD40 | Low/- | YES (Ref. 39) |
| IL-15 | 2F/5M | 6 ± 3 5 |
0 | 0 | IL15RA | Medium/Low | - | ||
| IL-1β | 2F/1M | 22 ± 17 12 |
0 | 0 | IL1R1 | High/High | YES (Ref. 40) | ||
| IL-2 | 1M | 103 103 |
0 | 0 | IL2RA,B,G | -/- | - | ||
| IL-17 | 1M | 12.5 12.5 |
0 | 0 | IL17R | medium/- | YES (Ref. 41) | ||
| TNFβ | 1M | 8.8 8.8 |
0 | 0 | TNFRSF1B | High/- | - | ||
| TNFα | 7F/5M | 24 ± 55 8 |
1F/2M | 6 ± 0.5 7 |
0 | TNFRSF1A | Medium/Low | YES (Ref. 42) | |
| IL-10 | 2F/2M | 114 ± 218 7 |
2F | 208 ± 287 208 |
0 | IL10Rb | Medium/- | - | |
| IL-8 | 12F/8M | 21 ± 19 13 |
9F/11M | 13 ± 15 10 |
7F/5M | 6 ± 3 4 |
CXCR1 | Medium/- | - |
| IL-4 | 9F/5M | 74 ± 35 68 |
6F/6M | 53 ± 29 42 |
1F/2M | 59 ± 14 59 |
IL4R | Medium/- | YES (Ref. 43) |
| CCL2 | 13F/8M | 739 ± 1 303 444 |
10F/12M | 427 ± 124 426 |
11F/8M | 305 ± 76 292 |
CCR2 | Medium/- | YES (Ref. 44) |
| TGFα | 10F/6M | 8 ± 3 8 |
6F/8M | 7 ± 3 6 |
2F/3M | 7 ± 3 6 |
EGFR | High/Medium | - |
| CXCL10 | 12F/7M | 174 ± 88 165 |
10F/12M | 141 ± 129 97 |
11F/8M | 68 ± 20 67 |
CXCR3 | -/- | - |
Notes:.(A) Cytokines that in the overall plasma analyzed (38 frail, 34 non-frail, 36 young plasma) are exclusively present in some frail or frail and non-frail plasma (Supplementary Table 7A). (B) Subgroup of 21 frail, 22 non-frail, 19 young plasma used for treating NS cultures (Supplementary Table 7B), are shown some cytokines, prevalent in the frail plasma subgroup and with dominating role in PCA analysis. The expression level of the specific cytokine receptors on renal cells, based on “The Human Protein Atlas” database (https://www.proteinatlas.org/), and the cytokine capacity to induce intracellular ROS (YES) were reported.
Gender, F = Female; M = Male; Ref = reference number; -/- or - = negative result.
These results suggest that in frail subjects, the plasma profile of the studied inflammatory cytokines and their capacity to induce oxidative stress could have a role in modulating the stem characteristics in not only the renal tissue but also the tissues of the organism more broadly.
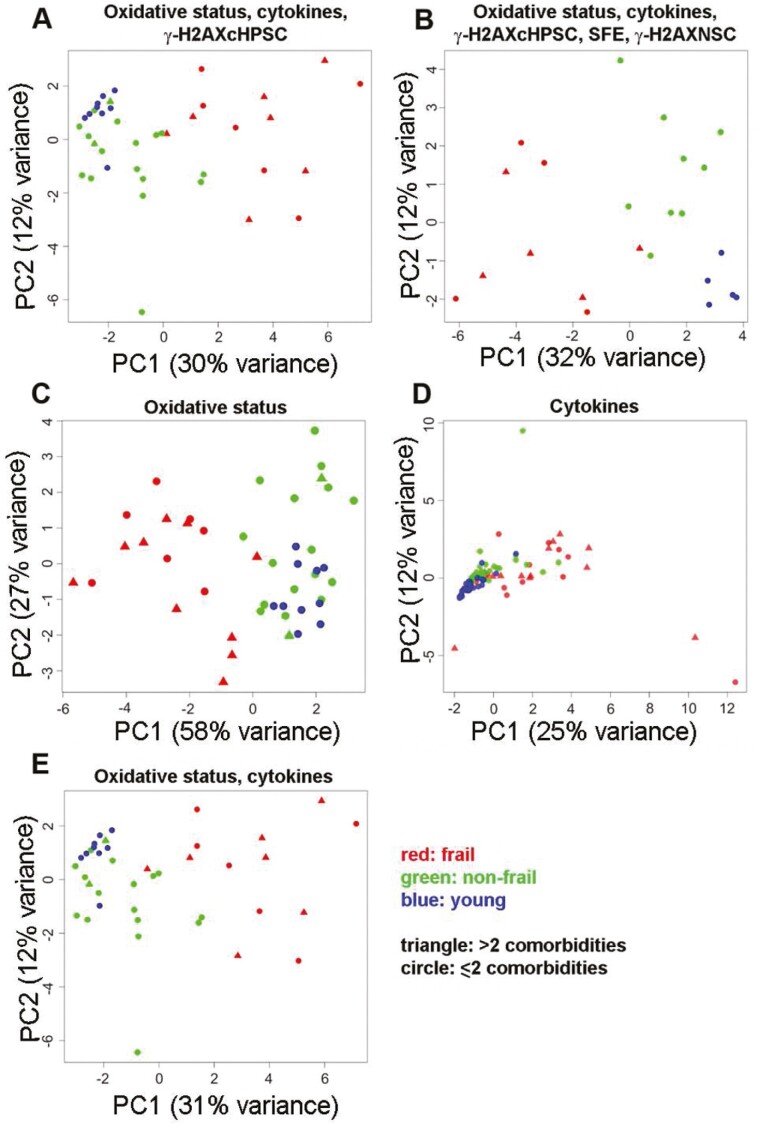
Whole Integration of the Studied Variables
After examining the individual variables for their differences, as presented in the previous paragraphs, we performed a PCA to analyze them together. The variables were classified into different domains represented by the following: (i) plasma oxidative status, (ii) plasma inflammatory cytokines, (iii) DNA-damaged homologous cHPSC (herein referred as to γ-H2AXcHPSC), (iv) SFE, and (v) DNA-damaged allogeneic NS cells (herein referred as to γ-H2AXNSC; see Supplementary Methods). Incorporating age and sex variables into the PCA did not significantly alter the explained variance or the overall data structure, confirming that the primary patterns of variability are predominantly driven by the biological experimental data. The aim was to evaluate whether this analysis would reveal a distribution of individuals who aligned with their clinical classification (Figure 4, Supplementary Table 8). The distribution of individuals whose plasma variables were analyzed within the oxidative status, cytokines, and γ-H2AXcHPSC domains is summarized in Figure 4A. The distribution of individuals based on merging of all the plasma variables considered in Figure 4A, with the addition of SFE and γ-H2AXNSC for documentation of the effects of the different plasmas on NS cells, is shown in Figure 4B. Both panels along PC1 seemed to exhibit a trend toward a distinct distribution of the frail group, although with broad variability. Non-frail individuals spanned mainly PC2, particularly in Figure 4B. The young group in Figure 4A had some overlaps between non-frail and young individuals, indicating the presence of some similarities in the profiles of their variables. Instead, incorporating the 2 variables associated with the effects of plasma on NS cells (Figure 4B), young individuals predominantly occupied the quadrant characterized by high PC1 and low PC2 values, exhibiting a more distinct segregation compared to the non-frail individuals. The distinct groupwise segregation and variability within groups suggested, with caution due to the relatively small sample size, a possible differential role that the studied variables might play in the context of frailty. The distribution of frail individuals in these analyses was dominated by variables reported in Supplementary Table 8. All these variables had a significant biological role in frailty, as reported in the previous paragraphs. Of note, the comorbidities did not seem to influence the organization of frail individuals in the space of the first 2 PCs. Figure 4A and B show the endpoints of the steps in which the different variable combinations were progressively analyzed. The oxidative status and cytokine variables were first analyzed separately and then together (Figure 4C–E). In the plot of the oxidative status variables (Figure 4C), frail individuals were in a different quadrant than the other 2 groups. Of note, there seemed to be less diversity within the young and non-frail groups, which were located in the same portion of the plot, distributed across PC1. The presence of overlaps between non-frail and young individuals indicated some similarities in their profiles. Frail and non-frail individuals had a more diverse within-group oxidative stress profile. The cytokine plot (Figure 4D) did not show separation; the overlap of the young and non-frail individuals suggested more similar profiles, while the frail individuals had a discernible rightward spread distribution, indicating a larger variability. When oxidative status and cytokines were analyzed together (Figure 4E), the frail subjects returned to occupy a separate portion of the space, maintaining a broader spread in both PC1 and PC2. Non-frail individuals were located mostly between frail and young individuals and showed a tighter grouping. The young individuals displayed minimal spread and did not overlap with frailty along PC1. From the PCA, it was evident that oxidative status variables were paramount in capturing the intrinsic variability within the data. Of note, even in Figure 4C and E the distribution of frail individuals appeared to be independent of comorbidities.
Figure 4.
PCA results, distribution of subjects along the first 2 principal components (PC1 and PC2). (A) Distribution of 13 frail, 18 non-frail, and 9 young individuals based on the merged data of the oxidative status, cytokines, and γ-H2AXcHPSC variables of their own plasma. (B) Distribution of 9 frail, 9 non-frail, and 5 young individuals based on the merged data of the oxidative status, cytokines, and γ-H2AXcHPSC variables of their own plasma and the variables SFE and γ-H2AXNSC for the effects of the same plasma on NS cells. (C) Distribution of 17 frail, 18 non-frail, and 10 young individuals based on data on their own plasma oxidative status variables. (D) Distribution of 36 frail, 34 non-frail, and 36 young individuals based on data on their plasma cytokine variables. (E) Distribution of 13 frail, 18 non-frail, and 9 young individuals based on the merged data of their plasma oxidative status and cytokine variables. Each point represents a subject. Color-coded: red, frail; green, non-frail; blue, young. Point shape represents the number of comorbidities of the subject: triangles, more than 2; circles, 2 or less than 2. Supplementary Table 1B shows the individual subjects whose data were used for this integrated analysis.
Discussion
In the studied population, we investigated whether biological features could influence the stem cell behavior of the human renal stem/progenitor cell compartment and contribute to defining frailty status in frail older adults. Here, we showed that the plasma of frail patients was able to reduce the SFE of allogeneic human adult renal stem/progenitor cells growing to form NSs. The reduced SFE matched with increased DNA damage in NS cells and impaired mitochondrial function, as observed in frail plasma. The ensuing possible accumulation of ROS, with oxidative stress activation, is also supported by the significant increase in some autooxidation forms of cholesterol (14), previously analyzed in the plasma of the same frail subjects. Our findings also suggested that the alterations observed in allogeneic NS cells treated with frail plasma were significant responses to stimuli determined by factors present in these plasma samples. These factors may pose a risk to various tissues, potentially affecting the self-renewal capacity in their stem compartments (8). In this context of frailty, cytokine profiles can also play a role, as they are closely interconnected with other biological parameters, such as oxidative activity and DNA integrity (1,5,6). Our analysis of 38 inflammatory cytokines showed that some of them were significantly more abundant or exclusive in frail plasma, and a few were absent in plasma from young individuals. Among these cytokines, IL-6 and IL-1β are described as characteristically associated with inflammaging (1,45). It was also shown that the levels of IL-6 are more heterogeneous in frail individuals than in young individuals (12). In our study, the levels of the cytokines analyzed were more heterogeneous in frail individuals than in young and non-frail subjects, as also evidenced by the PCA of our data. It was not surprising that some of the more concentrated cytokines, even present in the randomly selected frail plasma used for our treatments, were able to elicit intracellular ROS, as reported in the literature (36–44). This characteristic is clinically relevant, as it can disrupt the homeostasis of different tissues due to in vivo interactions between cytokines and their specific receptors expressed on differentiated cells or stem cells. Our data, showing the alterations in intracellular ROS, DNA damage, and SFE in treated allogeneic NS cells, have substantiated the role that the combination of mitochondrial functional impairment and increased oxidative stress with the peculiar profiles of inflammatory plasma cytokines could play in frail patients. In addition, in the same frail individuals, donors of plasma that significantly affected DNA and SFE in allogeneic NS cells, there was a significant in vivo increase in DNA-damaged autologous cHPSCs, which likely may also have functional alterations (15,16). Our data, showing that the same plasma has relation with DNA damage of both autologous and allogeneic cells, has enhanced the possibility that in frail patients various stem/progenitor cell compartments may be targets induced to exhaustion, contributing to the development of frailty. Our study was also motivated by the frequent involvement of the kidney in frailty, and due to ethical considerations, we only indirectly examined the kidney compartment, drawing upon our experience with NS cells (21–23). Regardless, the data obtained can serve as a motivating starting point for broader research analyzing whether the concomitant deterioration of the functions of diverse stem cells is associated with frailty in older adults. In this regard, an investigation of the alterations in the muscle stem cell compartment and their connections with sarcopenia, often associated with frailty (11,46), would yield intriguing results if other stem cells were also studied in such research. This could further support the hypothesis of concomitant deterioration across multiple stem cell compartments in frailty. PCA used to integrate the data concerning the variables of the different domains indicated the potential to delineate a biological difference in frailty status in comparison with the non-frail and young status. The spatial distribution in the PCA plots containing oxidative status variables underscored differences among frail, non-frail, and young groups, and the consistent positioning of these groups, even upon inclusion of other variables, attested to the dominance of oxidative stress factors. In addition, the stratification of individuals based on comorbidities maintained the observed difference between frail and other groups. This finding was in agreement with the description that frailty can occur independently of the presence of comorbidities (1,2) and is not synonymous with either comorbidity or disability. The integration of variables, while not allowing a link to the variable specification of the single patient, revealed more quantitatively pronounced molecular profiles in frail individuals than non-frail individuals. Therefore, these data can pave the way for further characterization of cellular and molecular alterations that may be important if they precede overt clinical symptoms, as early frailty can be managed (47). New approaches to develop ameliorating therapeutic strategies that act on damaged cells, oxidative stress, and inflammatory profiles are encouraged (8,46,48,49).
In conclusion, the data obtained from our analysis of a frail population converged toward the existence of biological conditions capable of defining frailty and causing stem cell exhaustion, highlighting the possibility of concomitant impairment in several stem compartments. Our data from a clinical setting indicate the possibility that the exhaustion of stem cells can be the “last culprit” (5), which acts as a “shared biological driver for parallel dysregulation” (1) of multiple systems in different tissues.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Acknowledgments
Thanks to Domenico Ciceri MPhys for helpful discussion. C.G. and V.V. were supported by PhD fellowships, and F.V. by residency fellowship, of Italian Minister of University. I.M. by fellowship of University of Milano-Bicocca. S.D.M. by fellowship of Fondazione Cariplo-project 2017-0577.
Contributor Information
Silvia Bombelli, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Chiara Grasselli, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Paolo Mazzola, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Acute Geriatric Unit, IRCCS San Gerardo, Monza, Italy.
Valentina Veronesi, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Bicocca Bioinformatics Biostatistics and Bioimaging Center - B4, University of Milano-Bicocca, Monza, Italy.
Ivana Morabito, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Nicola Zucchini, Pathology Unit, IRCCS San Gerardo, Monza, Italy.
Chiara M Scollo, Immunotransfusional Unit, Laboratory of Hematology, IRCCS San Gerardo, Monza, Italy.
Salvatore I Blanco, Urology Unit, IRCCS San Gerardo, Monza, Italy.
Sofia De Marco, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Barbara Torsello, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Federica Vitarelli, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Laboratory of Clinical Pathology and Toxicology, Pio XI Hospital, ASST-Brianza, Desio, Italy.
Laura Antolini, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Bicocca Bioinformatics Biostatistics and Bioimaging Center - B4, University of Milano-Bicocca, Monza, Italy.
Cristina Bianchi, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Valerio Leoni, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Laboratory of Clinical Pathology and Toxicology, Pio XI Hospital, ASST-Brianza, Desio, Italy.
Giuseppe Bellelli, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Acute Geriatric Unit, IRCCS San Gerardo, Monza, Italy.
Roberto A Perego, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Gustavo Duque, (Biological Sciences Section).
Funding
The entire project and the open access was funded by Fondazione Cariplo Milano (Italy), grant [2017-0577] to R.A.P.
Conflict of Interest
None.
Author Contributions
S.B., C.G.: nephrosphere treatments, experimental design, data analysis, writing manuscript, and figure preparation. P.M.: recruiting the frail and non-frail individuals, experimental design, clinical data analysis, and writing manuscript. V.V.: statistical analysis, data integration, writing manuscript, and figure preparation. I.M., S.D.M., B.T.: experiment participation and figure preparation. N.Z.: providing renal tissues and histology characterization. C.M.S.: recruiting young individuals. S.I.B.: recruiting patients for nephrectomy. V.L., F.V.: oxidative stress analysis, data analysis, and writing the manuscript. G.B., L.A., C.B.: Scientific support and revision of manuscript. R.A.P.: responsibility of the project, management of collaborations, experimental design, data analysis, writing manuscript, revision and final version of manuscript, and funding.
References
- 1. Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1:36–46. 10.1038/s43587-020-00017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 3. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. 10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 4. Beard JR, Officer A, Araujo de Carvalho I, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. 10.1016/S0140-6736(15)00516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmauck-Medina T, Molière A, Lautrup S, et al. New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging. 2022;14:6829–6839. 10.18632/aging.204248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Passos JF, Saretzki G, Ahmed S, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Bio. 2007;5:e110. 10.1371/journal.pbio.0050110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams PD, Jasper H, Rudolph KL. Aging-induced stem cell mutations as drivers for disease and cancer. Cell Stem Cell. 2015;16:601–612. 10.1016/j.stem.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8:1960. 10.3389/fimmu.2017.01960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutenberg AD, Mitnitski AB, Farrell SG, Rockwood K. Unifying aging and frailty through complex dynamical networks. Exp Gerontol. 2018;107:126–129. 10.1016/j.exger.2017.08.027 [DOI] [PubMed] [Google Scholar]
- 11. Bernabeu-Wittel M, Gómez-Díaz R, González-Molina A, Vidal-Serrano S, Díez-Manglano J, Salgado F, Soto-Martín M, Ollero-Baturone M; On Behalf of the Proteo Researchers. Oxidative stress, telomere shortening, and apoptosis associated to sarcopenia and frailty in patients with multimorbidity. J Clin Med. 2020;9:2669. 10.3390/jcm9082669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alberro A, Iribarren-Lopez A, Sáenz-Cuesta M, Matheu A, Vergara I, Otaegui D. Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency. Sci Rep. 2021;11:4358. 10.1038/s41598-021-83991-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tümpel S, Rudolph KL. Quiescence: good and bad of stem cell aging. Trends Cell Biol. 2019;29:672–685. 10.1016/j.tcb.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 14. Grasselli C, Bombelli S, Eriani S, et al. DNA damage in circulating hematopoietic progenitor stem cells as promising biological sensor of frailty. J Gerontol A Biol Sci Med Sci. 2022;77:1279–1286. 10.1093/gerona/glac034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schiroli G, Conti A, Ferrari S, et al. Precise gene editing preserves hematopoietic stem cell function following transient p53-mediated DNA damage response. Cell Stem Cell. 2019;24:551–565.e8. 10.1016/j.stem.2019.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu L, Yin X, Zhang Y, et al. Radiation-induced bystander effects impair transplanted human hematopoietic stem cells via oxidative DNA damage. Blood. 2021;137:3339–3350. 10.1182/blood.2020007362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilhelm-Leen ER, Hall YN, Tamura MK, Chertow GM. Frailty and chronic kidney disease: the Third National Health and nutrition evaluation survey. Am J Med. 2009;122:664–671.e2. 10.1016/j.amjmed.2009.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kojima G. Prevalence of frailty in end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2017;49:1989–1997. 10.1007/s11255-017-1547-5 [DOI] [PubMed] [Google Scholar]
- 19. Kennard A, Glasgow N, Rainsford S, Talaulikar G. Frailty in chronic kidney disease: challenges in nephrology practice. A review of current literature. Intern Med J. 2023;53:465–472. 10.1111/imj.15759 [DOI] [PubMed] [Google Scholar]
- 20. Chu NM, Chen X, Norman SP, et al. Frailty prevalence in younger ESKD patients undergoing dialysis and transplantation. Am J Nephrol. 2020;51:501–510. 10.1159/000508576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bombelli S, Zipeto MA, Torsello B, et al. PKHhigh cells within clonal human nephrospheres provide a purified adult renal stem cell population. Stem Cell Res. 2013;11:1163–1177. 10.1016/j.scr.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 22. Bombelli S, Meregalli C, Scalia C, et al. Nephrosphere-derived cells are induced to multilineage differentiation when cultured on human decellularized kidney scaffolds. Am J Pathol. 2018;188:184–195. 10.1016/j.ajpath.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 23. Bombelli S, Meregalli C, Grasselli C, et al. PKHhigh/CD133+/CD24− renal stem-like cells isolated from human nephrospheres exhibit in vitro multipotency. Cells. 2020;9:1805. 10.3390/cells9081805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnston MC, Crilly M, Black C, Prescott GJ, Mercer SW. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health. 2019;29:182–189. 10.1093/eurpub/cky098 [DOI] [PubMed] [Google Scholar]
- 25. Bianchi C, Bombelli S, Raimondo F, et al. Primary cell cultures from human renal cortex and renal-cell carcinoma evidence a differential expression of two spliced isoforms of Annexin A3. Am J Pathol. 2010;176:1660–1670. 10.2353/ajpath.2010.090402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leoni V, Nury T, Vejux A, et al. Mitochondrial dysfunctions in 7-ketocholesterol-treated 158N oligodendrocytes without or with α-tocopherol: impacts on the cellular profile of tricarboxylic cycle-associated organic acids, long chain saturated and unsaturated fatty acids, oxysterols, cholesterol and cholesterol precursors. J Steroid Biochem Mol Biol. 2017;169:96–110. 10.1016/j.jsbmb.2016.03.029 [DOI] [PubMed] [Google Scholar]
- 27. Jolliffe IT. Principal Component Analysis. 2nd ed. Springer; 2002. [Google Scholar]
- 28. Sobecki M, Mrouj K, Colinge J, et al. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res. 2017;77:2722–2734. 10.1158/0008-5472.CAN-16-0707 [DOI] [PubMed] [Google Scholar]
- 29. Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. 10.1038/nature05862 [DOI] [PubMed] [Google Scholar]
- 30. Siddiqui MS, Francois M, Fenech MF, Leifert WR. Persistent γH2AX: a promising molecular marker of DNA damage and aging. Mutat Res Rev Mutat Res. 2015;766:1–19. 10.1016/j.mrrev.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 31. Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S. γH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat Res. 2013;753:24–40. 10.1016/j.mrrev.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 32. Johansson P, Fasth A, Ek T, Hammarsten O. Validation of a flow cytometry-based detection of γ-H2AX, to measure DNA damage for clinical applications. Cytometry B Clin Cytom. 2017;92(B):534–540. 10.1002/cyto.b.21374 [DOI] [PubMed] [Google Scholar]
- 33. Leoni V, Caccia C. 24S-hydroxycholesterol in plasma: a marker of cholesterol turnover in neurodegenerative diseases. Biochimie. 2013;95:595–612. 10.1016/j.biochi.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 34. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 35. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forcina L, Miano C, Scicchitano BM, et al. Increased circulating levels of interleukin-6 affect the redox balance in skeletal muscle. Oxid Med Cell Longev. 2019;3018584:1–13. 10.1155/2019/3018584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park J, Song KH, Ha H. Fractalkine increases mesangial cell proliferation through reactive oxygen species and mitogen-activated protein kinases. Transplant Proc. 2012;44:1026–1028. 10.1016/j.transproceed.2012.03.045 [DOI] [PubMed] [Google Scholar]
- 38. Bretheau F, Castellanos-Molina A, Bélanger D, et al. The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury. Nat Commun. 2022;13:5786. 10.1038/s41467-022-33463-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nadeau PJ, Roy A, Gervais-St-Amour C, Marcotte ME, Dussault N, Néron S. Modulation of CD40-activated B lymphocytes by N-acetylcysteine involves decreased phosphorylation of STAT3. Mol Immunol. 2012;49:582–592. 10.1016/j.molimm.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 40. Hwang YS, Jeong M, Park JS, et al. Interleukin-1β stimulates IL-8 expression through MAP kinase and ROS signaling in human gastric carcinoma cells. Oncogene. 2004;23:6603–6611. 10.1038/sj.onc.1207867 [DOI] [PubMed] [Google Scholar]
- 41. Dhillion P, Wallace K, Herse F, et al. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2012;303:R353–R358. 10.1152/ajpregu.00051.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chandel NS, Schumacker PT, Arch RH. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem. 2001;276:42728–42736. 10.1074/jbc.M103074200 [DOI] [PubMed] [Google Scholar]
- 43. Zhang B, Yang Y, Yi J, Zhao Z, Ye R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J Periodontal Res. 2021;56:991–1005. 10.1111/jre.12912 [DOI] [PubMed] [Google Scholar]
- 44. Gu H, Deng W, Zheng Z, Wu K, Sun F. CCL2 produced by pancreatic ductal adenocarcinoma is essential for the accumulation and activation of monocytic myeloid‐derived suppressor cells. Immun Inflamm Dis. 2021;9:1686–1695. 10.1002/iid3.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 46. Behrens A, Van Deursen JM, Rudolph LK, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol. 2014;16:201–207. 10.1038/ncb2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69:e61–e69. 10.3399/bjgp18X700241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhuang Q, Ou J, Zhang S, Ming Y. Crosstalk between the CX3CL1/CX3CR1 axis and inflammatory signaling pathways in tissue injury. Curr Protein Pept Sci. 2019;20:844–854. 10.2174/1389203720666190305165722 [DOI] [PubMed] [Google Scholar]
- 49. Faridvand Y, Nemati M, Zamani-Gharehchamani E, et al. Dapagliflozin protects H9c2 cells against injury induced by lipopolysaccharide via suppression of CX3CL1/CX3CR1 Axis and NF-κB activity. Curr Mol Pharmacol. 2022;15:862–869. 10.2174/1874467214666211008142347 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.