Abstract
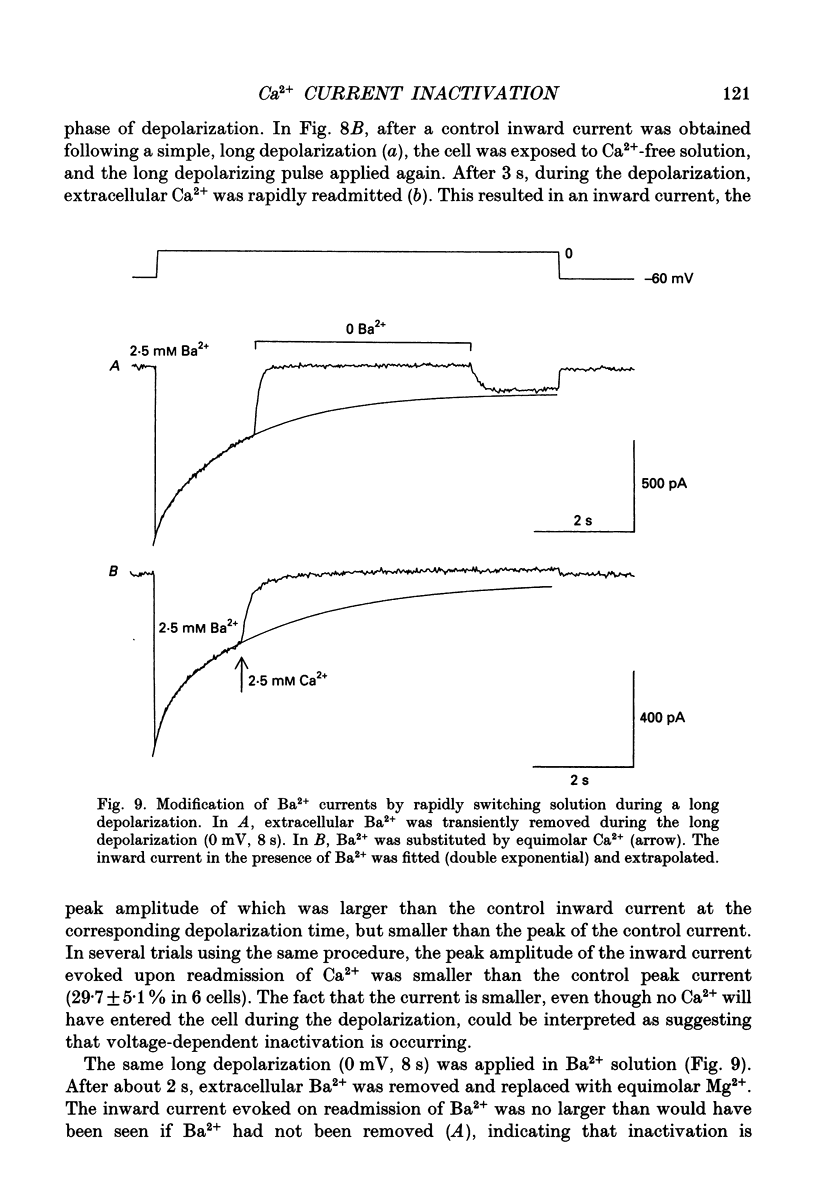
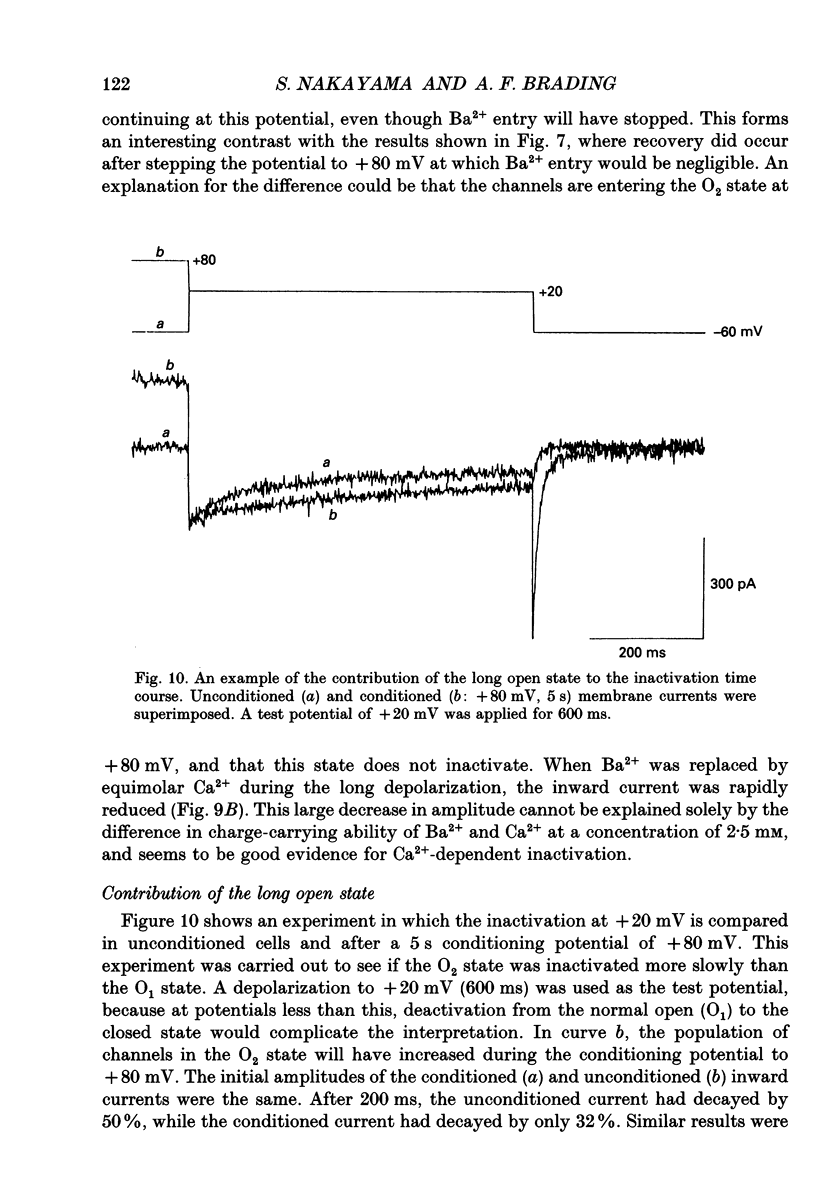
1. Whole-cell voltage clamp techniques were applied to single smooth muscle cells enzymatically dissociated from guinea-pig urinary bladder. The inactivation and recovery of voltage-dependent Ca2+ channel currents were examined by manipulating the membrane potential over a wide range and by changing the extracellular divalent cation concentrations. 2. After exposing the cells to conditioning potentials (-100 to +80 mV in 20 mV increments), the degree of inactivation was estimated by stepping to a 0 mV test potential. In the presence of 2.5 mM Ca2+, the inactivation of the current was U-shaped with respect to the conditioning potential, with maximum inactivation at 0 mV. The maximal inactivation was 60 and 90% after conditioning durations of 0.8 and 5 s, respectively. The U-shaped curve is characteristic of Ca(2+)-dependent inactivation. When conditioning potentials of +80 mV with either duration were applied, the inward current at the test potential and the subsequent tail current on returning to the holding potential were larger than in control conditions (when the conditioning potential = the holding potential, -60 mV). 3. A U-shaped inactivation curve was also observed in the presence of 2.5 mM Ba2+. The inactivation was maximal with a conditioning potential of about -20 mV, and the inactivation was smaller than seen with Ca2+ entry. 4. Paired-pulse protocols were applied to examine the voltage dependence of recovery of the Ca2+ inward current. After the inward current had been inactivated during a 100 ms depolarization at 0 mV, it took 700 ms at -60 mV for nearly complete recovery of the current. Recovery was also observed at +80 mV. When the potential of the paired pulses was increased to +20 mV, less recovery was seen when the interpulse potential was at +80 mV. When a longer (3 s) depolarization was applied, the peak amplitude of the inward current took much longer to recover, and had not completely recovered after 4 s at either of the interpulse potentials, although recovery was greater with an interpulse potential of -60 mV than with one of +80 mV. Similar recoveries were observed in the presence of Ba2+. 5. During a long depolarization (8 s, 0 mV), the effects of rapid changes in the extracellular solution were examined. Partial recovery of the inward current occurred after a period in which Ca2+ was replaced with Mg2+. This recovery was not observed in the presence of Ba2+.(ABSTRACT TRUNCATED AT 400 WORDS)
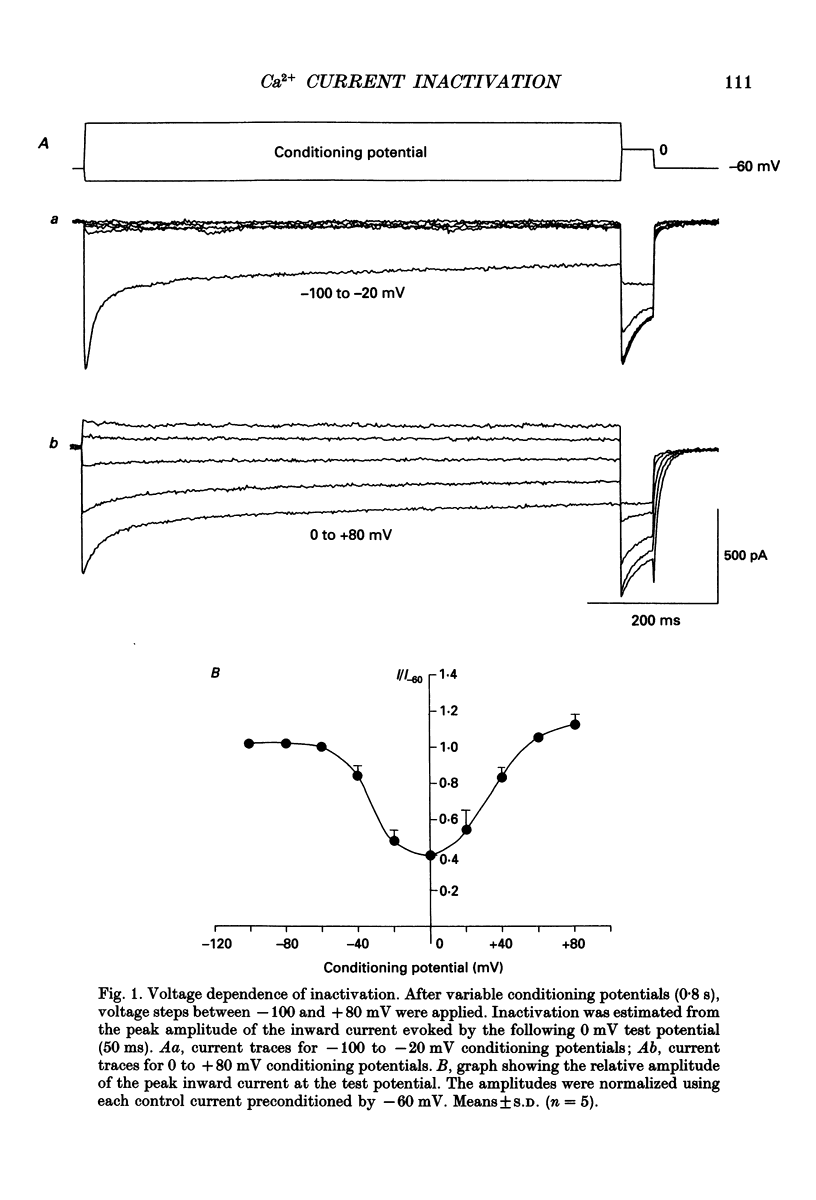
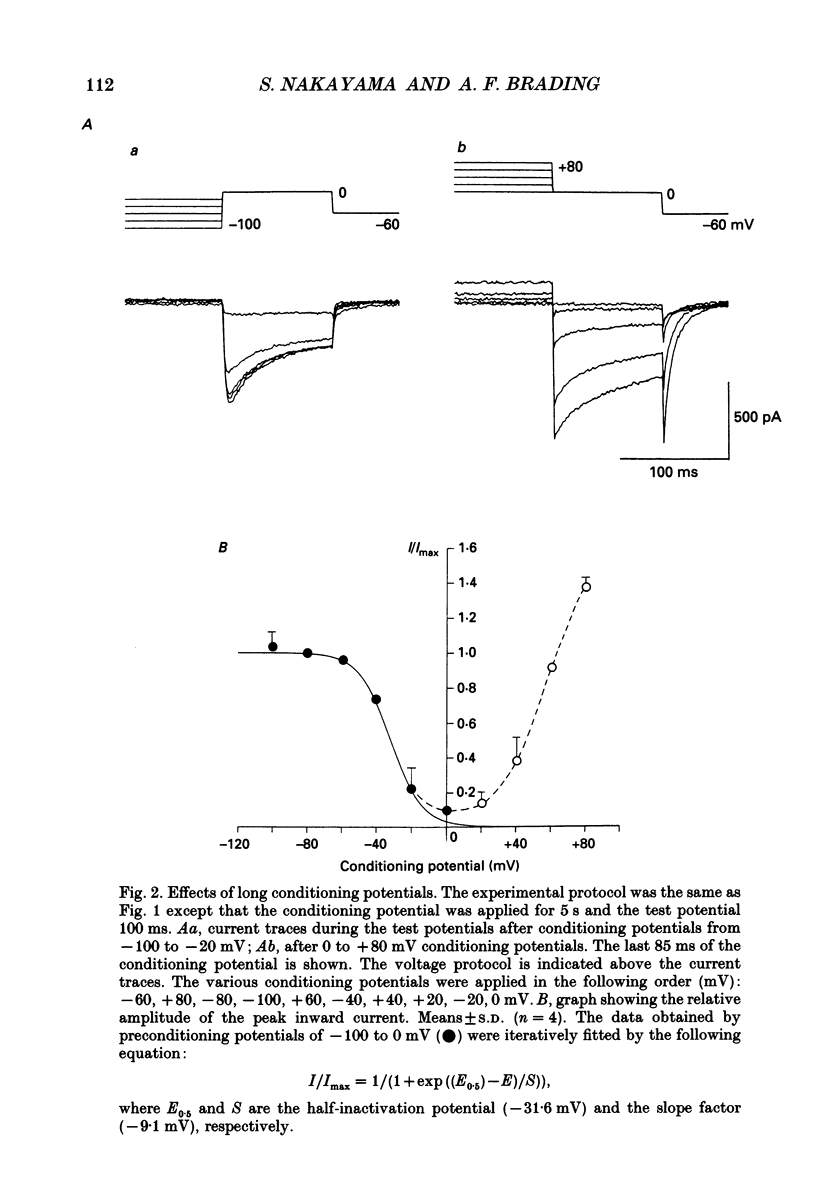
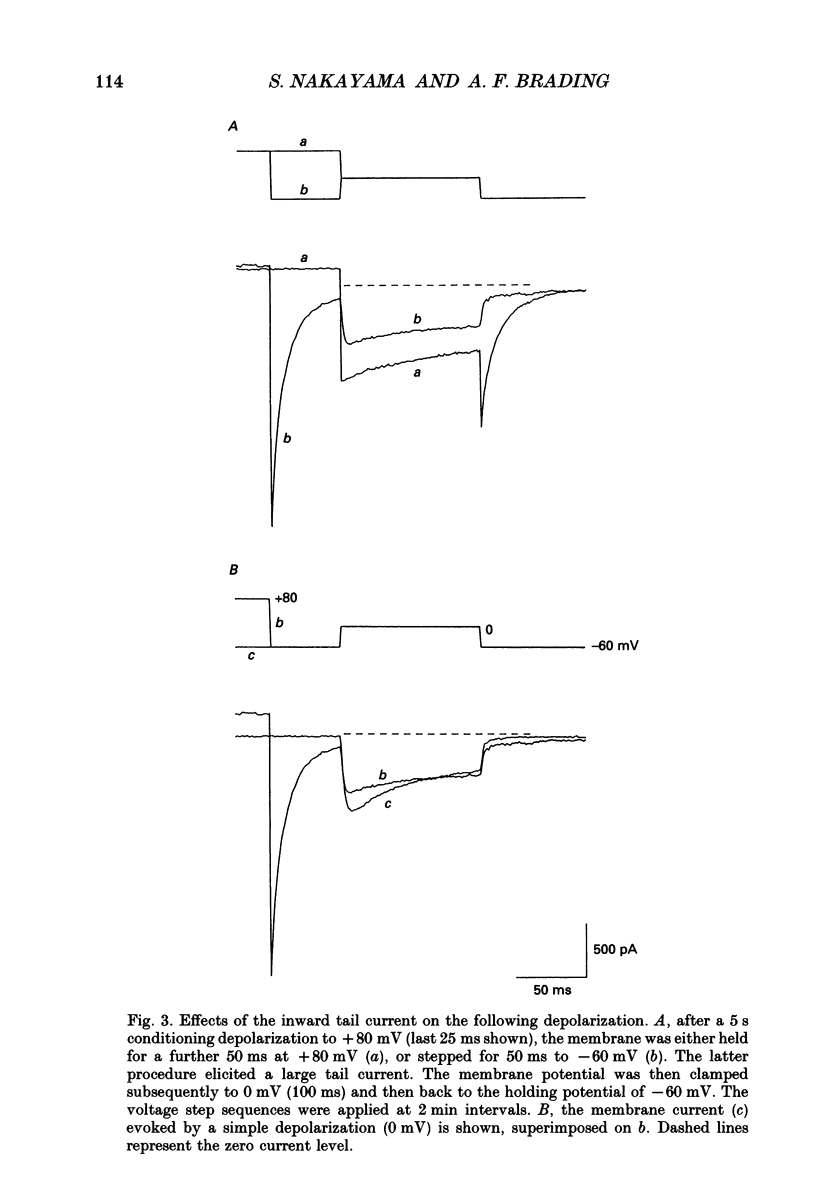
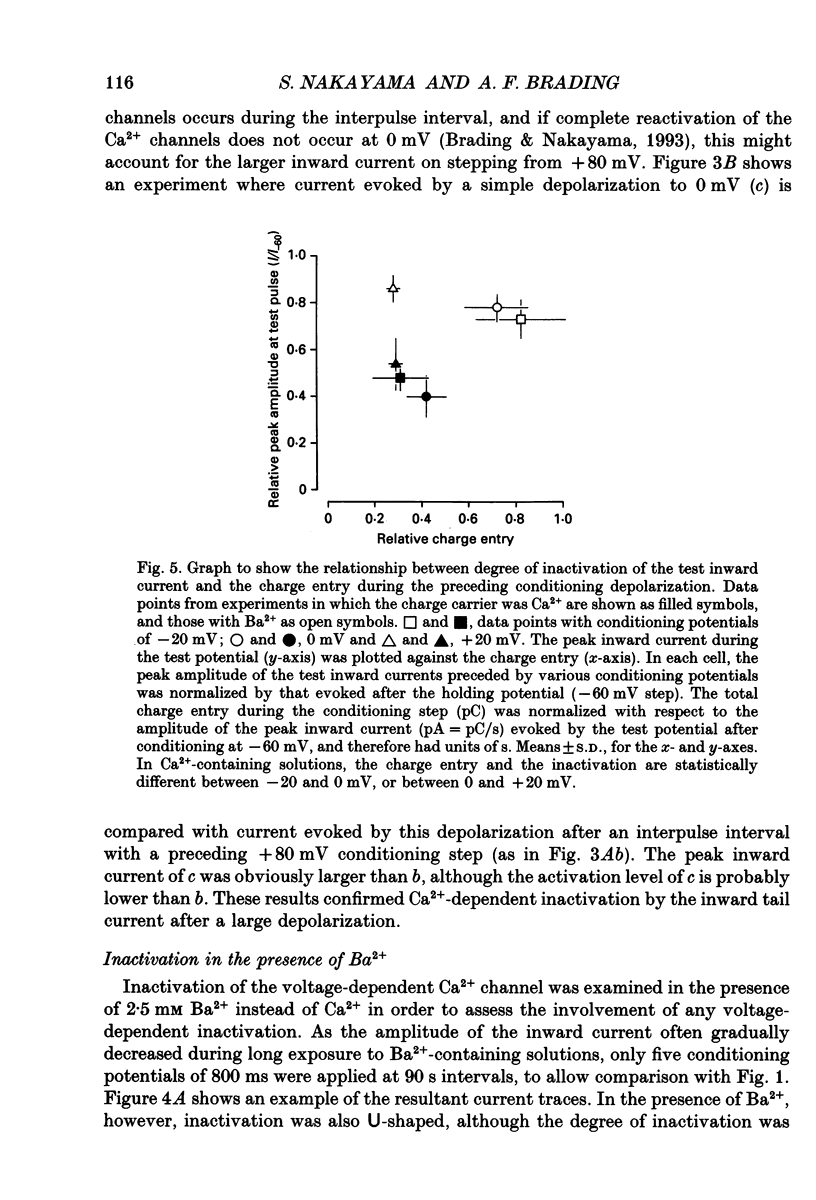
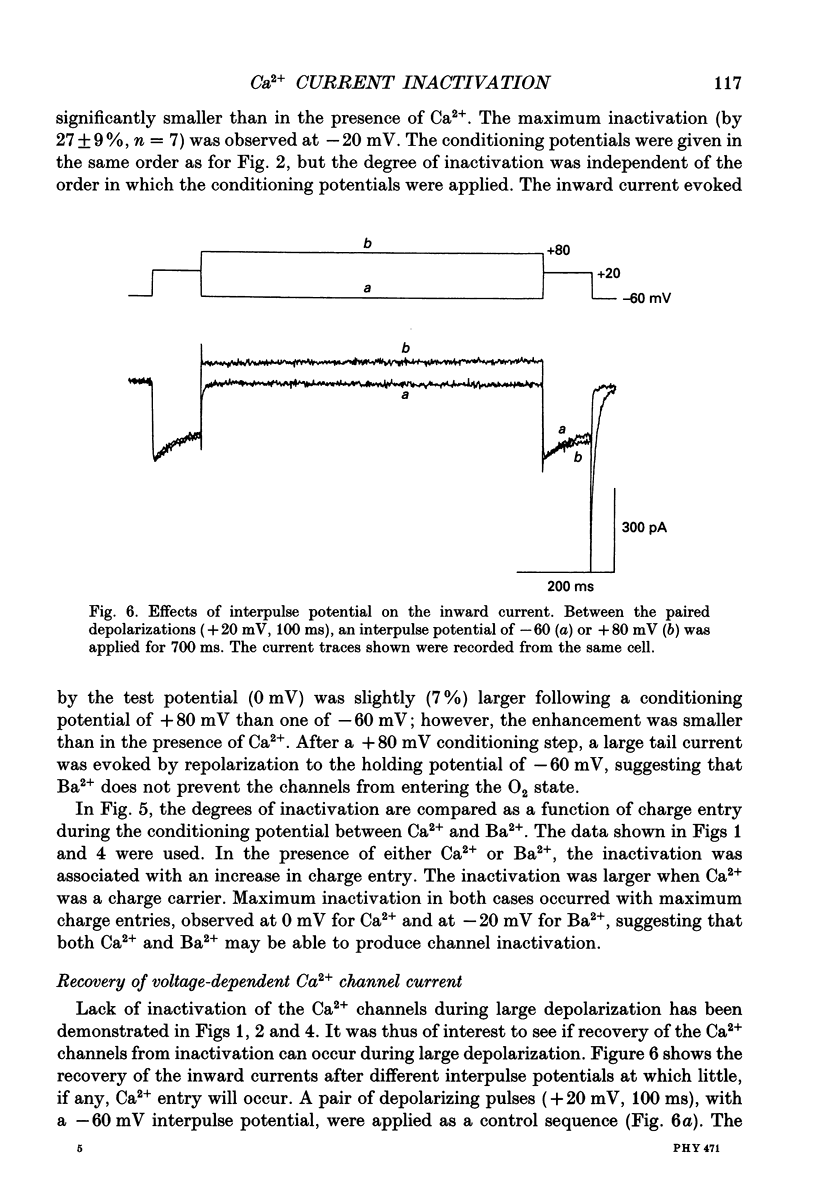
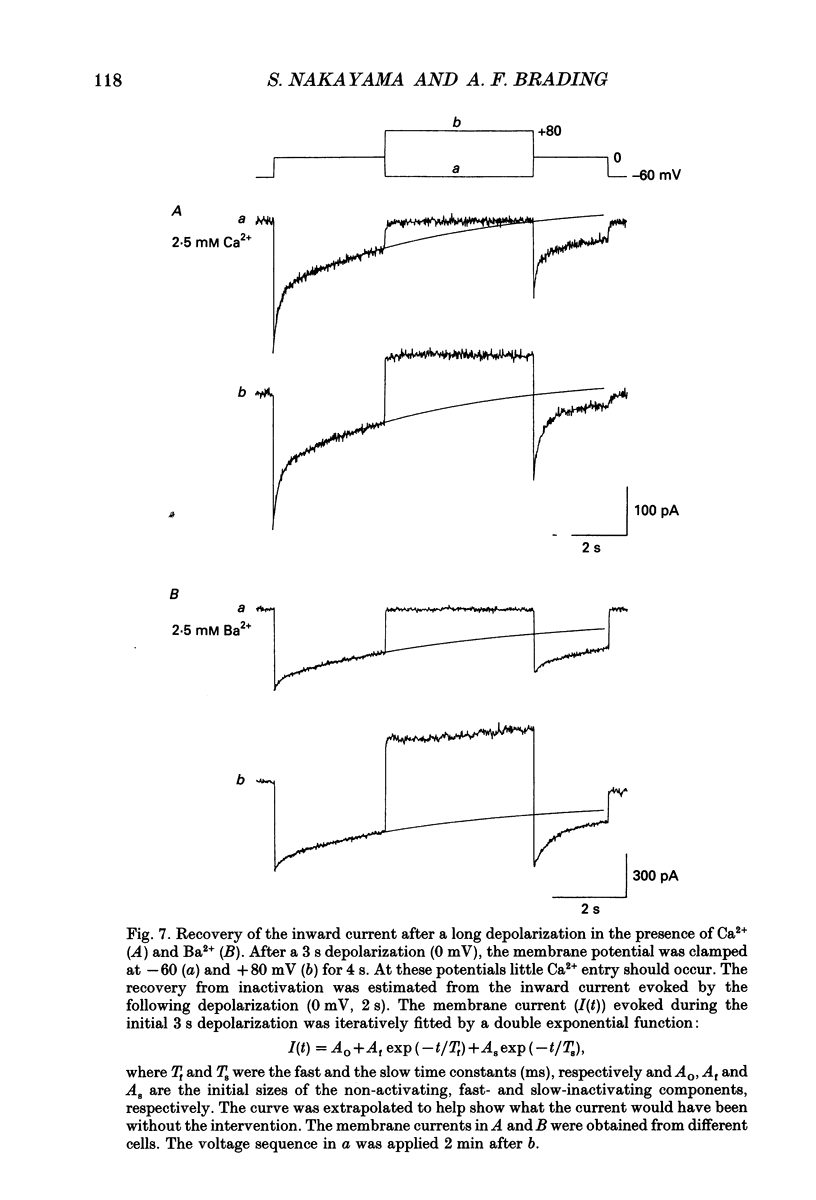
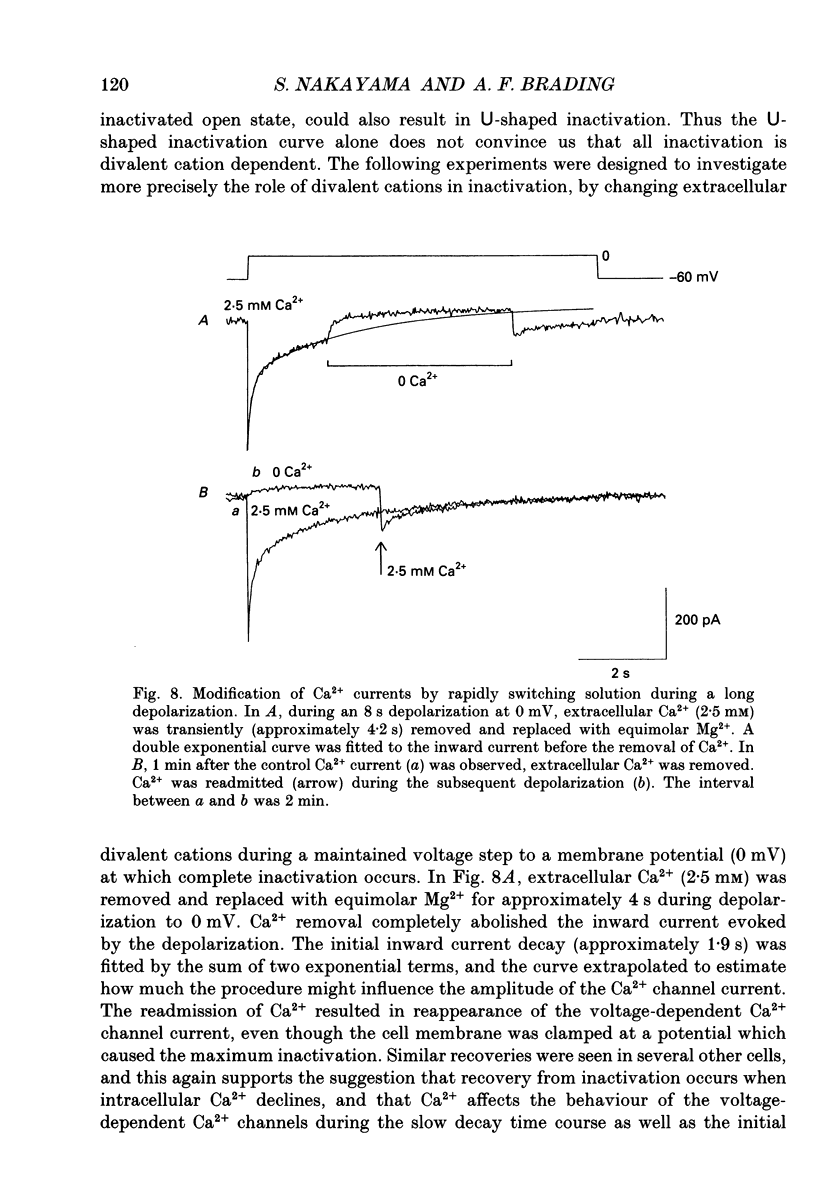
Full text
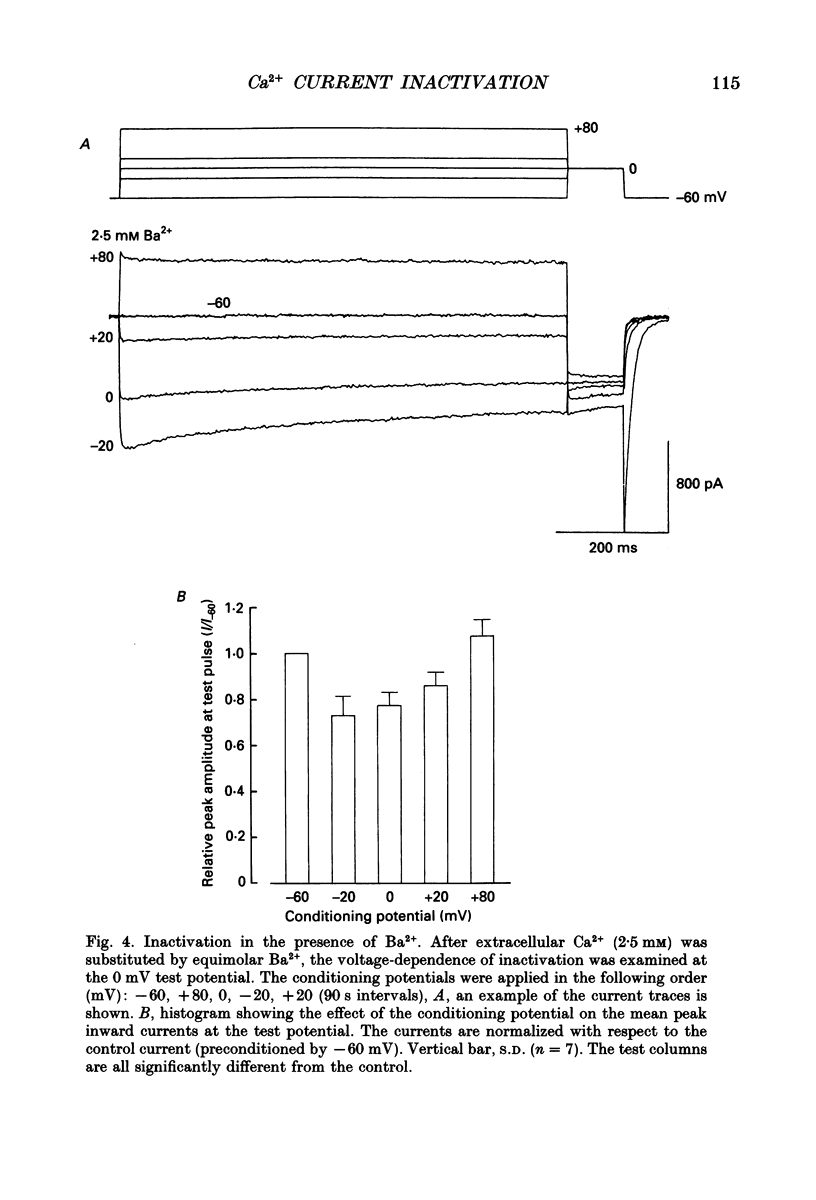
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P. I., Bolton T. B., Lang R. J., MacKenzie I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal-calcium and high-barium solutions. J Physiol. 1988 Nov;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V Y. a., Isenberg G. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. J Physiol. 1991 Apr;435:187–205. doi: 10.1113/jphysiol.1991.sp018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987 Nov;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Potential-dependent calcium inward current in a single isolated smooth muscle cell of the guinea-pig taenia caeci. J Physiol. 1986 Nov;380:1–16. doi: 10.1113/jphysiol.1986.sp016268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Hume J. R. An intrinsic potential-dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. J Physiol. 1987 Aug;389:205–222. doi: 10.1113/jphysiol.1987.sp016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991 Dec;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R. Effect of external Cd2+ and other divalent cations on carbachol-activated non-selective cation channels in guinea-pig ileum. J Physiol. 1991 Oct;442:447–463. doi: 10.1113/jphysiol.1991.sp018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jmari K., Mironneau C., Mironneau J. Inactivation of calcium channel current in rat uterine smooth muscle: evidence for calcium- and voltage-mediated mechanisms. J Physiol. 1986 Nov;380:111–126. doi: 10.1113/jphysiol.1986.sp016275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jmari K., Mironneau C., Mironneau J. Selectivity of calcium channels in rat uterine smooth muscle: interactions between sodium, calcium and barium ions. J Physiol. 1987 Mar;384:247–261. doi: 10.1113/jphysiol.1987.sp016453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Lang R. J. The whole-cell Ca2+ channel current in single smooth muscle cells of the guinea-pig ureter. J Physiol. 1990 Apr;423:453–473. doi: 10.1113/jphysiol.1990.sp018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt F., Nilius B. Modulation of calcium channel currents in guinea-pig single ventricular heart cells by the dihydropyridine Bay K 8644. J Physiol. 1988 May;399:559–575. doi: 10.1113/jphysiol.1988.sp017096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J. J., Volk K. A., Shibata E. F. Calcium currents in isolated rabbit coronary arterial smooth muscle myocytes. J Physiol. 1990 Aug;427:657–680. doi: 10.1113/jphysiol.1990.sp018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Brading A. F. Evidence for multiple open states of the Ca2+ channels in smooth muscle cells isolated from the guinea-pig detrusor. J Physiol. 1993 Nov;471:87–105. doi: 10.1113/jphysiol.1993.sp019892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1988 Feb;62(2):375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Pelzer S., McDonald T. F. Properties and regulation of calcium channels in muscle cells. Rev Physiol Biochem Pharmacol. 1990;114:107–207. doi: 10.1007/BFb0031019. [DOI] [PubMed] [Google Scholar]
- Sham J. S., Cleemann L., Morad M. Gating of the cardiac Ca2+ release channel: the role of Na+ current and Na(+)-Ca2+ exchange. Science. 1992 Feb 14;255(5046):850–853. doi: 10.1126/science.1311127. [DOI] [PubMed] [Google Scholar]
- Smirnov S. V., Aaronson P. I. Ca2+ currents in single myocytes from human mesenteric arteries: evidence for a physiological role of L-type channels. J Physiol. 1992 Nov;457:455–475. doi: 10.1113/jphysiol.1992.sp019387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F., Publicover N. G., Sanders K. M. Regulation of calcium current by voltage and cytoplasmic calcium in canine gastric smooth muscle. Am J Physiol. 1992 Mar;262(3 Pt 1):C691–C700. doi: 10.1152/ajpcell.1992.262.3.C691. [DOI] [PubMed] [Google Scholar]


