Abstract
During the coronavirus disease 2019 (COVID-19) pandemic, hospitals and households have used personal protective equipment (PPE), such as masks and gloves. Some of these potentially infectious materials were discarded with other household wastes in garbage dumping sites. Thus, this study aimed to detect the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in contaminated wastes, environments, and mammals scavenging around these sites. From September to October 2022, we visited three garbage dumping sites located in Bangkok, Nakhon Pathom, and Nonthaburi provinces of Thailand. Oral, nasal, rectal swabs, and blood samples were collected from small mammals, stray dogs, and cats. Masks, gloves, soil, and water samples from the sites were additionally collected. Of the 582 samples collected from 238 animals, none tested positive for SARS-CoV-2 in the virus isolation, real-time reverse-transcription polymerase chain reaction, and neutralizing antibody detection. However, one sample (1.18 %; 1/85) from a rat (Rattus spp.) captured in Nonthaburi was serologically positive in the indirect enzyme-linked immunosorbent assay. The surveillance of coronaviruses in rats is strongly encouraged because rats may harbor different zoonotic pathogens, including unknown potentially zoonotic coronaviruses. Moreover, two face mask samples (4.65 %; 2/43) collected from the dumping site in Nakhon Pathom tested positive for SARS-CoV-2 by real-time RT-PCR. To reduce environmental contamination, detecting the SARS-CoV-2 viral genome in contaminated face masks highlights the critical need for proper waste management in households and communities in Thailand. Thus, to minimize exposure and prevent onward transmission, waste management personnel, including garbage dump staff and waste pickers, should be equipped with appropriate PPE and receive regular training on safe handling and disposal.
Keywords: Face mask, Garbage dumping site, SARS-CoV-2, Serology, Small mammals
1. Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), was first reported in Wuhan, China, in December 2019 [1]. This novel virus is a single-stranded RNA virus that belongs to beta coronaviruses. RNA viruses, which lack the polymerase-proofreading capability, have high mutation rates. This trait enhances virulence, adaptability, and evolvability [2]. The rapid dissemination of the pathogen prompted the World Health Organization (WHO) to declare it a public health emergency of international concern in 2020. This was followed by a dramatic surge in the number of cases worldwide. As of March 2024, more than 774 million confirmed cases and 7 million deaths have been reported globally [3].
The virus can rapidly transmit from among humans and any contaminated surfaces. During the pandemic, massive quantities of personal protective equipment (PPE), such as masks and gloves, were used in hospitals and households, generating unusual amounts of waste [4], which may be contaminated with live viruses. Accordingly, the virus may be found in the environment because of improper waste disposal [5]. SARs-CoV-2 can be stable in different environmental conditions. In addition, genetic materials can be detected on various surfaces and wastewater [[6], [7], [8], [9]].
Despite robust management of infectious wastes in healthcare settings, concerns arise regarding potential contamination from home isolation and asymptomatic cases, particularly in the absence of proper waste disposal protocols before treatment. Notably, the omicron variant and the associated increase in asymptomatic infections likely contribute to the extensive viral spread [10]. Massive accumulation of waste increases the risk of infectious disease transmission [11]. Pathogen contamination is related to the characteristics of the environment. Moreover, garbage dumping sites contain considerable organic waste, which can be a food source for various mammals [12]. The availability of resources in garbage sites significantly influences the host species, particularly small mammals such as rodents, which are consistently found in these environments [13]. The areas with large amounts of garbage where animals congregate, like garbage dumping sites, may serve as a reservoir for zoonotic diseases.
However, evidence remains to be clarified. The zoonotic origin of COVID-19, with a probable spillover from wild animals, is a widely accepted hypothesis. Whole-genome studies revealed the high sequence identity with the bat coronavirus RaTG13 (BatCoV RaTG13) and Pangolin-CoV [14,15]. The structural proteins of the coronavirus virion are composed of nucleocapsid (N), membrane, envelope, and spike. The virus enters the host cells by binding to angiotensin-converting enzyme (ACE) 2 with a spike protein [16]. Thus, animals possessing ACE receptors are highlight susceptible to infection and may serve as disease reservoirs.
Furthermore, the number of cases and wildlife species, companion, and exotic animals increased with close exposure to humans infected with SARS-CoV-2, as observed overseas. Since the SARS-CoV-2 pandemic, studies have reported anthroponotic transmission cases in 29 species, including cats, dogs, minks, ferrets, and various wild animals such as tigers, gorillas, and white-tailed deer worldwide [17]. In mammalian species at garbage dumps, reverse virus transmission from human wastes to animals at dump sites may occur. Thus, diseases in mammalian species must be explored because most emerging infectious diseases originate from animals, which are sources of various zoonotic viruses. Infected animals could become amplified hosts, which might cause a future outbreak. This study aimed to detect SARS-CoV-2 in contaminated wastes, environment, and mammals scavenging at garbage dumping sites to increase awareness of proper waste management in order to reduce probable animal and environmental contamination.
2. Materials and methods
2.1. Field sample collection
From September to October 2022, we visited three garbage dumping sites located in Bangkok, Nonthaburi, and Nakhon Pathom, Thailand (Fig. 1). The dump in Bangkok receives approximately 3500 tons of waste per day (one-third of the total waste generated in Bangkok). Most of the waste generated in Nonthaburi Province (1231 tons/day) is moved to the garbage dumping sites we studied, and the dump in Nakhon Pathom, the largest garbage dumping site in the province, receives 218.62 tons of waste per day. Samples were collected from three distinct categories: animal specimens, potentially infectious wastes (masks and gloves), and environmental samples (soil and water).
Fig. 1.
Geographical locations of the provinces of the studied garbage dumping sites. A. Map of Thailand highlights the provinces in purple. B. Magnified boundaries of the three provinces, namely, Bangkok, Nakhon Pathom, and Nonthaburi. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Animal sampling
Animals foraging near waste disposal sites, including small mammals, stray dogs, and cats, were documented. A field assessment was conducted at each location to identify suitable line transects encompassing critical zones with anticipated interactions among humans, animals, and the environment. In each study site, 100 trapping cages for small mammals, which measured 15 × 30 × 15 cm, were placed at night for three consecutive nights to capture small mammals residing around the garbage dumping sites (Fig. 2). These traps were baited with fruits or vegetables at approximately 6 p.m. and inspected at around 6 a.m. The captured animals were then placed in a glass chamber and anesthetized with isoflurane at a 5 % gas flow rate. After complete anesthetization, the animals were taken out of the chamber, and the anesthetic level was maintained using an anesthetic mask. The sex and weight of the animals were recorded. The animal species were classified according to a referencing manual [18]. Oral, nasal, and rectal samples were collected from each animal using cotton swabs and placed in transport media. Blood samples were collected from each animal's saphenous vein or orbital sinus in a volume not exceeding 1 % of body weight. Upon arrival at our laboratory, we centrifuged the samples at 5000 xg, 4°C for 10 min, and the serum was then stored at −20°C until analysis.
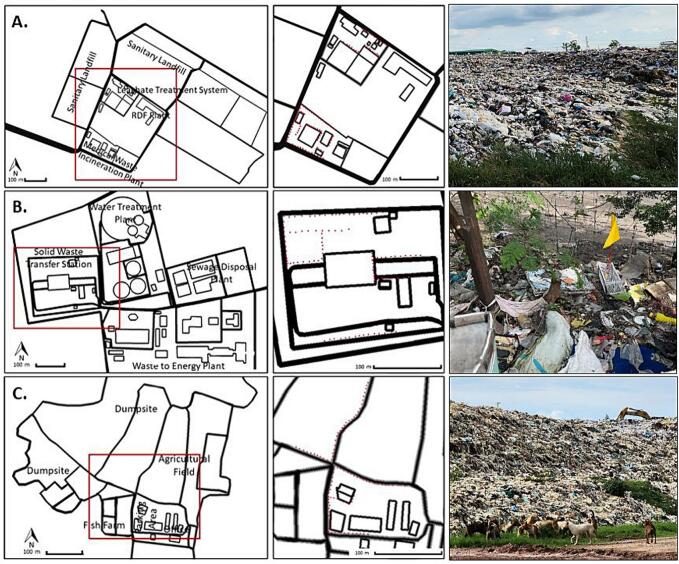
Fig. 2.
Garbage dumping sites where samples were collected. A. Nonthaburi, B. Bangkok, and C. Nakhon Pathom. The red boxes and red dots (left panels) represent areas and caging locations magnified to the figures in the middle panels, and the right panels, illustrating the actual scenes of the study sites. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3. Potentially infectious waste and environmental sampling
Samples of suspected viral contamination items such as gloves, masks, soil, and water were collected. Water samples of 50–100 mL were taken from nearby puddles, and approximately 20 g of soil (<5 cm depth), as well as some gloves or masks found at the points, was collected along line transects. All samples were transported under cool chain conditions, refrigerated after arrival, and tested for SARS-CoV-2 genome within 24 h.
2.4. Laboratory examinations
2.4.1. Virus culture and isolation from animal samples
African green monkey kidney (Vero) cells (CCL-81, American Type Culture Collection) were cultured in Eagle's minimum essential medium (EMEM) (Gibco, Grand Island, NY, USA) supplemented with 10 % heat-inactivated fetal bovine serum (FBS), 200 IU/mL penicillin, 200 μg/mL streptomycin, 75 μg/mL gentamicin sulfate, and 6 μg/mL amphotericin B. The growth of Vero cells was incubated at 37 °C with 5 % CO2.
Oral, nasal, or rectal swabs processed in viral transport media were inoculated onto monolayers of Vero cells for two hours and gently agitated at 37 °C. Subsequently, a fresh maintenance medium with 2 % FBS was added. For 5–7 days, the inoculated cells were incubated at 37 °C with 5 % CO2 [19]. The cells were observed daily for a cytopathic effect (CPE). Virus isolation was performed for three blind passages before concluding as negative if the CPE was not observed. All infection experiments were performed in a biosafety level 3 (BSL-3) laboratory.
2.4.2. Neutralizing antibody detection in animal samples
Two SARS-CoV-2 strains were used as the tested antigen in the microneutralization test (MNT) for neutralizing antibody detection, namely, (1) delta variant of hCoV-19/Thailand/Nan_SEQ7413/2021 (GISAID Accession no. EPI_ISL_3797061) and (2) Omicron BA.2 subvariant of hCoV-19/Thailand/NIC_BKK_SEQ4804/2022 (GISAID Accession no. EPI_ISL_9611330). These viruses were primarily isolated from an individual with COVID-19 and then propagated in Vero cells. The viral titers were determined by a 50 % tissue culture infectious dose (TCID50) assay.
All serum samples were subjected to the in-house MNT for SARS-CoV-2 neutralizing antibodies against the delta variant and omicron BA.2 subvariant. An equal volume (60 μL) of serial two-fold dilutions of heat-inactivated sera (56 °C, 30 min) and 100 TCID50 of SARS-CoV-2 were mixed. After 1 h of incubation at 37 °C, 100 μL of the serum–virus mixture was transferred onto Vero cell monolayers (2 × 104 cells/well) maintained in EMEM supplemented with 2 % FBS at 37 °C with 5 % CO2 for 3 days. The presence of a CPE in each sample was observed. The titer of a sample was recorded as the reciprocal of the highest serum dilution that provided at least 100 % neutralization of the tested virus, as determined by the presence of the CPE. Each serum sample was tested in duplicate in 96-well plates. The MNT was performed in a BSL-3 facility.
2.4.3. Indirect ELISA for SARS-CoV-2 antibody detection
All sera were tested for SARS-CoV-2 antibodies using a commercially species-independent test kit, ID Screen® SARS-CoV-2 Double Antigen Multispecies ELISA Kit (IDvet, Grabels, France), which detects the presence of IgG anti-SARS-CoV-2 N protein in the tested animal sera. The test was performed according to the manufacturer's instructions. Briefly, 25 μL of each sample and positive and negative control samples were diluted in 25 μL of a dilution buffer. The plate was incubated for 45 min at 37 °C and rinsed with a washing solution. A 100 μL of horseradish peroxidase-conjugated N protein recombinant antigen was added into each well, incubated for 30 min at 25 °C, and washed again. Then, 100 μL of the substrate solution was dispensed into each well, incubated for 20 min at 25 °C in a dark area, and then added with 100 μL of the stop solution. At 450 nm, the microplate was read using an 800 TS microplate reader (Biotek Instruments Inc., Winooski, VT, USA). The Optical Density of each sample was calculated as the S/P percentage (S/P%). A serum with an S/P% of >60 % was considered positive, 50 %–60 % was suspected, and < 50 % was negative.
2.5. RNA extraction
According to the manufacturer's instructions, total RNA was extracted from nasal and rectal swabs collected from animals foraging near waste disposal sites using the Total RNA Mini Kit (Geneaid Biotech Ltd., Taiwan).
For water and soil, RNA was extracted from 45 mL of water samples using the NaCl/PEG precipitation method, and RNA was extracted using a commercial kit (Total RNA Mini Kit; Geneaid Biotech Ltd.). A 45-mL water sample was centrifuged at 3000 ×g for 30 min at 4 °C to pellet bacteria, sediment, and large particles. The virus in clarified water samples was precipitated overnight by gentle agitation at 4 °C with 10 % polyethylene glycol 8000 and 2.25 % NaCl. The precipitated virus was recovered in a pellet by centrifugation at 15,000 ×g for 60 min at 4 °C [20]. Then, the pellet was resuspended in 400 μL of lysis buffer, and the RNA was extracted using a Total RNA Mini Kit (Geneaid Biotech Ltd., Taiwan). Similarly, up to 2 g of soil sample was used for RNA extraction using the RNeasy PowerSoil Total RNA Kit (QIAGEN, Germany) following the manufacturer's protocol.
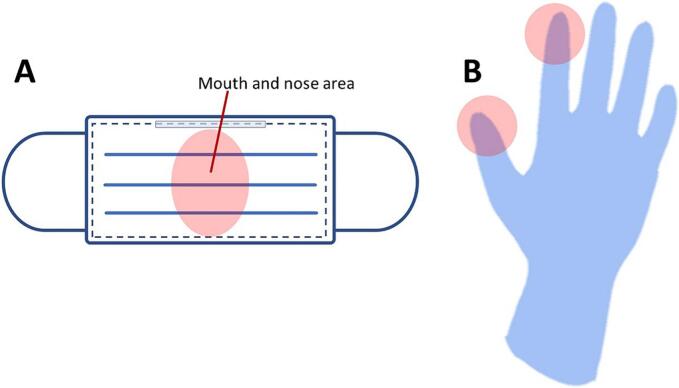
For masks and gloves, the RNA extraction method was modified from the procedure used for water samples [20]. The nose and mouth areas of the mask and areas of the thumb and index fingers were cut (Fig. 3) and soaked in 20 mL of phosphate-buffered saline, pH 7.5, overnight. The virus was precipitated using the PEG/NaCl method described earlier, and the RNA was extracted using a Total RNA Mini Kit (Geneaid Biotech Ltd.).
Fig. 3.
Areas on the mask and glove where the cut material was examined for RNA extraction (pink areas): A. mask and B. glove. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.6. RNA detection
Viral RNA was detected by real-time RT-PCR using a forward primer (5’ CGCATACAGTCTTRCAGGCT 3′), a reverse primer (5’ GTGTGATGTTGAWATGACATGGTC 3′), and probe (5’ FAM-TTAAGATGTGGTGCTTGCATACGTAGAC-lABkFQ 3′), and targeting RNA-dependent RNA polymerase/helicase gene (Chan et al., 2020). The real-time RT-PCR mixture contained 5 μL of the template RNA, 10 μL of the 2× Reaction Mix, 0.2 μM of each primer, 0.1 μM of the probe, 0.4 μL of the SuperScript™ III RT/Platinum™ Taq Mix (Invitrogen, USA), and 0.4 μL of ROX™ Reference Dye. Up to 20 μL of nuclease-free water was added. The PCR reactant was incubated at 50 °C for 15 min, 95 °C for 3 min, and followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 55 °C for 30 s. A cycle threshold (Ct) of <38 was considered positive.
3. Results
A total of 582 samples were collected from 238 animals, and house rats (Rattus tanezumi) were the most common (58.8 %), followed by Norway rats (Rattus norvegicus) (14.7 %) and Asian house shrews (Suncus murinus) (9.2 %). No samples tested positive for SARS-CoV-2 by real-time RT-PCR, MNT, and virus isolation (Table 1).
Table 1.
SARS-CoV-2 and serological detection in animal samples collected from garbage dumping sites.
| Animals (scientific name) | No. of animals | No. of samples collected | No. of positive sample/total tested |
|||
|---|---|---|---|---|---|---|
| Real-time RT-PCR | Virus isolation | MNT | Indirect ELISA | |||
| House rats (Rattus tanezumi) | 128 | 317 | 0/256 | 0/256 | 0/61 | 0/58 |
| Norway rats (Rattus norvegicus) | 35 | 87 | 0/70 | 0/70 | 0/17 | 0/7 |
| Asian house shrews (Suncus murinus) | 22 | 47 | 0/44 | 0/44 | 0/3 | 0/1 |
| Pacific rats (Rattus exulans) | 7 | 16 | 0/14 | 0/14 | 0/2 | 0/0 |
| Unidentified rats (Rattus spp.) | 26 | 62 | 0/52 | 0/52 | 0/10 | 1/8 |
| Cats (Felis catus) | 6 | 13 | 0/12 | 0/12 | 0/1 | 0/0 |
| Dogs (Canis familiaris) | 13 | 38 | 0/26 | 0/26 | 0/12 | 0/11 |
| Northern treeshrews (Tupaia belangeri) | 1 | 2 | 0/2 | 0/2 | 0/0 | 0/0 |
| Total | 238 | 582 | 0/476 | 0/476 | 0/106 | 1/85 |
Of the 85 serum samples collected, 1 (1.18 %) was ELISA-positive (S/P% of 253 %), and one serum sample (1.18 %) was inconclusive (S/P% of 56 %). The ELISA-positive sample was derived from an unidentified rat (Rattus spp.) at a garbage dumping site in Nonthaburi. Meanwhile, the sample suspected to be SARS-CoV-2 positive by ELISA was derived from a house rat (Rattus tanezumi) from the same location.
Of the 150 potentially infectious waste (face masks and gloves) and environmental samples (soil and water), two face mask samples (1.33 %, 2/150; 4.65 %, 2/43, for total and mask samples, respectively) collected from Nakhon Pathom tested positive for SARS-CoV-2 by real-time RT-PCR with Ct values of 36.57 and 37.12 (Table 2).
Table 2.
SARS-CoV-2 detection by real-time RT-PCR in potentially infectious waste and environmental samples collected from garbage dumping sites.
| Sample type | Number of positive samples by real-time RT-PCR (%) |
|---|---|
| Water | 0 /40 (0) |
| Soil | 0/50 (0) |
| Mask | 2/43 (4.65) |
| Glove | 0 /17 (0) |
| Total | 2 /150 (1.33) |
4. Discussion
This study explored the potential of small mammals inhabiting areas surrounding garbage dumping sites, where infectious wastes are disposed of, to carry SARS-CoV-2. In addition, the risk of environmental contamination from these sites was assessed. Although SARS-CoV-2 was not detected in the animal or environmental samples, genomic RNA was identified in some face mask samples. This finding suggests that face masks may harbor and retain the SARS-CoV-2 genome even after prolonged periods because of their proximity to the respiratory tract. A previous study reported that under the experimental condition of 37 °C, the virus would be undetectable after up to 2 days [21]. In Central Thailand, the average temperatures in September and October 2022 were 27.9 °C and 27.5 °C, respectively [22]. In such conditions, the virus likely remained in the mask and may stay viable for hours to days. Although some viral genomes may be deactivated or degraded by sunlight in outdoors, potentially resulting in low copies of the RNA genome of the virus, animals scavenging around garbage dumping sites may be exposed to the remaining virus in contaminated masks.
In this study, although no virus was isolated, immunological responses could be detected from a rat. This serological positivity indicated that the rat was previously exposed to the virus. A study conducted in New York City in the fall of 2021 also discovered rats serologically positive for SARS-CoV-2 [23]. By contrast, all rat samples tested negative serologically in other studies in Belgium [24] and Canada [25]. Nonetheless, the surveillance of coronaviruses in rats is still encouraged because rats are ubiquitous animals living closely with humans and may harbor different zoonotic pathogens, including unknown potentially zoonotic coronaviruses.
Nevertheless, the persistence of SARS-CoV-2 in garbage dumping sites is likely influenced by environmental factors, particularly the high temperature and humidity in Thailand. These conditions may limit the viability of the virus, reducing the likelihood of long-term survival and potential transmission to animals. Even if the virus remains viable on discarded materials such as face masks, direct contact with animals may be limited by the perceived food source value of these items. The more probable route of animal exposure would be through contaminated soil or water, where viral concentrations may be diluted significantly, potentially diminishing the infectious risk for small mammals. In addition, the captured small mammals do not appear to be primary reservoirs of SARS-CoV-2 [26].
However, as our capturing methods presumably trapped only healthy animals, the absence of clinical signs in the captured animals does not preclude the susceptibility of these species to SARS-CoV-2. To further investigate the potential transmission of SARS-CoV-2 through small mammals, future studies should focus on trapping individuals in urban areas where they may have more direct contact with freshly contaminated materials. A network analysis of garbage truck movement could also provide valuable insights into the routes and potential sources of infectious wastes reaching garbage dumping sites. This information could be instrumental in identifying and monitoring high-risk areas in future outbreaks. Although this study did not assess waste pickers working at garbage dumping sites, they should be considered at high risk for SARS-CoV-2 because of their close contact with potentially contaminated materials [27]. Expanding the scope of future studies by including waste pickers could provide valuable data on their exposure risks and potential role in disease transmission. Beyond SARS-CoV-2, the role of small mammals as reservoirs for other infectious diseases prevalent in garbage disposal sites, such as leptospirosis, must be also explored [28].
This study has some potential limitations. First, only three dumping sites in Bangkok and its vicinity were surveyed. A future study may include more study locations across Thailand. However, the COVID-19 pandemic is now over. The likelihood of detecting the virus is lower than that in this study. Second, the virus from the face mask samples was not cultured, resulting in the lack of information regarding the infectivity of the virus detected by real-time RT-PCR.
5. Conclusions
The detection of SARS-CoV-2 viral particles in contaminated protective gear, such as face masks, highlights the critical need for proper waste segregation in households and communities. Potentially infectious waste streams should be handled according to established guidelines, including appropriate segregation, collection, and disposal. Establishing designated disposal sites throughout the city and implementing incineration rather than dumping would eliminate the risk of environmental contamination and potential transmission. Moreover, to minimize exposure and prevent onward transmission, waste management personnel, including garbage dump staff and waste pickers, should be equipped with appropriate PPE and receive regular training on safe handling and disposal.
Ethics statement
The use of animals in the study was approved by the Institutional Animal Care and Use Committee, Faculty of Veterinary Science, Mahidol University (MUVS-2002-03-09). The use of biological materials was approved by the Mahidol University Biosafety Committee (Approval no. MU 2022-008). No human subjects were included in this study.
Funding
This study was funded by Health Security Partners, U.S. CDC, and the Thailand MoPH-U.S. CDC Collaboration under the project “Assessing the likelihood of SARS-CoV-2 environmental contamination: cases of exotic pets and animals scavenging at garbage dumping sites in Bangkok Metropolitan and its suburbs.”
Author contributions
Conceptualization and methodology: AW, SS, and NS. Research design: AW, SS, and NS. Investigation and data collection: AW, SS, TC, PW, KT, and NS. Data analysis and interpretation: AW, SS, LS, WP, ST, BB, and NS. Discussion of the results: AW, SS, WS, WW, and NS. Original draft preparation: AW and NS. Reviewing and editing: AW, LS, WP, SS, TC, ST, BB, PW, KT, WS, WW, and NS. Project administration: SS and WS. All authors have read and agreed to the published version of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention.
Declaration of generative AI in scientific writing
During the preparation of this work, the authors used Gemini to enhance the readability and clarity of the manuscript. The AI assistance was limited to language editing and did not contribute to the research design, data analysis, interpretation, or authorship of the content. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
CRediT authorship contribution statement
Anuwat Wiratsudakul: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ladawan Sariya: Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation, Formal analysis. Weena Paungpin: Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation, Formal analysis. Sarin Suwanpakdee: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Tatiyanuch Chamsai: Writing – review & editing, Writing – original draft, Visualization, Resources, Investigation, Data curation. Siriporn Tangsudjai: Writing – review & editing, Resources, Methodology, Investigation. Benjaporn Bhusri: Writing – review & editing, Resources, Investigation. Peerawat Wongluechai: Writing – review & editing, Resources, Investigation, Data curation. Kanittha Tonchiangsai: Writing – review & editing, Resources, Investigation, Data curation. Walasinee Sakcamduang: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Witthawat Wiriyarat: Writing – review & editing, Supervision, Methodology, Conceptualization. Nareerat Sangkachai: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that the research was conducted without commercial or financial relationships that could create a conflict of interest.
Acknowledgments
SARS-CoV-2 strains were kindly provided by the National Institute of Health, Department of Medical Sciences, Thailand, through Dr. Pilailuk Akkapaiboon Okada. We thank the management of the three dump sites in Bangkok, Nonthaburi, and Nakhon Pathom.
Data availability
Raw data supporting the conclusions of this study will be made available by the authors without undue reservation.
References
- 1.Na Zhu, Dingyu Zhang, Wenling Wang, Xingwang Li, Bo Yang, Jingdong Song, Xiang Zhao, Baoying Huang, Weifeng Shi, Roujian Lu, Peihua Niu, Faxian Zhan, Xuejun Ma, Dayan Wang, Wenbo Xu, Guizhen Wu, Gao George F., Wenjie Tan. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO COVID-19 Epidemiological Update – 15 March 2024. 2024. https://www.who.int/publications/m/item/covid-19-epidemiological-update-15-march-2024 (accessed April 5, 2024)
- 4.Fan Y.V., Jiang P., Hemzal M., Klemeš J.J. An update of COVID-19 influence on waste management. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangkham S. Face mask and medical waste disposal during the novel COVID-19 pandemic in Asia. Case Stud. Chem. Environ. Eng. 2020;2 doi: 10.1016/j.cscee.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, greater Paris, France, 5 March to 23 April 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Bonneau R., Brown M.A., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., Matus M., McElroy K.A., Nagler J., Rhode S.F., Santillana M., Tucker J.A., Wuertz S., Zhao S., Thompson J., Alm E.J. SARS-CoV-2 RNA concentrations in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrett N., Tapley A., Andriesen J., Seocharan I., Fisher L.H., Bunts L., Espy N., Wallis C.L., Randhawa A.K., Ketter N., Yacovone M., Goga A., Bekker L.-G., Gray G.E., Corey L. 2022. High Rate of Asymptomatic Carriage Associated with Variant Strain Omicron. 2021.12.20.21268130. [DOI] [Google Scholar]
- 11.Nzediegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020;161 doi: 10.1016/j.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krystosik A., Njoroge G., Odhiambo L., Forsyth J.E., Mutuku F., LaBeaud A.D. Solid wastes provide breeding sites, burrows, and food for biological disease vectors, and urban zoonotic reservoirs: a call to action for solutions-based research. Front. Public Health. 2020;7 doi: 10.3389/fpubh.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray M.H., Fidino M., Fyffe R., Byers K.A., Pettengill J.B., Sondgeroth K.S., Killion H., Magle S.B., Rios M.J., Ortinau N., Santymire R.M. City sanitation and socioeconomics predict rat zoonotic infection across diverse neighbourhoods. Zoonoses Public Health. 2020;67:673–683. doi: 10.1111/zph.12748. [DOI] [PubMed] [Google Scholar]
- 14.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WOAH SARS-CoV-2, WOAH - World Organisation for Animal Health. 2024. https://www.woah.org/en/disease/sars-cov-2/ (accessed April 5, 2024)
- 18.Francis C. 1st edition. New Holland; London: 2008. A Field Guide to the Mammals of South-East Asia. [Google Scholar]
- 19.McAloose D., Laverack M., Wang L., Killian M.L., Caserta L.C., Yuan F., Mitchell P.K., Queen K., Mauldin M.R., Cronk B.D., Bartlett S.L., Sykes J.M., Zec S., Stokol T., Ingerman K., Delaney M.A., Fredrickson R., Ivančić M., Jenkins-Moore M., Mozingo K., Franzen K., Bergeson N.H., Goodman L., Wang H., Fang Y., Olmstead C., McCann C., Thomas P., Goodrich E., Elvinger F., Smith D.C., Tong S., Slavinski S., Calle P.P., Terio K., Torchetti M.K., Diel D.G. From people to panthera: natural SARS-CoV-2 infection in tigers and lions at the bronx zoo. mBio. 2020;11 doi: 10.1128/mbio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farkas K., Hillary L.S., Thorpe J., Walker D.I., Lowther J.A., McDonald J.E., Malham S.K., Jones D.L. Concentration and quantification of SARS-CoV-2 RNA in wastewater using polyethylene glycol-based concentration and qRT-PCR. Methods Protoc. 2021;4:17. doi: 10.3390/mps4010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwok C.S., Dashti M., Tafuro J., Nasiri M., Muntean E.-A., Wong N., Kemp T., Hills G., Mallen C.D. Methods to disinfect and decontaminate SARS-CoV-2: a systematic review of in vitro studies. Ther. Adv. Infect. 2021;8 doi: 10.1177/2049936121998548. 2049936121998548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thailand Meteorological Department Weather condiitions in Thailand, 2022. 2023. http://climate.tmd.go.th/content/category/17
- 23.Wang Y., Lenoch J., Kohler D., DeLiberto T.J., Tang C.Y., Li T., Tao Y.J., Guan M., Compton S., Zeiss C., Hang J., Wan X.-F. SARS-CoV-2 exposure in Norway rats (Rattus norvegicus) from New York city. mBio. 2023;14:e03621–e03622. doi: 10.1128/mbio.03621-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo V.C., Sluydts V., Mariën J., Vanden Broecke B., Van Houtte N., Leirs W., Jacobs L., Iserbyt A., Hubert M., Heyndrickx L., Goris H., Delputte P., De Roeck N., Elst J., Ariën K.K., Leirs H., Gryseels S. SARS-CoV-2 surveillance in Norway rats (Rattus norvegicus) from Antwerp sewer system, Belgium. Transbound. Emerg. Dis. 2022;69:3016–3021. doi: 10.1111/tbed.14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson S.J., Kotwa J.D., Jeeves S.P., Himsworth C.G., Pearl D.L., Weese J.S., Lindsay L.R., Dibernardo A., Toledo N.P.L., Pickering B.S., Goolia M., Chee H.-Y., Blais-Savoie J., Chien E., Yim W., Yip L., Mubareka S., Jardine C.M. Surveillance for SARS-CoV-2 in Norway rats (Rattus norvegicus) from southern Ontario. Transbound. Emerg. Dis. 2023;2023 doi: 10.1155/2023/7631611. [DOI] [Google Scholar]
- 26.Sharun K., Dhama K., Pawde A.M., Gortázar C., Tiwari R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., de la Fuente J., Michalak I., Attia Y.A. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet. Q. 2021;41:181–201. doi: 10.1080/01652176.2021.1921311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazemi Moghaddam V., Walker T.R., Pakdel M., Ahmadinejad P., Mohammadi A.A. Waste workers and pickers: neglected highrisk groups in developing countries during the COVID-19 pandemic. J. Health Sci. Surveill. Syst. 2023;11:252–259. doi: 10.30476/jhsss.2021.93040.1410. [DOI] [Google Scholar]
- 28.Mohd-Taib F.S., Ishak S.N., Yusof M.A., Azhari N.N., Md-Lasim A., Md Nor S., Mohd-Sah S.A., Neela V.K. Leptospirosis: an insight into community structure of small mammal’s host in urban environment. Trop. Biomed. 2020;37:142–154. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data supporting the conclusions of this study will be made available by the authors without undue reservation.